2. Materials and Methods
Spherical Au NPs of about 13 nm in diameter were synthesized with the Turkevich method by reducing Au ions in water at 100 °C with sodium citrate. The whole synthetic and purification protocol has been described previously [
10].
To deposit a TiO2 layer, a solution of Ethanol (0.413 mL), titanium butoxide (0.447 mL), and acetylacetone (0.216 mL) was prepared under vigorous stirring at room temperature. After 10 min, 0.1 mL Milli-Q water were added and were let stir for additional 20 min. Then, 1.83 mL ethanol was added, the total solution was let stir for 5 more minutes, and then it was used for films deposition.
To deposit the NiO layer, 300 mg of Nickel Acetate tetrahydrate were dissolved in 2 mL methanol, and subsequently 0.18 mL diethanolamine were added under stirring. After 40 min, 1.4 mL ethanol were added, and after additional 5 min, the solution was used for films deposition.
To deposit the ZnO layer, 200 mg of Zinc acetate dehydrate were dissolved in 0.9 mL ethanol, and subsequently 0.066 mL monoethanolamine (MEA) were added under stirring. After 30 min, 0.35 mL ethanol are added and the solution was used for film depositions after five more minutes of stirring.
The bottom oxide coating was deposited on either Si or SiO
2 (fused silica) substrates by spin coating with rotating speed ranging from 2000 rpm to 3000 rpm for 30 s, and then the sample was annealed directly at 500 °C for 10 min. The spinning rate was calibrated and adjusted in order to obtain films of about 45 nm after the 500 °C annealing for all of the three oxides used. The accuracy and reproducibility of the spinning procedure was tested after repeated depositions and gave a ±5 nm error on the sample thickness. To promote Au NPs bonding, the outer oxide surface is functionalized with aminopropyltrimethoxsilane (APS), after re-activation of the surface to promote formation of hydroxyl groups, which are necessary for the reaction with APS molecules (as a consequence of the thermal annealing, all of the hydroxyl groups were removed). The optimized activating procedure for NiO and TiO
2 films consisted in dipping the samples into a 4% H
2O
2 aqueous solution at room temperature for 1 min, followed by a thorough rinsing with deionized water. After this procedure, the previously reported protocols of substrate functionalization and Au NPs layer deposition were performed [
10], followed by the deposition of the top oxide layer using the sol-gel recipes described earlier; eventually, the samples were thermally treated at 500 °C for one hour. The surface coverage of Au NPs was tailored simply diluting the Au colloidal solution: in this study we prepared samples with three different Au surface coverages, hereafter indicated as low (L, 6%), medium (M, 19%), and high (H, 35%). The surface coverage was estimated from SEM images, as reported in reference [
10]. ZnO was not used as bottom layer because the hydroxylation protocol caused etching of the porous ZnO films, even if it was performed in milder conditions.
The films were characterized by XRD using a Philips PW1710 diffractometer (Amsterdam, The Netherlands) equipped with glancing-incidence X-ray optics. The analysis was performed at 0.5° incidence, using CuKα Ni filtered radiation at 30 kV and 40 mA. Optical absorption spectra of samples that were deposited on fused silica substrates were measured in the 300–2000 nm range using a Jasco V-570 spectrophotometer (Japan) Ellipsometry measurements were carried out on a J.A. Woollam V-VASE Spectroscopic Ellipsometer (Lincoln, NE, USA) in vertical configuration, in the 300–1500 nm range at three different angles of incidence (65°, 70°, 75°). The nanocomposites were modeled with Cauchy dispersions for the non-absorbing region, while Gaussian or Tauc-Lorentz oscillators were used for the UV absorption onset fitting. Rutherford backscattering spectrometry (RBS) was performed with an electrostatic accelerator, Van de Graaff type, using single-charged alpha particles (4He+) at 2.0 MeV and 20 nA. RBS analysis was performed on samples deposited on Si substrates. The incident beam was perpendicular to the sample, while the scattering angle was 160°. The surface and cross-sectional structure of the nanocomposite films were investigated with a xT Nova NanoLab Scanning Electron Microscopy (SEM). Secondary Ion Mass Spectrometry (SIMS) was exploited to measure the elemental in-depth profiles of chemical species in the deposited film. SIMS measurements were carried out by means of an IMS 4f mass spectrometer (Cameca, Padova, Italy), using a 14.5 KeV Cs+ primary beam and by negative secondary ion detection. The charge build up while profiling the insulating samples was compensated by an electron gun without any need to cover the surface with a metal film. The SIMS spectra were carried out at different primary beam intensity (20 nA, stability 0.2%) rastering over a 150 × 150 µm2 area and detecting secondary ions from a sub region close to 7 × 7 µm2 to avoid crater effects. The primary beam was chosen in order to optimise the depth resolution and the multilayer interface determination. The signals were detected in beam blanking mode (i.e., interrupting the sputtering process during magnet stabilization periods) in order to improve the in-depth resolution. Moreover, the dependence of the erosion speed on the matrix composition was taken into account by measuring the erosion speed at various depths for each sample. The erosion speed was then evaluated by measuring the depth of the erosion crater at the end of each analysis by means of a Tencor Alpha Step profilometer with a maximum uncertainty of few nanometers (final value given by the average on 8 measures). The measurements were performed in High Mass Resolution configuration to avoid mass interference artifacts. The film thickness was determined by analysing the element signal dynamics. The error of the film thickness contains, therefore, contributes of the element inter-diffusion, of the film roughness, and finally of the technique artefacts. Optical gas sensing tests were performed by making optical absorption measurements in the 350–1500 nm wavelength range on films deposited on SiO2 glass substrates using a Harrick gas flow cell (with an optical path length of 5.5 cm), coupled with a Jasco V-570 spectrophotometer. The operating temperature (OT) was set at 300 °C and gases at concentrations of 1 vol% for H2 and of 1 vol% for CO in dry air at a flow rate of 0.4 L/min were used. The incident spectrophotometer beam was set normal to the film surface and illuminated an area of ~13 mm2.
3. Results and Discussion
As described in the experimental section, the different samples that were prepared consist on a bottom layer (TiO2, NiO), an intermediate layer of Au NPs with different surface coverage, and a top layer (TiO2, NiO, ZnO). The Au colloids surface coverage can be easily tuned by changing the NPs concentration in the spin coating solution (more concentrated solutions lead to greater surface coverages) or by modifying the spinning speed (increasing the rotational speed leads to lower surface coverages).
Optical spectroscopy is a powerful tool to investigate Au NPs amount and organization in between the two oxide layers.
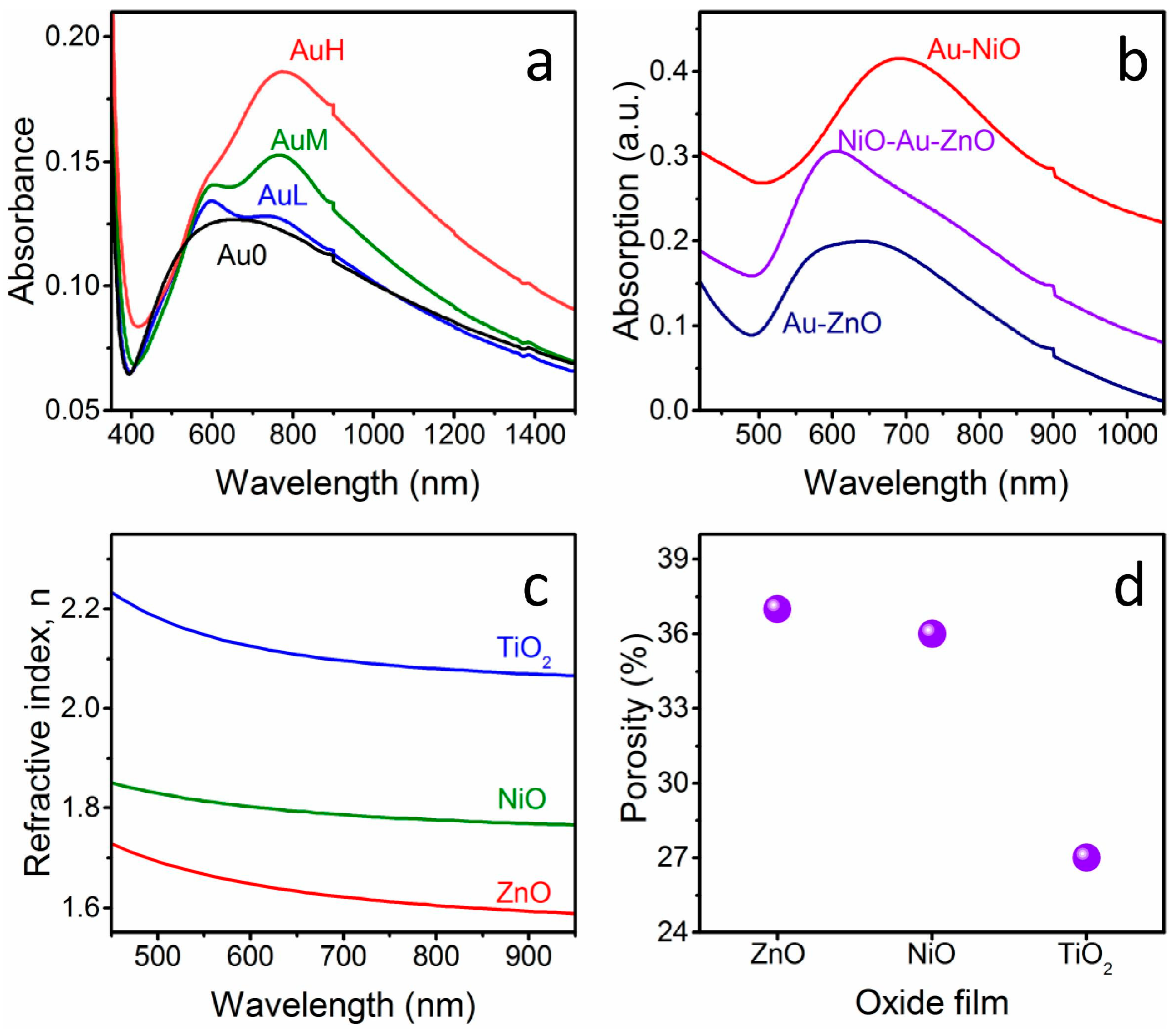
Figure 1 shows the absorption spectra of some of the prepared nanostructures: for all of the NPs-containing samples the SPR peak appears in the visible-near IR range (
Figure 1a,b). As can be seen in
Figure 1a, an increase in intensity of the SPR peak component at higher wavelengths with increasing Au NPs surface coverage is observed, as already reported previously for bare Au NPs layers [
8,
9], and for Au NPs layers that were covered with metal oxides [
10]. This effect is due to the reduced mutual distance between close-packed Au NPs, which leads to a stronger coupling of the plasmon resonances [
6]. The broad absorption feature of the Au-free sandwich structure is due to optical interference because of the high refractive index of the oxide films (see below).
By comparing a sandwich structure that was prepared in this study with Au NPs layers that were deposited on glass with the same particles density and covered with only one metal oxide (
Figure 1b), it can be noticed that the optical features of Au NPs that are embedded in between two metal oxides (in this case NiO as bottom layer and ZnO as top layer) are effectively in between the properties of the Au-NiO and Au-ZnO systems: in fact, the SPR peak of Au NPs deposited on glass and covered with ZnO is registered at about 605 nm, while when the Au colloids deposited on glass are covered with NiO, the SPR frequency is 690 nm. The plasmon peak of the Au NPs embedded in between the two oxides is definitely blue shifted compared to Au-NiO films, while due to the low frequency component related to plasmon coupling of neighboring NPs, it is difficult to appreciate the red shift compared to Au-ZnO. Nevertheless, the low frequency component of the SPR band is definitely red shifted in the sandwich structure compared to the Au-ZnO composite.
The blue or red shift observed in the SPR band is related to the difference in refractive index between the two oxides: NiO has a higher refractive index value when compared to ZnO, as will be discussed later on along with the ellipsometry measurements. So, according to Mie theory [
16], the greater the refractive index value of the matrix in which the Au NPs are embedded, the greater the SPR wavelength arising from the metal NPs.
The actual refractive index n and the thickness of the oxide layers that were deposited on glass substrates have been measured using spectroscopic ellipsometry, and the results are presented in
Figure 1c. As can be seen, the refractive index values for the three oxides that are used are rather different between each other, but also they differ from the bulk values for the respective oxides. This difference is ascribed to the residual porosity of the thin films, which is a well-known effect for oxides films that are prepared from sol-gel solutions and annealed at relatively low temperatures, outside the sintering range [
17,
18,
19]. For this reason, the oxide layer is modeled as an effective medium that is composed of dense matrix and pores, and through effective medium approximation (EMA) models, it is possible to evaluate the porosity amount. Using the bulk refractive index values at ~600 nm for the three oxides (n
ZnO = 2.01 [
20]; n
NiO = 2.33 [
21]; n
TiO2 = 2.51 [
20]), the pores volume fraction evaluated with the Bruggeman [
22] relationship are 37%, 36%, and 27% for ZnO, NiO, and TiO
2, respectively. As a consequence, as can be also visualized in
Figure 1c,d, the actual refractive index of the prepared samples follows the order of the bulk and dense materials, but the porosity of the TiO
2 layer is lower when compared to the other two oxides. In fact, anatase layers are more compact and smooth as compared to NiO and ZnO, as will be clarified later along with SEM characterization.
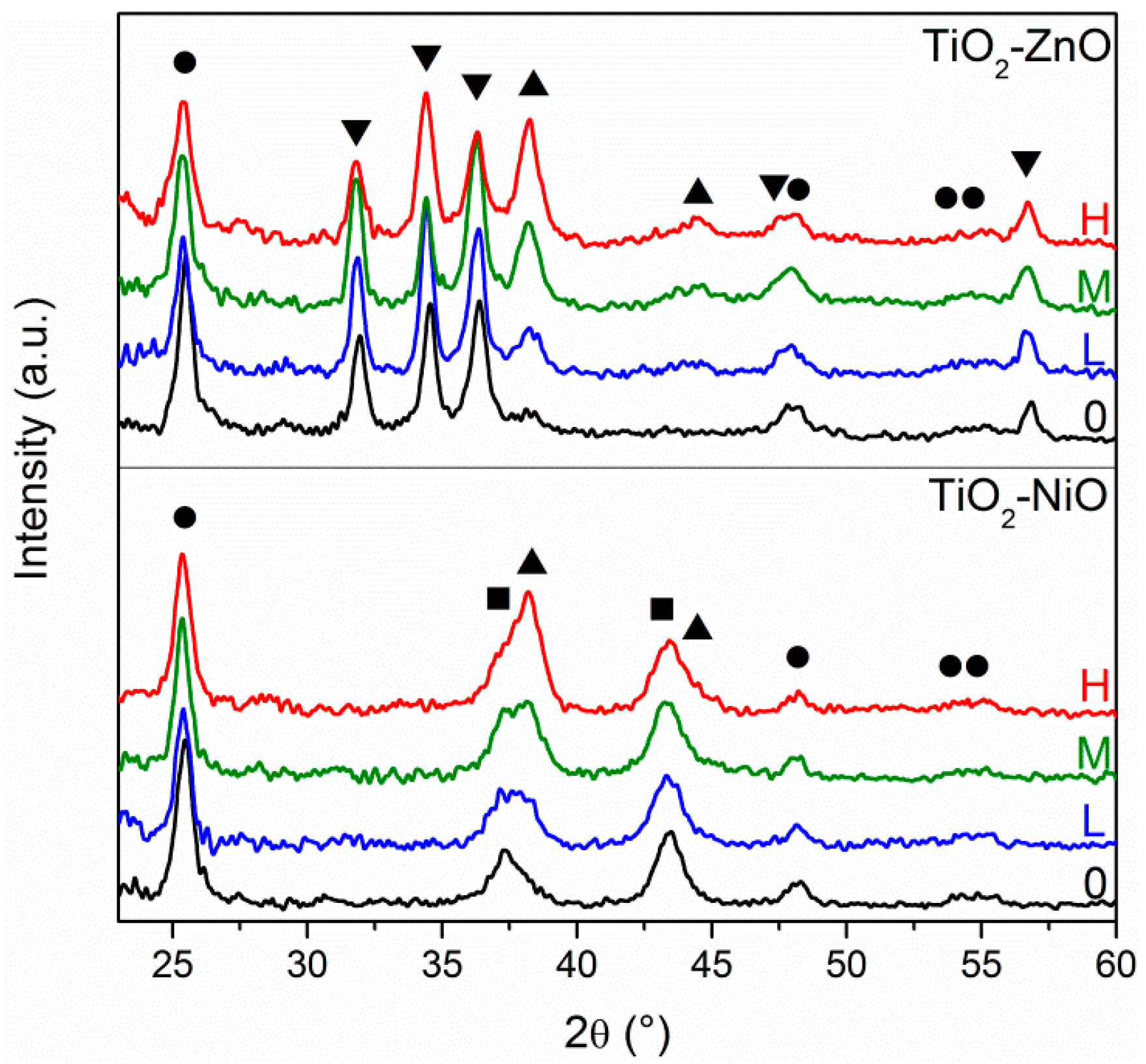
X-ray diffraction analysis gives a confirmation of the different Au amount according to the concentration of the solutions that are used for the Au layer deposition, and also verifies the crystallinity of the three oxides: all of these results are reported in
Figure 2. Typical diffraction patterns for anatase TiO
2 (ICDD No. 86-1157, highlighted with ●), bunsenite NiO (ICDD No. 47-1049, highlighted with ■), wurtzite ZnO (ICDD No. 36-1451, highlighted with ▼) and cubic Au (ICDD No.04-0784, highlighted with ▲) can be easily identified in the prepared samples, according to their respective composition. Analyzing the oxide diffraction peaks, they do not undergo any relevant change from one sample to another, nor in the intensity or broadening (the full width at half maximum, FWHM, is related to the crystallite size, according to the Scherrer equation), validating the reproducibility of the different sol-gel recipes adopted. However, it has to be said that such a comparison, especially for the intensity of the diffraction peaks, is merely qualitative. In fact, although all of the samples had approximately the same films thickness and the same substrate size, the difference in XRD peaks intensity is strongly related to the thickness of the samples, the X-ray beam spot size, the careful alignment of the sample stage, because the measurements have been performed at glancing angle (0.5°), and so a quantitative comparison would be rather speculative. As far as Au diffraction peaks are concerned, few differences can be observed among the different samples: by increasing the Au NPs amount (from Low, to Medium, to High), a clear progressive increase in Au peaks intensity is detected, confirming the different surface coverage.
XRD has also been adopted to evaluate the effect of the activation of the bottom oxide layer before performing the APS functionalization process: as described in the experimental section, NiO and TiO2 films were immersed in a hydrogen peroxide dilute solution in order to create –OH surface bonds. XRD measurements performed before and after the etching treatment (not reported) do not show any modification of the oxide diffraction peaks, nor in intensity or FWHM, excluding any change in the morphology and chemical composition of the oxide layers.
NiO-TiO
2 sandwich structures—with NiO as bottom layer and TiO
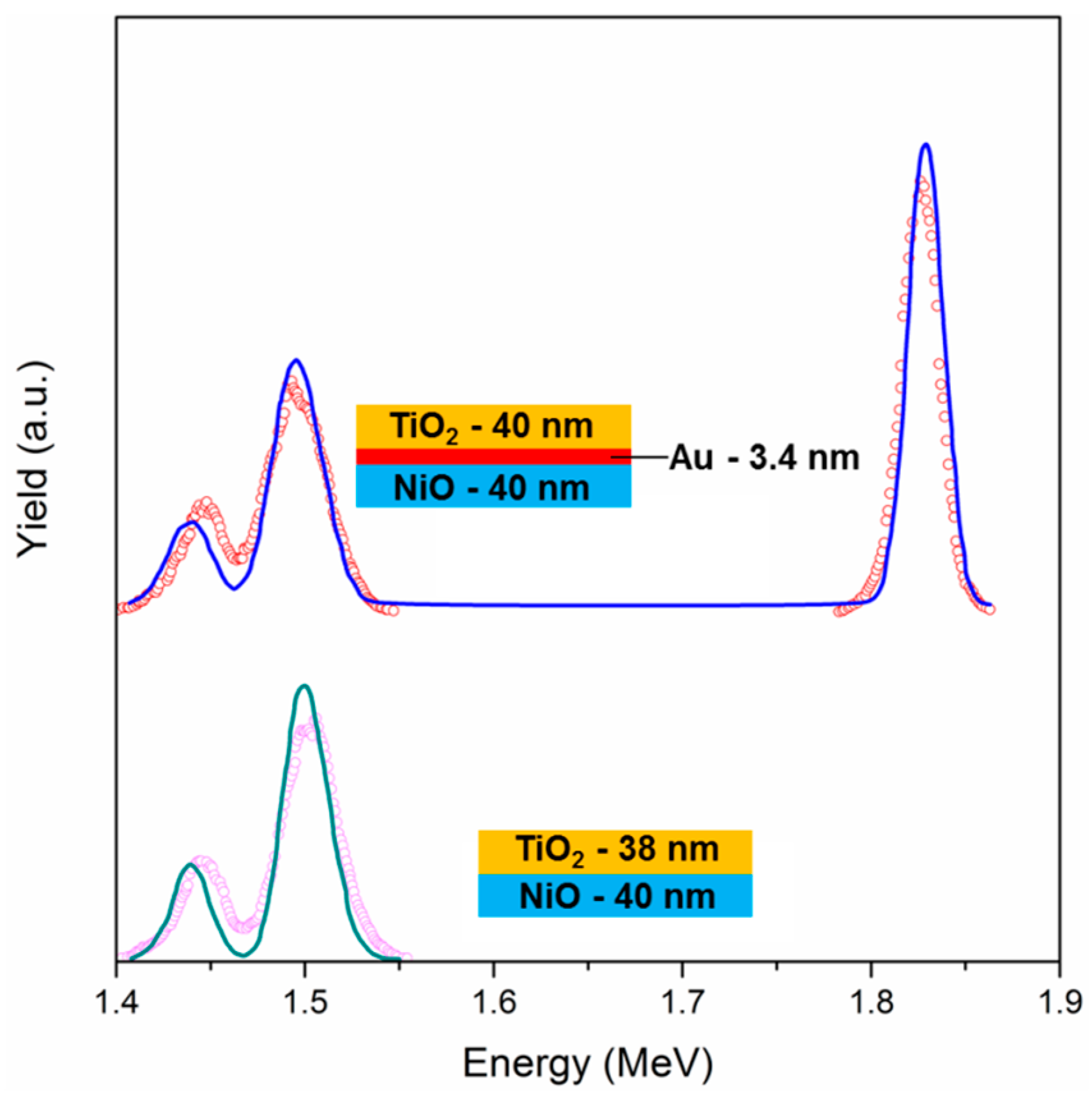
2 as top layer—with and without Au NPs, have been characterized with Rutherford Backscattering Spectrometry (RBS): This technique is useful to gain information about thickness, composition, and spatial distribution over thickness of the different components. The spectrum of the Au-free sample (
Figure 3) shows two distinct peaks, which are centered at about 1.44 MeV and 1.5 MeV, which can be ascribed to Ti and Ni signals, respectively. The predicted energy positions for Ti and Ni (with the experimental setup used) are 1.44 MeV and 1.53 MeV, respectively: Ni signal is found at lower energies because the NiO layer is slightly far from the surface, so it is probed after the TiO
2 film. A simulation has been performed when considering a simple sandwich structure composed of a bottom layer of NiO and a top layer of TiO
2, letting the thickness vary: the best fit was obtained with a TiO
2 layer of 38 nm and a NiO film of 40 nm. The two values are extremely close to each other, confirming the correct choice of the deposition parameters in order to get similar thicknesses, even if the thickness values are slightly lower when compared to the expected ones (about 45 nm), as measured by ellipsometry and SEM analyses (see below): this is because both SEM and ellipsometry take into account the porosity of the films, while the RBS technique is based on nominal density for the different materials, and measuring the atoms/cm
2 values, the apparent thickness evaluated with RBS is reliable only if measuring fully dense materials. Nonetheless, having obtained similar thicknesses for both oxide coatings is a further proof of the accuracy of the experimental procedure.
The same sample architecture, but with a layer of Au NPs in between the two oxides (
Figure 3), shows the same two peaks at 1.44 MeV and 1.5 MeV, and an additional peak at 1.82 MeV, due to metallic Au. These experimental data have been modeled using a bottom layer of fully dense NiO of 40 nm thickness, an intermediate layer of Au (3.4 nm) and a top layer of dense TiO
2 of 40 nm. Again, the two oxide films are of the same thickness, slightly lower than the expectations due to the porosity effect described before. Since the software that is used for the fitting procedure does not take into account the possibility of having a layer composed of NPs, the simulation has been carried out with a bulk gold layer, obtaining a thickness of 3.4 nm as best fit. Using the integral of the Au peak, the dose of Au atoms can be estimated, being it 2 × 10
16 at/cm
2; knowing the actual size of the Au NPs (13 nm), and using simple mathematics it is possible to estimate the Au NPs surface coverage, being it about 2.9 × 10
11 NPs/cm
2. When considering the area of a single Au NP having a diameter of 13 nm, the estimated surface coverage is about 38%. This value, although being affected by a considerable error due to the simple calculations that for example do not take into account NPs size dispersity and crystalline structure, is quite close to the surface coverage value that was evaluated from a bare Au NPs layer deposited using the same experimental parameters (34%). So, the surface coverage of Au NPs is thereby qualitatively confirmed.
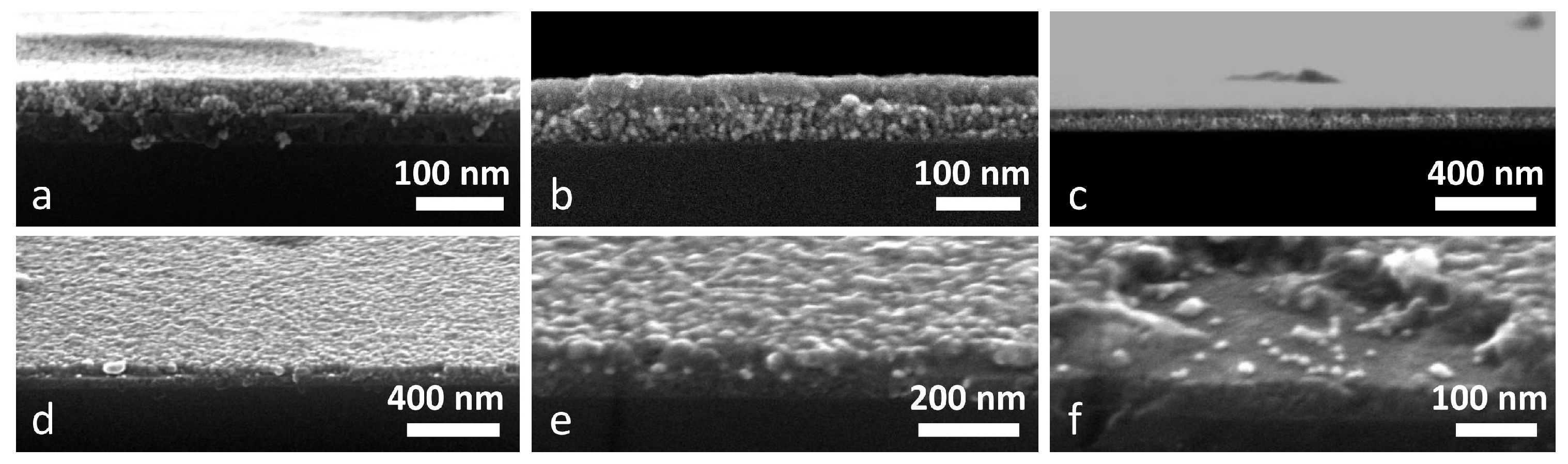
SEM characterization has been carried out performing the measurements in top view and in cross section, in order to evaluate the presence of the two layers, their thickness and morphology, and to examine the Au NPs distribution across the samples; all of the results are reported in
Figure 4.
Figure 4a–c shows the Au NPs layer embedded between TiO
2 and NiO films: anatase film is the bottom layer in
Figure 4a, and the top layer in
Figure 4b,c. The morphology of the two oxides is clearly different: TiO
2 films are more compact and smooth, while NiO layers have a more structured morphology, with the crystalline grains being clearly identifiable. Moreover, from the morphological difference between the two oxides, it seems that the NiO film has a higher porosity when compared to the TiO
2 layer, and effectively this has been confirmed by the ellipsometric evaluation discussed before. The thicknesses evaluated from the SEM images is in good agreement with the predicted values: In
Figure 4a, TiO
2 and NiO films have been measured to be around 45 nm and 47 nm thick, respectively, while in
Figure 4b, samples the evaluated thickness is 43 nm and 46 nm, respectively. Therefore, the target thickness of ~45 nm is confirmed. Au NPs can be seen as brighter spots, but since the difference in contrast with NiO crystals is quite low, it is sometimes difficult to distinguish them. Nevertheless, especially in
Figure 4b, few bright particles in between the two oxide films can be recognized. From the low magnification image (
Figure 4c), the high quality of the sandwich structure over few microns can be appreciated, and also Au NPs as brighter spots can be seen throughout the whole image, in between the two oxides.
Figure 4d–f show some images of a sample composed of Au NPs that are embedded between a bottom TiO
2 layer and a top ZnO layer. Again, the difference in morphology between the two oxides can be seen, being ZnO rougher and TiO
2 smoother, but also, Au NPs can be clearly seen due to the higher contrast difference. Bright circular spots exactly at the ZnO-TiO
2 interface are seen in all three images, and their size has been estimated in the 10 nm–15 nm range, as consistent with the value of the as-synthesized particles. We previously reported that Au NPs deposited on glass tend to sinter upon thermal treatment, but when the Au NPs are covered with a metal oxide film, this provides a physical barrier that strongly reduces the temperature-driven sintering [
10].
Figure 4f shows a picture of the double layer where a portion of the ZnO layer is missing (probably as a consequence of the sample cutting and handling): few Au NPs that are deposited over the TiO
2 film can be easily seen, giving another proof of the metal NPs presence at the interface between the two oxides. Moreover, the thickness of TiO
2 and ZnO layers has been evaluated as well, being 46 nm and 51 nm, respectively; again, the predicted thickness is hereby confirmed, even if the ZnO film is slightly thicker than expected, possibly because its high surface roughness makes the precise evaluation of the thickness quite challenging.
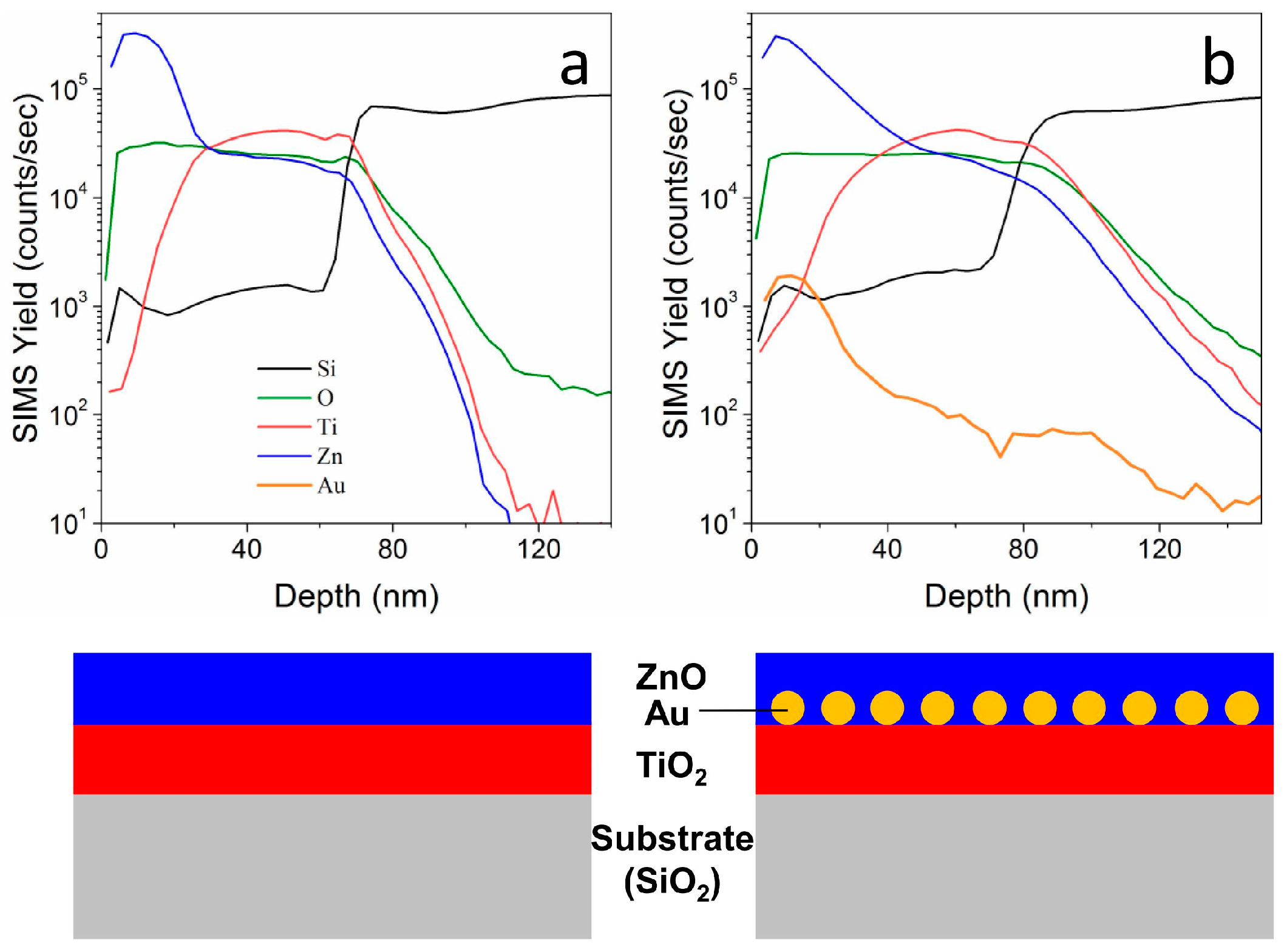
SIMS provides another confirmation of the actual structure of the layered films (
Figure 5): we evaluated the compositional depth profiles for two TiO
2-ZnO films that were deposited on silicon substrates, with (b) and without (a) an Au NPs layer that is embedded in between. The total samples thickness has been estimated to be around 80 nm (based on Si and O signals), in good agreement with previous characterizations. The Zn signal is detected in both samples at the surface, while the Ti signal is centered around 40–60 nm far from the surface. The sample containing Au NPs (
Figure 5b) shows Au signal that is centered about 20 nm from the surface, not exactly in between the two oxides, but slightly closer to the surface. This is understandable when considering the structure and morphology of the sample (
Figure 4d–f), where it can be seen that Au NPs are laying on top of the anatase layer, and they are surrounded and submerged by the top ZnO coating. The schematic presented in
Figure 5c shows the sample structure, highlighting the position of Au NPs. Moreover, with such thin layered structures the thickness estimation using SIMS is affected by a substantial error. However, a further confirmation of the results that were presented in the previous characterizations has been obtained.
We already studied the optical gas sensing properties of NiO, ZnO, and TiO
2 film containing Au NPs [
23,
24], showing how the interaction of the target gas with the metal oxide matrix can be monitored by looking at the SPR of the Au NPs. Here, we embedded the Au NPs between two different metal oxide layers for studying the effect, if any, of their coupling on the optical gas sensing properties. For this preliminary study, H
2 and CO have been tested as target gas because they were also used in our previous study on single metal oxides.
Among the different synthetized multilayer structures, the NiO-Au-TiO
2 (NAT) and TiO
2-Au-NiO (TAN) multilayers have been selected for the gas sensing measurements, because in our previous studies, the NiO-Au and TiO
2-Au films showed good optical gas sensing properties toward H
2 and CO [
23,
24].
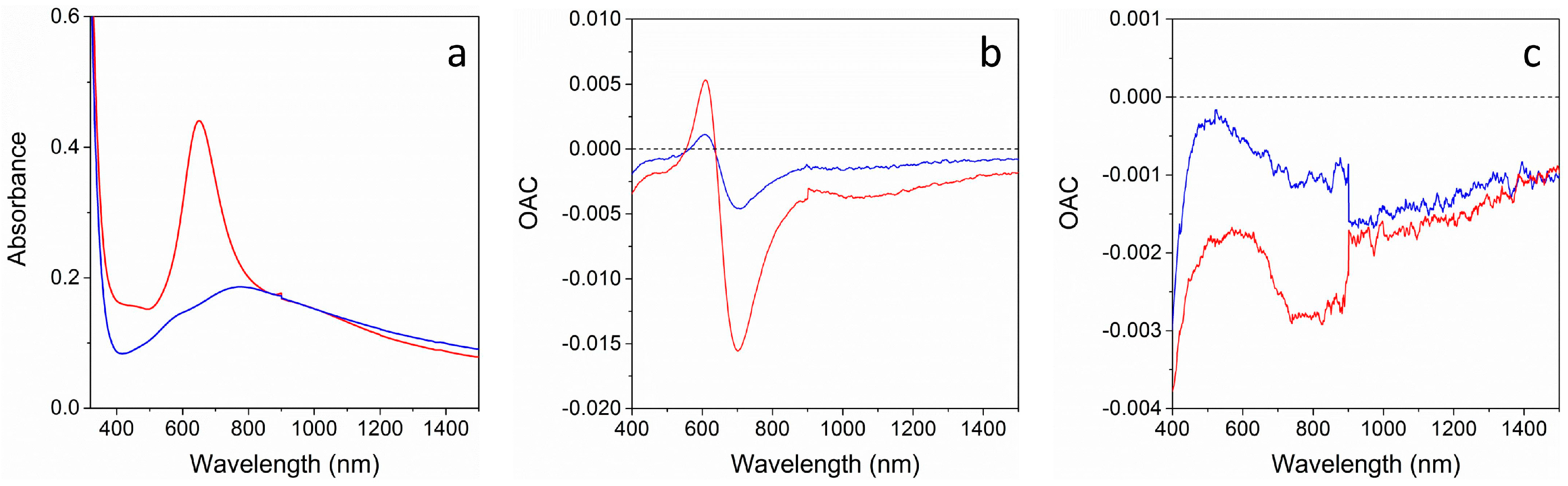
Figure 6 shows the absorption spectra of the two multi-layer structures and their Optical Absorbance Change (OAC) parameter, defined as the difference between absorbance during gas exposure and absorbance in air (OAC = Abs
Gas − Abs
Air). The two samples respond rather differently: outside the 600–900 nm range, a decrease in absorption when exposed to both gases is seen for both samples, because of the interaction of the reducing gas with the NiO film [
23]. Inside the 600–900 nm range, the NAT sample shows a sharp and strongly wavelength dependent signal, which is more intense for H
2 when compared to CO, while for the TAN sample, only a weak modulation of the OAC curve is observed. This large difference can be related to the optical absorption spectra of the two samples (
Figure 6a): NAT sample shows a narrow and sharp Au SPR peak, possibly due to a partial detachment of Au NPs when depositing the top TiO
2 layer, while TAN optical spectrum presents a much broader, weaker, and red shifted plasmon peak. As a consequence, the difference from the spectra collected during gas exposure and the spectra collected in air, i.e., the OAC parameter, is strongly affected, being much higher when the optical spectrum has steep features, and much lower when the optical spectrum has plainer features.
In any case, for both of the samples, a reversible response for both gases is observed and some distinctive wavelengths corresponding to maximum and minimum (or null) response can be identified, theoretically permitting selective gas recognition through an appropriate choice of the analysis wavelength [
25,
26].
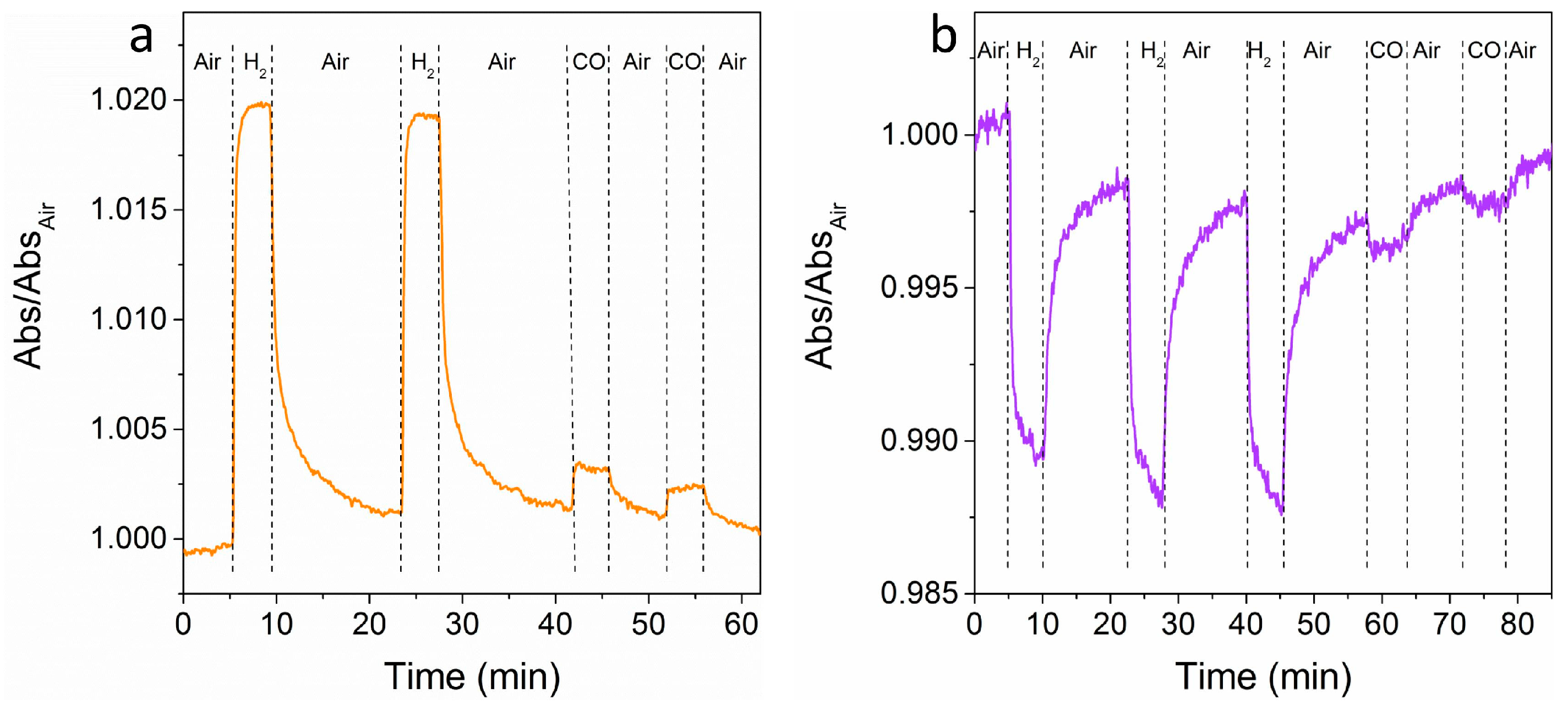
Figure 7 shows time-resolved tests at a fixed wavelength for multiple air-gas-air cycles. The wavelengths have been selected for obtaining a very high signal for H
2 and the smallest signal for CO, in order to demonstrate the selectivity of the sensor. An easily detectable signal for both of the gases is observed, which is much higher for hydrogen when compared to CO, as predicted from OAC curves (see
Figure 6b,c), with relatively fast response times (between 30 s and 60 s) and acceptable recovery times (between 60 s and 90 s).