Microporosity and CO2 Capture Properties of Amorphous Silicon Oxynitride Derived from Novel Polyalkoxysilsesquiazanes
Abstract
:1. Introduction
2. Experimental Section
2.1. Precursor Synthesis
2.2. Pyrolysis and Heat Treatment
2.3. Characterizations
3. Results and Discussions
3.1. ROSZ Preceramic Polymers
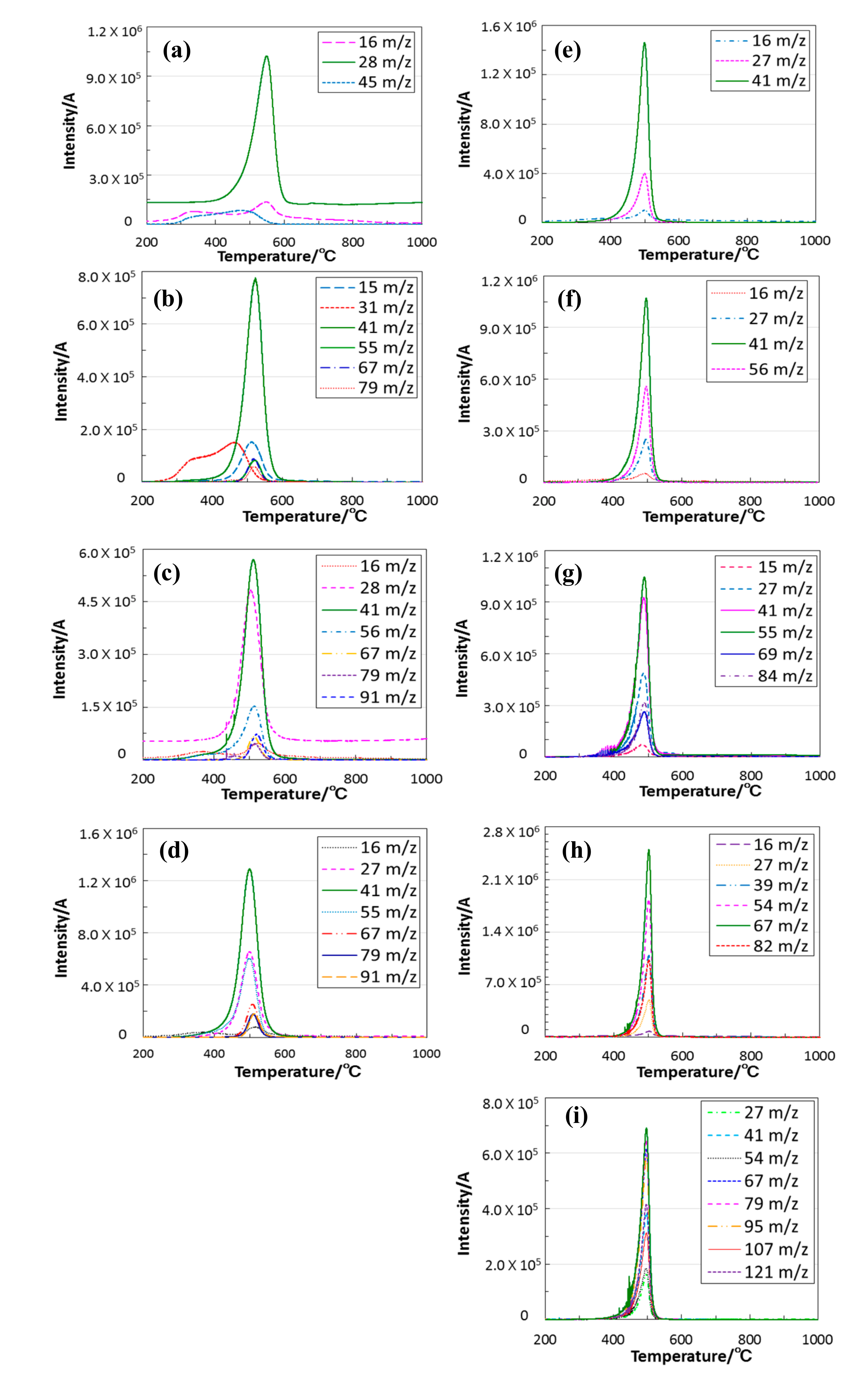
3.2. Thermal Conversion to Inorganic Compound
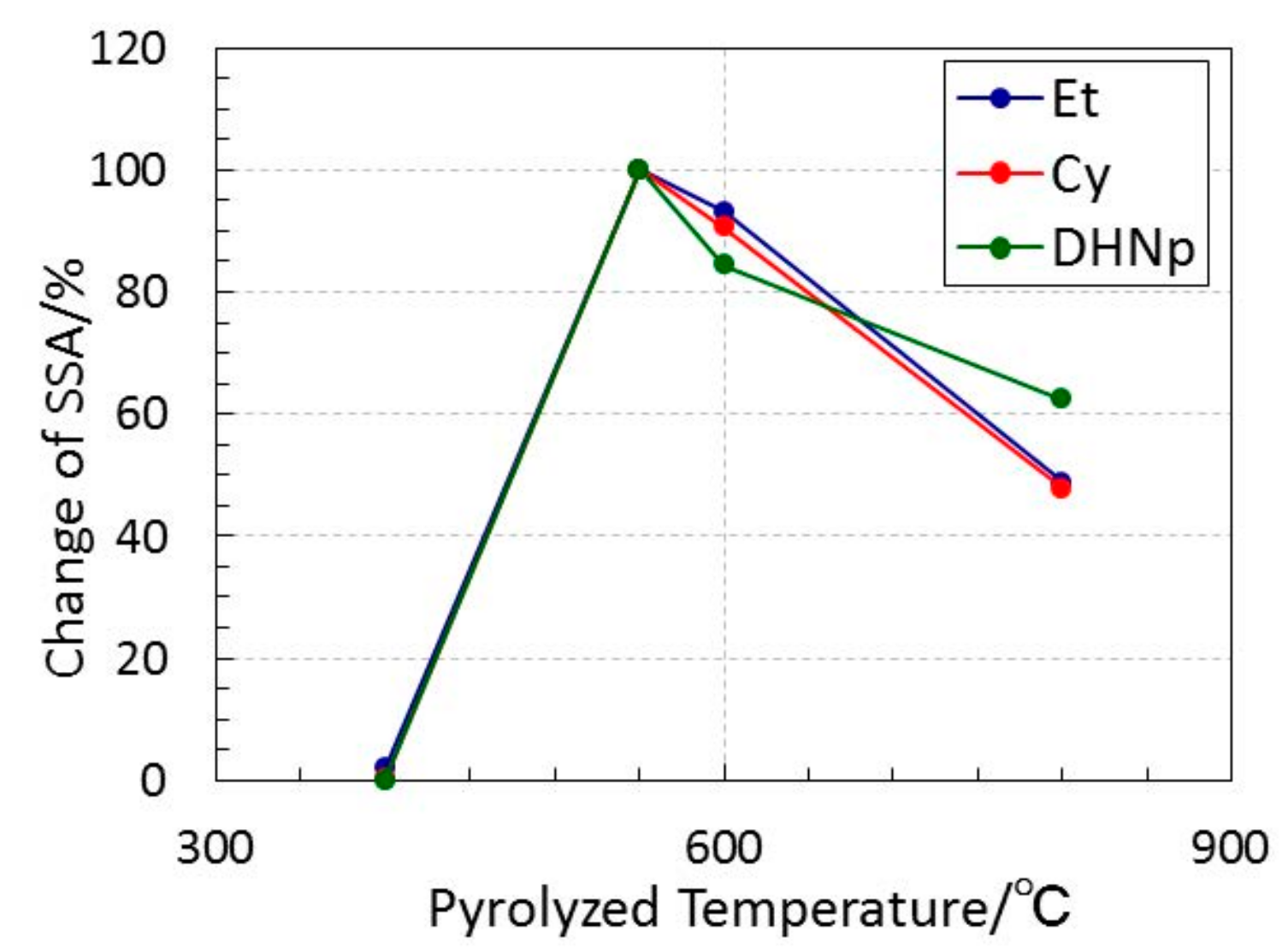
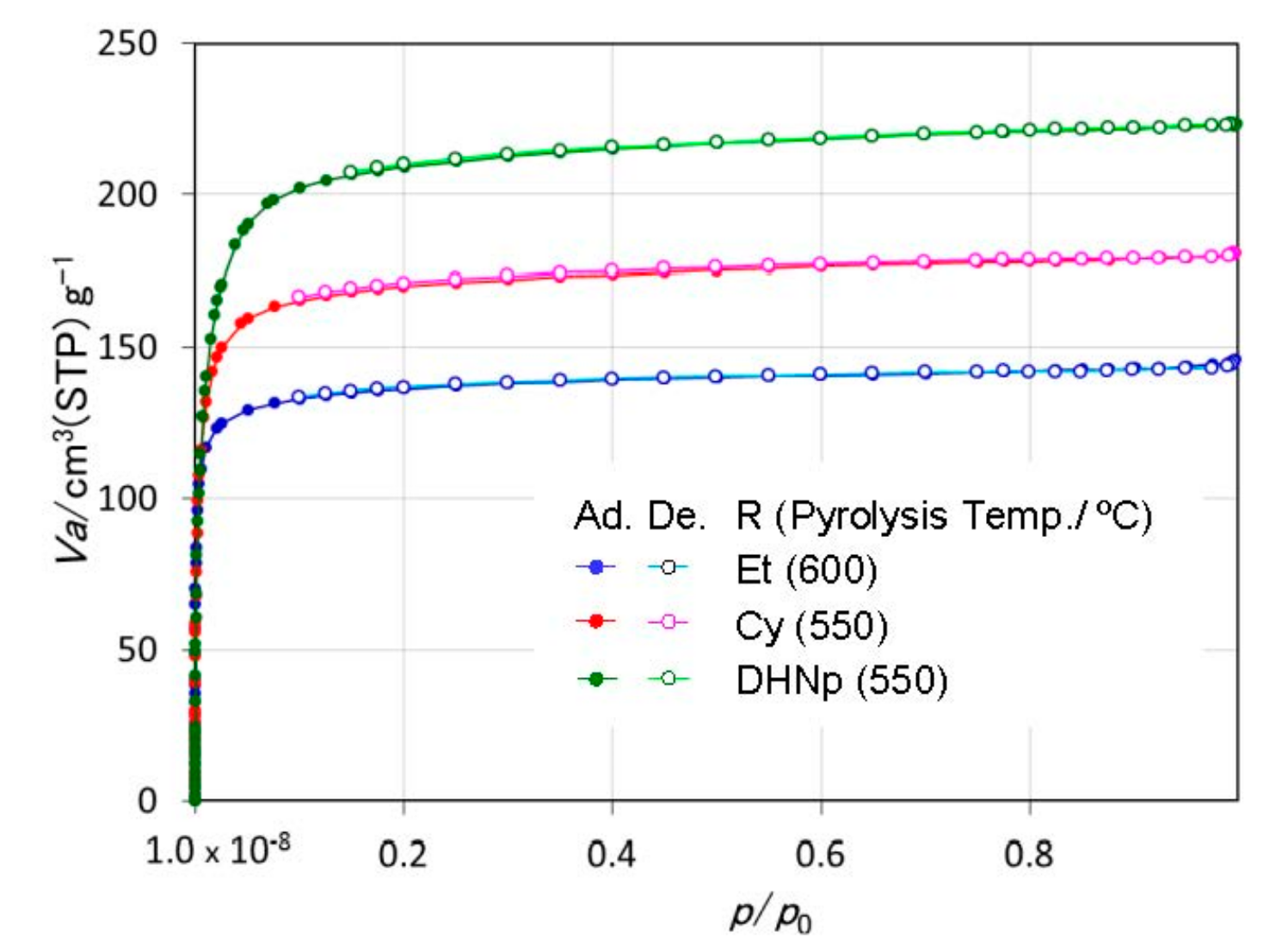
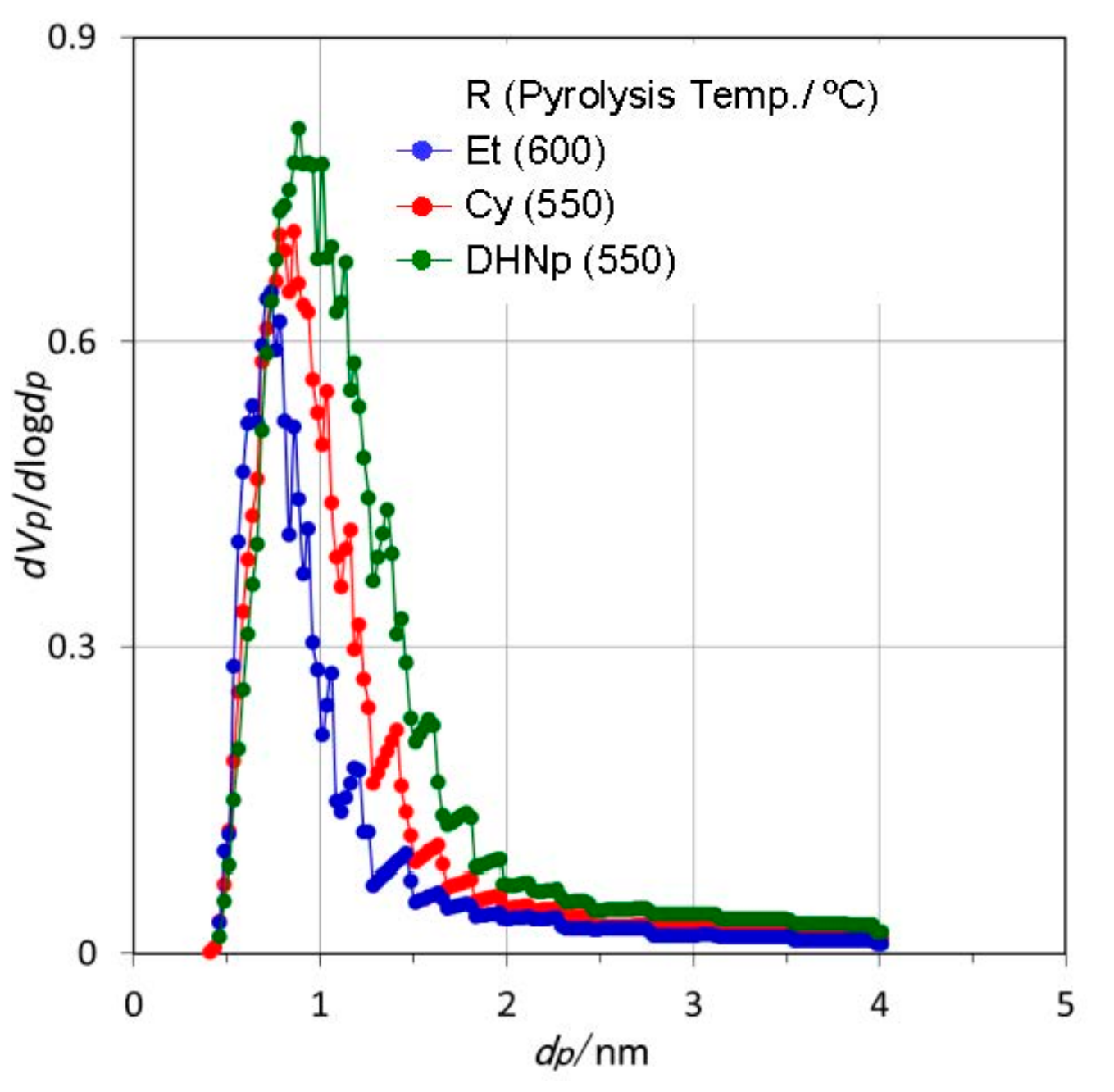
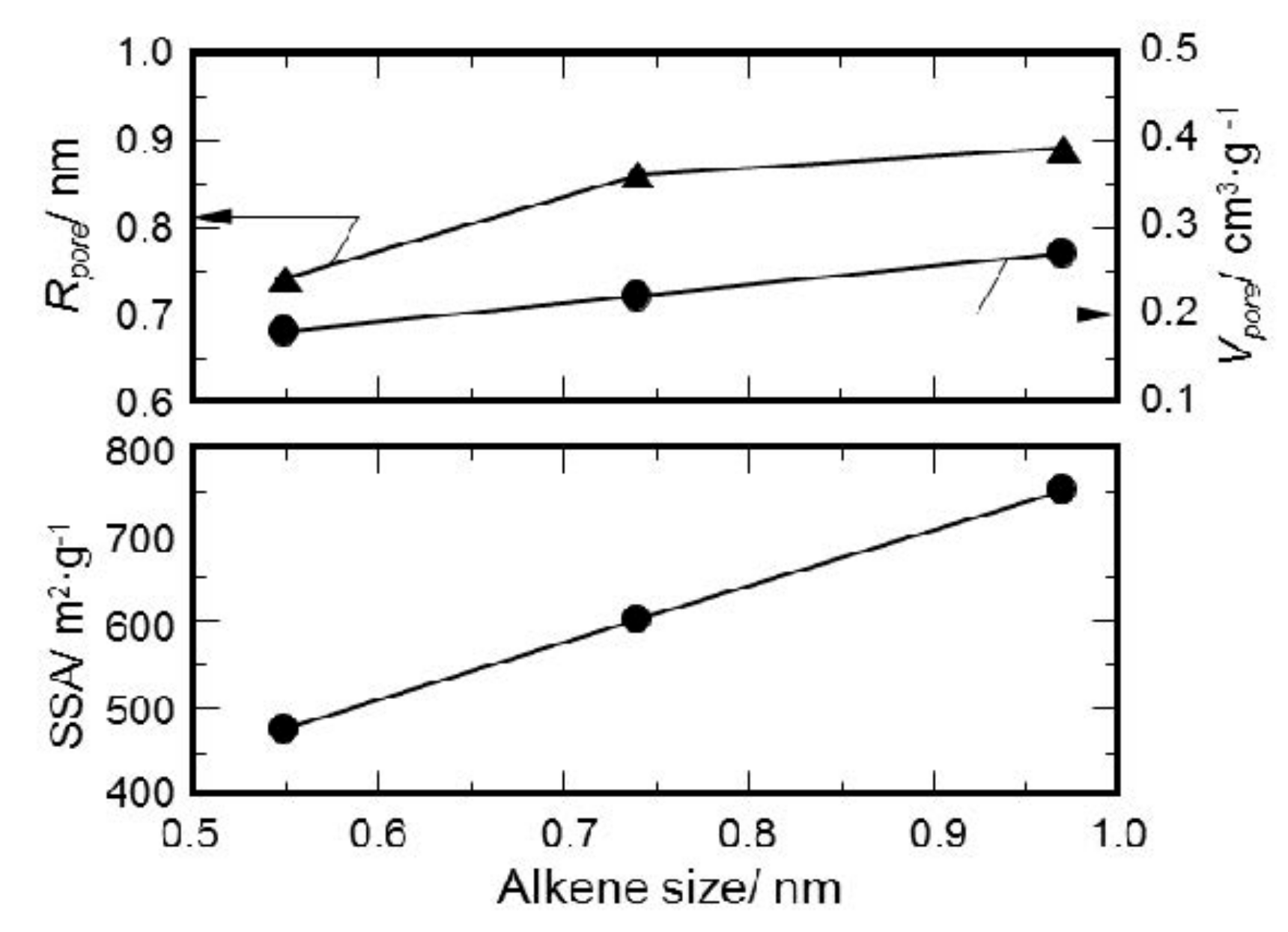
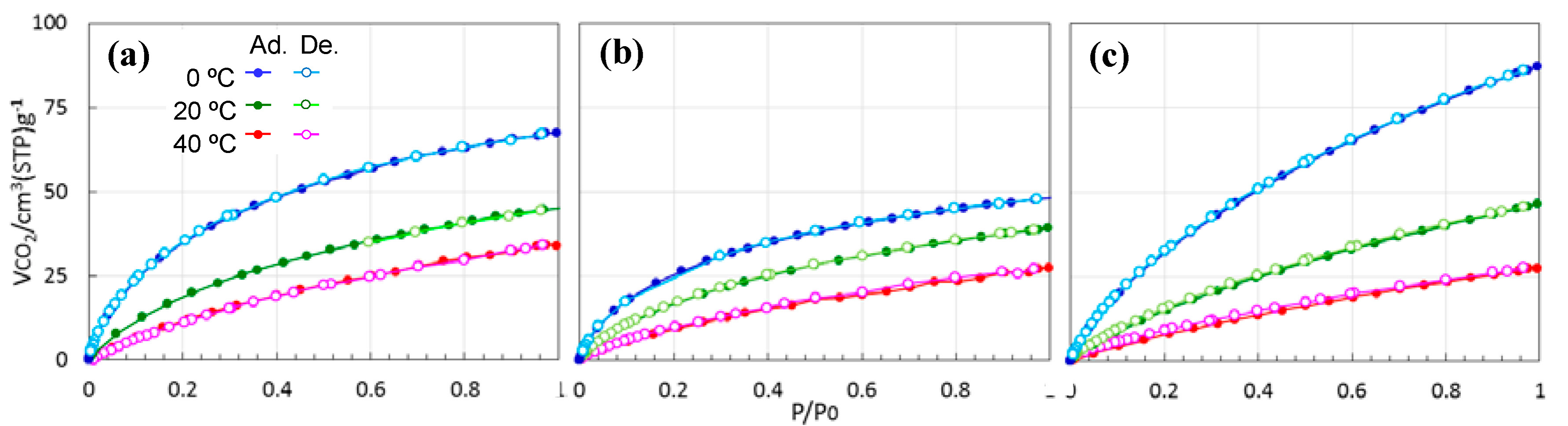
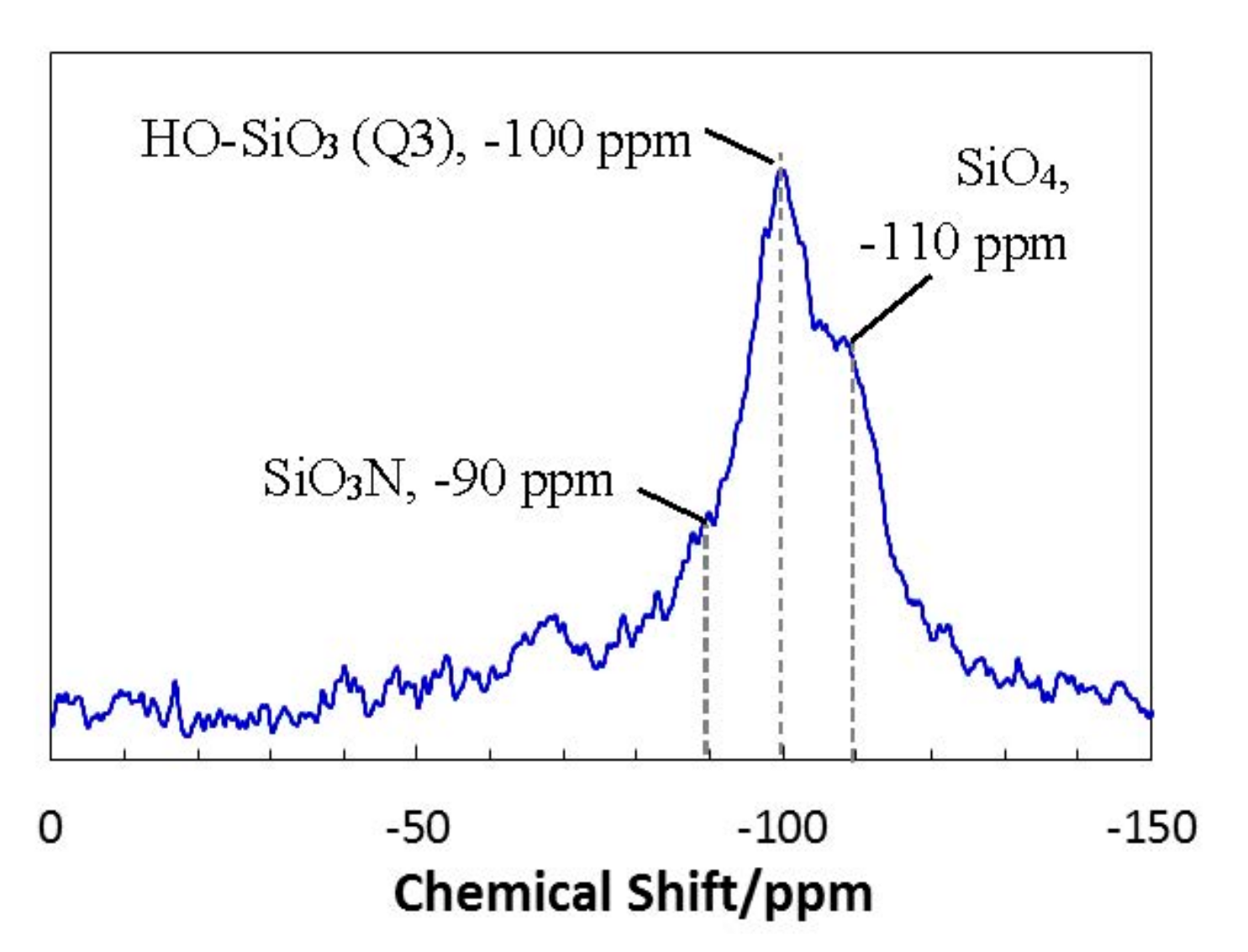
3.3. Textural Properties of ROSZ-Derived Amorphous Si–O–N
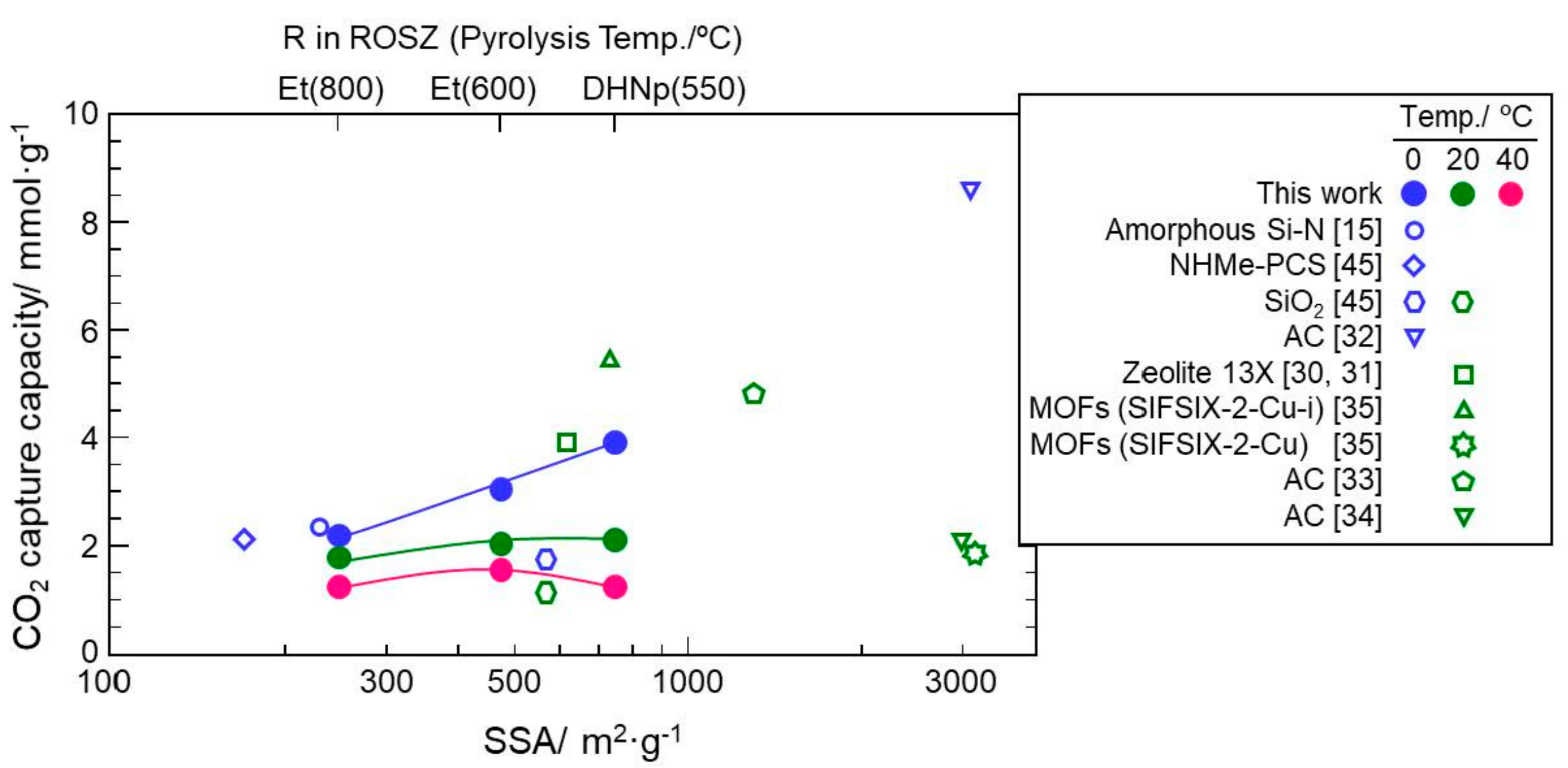
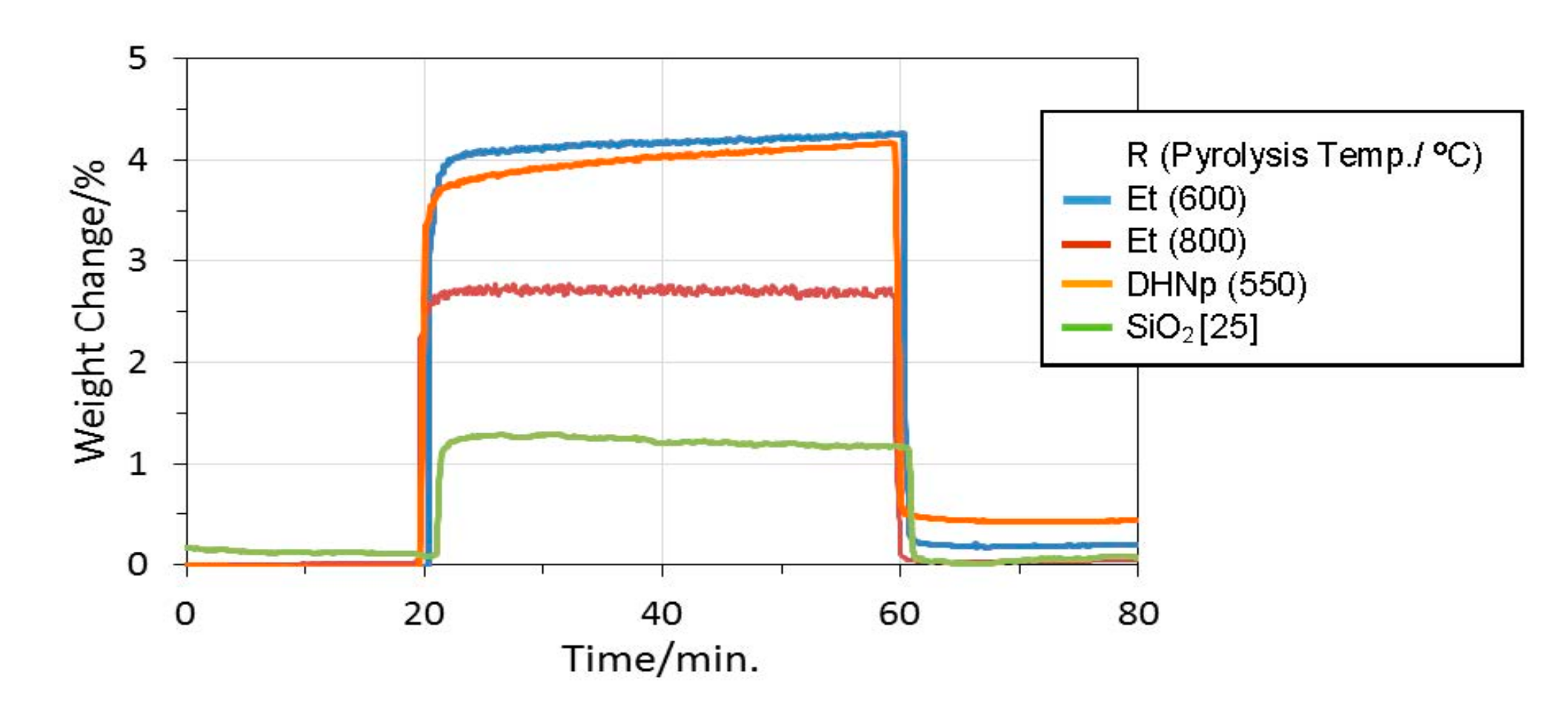
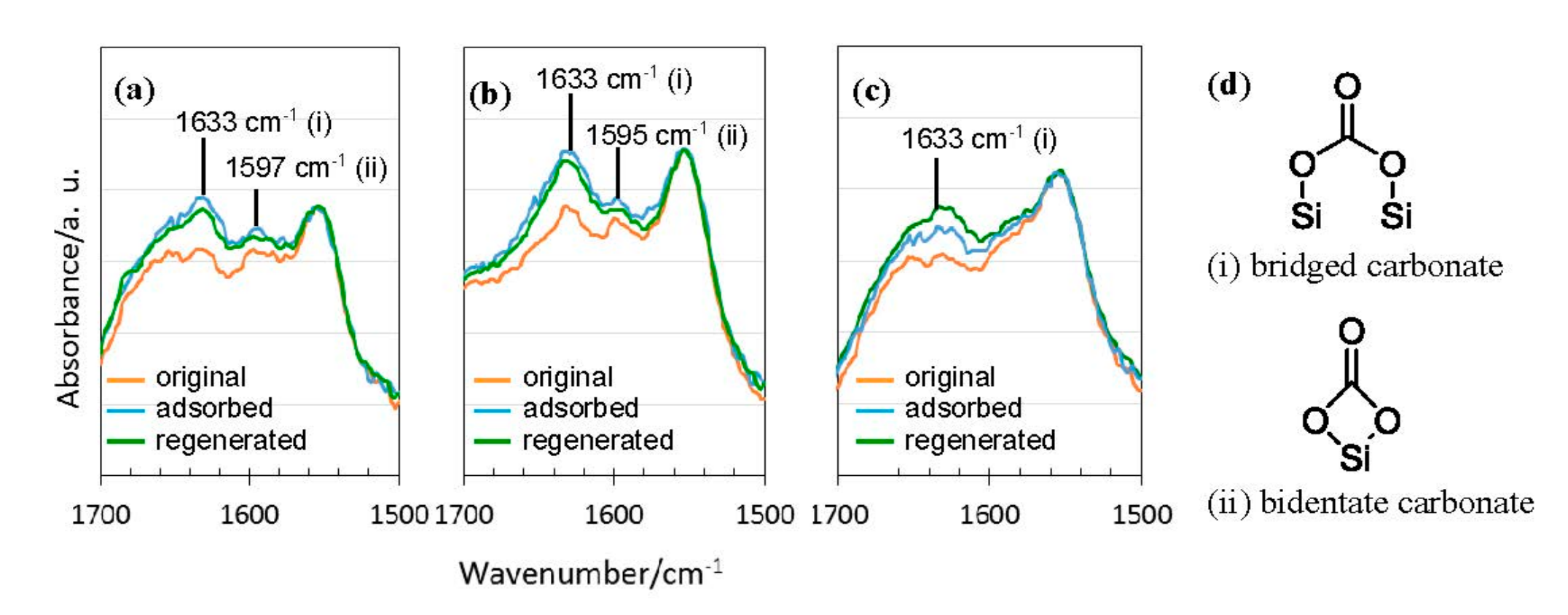
3.4. CO2 Capture Properties
4. Summary
- (1)
- ATR–IR, 13C– and 29Si–NMR spectroscopic analyses revealed that a series of polyalkoxysilsesquiazanes ([ROSi(NH)1.5]n, ROSZ, R = Et, nPr, iPr, nBu, sBu, nHex, sHex, cHex, DHNp) were successfully synthesized in a good yield via two simple steps: reaction of SiCl4 with ROH to afford ROSiCl3, followed by ammonolysis at −78 °C.
- (2)
- The simultaneous TG-MS analyses of the ROSZs under a He flow revealed that the cleavage of the oxygen–carbon bond of the RO group was a common and dominant decomposition reaction. The subsequent evolution of alkene as a main gaseous species formed in-situ lead to the formation at 550 to 800 °C of the X-ray amorphous microporous Si–O–N.
- (3)
- The peak of the pore size distribution curve located within the micropore size range, and the total micropore volume as well as the SSA of the resulting X-ray amorphous Si–O–N increased consistently with the molecular size estimated for the alkene formed in-situ during the pyrolysis.
- (4)
- The CO2 capture capacity at 0 °C of the Si–O–N material increased consistently with the SSA, and reached 3.9 mmol·g−1 at the SSA of 750 m2·g−1, which was achieved for the 550 °C-pyrolyzed DHNpOSZ.
- (5)
- DRIFTS monitoring of the CO2 adsorption under continuous Ar–CO2–Ar run at 40 °C revealed carbonate formations at the CO2 chemisorption site provided by the surface OH groups formed in-situ during the polymer/ceramics thermal conversion at around 550 °C.
- (6)
- The CO2 capture capacity measured at the CO2 partial pressure, p/p0 = 1 could be enhanced by the initial CO2 chemisorption at very low CO2 partial pressures.
Author Contributions
Conflicts of Interest
References
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic Nanocomposites from Tailor-Made Preceramic Polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Gallei, M.; Ionescu, E. Polymer-Ceramic Nanohybrid Materials. Adv. Polym. Sci. 2015, 267, 143–185. [Google Scholar]
- Miyajima, K.; Eda, T.; Ohta, H.; Ando, Y.; Nagaya, S.; Ohba, T.; Iwamoto, Y. Development of Si-N Based Hydrogen Separation Membrane. Ceram. Trans. 2010, 213, 87–94. [Google Scholar]
- Kusakabe, K.; Li, Z.Y.; Maeda, H.; Morooka, S. Preparation of supported composite membrane by pyrolysis of polycarbosilane for gas separation at high temperature. J. Membr. Sci. 1995, 103, 175–180. [Google Scholar] [CrossRef]
- Li, Z.Y.; Kusakabe, K.; Morooka, S. Preparation of thermostable amorphous Si-C-O membrane and its application to gas separation at elevated temperature. J. Membr. Sci. 1996, 118, 159–168. [Google Scholar]
- Nagano, T.; Sato, K.; Saito, T.; Iwamoto, Y. Gas permeation properties of amorphous SiC membranes synthesized from polycarbosilane without oxygen-curing process. J. Ceram. Soc. Jpn. 2006, 114, 533–538. [Google Scholar] [CrossRef]
- Suda, H.; Yamauchi, H.; Uchimaru, Y.; Fujiwara, I.; Haraya, K. Preparation and gas permeation properties of silicon carbide-based inorganic membranes for hydrogen separation. Desalination 2006, 193, 252–255. [Google Scholar] [CrossRef]
- Mourhatch, R.; Tsotsis, T.T.; Sahimi, M. Network model for the evolution of the pore structure of silicon-carbide membranes during their fabrication. J. Membr. Sci. 2010, 356, 138–146. [Google Scholar] [CrossRef]
- Takeyama, A.; Sugimoto, M.; Yoshikawa, M. Gas permeation property of SiC membrane using curing of polymer precursor film by electron beam irradiation in helium atmosphere. Mater. Trans. 2011, 52, 1276–1280. [Google Scholar] [CrossRef]
- Völger, K.W.; Hauser, R.; Kroke, E.; Riedel, R.; Ikuhara, Y.H.; Iwamoto, Y. Synthesis and characterization of novel non-oxide sol-gel derived mesoporous amorphous Si-C-N membranes. J. Ceram. Soc. Jpn. 2006, 114, 567–570. [Google Scholar] [CrossRef]
- Soraru, G.D.; Liu, Q.; Interrante, L.V.; Apple, T. Role of precursor molecular structure on the microstructure and high temperature stability of silicon oxycarbide glasses derived from methylene-bridged polycarbosilanes. Chem. Mater. 1998, 10, 4047–4054. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, W.; Babonneau, F.; Interrante, L.V. Synthesis of Polycarbosilane/Siloxane Hybrid Polymers and Their Pyrolytic Conversion to Silicon Oxycarbide Ceramics. Chem. Mater. 1997, 9, 2434–2441. [Google Scholar] [CrossRef]
- Lee, L.; Tsai, D.S. A hydrogen-permselective silicon oxycarbide membrane derived from polydimethylsilane. J. Am. Ceram. Soc. 1999, 82, 2796–2800. [Google Scholar] [CrossRef]
- Schitco, C.; Bazarjani, M.S.; Riedel, R.; Gurlo, A. NH3-assisted synthesis of microporous silicon oxycarbonitride ceramics from preceramic polymers: A combined N2 and CO2 adsorption and small angle X-ray scattering study. J. Mater. Chem. A 2015, 3, 805–818. [Google Scholar] [CrossRef]
- Schitco, C.; Bazarjani, M.S.; Riedel, R.; Gurlo, A. Ultramicroporous silicon nitride ceramics for CO2 capture. J. Mater. Res. 2015, 30, 2958–2966. [Google Scholar] [CrossRef]
- Schitco, C.; Turdean-Ionescu, C.; Seifollahi Bazarjani, M.; Tai, C.-W.; Li, D.; Fasel, C.; Donner, W.; Shen, J.; Riedel, R.; Gurlo, A.; et al. Silicon oxycarbonitrides synthesized by ammonia-assisted thermolysis route from polymers: A total X-ray scattering, solid-state NMR, and TEM structural study. J. Eur. Ceram. Soc. 2016, 36, 979–989. [Google Scholar] [CrossRef]
- Hauser, R.; Nahar-Borchard, S.; Riedel, R.; Ikuhara, Y.H.; Iwamoto, Y. Polymer-derived SiBCN ceramic and their potential application for high temperature membranes. J. Ceram. Soc. Jpn. 2006, 114, 524–528. [Google Scholar] [CrossRef]
- Prasad, R.M.; Iwamoto, Y.; Riedel, R.; Gurlo, A. Multilayer amorphous Si-B-C-N/γ-Al2O3/α-Al2O3 membranes for hydrogen purification. Adv. Eng. Mater. 2010, 12, 522–528. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Müller, M.M.; Kleebe, H.-J.; Jüttke, Y.; Voigt, I.; Yazdi, M.B.; Alff, L.; Riedel, R.; Gurlo, A. High-temperature stability and saturation magnetization of superparamagnetic nickel nanoparticles in microporous polysilazane-derived ceramics and their gas permeation properties. Appl. Mater. Interfaces 2014, 6, 12270–12278. [Google Scholar] [CrossRef] [PubMed]
- Kroke, E.; Li, Y.-L.; Konetschny, C.; Lecomte, E.; Fasel, C.; Riedel, R. Silazane derived ceramics and related materials. Mater. Sci. Eng. R. 2000, 26, 97–199. [Google Scholar] [CrossRef]
- Weinmann, M. Chapter 7 Polysilazanes. In Inorganic Materials; De Jaeger, R., Gleria, M., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2007; pp. 371–413. [Google Scholar]
- Sokri, M.N.M.; Onishi, T.; Mouline, Z.; Daiko, Y.; Honda, S.; Iwamoto, Y. Polymer-derived amorphous silica-based inorganic-organic hybrids having alkoxy groups: Intermediates for synthesizing microporous amorphous silica materials. J. Ceram. Soc. Jpn. 2015, 123, 732–738. [Google Scholar] [CrossRef]
- Sokri, M.N.M.; Daiko, Y.; Mouline, Z.; Honda, S.; Iwamoto, Y. Formation of micro and mesoporous amorphous silica-based materials from single source precursors. Inorganics 2016, 4, 5. [Google Scholar] [CrossRef]
- Iwase, Y.; Horie, Y.; Daiko, Y.; Honda, S.; Iwamoto, Y. Synthesis of a novel polyethoxysilsesquiazane and thermal conversion into ternary silicon oxynitride ceramics with enhanced thermal stability. Materials 2017, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Sato, K.; Kato, T.; Inada, T.; Kubo, Y. A hydrogen-permselective amorphous silica membrane derived from polysilazane. J. Eur. Ceram. Soc. 2005, 25, 257–264. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al‑Maythalony, A.B.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Numaguchi, R.; Fujiki, J.; Yamada, H.; Chowdhury, F.A.; Kida, K.; Goto, K.; Okumura, T.; Yoshizawa, K.; Yogo, K. Development of post-combustion CO2 capture system using amine-impregnated solid sorbent. Energy Procedia 2017, 114, 2304–2312. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Yu, C.-H; Huang, C.-H; Tan, C.-S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar]
- Jadhav, P.D.; Chatti, R.V.; Biniwale, R.B.; Labhsetwar, N.K.; Devotta, S.; Rayalu, S.S. Monoethanol Amine Modified Zeolite 13X for CO2 Adsorption at Different Temperatures. Energy Fuels 2007, 21, 3555–3559. [Google Scholar] [CrossRef]
- Liang, Z.; Marshall, M.; Chaffee, A.L. CO2 Adsorption-Based Separation by Metal Organic Framework (Cu-BTC) versus Zeolite (13X). Energy Fuels 2009, 23, 2785–2789. [Google Scholar] [CrossRef]
- Wahby, A.; Ramos-Fernandez, J.M.; Martinez-Escandell, M.; Sepulveda-Escribano, A.; Silvestre-Albero, J.; Rodriguez-Reinoso, F. High-Surface-Area Carbon Molecular Sieves for Selective CO2 Adsorption. ChemSusChem 2010, 3, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Zhang, C.; Song, W.; Sun, G.; Xie, L.; Wang, J.; Li, K.; Sun, L.C.; Liu, H.; Snape, C.E.; Drage, T. CO2 Capture with Activated Carbon Grafted by Nitrogenous Functional Groups. Energy Fuels 2013, 27, 4818–4823. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- CO2 Ultimate Reduction in Steel-Making Process by Innovative Technology for Cool Earth 50 (COURSE50) Project Promoted by New Energy and Industrial Technology Development Organization (NEDO), Japan. Available online: http://www.jisf.or.jp/course50/index.html (accessed on 1 March 2018).
- Waade, W.; Farnand, S.; Hutchison, R.; Welch, K. CO2 Capture from SMRs: A Demonstration project. Hydrocarb. Process. 2012, 9, 63–68. [Google Scholar]
- Grande, C.A.; Blom, R.; Andreassen, K.A.; Stensrød, R.E. Experimental Results of Pressure Swing Adsorption (PSA) for Pre-combustion CO2 Capture with Metal Organic Frameworks. Energy Procedia 2017, 114, 2265–2270. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Enhancing Capacity of Activated Carbons for Hydrogen Purification. Ind. Eng. Chem. Res. 2009, 48, 3978–3990. [Google Scholar] [CrossRef]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.-S.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef]
- Saito, A.; Foley, H. Curvature and parametric sensitivity in models for adsorption in micropores. AIChE J. 1991, 37, 429–436. [Google Scholar] [CrossRef]
- Mouline, Z.; Asai, K.; Kawai, A.; Sekimoto, K.; Onishi, T.; Daiko, Y.; Honda, S.; Iwamoto, Y. Synthesis and characterization of organoamine-functionalized amorphous silica materials for CO2-selective membranes. J. Ceram. Soc. Jpn. 2015, 123, 779–784. [Google Scholar] [CrossRef]
- Mouline, Z.; Asai, K.; Daiko, Y.; Honda, S.; Bernard, S.; Iwamoto, Y. Amine-functionalized polycarbosilane hybrids for CO2-selective membrane. J. Eur. Ceram. Soc. 2017, 37, 5213–5221. [Google Scholar] [CrossRef]
- Seyferth, D.; Wiseman, G.; Prud’homme, C. A liquid silazane precursor to silicon nitride. J. Am. Ceram. Soc. 1983, 66. [Google Scholar] [CrossRef]
- Alkenes Appropriate for ROSZ, R = Et~Cy and R = DHNp Were Identified Using the NIST 05 Database (NIST/EPA/NIH Mass Spectral Library with Search Program, Data Version: NIST v17); Mass Spectrometry Data Center, National Institute of Standards and Technology: Gaithersburg, MD, USA.
- Santoro, M.; Gorelli, F.; Haines, J.; Cambon, O.; Levelut, C.; Garbarino, G. Silicon carobonate phase formed from carbon dioxide and silica under pressure. PNAS 2011, 108, 7689–7692. [Google Scholar] [CrossRef] [PubMed]
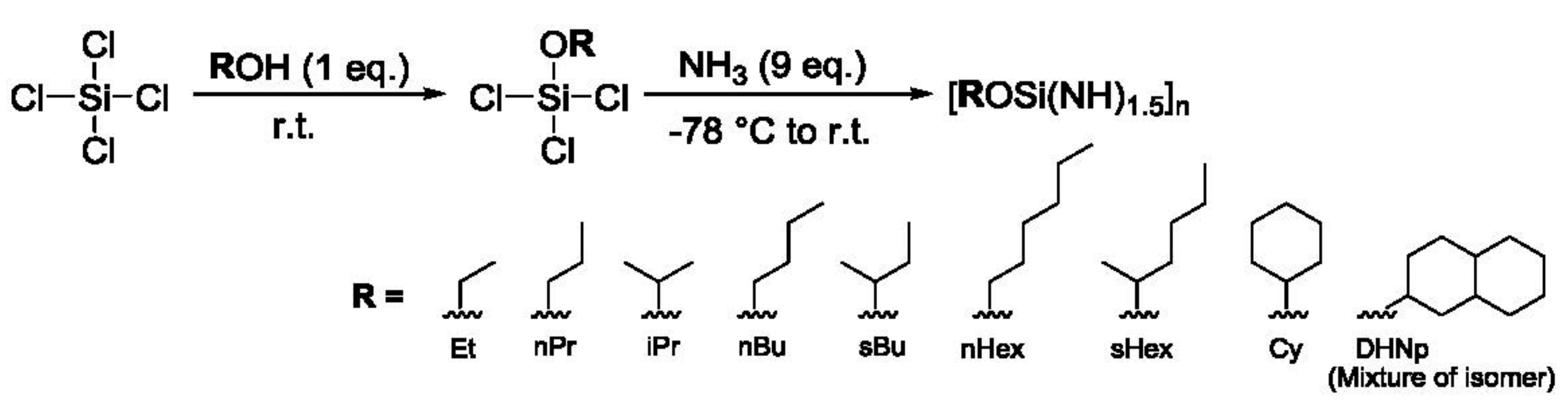
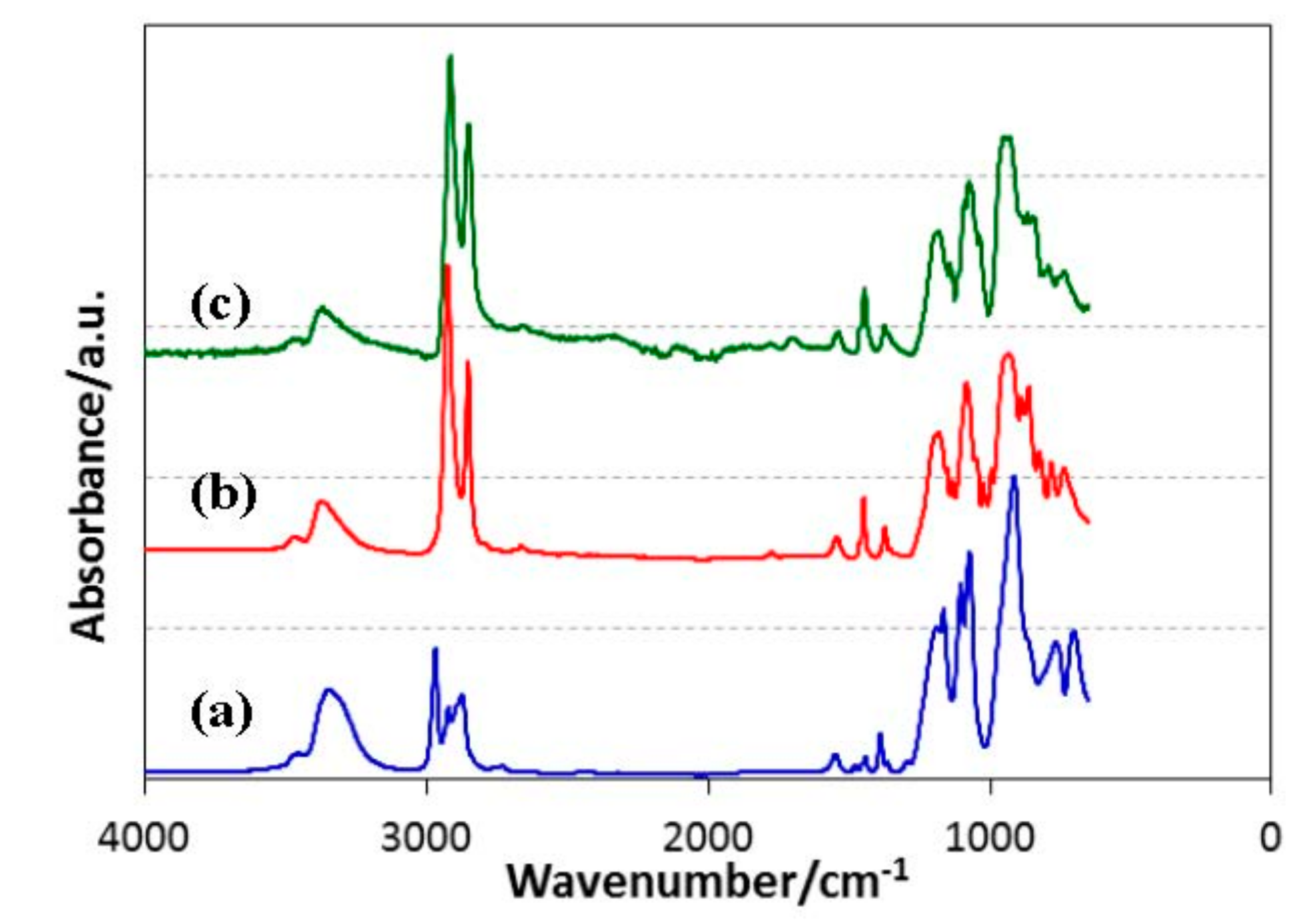

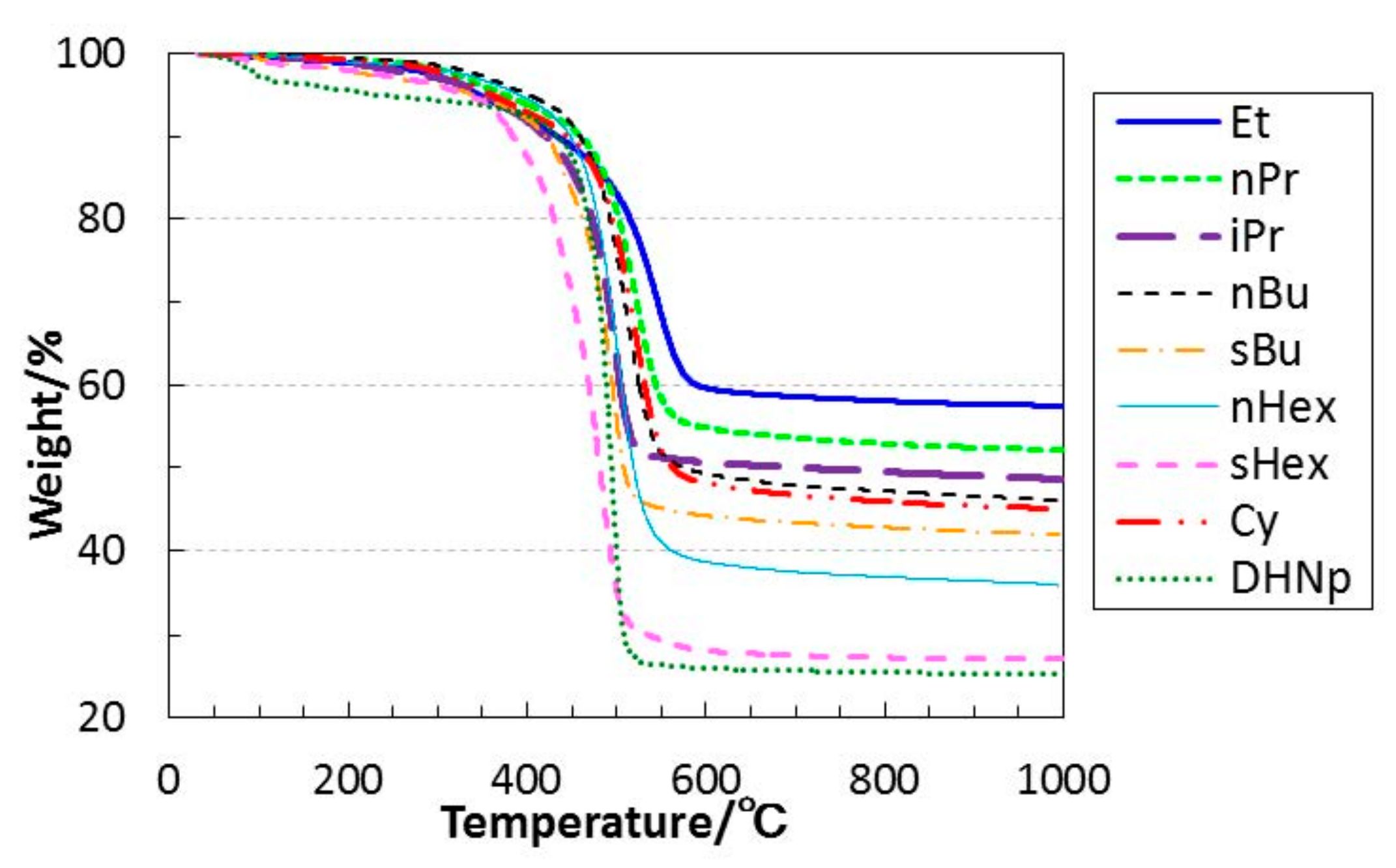

| R | ROSiCl3 | [ROSi(NH)1.5]n | Total Yield/% | ||
|---|---|---|---|---|---|
| b.p. | Recovery Rate/% | Appearance | Yield/% | ||
| Et | 102 °C/760 mmHg | 60 | Colorless solid | 95 | 57 |
| nPr | 123 °C/760 mmHg | 51 | Colorless solid | 87 | 44 |
| iPr | 116 °C/760 mmHg | 61 | Colorless solid | 93 | 57 |
| nBu | 150 °C/760 mmHg | 53 | Colorless solid | 94 | 50 |
| sBu | 175 °C/760 mmHg | 64 | Colorless paste | 73 | 47 |
| nHex | 135 °C/32 mmHg | 57 | Colorless paste | 86 | 49 |
| sHex | 120 °C/24 mmHg | 54 | Colorless paste | 88 | 48 |
| Cy | 78 °C/11 mmHg | 53 | Colorless solid | 98 | 52 |
| DHNp | 185 °C/16.5 mmHg | 52 | Colorless solid | 97 | 51 |
| R | Yield/% | Appearance |
|---|---|---|
| Et | 58 | Brown solid |
| nPr | 53 | Black solid |
| iPr | 50 | Colorless solid |
| nBu | 47 | Black solid |
| sBu | 43 | brown solid |
| nHex | 37 | Black solid |
| sHex | 28 | Brown solid |
| Cy | 46 | Pale brown solid |
| DHNp | 26 | Black solid |
| R | Treated Temperature | Composition/wt % | Empirical Ratio | ||||
|---|---|---|---|---|---|---|---|
| Si | C | O | N | H | |||
| Et | As-synthesized | 29.3 | 25.1 | 16.8 | 22.0 | 6.8 | Si1.0C2.0O1.0N1.5H6.5 |
| 800 °C-pyrolysed | 51.3 | 1.1 | 32.4 | 13.5 | 1.7 | Si1.0C0.05O1.1N0.5H0.9 | |
| iPr | As-synthesized | 25.6 | 32.9 | 14.6 | 19.2 | 7.8 | Si1.0C3.0O1.0N1.5H8.5 |
| 800 °C-pyrolysed | 52.2 | 0.04 | 32.4 | 14.0 | 1.4 | Si1.0C0.0O1.1N0.5H0.8 | |
| nBu | As-synthesized | 22.7 | 38.9 | 13.0 | 17.0 | 8.5 | Si1.0C4.0O1.0N1.5H10.5 |
| 800 °C-pyrolysed | 55.1 | 2.6 | 27.2 | 13.7 | 1.4 | Si1.0C0.1O0.9N0.5H0.7 | |
| sBu | As-synthesized | 22.7 | 38.9 | 13.0 | 17.0 | 8.5 | Si1.0C4.0O1.0N1.5H10.5 |
| 800 °C-pyrolysed | 55.0 | 0.2 | 29.3 | 14.2 | 1.4 | Si1.0C0.01O0.9N0.5H0.7 | |
| nHex | As-synthesized | 18.5 | 47.5 | 10.6 | 13.9 | 9.5 | Si1.0C6.0O1.0N1.5H14.5 |
| 800 °C-pyrolysed | 55.2 | 2.7 | 26.4 | 14.6 | 1.23 | Si1.0C0.1O0.8N0.5H0.6 | |
| sHex | As-synthesized | 18.5 | 47.5 | 10.6 | 13.9 | 9.5 | Si1.0C6.0O1.0N1.5H14.5 |
| 800 °C-pyrolysed | 52.3 | 0.8 | 28.2 | 17.0 | 1.3 | Si1.0C0.03O0.9N0.6H0.7 | |
| Cy | As-synthesized | 18.7 | 48.2 | 10.7 | 14.0 | 8.4 | Si1.0C6.0O1.0N1.5H12.5 |
| 800 °C-pyrolysed | 49.9 | 0.10 | 32.9 | 15.7 | 1.4 | Si1.0C0.03O0.9N0.6H0.8 | |
| DHNp | As-synthesized | 13.8 | 59.0 | 7.9 | 10.3 | 9.1 | Si1.0C10.0O1.0N1.5H18.5 |
| 800 °C-pyrolysed | 51.1 | 1.92 | 33.4 | 12.1 | 1.4 | Si1.0C0.08O1.1N0.5H0.8 | |
| R | Completed Temp/°C | m/z+ Ratios (Assignment) |
|---|---|---|
| Et [24] | 600 | 16 (NH2+), 45 (SiNH3+) 28 (ethylene) |
| nPr | 600 | 15 (NH+) 41 (propylene) 31, 55, 67, 79 (unknown) |
| iPr | 550 | 16 (NH2+) 27, 41 (propylene) |
| nBu | 600 | 16 (NH2+) 28, 41, 56 (1-butene) 67, 79, 91 (unknown) |
| sBu | 550 | 16 (NH2+) 27, 41, 56 (2-butene) |
| nHex | 600 | 16 (NH2+) 27, 41, 55 (1- or 2-hexene) 67, 79, 91 (unknown) |
| sHex | 550 | 15 (NH+) 27, 41, 55, 69, 84 (3-hexene) |
| Cy | 550 | 16 (NH2+) 27, 39, 54, 67, 82 (cyclohexene) |
| DHNp | 550 | 27, 41, 67, 79, 95, 121, 136 (1,2,3,4,4a,5,6,8a-octahydronaphthalene isomers) 54, 107 (unknown) |
| R | Pyrolyzed Temp/°C | SSA/m2·g−1 | Rpore/nm | Vpore/cm3·g−1 |
|---|---|---|---|---|
| Et | 600 | 476 | 0.74 | 0.18 |
| Cy | 550 | 601 | 0.86 | 0.22 |
| DHNp | 550 | 750 | 0.89 | 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwase, Y.; Horie, Y.; Honda, S.; Daiko, Y.; Iwamoto, Y. Microporosity and CO2 Capture Properties of Amorphous Silicon Oxynitride Derived from Novel Polyalkoxysilsesquiazanes. Materials 2018, 11, 422. https://doi.org/10.3390/ma11030422
Iwase Y, Horie Y, Honda S, Daiko Y, Iwamoto Y. Microporosity and CO2 Capture Properties of Amorphous Silicon Oxynitride Derived from Novel Polyalkoxysilsesquiazanes. Materials. 2018; 11(3):422. https://doi.org/10.3390/ma11030422
Chicago/Turabian StyleIwase, Yoshiaki, Yoji Horie, Sawao Honda, Yusuke Daiko, and Yuji Iwamoto. 2018. "Microporosity and CO2 Capture Properties of Amorphous Silicon Oxynitride Derived from Novel Polyalkoxysilsesquiazanes" Materials 11, no. 3: 422. https://doi.org/10.3390/ma11030422
APA StyleIwase, Y., Horie, Y., Honda, S., Daiko, Y., & Iwamoto, Y. (2018). Microporosity and CO2 Capture Properties of Amorphous Silicon Oxynitride Derived from Novel Polyalkoxysilsesquiazanes. Materials, 11(3), 422. https://doi.org/10.3390/ma11030422





