Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Reagents
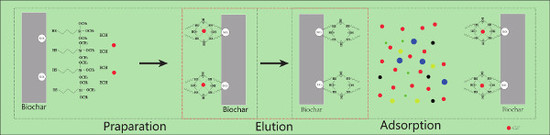
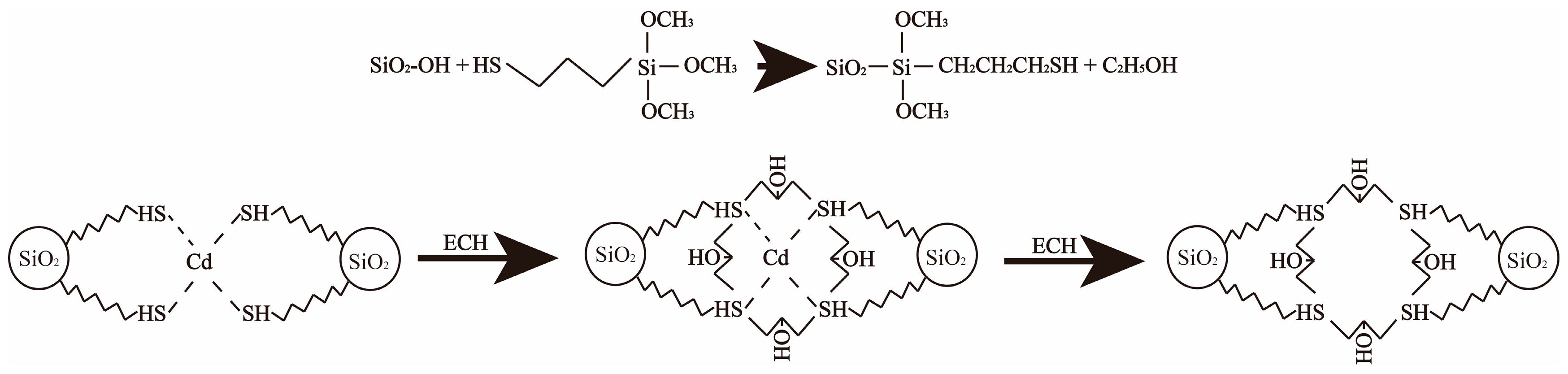
2.2. Biochar Modification
2.3. Adsorption Experiments
2.4. Characterizations
2.5. Data Analysis
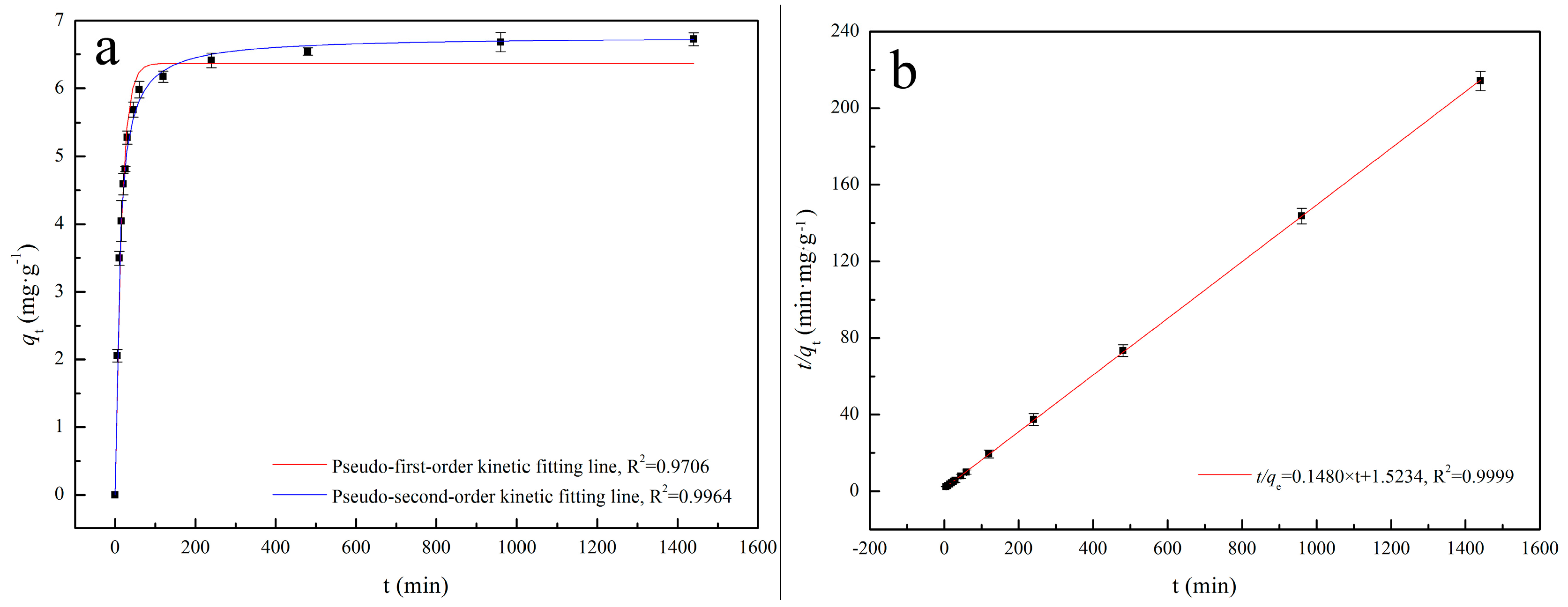
2.5.1. Adsorption Kinetics Analysis
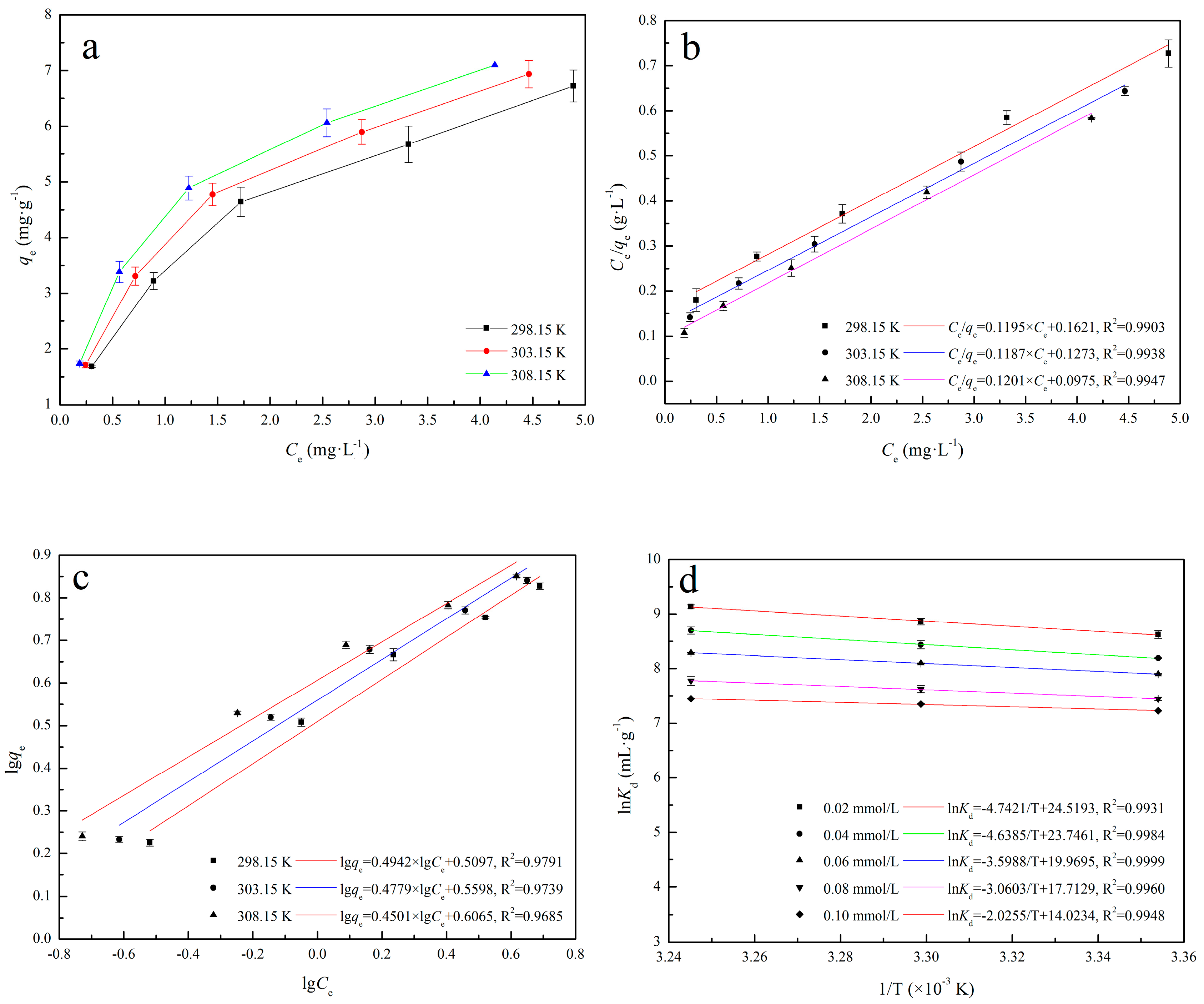
2.5.2. Adsorption Thermodynamics Analysis
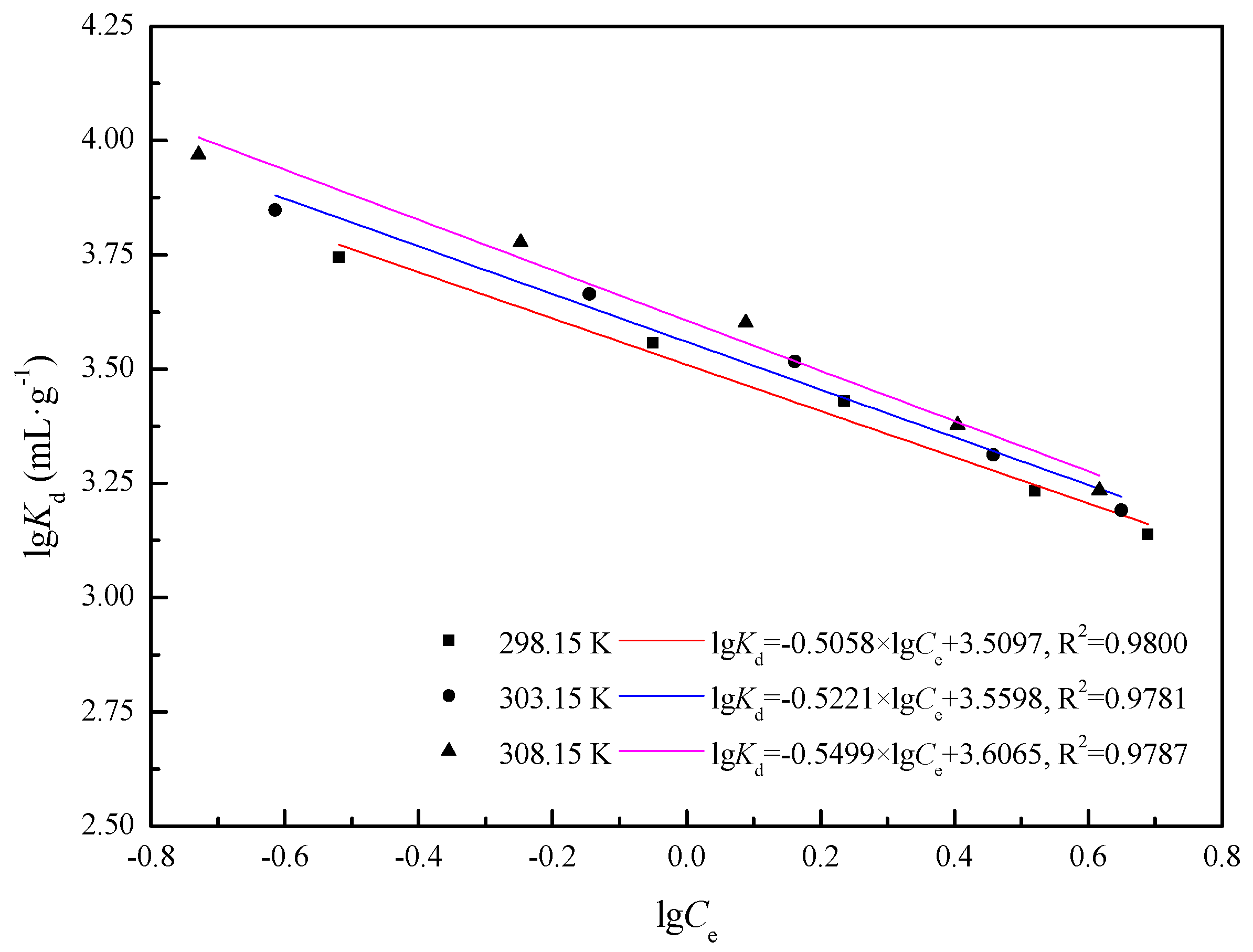
2.5.3. Stoichiometric Displacement Theory of Adsorption Analysis (SDT-A)
3. Results and Discussion
3.1. Biochar Characterization
3.1.1. XRD Analysis
3.1.2. Zeta Potential Analysis
3.1.3. FT-IR Analysis
3.1.4. XPS Analysis
3.2. Adsorption Experiment Results
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
3.2.3. Stoichiometric Displacement Theory of Adsorption
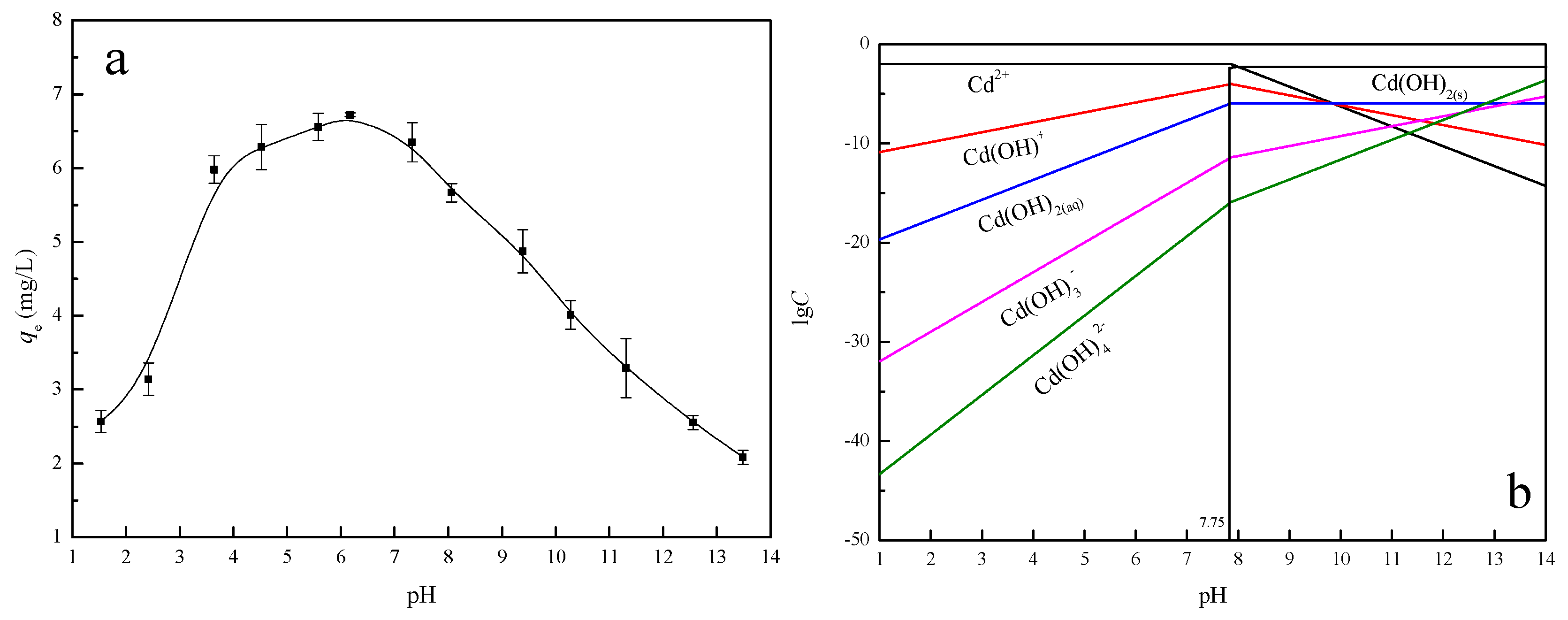
3.2.4. Effect of pH
3.2.5. Effect of Sorbent Dosage
3.2.6. Selective Adsorption
3.2.7. Desorption and Repeated Use
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gozde, D.; Cagdas, O.; Suat, U.; Ralph, S.; Jale, Y. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar]
- Chen, R.; Ye, C. Land management: Resolving soil pollution in China. Nature 2014, 505, 483. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Z.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Chu, C.L.; Li, T.; Xu, S.; Liu, L.; Ju, M. A water quality management strategy for regionally protected water through health risk assessment and spatial distribution of heavy metal pollution in 3 marine reserves. Sci. Total Environ. 2017, 599–600, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Almuktar, S.A.A.A.N.; Scholz, M.; Al-Isawi, R.H.K.; Sani, A. Recycling of domestic wastewater treated by vertical-flow wetlands for irrigating chillies and sweet peppers. Agric. Water Manag. 2015, 149, 1–122. [Google Scholar] [CrossRef]
- Huang, Y.; Qiu, W.; Yu, Z.; Song, Z. Toxic effect of cadmium adsorbed by different sizes of nano-hydroxyapatite on the growth of rice seedlings. Environ. Toxicol. Pharmacol. 2017, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 27–34. [Google Scholar] [CrossRef]
- Flanagan, P.R.; McLellan, J.S.; Haist, J.; Cherian, G.; Chamberlain, M.J.; Valberg, L.S. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology 1978, 74, 841–846. [Google Scholar] [PubMed]
- Kaewsarn, P.; Yu, Q. Cadmium(II) removal from aqueous solutions by pre-treated biomass of marine alga Padina sp. Environ. Pollut. 2001, 112, 209–213. [Google Scholar] [CrossRef]
- Singh, G.; Rana, D.; Matsuura, T.; Ramakrishna, S.; Narbaitz, R. Removal of disinfection byproducts from water by carbonized electrospun nanofibrous membranes. Sep. Purif. Technol. 2010, 74, 202–212. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.K.; Kobayashi, M.; Fumiaki, T.; Hideaki, S.; Takami, S.; Kentaro, T.; Ryusuke, H.; Takayoshi, K. Greenhouse gas emissions after a prescribed fire in white birch-dwarf bamboo stands in northern Japan, focusing on the role of charcoal. Eur. J. For. Res. 2011, 130, 1031–1044. [Google Scholar]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, G.G.; Chen, L.G.; Chen, Y.X.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A. Molecular imprinting polymers and their composites: A promising material for diverse applications. Biomater. Sci. 2017, 5, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.J.; Wang, J.; An, F.Q.; Liu, Q. Molecular imprinted material prepared by novel surface imprinting technique for selective adsorption of pirimicarb. Polymer 2008, 49, 1230–1238. [Google Scholar] [CrossRef]
- Fang, G.Z.; Tan, J.; Yan, X.P. An ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique combined with a sol-gel process for selective solid-phase extraction of cadmium(II). Anal. Chem. 2005, 77, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Cao, X.H.; Hua, R.; Wang, Y.Q.; Liu, Y.T.; Pang, C.; Wang, Y. Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy 2010, 104, 150–155. [Google Scholar] [CrossRef]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Gu, Z.N. Study on hydroxyl in quartz glass and quartz raw materials. J. Chin. Ceram. Soc. 2002, 30, 357–361. [Google Scholar]
- Rovetta, M.R.; Blacic, J.D.; Hervig, R.L.; Holloway, J.R. An experimental study of hydroxyl in quartz using infrared spectroscopy and ion microprobe techniques. J. Geophys. Res. Solid Earth 1989, 94, 5840–5850. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhu, Y.G.; Zhang, S.Q.; Deng, J.; Huang, Y.; Yan, W. Flotation behaviors of perovskite, titanaugite, and magnesium aluminate spinel using octyl hydroxamic acid as the collector. Minerals 2017, 7, 134. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, M.; Zhao, D. Synthesis and properties of magnetic Cu(II) ion imprinted composite adsorbent for selective removal of copper. Desalination 2008, 228, 135–149. [Google Scholar] [CrossRef]
- Arzu, E.; Rıdvan, S.; Adil, D. Ni(II) ion-imprinted solid-phase extraction and preconcentration in aqueous solutions by packed-bed columns. Anal. Chim. Acta 2004, 502, 91–97. [Google Scholar]
- Geng, X.D.; Zebolsky, D.M. The stoichiometric displacement model and Langmuir and Freundlich adsorption. J. Chem. Educ. 2002, 79, 385–388. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, X.D. A quantitative relationship between the affinity of component to adsorbent in liquid–solid system, βa, and composition of bulk solution. Thermochim. Acta 2003, 404, 109–115. [Google Scholar] [CrossRef]
- Song, Z.H.; Geng, X.D. A Study on the retention mechanism of solute of liquid-solid chromatography by stoichiometric displacement model. Acta Chim. Sin. 1990, 48, 237–241. [Google Scholar]
- Xu, R.; Zhou, G.Y.; Tang, Y.H.; Chu, L.; Liu, C.B.; Zeng, Z.B.; Luo, S.L. New double network hydrogel adsorbent: Highly efficient removal of Cd(II) and Mn(II) ions in aqueous solution. Chem. Eng. J. 2015, 275, 179–188. [Google Scholar] [CrossRef]
| Sample | Binding Energy (eV) | Surface Atomic Composition (%) | ||||
|---|---|---|---|---|---|---|
| C1s | Si2p | S2p | C1s | Si2p | S2p | |
| Initial biochar | 284.80 | 102.07 | 164.39 | 53.75 | 9.31 | 0.17 |
| Actived biochar | 284.79 | 102.69 | 164.41 | 65.29 | 13.92 | 0.29 |
| No-elution biochar | 284.79 | 103.72 | 163.01 | 67.75 | 12.63 | 2.84 |
| Imprinted biochar | 284.81 | 103.73 | 163.89 | 69.33 | 13.28 | 3.03 |
| C0 (mmol·L−1) | ΔH0 (kJ·mol−1) | ΔS0 (J·mol−1·K−1) | ΔG0 (kJ·mol−1) | ||
|---|---|---|---|---|---|
| 298.15 K | 303.15 K | 308.15 K | |||
| 0.02 | 39.43 | 203.87 | −21.35 | −22.37 | −23.39 |
| 0.04 | 38.57 | 197.44 | −20.30 | −21.29 | −22.27 |
| 0.06 | 29.92 | 166.04 | −19.58 | −20.41 | −21.24 |
| 0.08 | 25.44 | 147.27 | −18.46 | −19.20 | −19.94 |
| 0.10 | 16.84 | 116.60 | −17.92 | −18.51 | −19.09 |
| C0 (mmol·L−1) | ΔHT (kJ·mol−1) | ΔST (J·mol−1·K−1) | ΔGT (kJ·mol−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 298.15 | 303.15 | 308.15 | 298.15 | 303.15 | 308.15 | 298.15 | 303.15 | 308.15 | |
| 0.02 | 21.05 | 21.70 | 22.68 | 142.83 | 146.21 | 150.29 | −21.53 | −22.54 | −23.64 |
| 0.04 | 17.42 | 18.15 | 18.95 | 126.11 | 129.48 | 133.16 | −21.19 | −22.22 | −22.08 |
| 0.06 | 15.20 | 15.78 | 16.35 | 115.93 | 118.56 | 121.18 | −19.36 | −19.94 | −20.76 |
| 0.08 | 13.00 | 13.48 | 13.89 | 105.78 | 108.00 | 109.90 | −18.54 | −19.26 | −19.97 |
| 0.10 | 11.69 | 12.00 | 12.25 | 99.78 | 101.19 | 102.35 | −18.06 | −18.68 | −19.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials 2018, 11, 299. https://doi.org/10.3390/ma11020299
Zhang S, Yang X, Liu L, Ju M, Zheng K. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials. 2018; 11(2):299. https://doi.org/10.3390/ma11020299
Chicago/Turabian StyleZhang, Shiqiu, Xue Yang, Le Liu, Meiting Ju, and Kui Zheng. 2018. "Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater" Materials 11, no. 2: 299. https://doi.org/10.3390/ma11020299
APA StyleZhang, S., Yang, X., Liu, L., Ju, M., & Zheng, K. (2018). Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials, 11(2), 299. https://doi.org/10.3390/ma11020299