Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum
Abstract
1. Introduction
2. Results
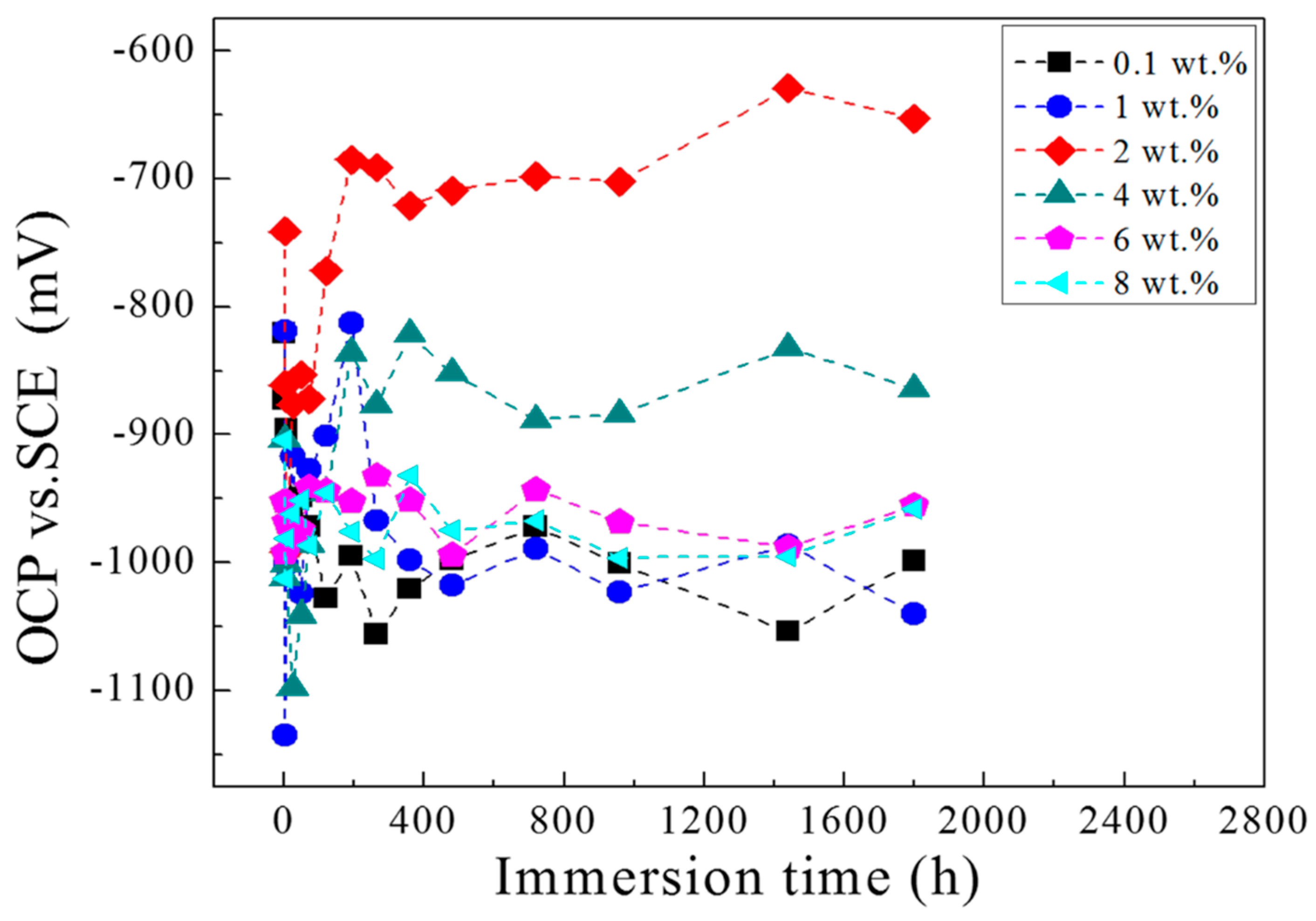
2.1. OCP Measurement
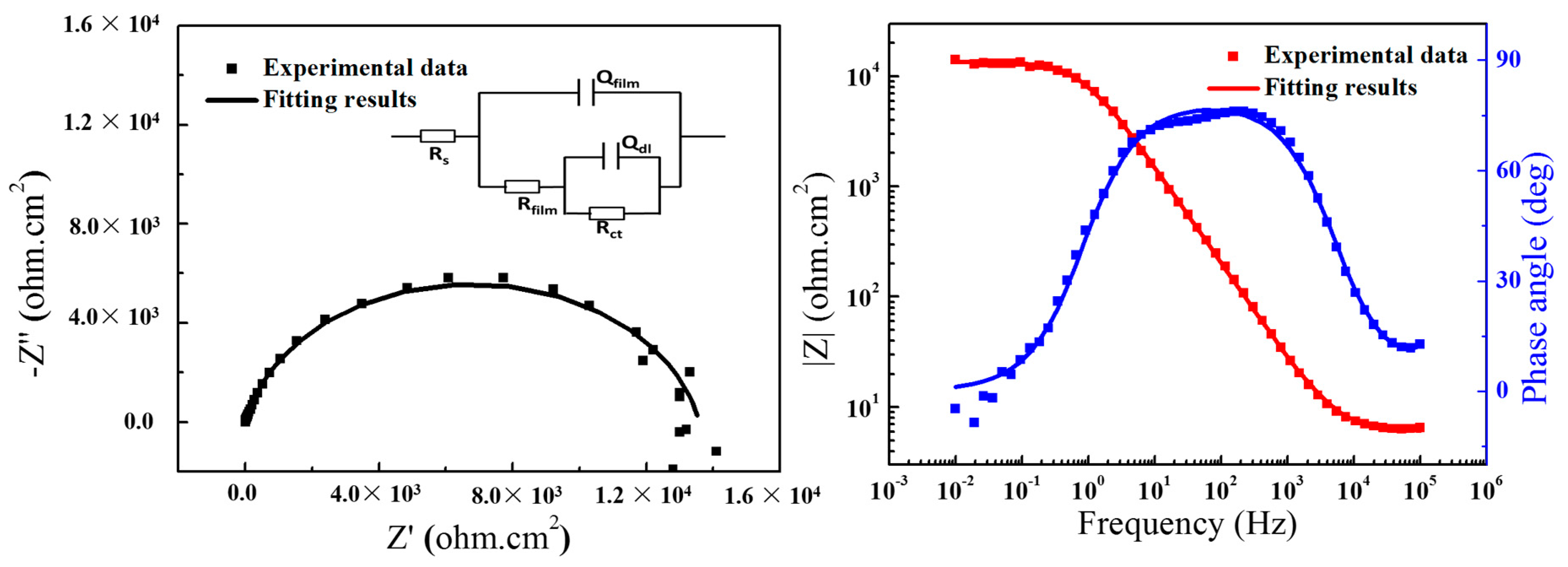
2.2. Electrochemical Impedance Spectroscopy (EIS)
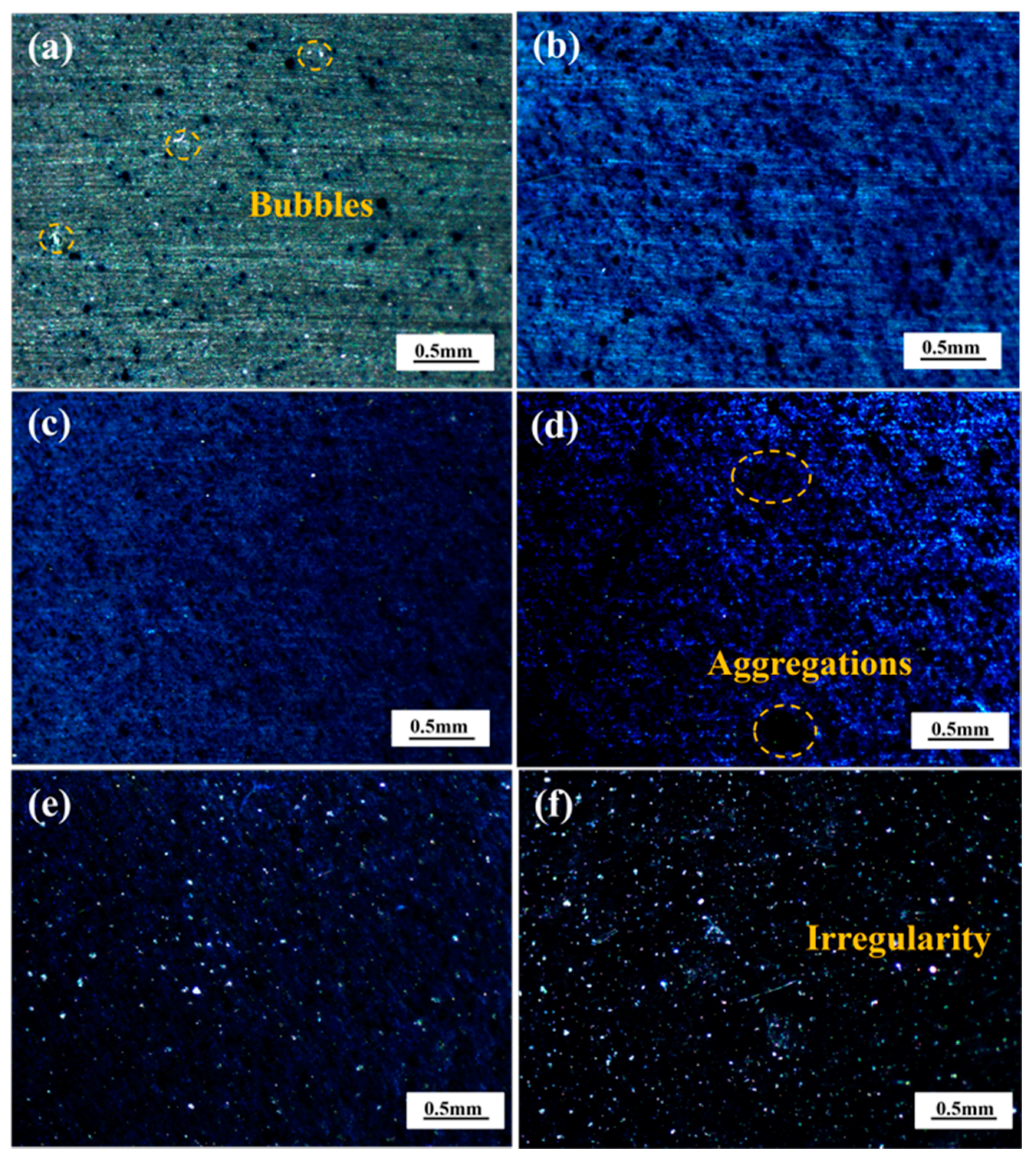
2.3. Surface Morphology of the Coatings
2.4. Gravimetric Results
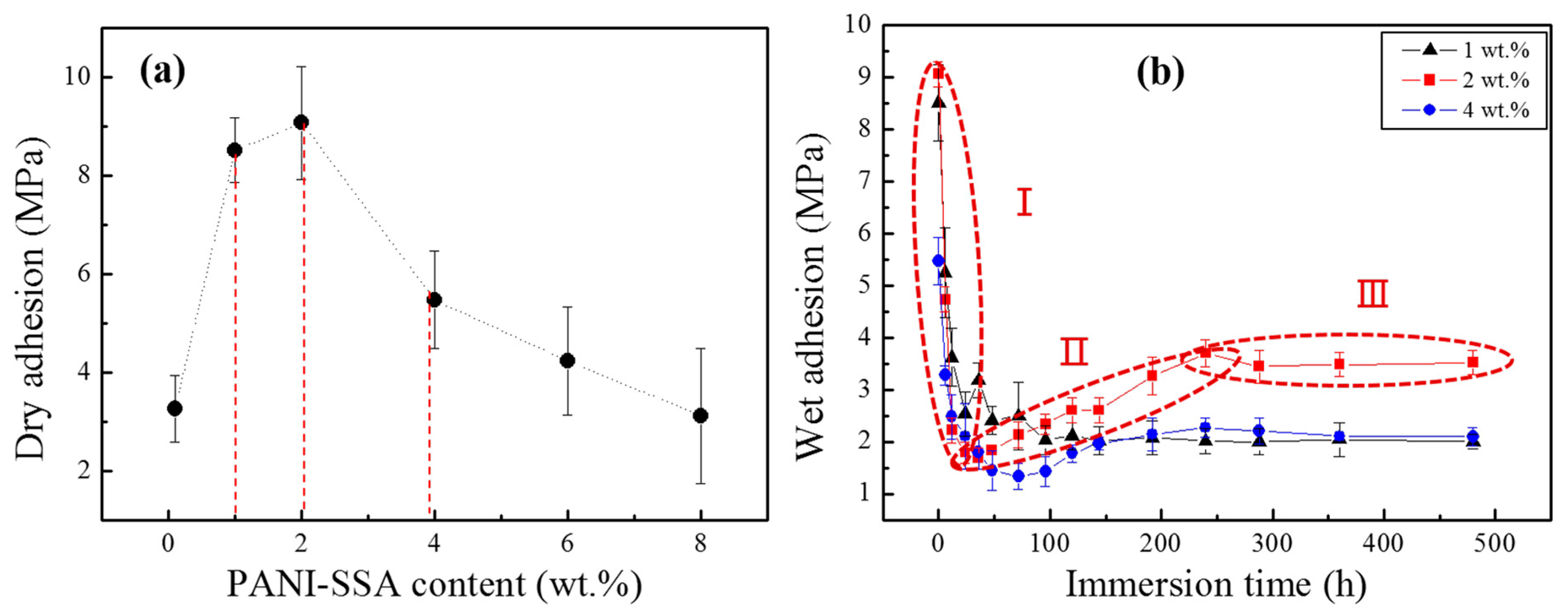
2.5. Adhesion Measurements
2.6. Tensile Results
2.7. Surface Analysis of the Based Aluminum
3. Discussion
3.1. Influence of PANI-SSA Content on Protection Properties of Coating
3.2. Influence of PANI-SSA Content on the Aluminum Surface Beneath Coating
3.3. The Fitting Content of PANI-SSA in Epoxy Coating
4. Materials and Methods
4.1. Sample Preparation
4.2. EIS Measurements
4.3. Adhesion Tests
4.4. Gravimetric Tests
4.5. Tensile Test
4.6. Analysis of Morphology of the Coatings and the Structure of Based Aluminum
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoshioka, H.; Yoshida, S.; Kawashima, A.; Asami, K.; Hashimoto, K. The Pitting Corrosion Behavior of Rapidly Solidified Aluminum-Alloys. Corros. Sci. 1986, 26, 795–807. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting corrosion of metals—A review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Insight into the Pitting Corrosion Behavior of Aluminum-Alloys. Corros. Sci. 1992, 33, 1193–1202. [Google Scholar] [CrossRef]
- Grilli, R.; Baker, M.A.; Castle, J.E.; Dunn, B.; Watts, J.F. Corrosion behaviour of a 2219 aluminium alloy treated with a chromate conversion coating exposed to a 3.5% NaCl solution. Corros. Sci. 2011, 53, 1214–1223. [Google Scholar] [CrossRef]
- Twite, R.L.; Bierwagen, G.P. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Tian, J.; Luo, Z.Z.; Qi, S.K.; Sun, X.J. Structure and antiwear behavior of micro-arc oxidized coatings on aluminum alloy. Surf. Coat. Technol. 2002, 154, 1–7. [Google Scholar] [CrossRef]
- Racicot, R.J.; Yang, S.C.; Brown, R. Evidence of a passive layer formation from a conductive polymer coating on aluminum alloys. Mater. Res. Soc. Symp. Proc. 1997, 458, 415–420. [Google Scholar] [CrossRef]
- Racicot, R.J.; Clark, R.L.; Liu, H.B.; Yang, S.C.; Alias, M.N.; Brown, R. Anti-corrosion studies of novel conductive polymer coatings on aluminum alloys. Mater. Res. Soc. Symp. Proc. 1996, 413, 529–534. [Google Scholar] [CrossRef]
- Seegmiller, J.C.; da Silva, J.E.P.; Buttry, D.A.; de Torresi, S.I.C.; Torresi, R.M. Mechanism of action of corrosion protection coating for AA2024-T3 based on poly(aniline)-poly(methylmethacrylate) blend. J. Electrochem. Soc. 2005, 152, B45–B53. [Google Scholar] [CrossRef]
- Cogan, S.F.; Gilbert, M.D.; Holleck, G.L.; Ehrlich, J.; Jillson, M.H. Galvanic coupling of doped poly-aniline and aluminum alloy 2024-T3. J. Electrochem. Soc. 2000, 147, 2143–2147. [Google Scholar] [CrossRef]
- Cecchetto, L.; Ambat, R.; Davenport, A.J.; Delabouglise, D.; Petit, J.P.; Neel, O. Emeraldine base as corrosion protective layer on aluminium alloy AA5182, effect of the surface microstructure. Corros. Sci. 2007, 49, 818–829. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Silva, T.M.E.; Montemor, M.F.; Fernandes, J.C.S.; Ferreira, M.G.S. Polyaniline coatings on aluminium alloy 6061-T6: Electrosynthesis and characterization. Electrochim. Acta 2010, 55, 3580–3588. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N. Polyaniline inhibition of filiform corrosion on organic coated AA2024-T3. Electrochim. Acta 2009, 54, 4245–4252. [Google Scholar] [CrossRef]
- Gupta, G.; Birbilis, N.; Cook, A.B.; Khanna, A.S. Polyaniline-lignosulfonate/epoxy coating for corrosion protection of AA2024-T3. Corros. Sci. 2013, 67, 256–267. [Google Scholar] [CrossRef]
- Tallman, D.E.; Pae, Y.; Bierwagen, G.P. Conducting polymers and corrosion: Polyaniline on steel. Corrosion 1999, 55, 779–786. [Google Scholar] [CrossRef]
- Tallman, D.E.; Pae, Y.; Bierwagen, G.P. Conducting polymers and corrosion: Part 2—Polyaniline on aluminum alloys. Corrosion 2000, 56, 401–410. [Google Scholar] [CrossRef]
- Spinks, G.M.; Dominis, A.J.; Wallace, G.G.; Tallman, D.E. Electroactive conducting polymers for corrosion control—Part 2. Ferrous metals. J. Solid State Electrochem. 2002, 6, 85–100. [Google Scholar] [CrossRef]
- Tallman, D.E.; Spinks, G.; Dominis, A.; Wallace, G.G. Electroactive conducting polymers for corrosion control Part 1. General introduction and a review of non-ferrous metals. J. Solid State Electrochem. 2002, 6, 73–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Zhang, T.; Meng, G.; Wang, F. The effect of epoxy coating containing emeraldine base and hydrofluoric acid doped polyaniline on the corrosion protection of AZ91D magnesium alloy. Corros. Sci. 2011, 53, 3747–3755. [Google Scholar] [CrossRef]
- Shah, K.G.; Akundy, G.S.; Iroh, J.O. Polyaniline coated on aluminum (Al-2024-T3): Characterization and electrochemical studies. J. Appl. Polym. Sci. 2002, 85, 1669–1675. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.; Venkatachari, G. Performance studies of phosphate-doped polyaniline containing paint coating for corrosion protection of aluminium alloy. J. Appl. Polym. Sci. 2008, 107, 2224–2230. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Ghoreishi, S.M.; Behpour, M. Electropolymerized polyaniline coatings on aluminum alloy 3004 and their corrosion protection performance. Electrochim. Acta 2009, 54, 6989–6995. [Google Scholar] [CrossRef]
- Kamaraj, K.; Devarapalli, R.; Siva, T.; Sathiyanarayanan, S. Self-healing electrosynthesied polyaniline film as primer coat for AA 2024-T3. Mater. Chem. Phys. 2015, 153, 256–265. [Google Scholar] [CrossRef]
- Jia, W.; Tchoudakov, R.; Segal, E.; Narkis, M.; Siegmann, A. Electrically conductive composites based on epoxy resin containing polyaniline-DBSA- and polyaniline-DBSA-coated glass fibers. J. Appl. Polym. Sci. 2004, 91, 1329–1334. [Google Scholar] [CrossRef]
- Jia, W.; Tchoudakov, R.; Segal, E.; Joseph, R.; Narkis, M.; Siegmann, A. Electrically conductive composites based on epoxy resin with polyaniline-DBSA fillers. Synth. Met. 2003, 132, 269–278. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Li, J.; Lu, J.; Wang, F. Long-term anticorrosion behaviour of polyaniline on mild steel. Corros. Sci. 2007, 49, 3052–3063. [Google Scholar] [CrossRef]
- Kendig, M.; Hon, M.; Warren, L. ‘Smart’ corrosion inhibiting coatings. Prog. Org. Coat. 2003, 47, 183–189. [Google Scholar] [CrossRef]
- Deberry, D.W. Modification of the Electrochemical and Corrosion Behavior of Stainless-Steels with an Electroactive Coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Talo, A.; Passiniemi, P.; Forsen, O.; Ylasaari, S. Polyaniline/epoxy coatings with good anti-corrosion properties. Synth. Met. 1997, 85, 1333–1334. [Google Scholar] [CrossRef]
- Fang, J.; Xu, K.; Zhu, L.; Zhou, Z.; Tang, H. A study on mechanism of corrosion protection of polyaniline coating and its failure. Corros. Sci. 2007, 49, 4232–4242. [Google Scholar] [CrossRef]
- Zhong, L.; Zhu, H.; Hu, J.; Xiao, S.H.; Gan, F.X. A passivation mechanism of doped polyaniline on 410 stainless steel in deaerated H2SO4 solution. Electrochim. Acta 2006, 51, 5494–5501. [Google Scholar] [CrossRef]
- Silva, R.S.; Aleman, C.; Ferreira, C.A.; Armelin, E.; Ferreira, J.Z.; Meneguzzi, A. Smart Paint for anodic protection of steel. Prog. Org. Coat. 2015, 78, 116–123. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. In situ synthesis of polyaniline-camphorsulfonate particles in an epoxy matrix for corrosion protection of mild steel in NaCl solution. Corros. Sci. 2014, 85, 204–214. [Google Scholar] [CrossRef]
- Shao, Y.; Huang, H.; Zhang, T.; Meng, G.; Wang, F. Corrosion protection of Mg-5Li alloy with epoxy coatings containing polyaniline. Corros. Sci. 2009, 51, 2906–2915. [Google Scholar] [CrossRef]
- Kraljic, M.; Mandic, Z.; Duic, L. Inhibition of steel corrosion by polyaniline coatings. Corros. Sci. 2003, 45, 181–198. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pasti, I.A.; Vujkovic, M.; Travas-Sejdic, J.; Ciric-Marjanovic, G.; Mentus, S.V. High-performance charge storage by N-containing nanostructured carbon derived from polyaniline. Carbon 2012, 50, 3915–3927. [Google Scholar] [CrossRef]
- Janosevic, A.; Pasti, I.; Gavrilov, N.; Mentus, S.; Ciric-Marjanovic, G.; Krstic, J.; Stejskal, J. Micro/mesoporous conducting carbonized polyaniline 5-sulfosalicylate nanorods/nanotubes: Synthesis, characterization and electrocatalysis. Synth. Met. 2011, 161, 2179–2184. [Google Scholar] [CrossRef]
- Morks, M.F.; Hamdy, A.S.; Fahim, N.F.; Shoeib, M.A. Growth and characterization of anodic films on aluminum alloys in 5-sulfosalicylic acid solution. Surf. Coat. Technol. 2006, 200, 5071–5076. [Google Scholar] [CrossRef]
- Luo, Y.Z.; Wang, X.H.; Guo, W.; Rohwerder, M. Growth Behavior of Initial Product Layer Formed on Mg Alloy Surface Induced by Polyaniline. J. Electrochem. Soc. 2015, 162, C294–C301. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, G.D.; Nishikata, A.; Tsuru, T. Anticorrosive behavior of hydroxyapatite as an environmentally friendly pigment. Corros. Sci. 2002, 44, 1087–1095. [Google Scholar] [CrossRef]
- Shi, A.; Koka, S.; Ullett, J. Performance evaluation on the weathering resistance of two USAF coating systems (standard 85285 topcoat versus fluorinated APC topcoat) via electrochemical impedance spectroscopy. Prog. Org. Coat. 2005, 52, 196–209. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Corrosion mechanisms of phosphated zinc layers on steel as substrates for automotive coatings. Prog. Org. Coat. 1996, 28, 59–75. [Google Scholar] [CrossRef]
- Murray, J.N. Electrochemical test methods for evaluating organic coatings on metals: An update. 1. Introduction and generalities regarding electrochemical testing of organic coatings. Prog. Org. Coat. 1997, 30, 225–233. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, L.; Li, Y.; Wang, F. Study of the failure mechanism of an epoxy coating system under high hydrostatic pressure. Corros. Sci. 2013, 74, 59–70. [Google Scholar] [CrossRef]
- Dalmoro, V.; Alemán, C.; Ferreira, C.A.; dos Santos, J.H.Z.; Azambuja, D.S.; Armelin, E. The influence of organophosphonic acid and conducting polymer on the adhesion and protection of epoxy coating on aluminium alloy. Prog. Org. Coat. 2015, 88, 181–190. [Google Scholar] [CrossRef]
- Dalmoro, V.; dos Santos, J.H.Z.; Armelin, E.; Aleman, C.; Schermann Azambuja, D. Phosphonic acid/silica-based films: A potential treatment for corrosion protection. Corros. Sci. 2012, 60, 173–180. [Google Scholar] [CrossRef]
- Dalmoro, V.; dos Santos, J.H.Z.; Baibich, L.M.; Butler, I.S.; Armelin, E.; Aleman, C.; Azambuja, D.S. Improving the corrosion performance of hybrid sol-gel matrix by modification with phosphonic acid. Prog. Org. Coat. 2015, 80, 49–58. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Y.; Liu, L.; Li, Y.; Wang, F. Studies on Mathematical Models of Wet Adhesion and Lifetime Prediction of Organic Coating/Steel by Grey System Theory. Materials 2017, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.K.; Mahato, K.K.; Ray, B.C. Water absorption behavior, mechanical and thermal properties of nano TiO2 enhanced glass fiber reinforced polymer composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 736–747. [Google Scholar] [CrossRef]
- Chatterjee, A.; Islam, M.S. Fabrication and characterization of TiO2–epoxy nanocomposite. Mater. Sci. Eng. A 2008, 487, 574–585. [Google Scholar] [CrossRef]
- Tian, W.; Liu, L.; Meng, F.; Liu, Y.; Li, Y.; Wang, F. The failure behaviour of an epoxy glass flake coating/steel system under marine alternating hydrostatic pressure. Corros. Sci. 2014, 86, 81–92. [Google Scholar] [CrossRef]
- Funke, W. Problems and progress in organic coatings science and technology. Prog. Org. Coat. 1997, 31, 5–9. [Google Scholar] [CrossRef]
- Santos, C.; Clarke, R.L.; Braden, M.; Guitian, F.; Davy, K.W.M. Water absorption characteristics of dental composites incorporating hydroxyapatite filler. Biomaterials 2002, 23, 1897–1904. [Google Scholar] [CrossRef]
- Wessling, B. Passivation of Metals by Coating with Polyaniline—Corrosion Potential Shift and Morphological-Changes. Adv. Mater. 1994, 6, 226–228. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Wang, X.; Li, J.; Wang, F. Growth kinetics of oxide films at the polyaniline/mild steel interface. Corros. Sci. 2011, 53, 4044–4049. [Google Scholar] [CrossRef]
- Fahlman, M.; Jasty, S.; Epstein, A.J. Corrosion protection of iron/steel by emeraldine base polyaniline: An X-ray photoelectron spectroscopy study. Synth. Met. 1997, 85, 1323–1326. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Ghoreishi, S.M.; Behpour, M. Direct electrosynthesis of polyaniline-montmorrilonite nanocomposite coatings on aluminum alloy 3004 and their corrosion protection performance. Corros. Sci. 2011, 53, 3035–3042. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 2808-2007, Paints and Varnishes-Determination of Film Thickness; Classification; International Organization for Standardization (ISO): Geneva, Switzerland, 2007. [Google Scholar]
- ASTM International. ASTMD 4541-02, Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L. Water up-take evaluation of new waterborne and high solid epoxy coatings: Part I: Measurements by means of gravimetrical methods. Prog. Org. Coat. 1999, 37, 57–67. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 37-2005, Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties; Classification; International Organization for Standardization (ISO): Geneva, Switzerland, 2005. [Google Scholar]



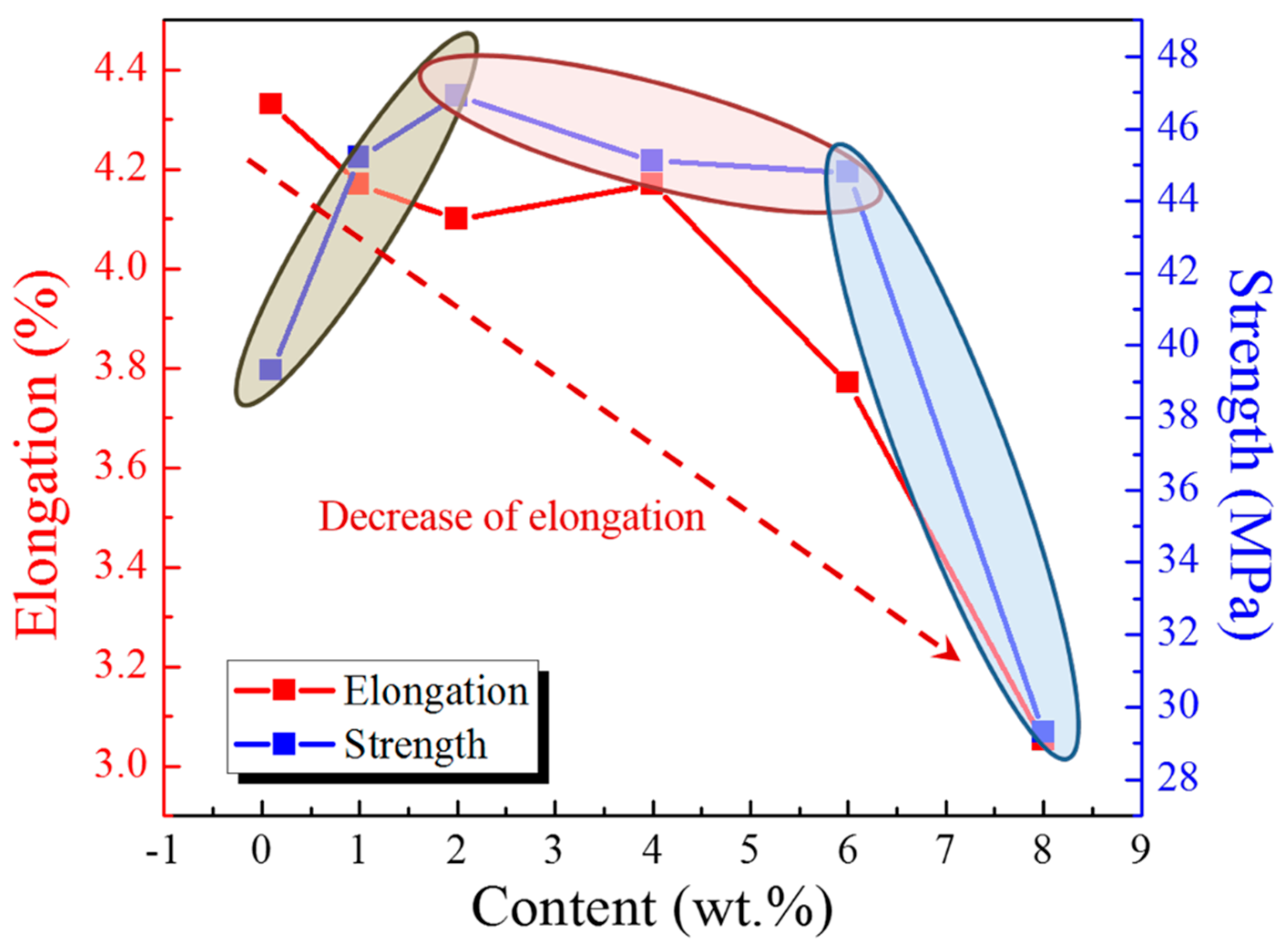
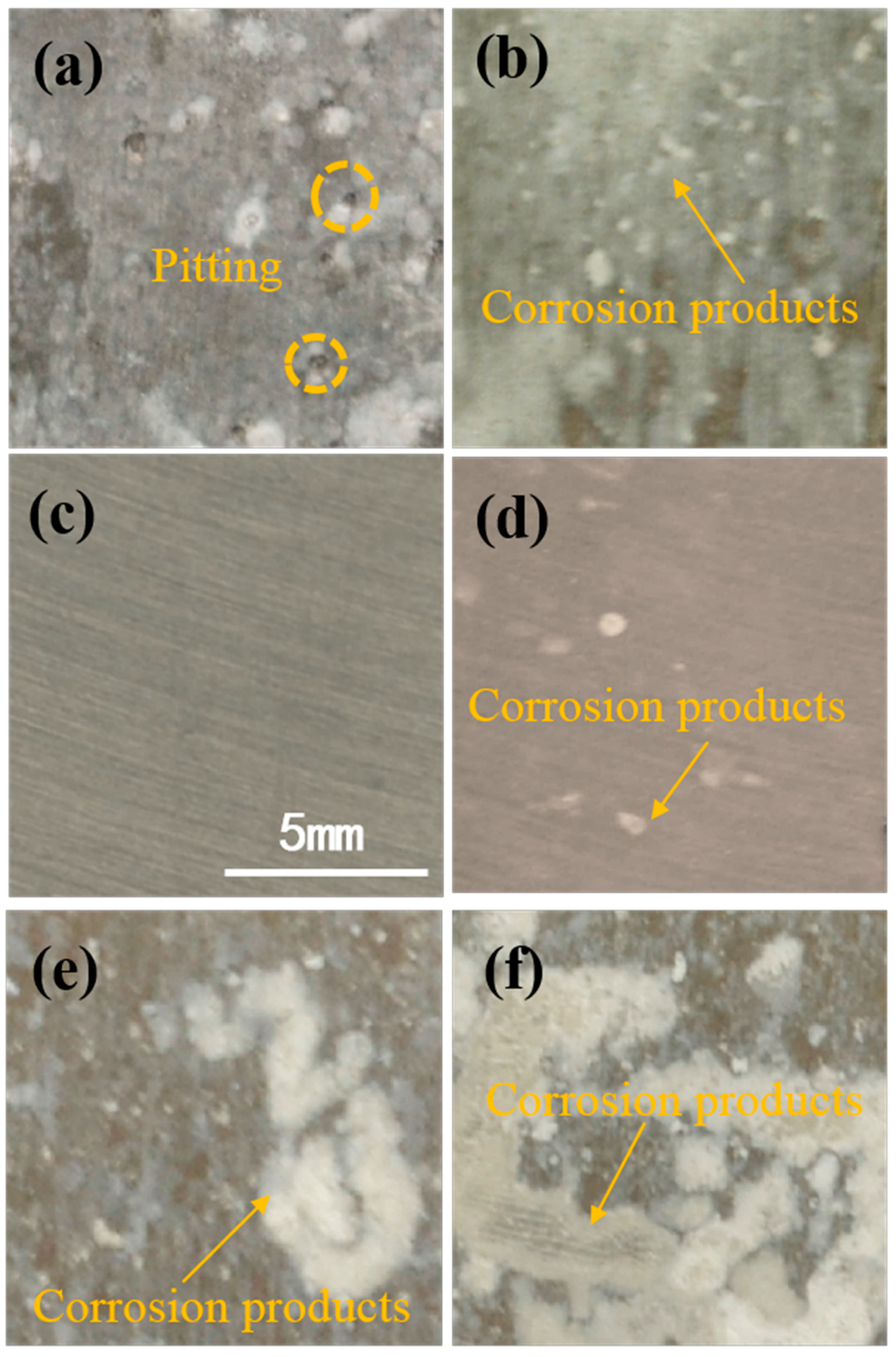
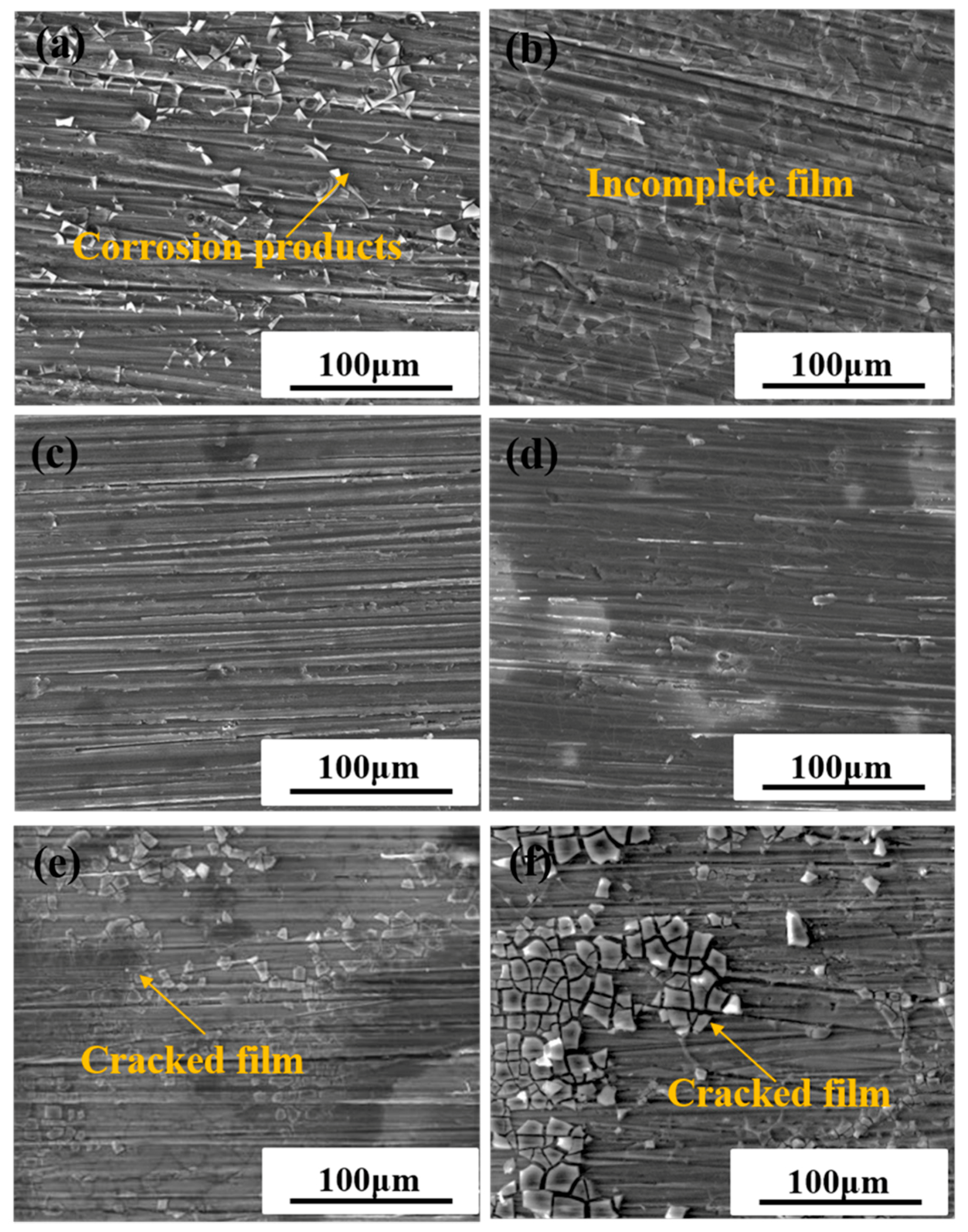

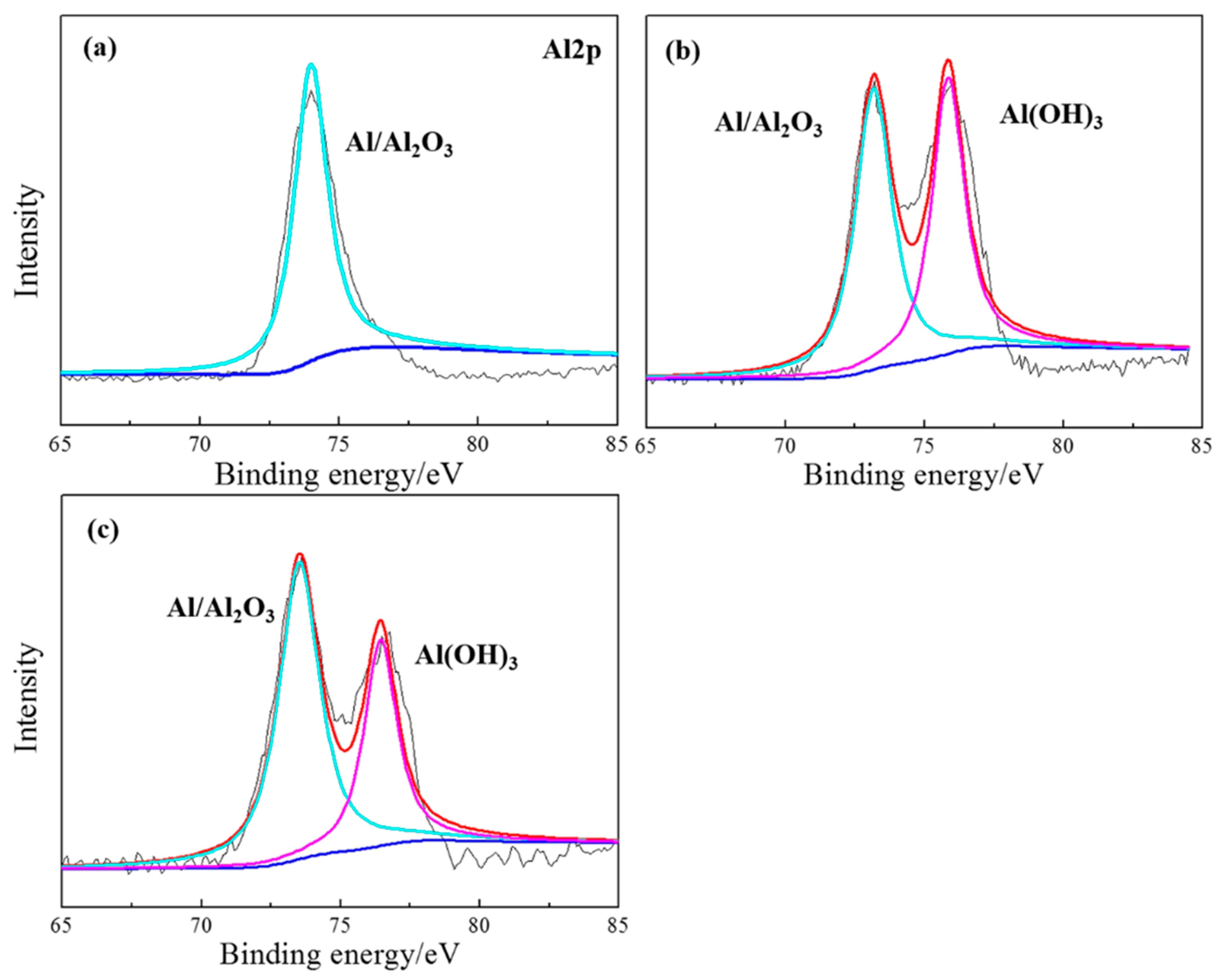

| Element | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Al |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 0.12 | 0.25 | 0.1 | 0.55 | 4.46 | 0.07 | 0.05 | 0.05 | bal |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, L.; Meng, F.; Li, Y.; Wang, F. Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum. Materials 2018, 11, 292. https://doi.org/10.3390/ma11020292
Liu S, Liu L, Meng F, Li Y, Wang F. Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum. Materials. 2018; 11(2):292. https://doi.org/10.3390/ma11020292
Chicago/Turabian StyleLiu, Suyun, Li Liu, Fandi Meng, Ying Li, and Fuhui Wang. 2018. "Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum" Materials 11, no. 2: 292. https://doi.org/10.3390/ma11020292
APA StyleLiu, S., Liu, L., Meng, F., Li, Y., & Wang, F. (2018). Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum. Materials, 11(2), 292. https://doi.org/10.3390/ma11020292





