HDPE/Chitosan Blends Modified with Organobentonite Synthesized with Quaternary Ammonium Salt Impregnated Chitosan
Abstract
1. Introduction
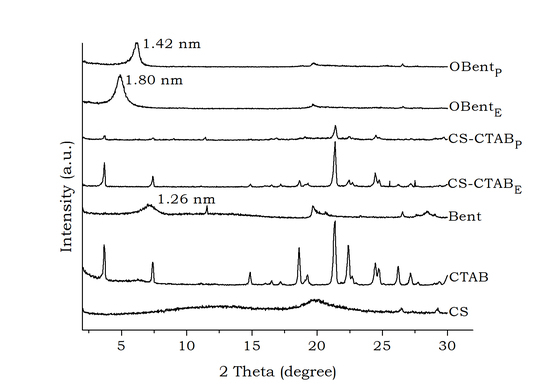
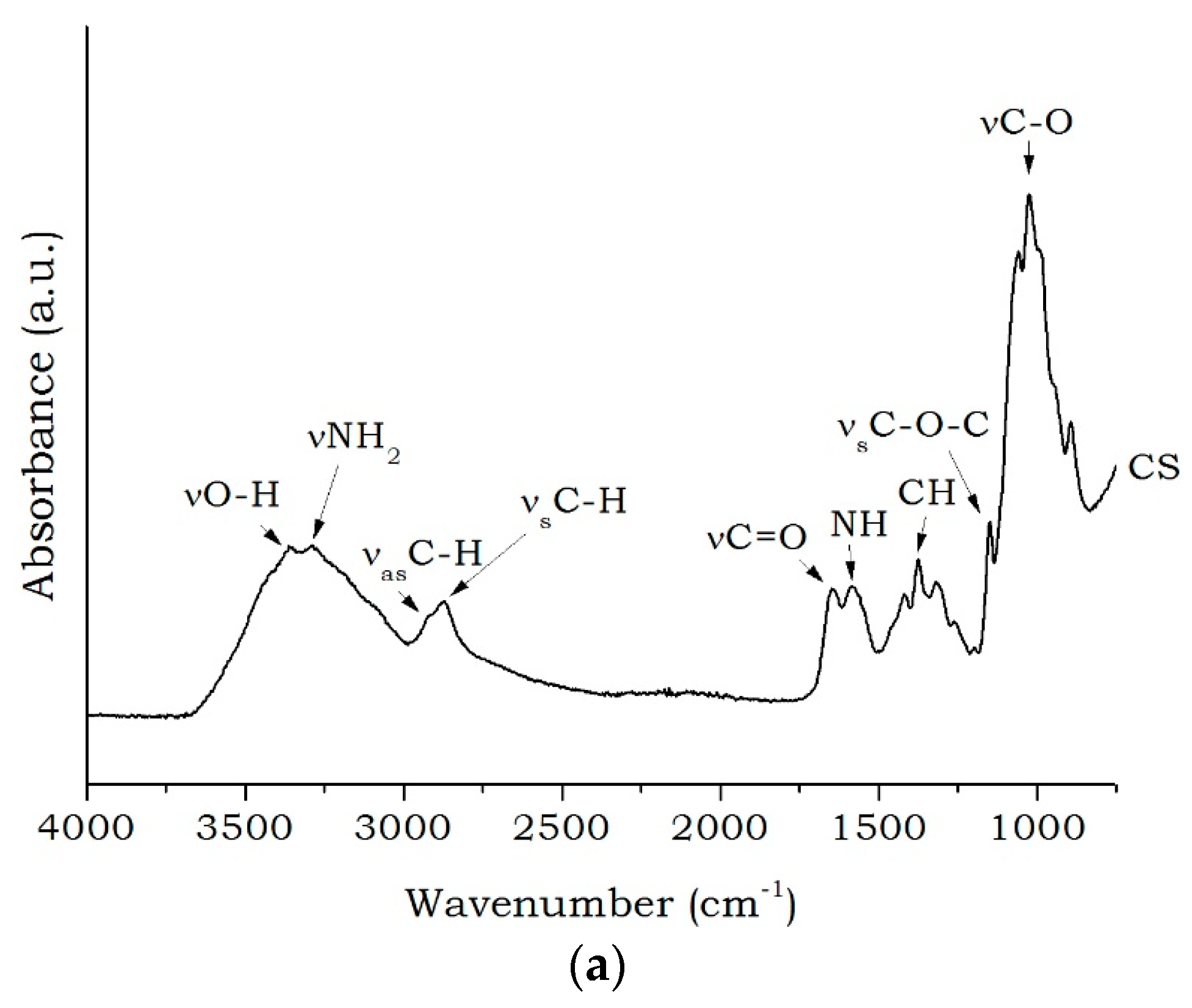
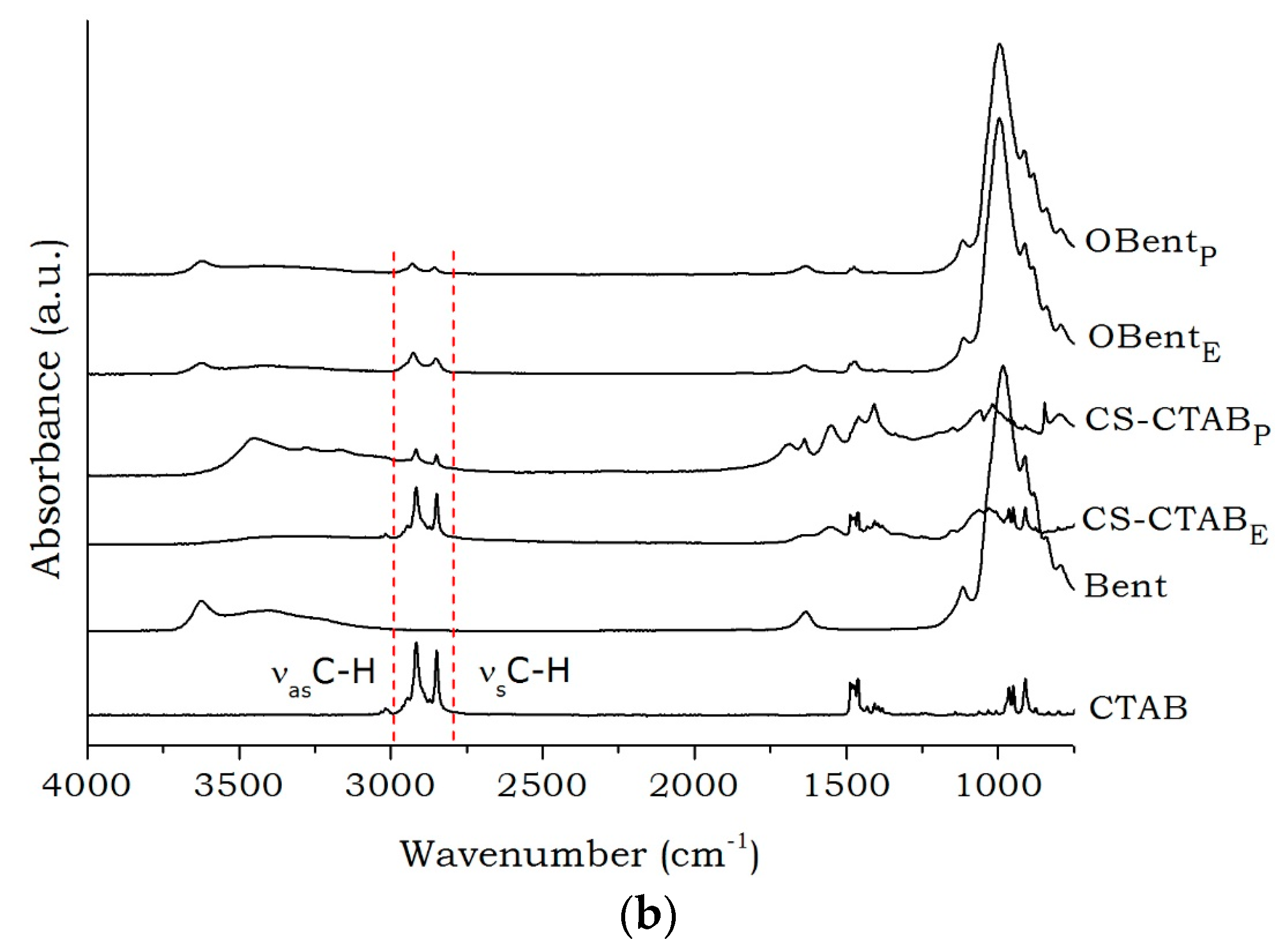
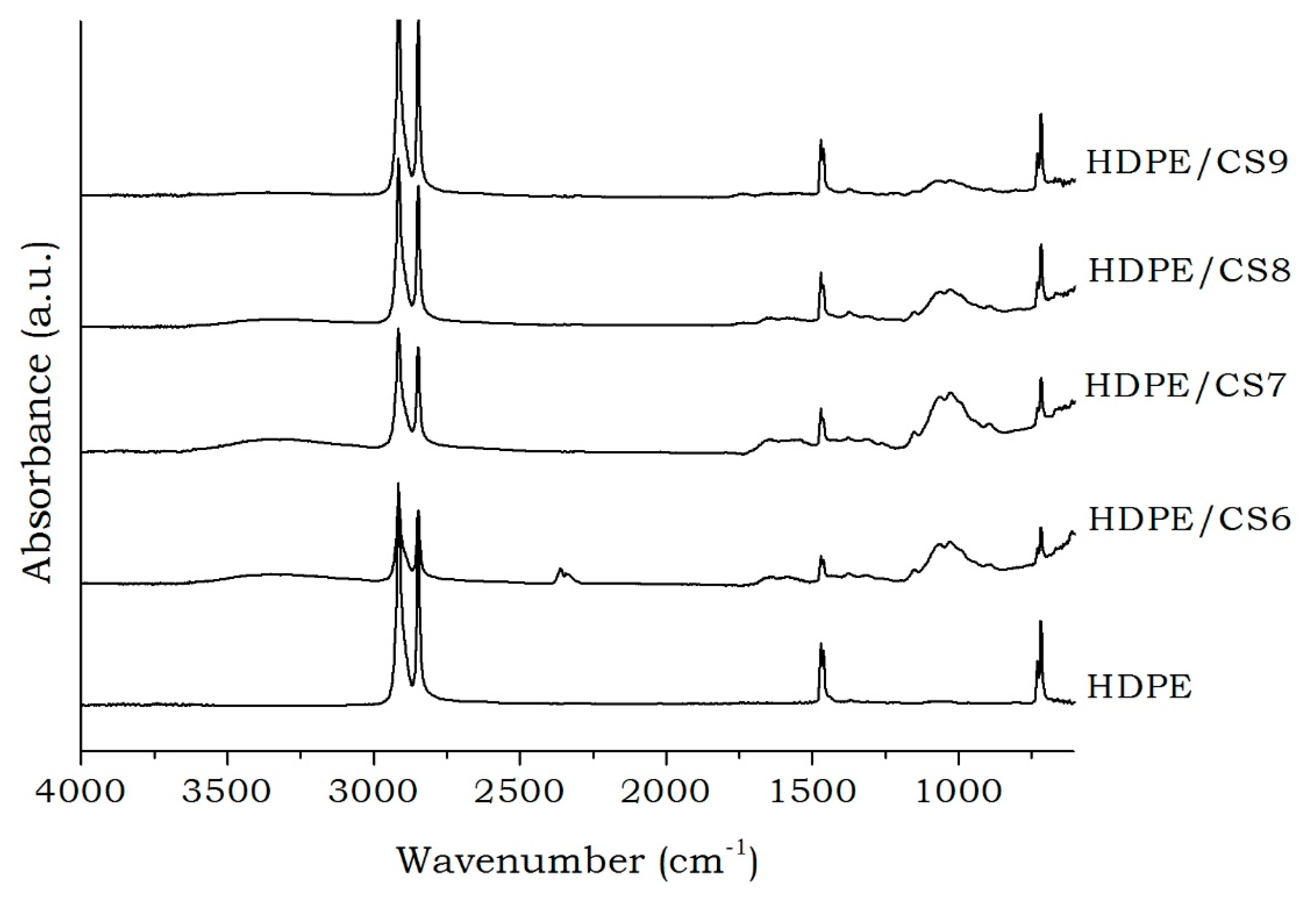
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of CTAB Impregnated Chitosan
3.3. Preparation of CS-CTAB Modified Bentonite
3.4. Preparation of HDPE Compounds
3.5. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peacock, A. Handbook of Polyethylene: Structures: Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Vasile, C.; Pascu, M. Practical Guide to Polyethylene; iSmithers Rapra Publishing: Akron, OH, USA, 2005. [Google Scholar]
- Malpass, D.B. Introduction to Industrial Polyethylene: Properties, Catalysts, and Processes; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 45. [Google Scholar]
- Bonhomme, S.; Cuer, A.; Delort, A.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Swift, G.; Wiles, D. Biodegradable and degradable polymers and plastics in landfill sites. In Encyclopedia of Polymer Science and Technology; Kroschwitz, J.I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Sudhakar, M.; Doble, M.; Murthy, P.S.; Venkatesan, R. Marine microbe-mediated biodegradation of low-and high-density polyethylenes. Int. Biodeterior. Biodegrad. 2008, 61, 203–213. [Google Scholar] [CrossRef]
- Ojeda, T.; Freitas, A.; Birck, K.; Dalmolin, E.; Jacques, R.; Bento, F.; Camargo, F. Degradability of linear polyolefins under natural weathering. Polym. Degrad. Stab. 2011, 96, 703–707. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Extruded films of blended chitosan, low density polyethylene and ethylene acrylic acid. Carbohydr. Polym. 2013, 91, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Kissin, Y.V. Polyethylene: End-Use Properties and Their Physical Meaning; Carl Hanser Verlag GmbH Co. KG: Munich, Germany, 2012. [Google Scholar]
- Tolinski, M. Additives for Polyolefins: Getting the Most Out of Polypropylene, Polyethylene and TPO; William Andrew: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Husseinsyah, S.; Azmin, A.N.; Ismail, H. Effect of Maleic Anhydride-Grafted-polyethylene (MAPE) and Silane on Properties of Recycled Polyethylene/Chitosan Biocomposites. Polym.-Plast. Technol. Eng. 2013, 52, 168–174. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Novikov, D.D.; Prut, E.V.; Rebrov, A.V. Synthesis and investigation of polyethylene blends with natural polysaccharides and their derivatives. Polym. Sci. Ser. A 2009, 51, 554–562. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Alexanyan, C.V.; Prut, E.V. Biodegradable blends based on chitin and chitosan: Production, structure, and properties. J. Appl. Polym. Sci. 2011, 121, 1850–1859. [Google Scholar] [CrossRef]
- Ismail, H.; Shaari, S.M.; Othman, N. The effect of chitosan loading on the curing characteristics, mechanical and morphological properties of chitosan-filled natural rubber (NR), epoxidised natural rubber (ENR) and styrene-butadiene rubber (SBR) compounds. Polym. Test. 2011, 30, 784–790. [Google Scholar] [CrossRef]
- Correlo, V.; Boesel, L.; Bhattacharya, M.; Mano, J.; Neves, N.; Reis, R. Properties of melt processed chitosan and aliphatic polyester blends. Mater. Sci. Eng. A 2005, 403, 57–68. [Google Scholar] [CrossRef]
- Ermolovich, O.; Makarevich, A. Effect of compatibilizer additives on the technological and performance characteristics of biodegradable materials based on starch-filled polyethylene. Russ. J. Appl. Chem. 2006, 79, 1526–1531. [Google Scholar] [CrossRef]
- Raghavan, D.; Emekalam, A. Characterization of starch/polyethylene and starch/polyethylene/poly (lactic acid) composites. Polym. Degrad. Stab. 2001, 72, 509–517. [Google Scholar] [CrossRef]
- Wu, C.-S. A comparison of the structure, thermal properties, and biodegradability of polycaprolactone/chitosan and acrylic acid grafted polycaprolactone/chitosan. Polymer 2005, 46, 147–155. [Google Scholar] [CrossRef]
- Husseinsyah, S.; Amri, F.; Husin, K.; Ismail, H. Mechanical and thermal properties of chitosan-filled polypropylene composites: The effect of acrylic acid. J. Vinyl Addit. Technol. 2011, 17, 125–131. [Google Scholar] [CrossRef]
- Salmah, H.; Faisal, A.; Kamarudin, H. Chemical modification of chitosan-filled polypropylene (PP) composites: The effect of 3-aminopropyltriethoxysilane on mechanical and thermal properties. Int. J. Polym. Mater. 2011, 60, 429–440. [Google Scholar] [CrossRef]
- Salmah, H.; Amri, F.; Kamarudin, H. Properties of chitosan-filled polypropylene (PP) composites: The effect of acetic acid. Polym.-Plast. Technol. Eng. 2012, 51, 86–91. [Google Scholar] [CrossRef]
- Amri, F.; Husseinsyah, S.; Hussin, K. Mechanical, morphological and thermal properties of chitosan filled polypropylene composites: The effect of binary modifying agents. Compos. Part A Appl. Sci. Manuf. 2013, 46, 89–95. [Google Scholar] [CrossRef]
- Carrasco-Guigón, F.J.; Rodríguez-Félix, D.E.; Castillo-Ortega, M.M.; Santacruz-Ortega, H.C.; Burruel-Ibarra, S.E.; Encinas-Encinas, J.C.; Plascencia-Jatomea, M.; Herrera-Franco, P.J.; Madera-Santana, T.J. Preparation and Characterization of Extruded Composites Based on Polypropylene and Chitosan Compatibilized with Polypropylene-Graft-Maleic Anhydride. Materials 2017, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-G.; Zheng, L.; Wang, Z.; Lee, C.-Y.; Park, H.-J. Molecular affinity and permeability of different molecular weight chitosan membranes. J. Agric. Food Chem. 2002, 50, 5915–5918. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Lee, J.; Lee, K.; Byun, M. Quality properties of pork sausage prepared with water-soluble chitosan oligomer. Meat Sci. 2001, 59, 369–375. [Google Scholar] [CrossRef]
- Reesha, K.V.; Panda, S.K.; Bindu, J.; Varghese, T.O. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Ogah, A.O.; Afiukwa, J.N.; Nduji, A.A. Characterization and Comparison of Rheological Properties of Agro Fiber Filled High-Density Polyethylene Bio-Composites. Open J. Polym. Chem. 2014, 4, 12–19. [Google Scholar] [CrossRef]
- Park, S.I.; Marsh, K.S.; Dawson, P. Application of chitosan-incorporated LDPE film to sliced fresh red meats for shelf life extension. Meat Sci. 2010, 85, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, D.E.; Quiroz-Castillo, J.M.; Grijalva-Monteverde, H.; Castillo-Castro, T.; Burruel-Ibarra, S.E.; Rodríguez-Félix, F.; Madera-Santana, T.; Cabanillas, R.E.; Herrera-Franco, P.J. Degradability of extruded polyethylene/chitosan blends compatibilized with polyethylene-graft-maleic anhydride under natural weathering. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Salmah, H.; Azieyanti, A.N. Properties of recycled polyethylene/chitosan composites: The effect of polyethylene-graft-maleic anhydride. J. Reinf. Plast. Compos. 2010, 30, 195–202. [Google Scholar] [CrossRef]
- Sunilkumar, M.; Francis, T.; Thachil, E.T.; Sujith, A. Low density polyethylene–chitosan composites: A study based on biodegradation. Chem. Eng. J. 2012, 204–206, 114–124. [Google Scholar] [CrossRef]
- Sunilkumar, M.; Gafoor, A.A.; Anas, A.; Haseena, A.P.; Sujith, A. Dielectric properties: A gateway to antibacterial assay—A case study of low-density polyethylene/chitosan composite films. Polym. J. 2014, 46, 422–429. [Google Scholar] [CrossRef]
- Vasile, C.; Darie, R.N.; Cheaburu-Yilmaz, C.N.; Pricope, G.-M.; Bračič, M.; Pamfil, D.; Hitruc, G.E.; Duraccio, D. Low density polyethylene—Chitosan composites. Compos. Part B Eng. 2013, 55, 314–323. [Google Scholar] [CrossRef]
- Wang, H.-S.; Chen, D.; Chuai, C.-Z. Mechanical and Barrier Properties of LLDPE/Chitosan Blown Films for Packaging. Packag. Technol. Sci. 2015, 28, 915–923. [Google Scholar] [CrossRef]
- Zhang, H.Z.; He, Z.C.; Liu, G.H.; Qiao, Y.Z. Properties of different chitosan/low-density polyethylene antibacterial plastics. J. Appl. Polym. Sci. 2009, 113, 2018–2021. [Google Scholar] [CrossRef]
- Quiroz-Castillo, J.; Rodríguez-Félix, D.; Grijalva-Monteverde, H.; del Castillo-Castro, T.; Plascencia-Jatomea, M.; Rodríguez-Félix, F.; Herrera-Franco, P. Preparation of extruded polyethylene/chitosan blends compatibilizedwith polyethylene-graft-maleic anhydride. Carbohydr. Polym. 2014, 101, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Yasin, T.; Halley, P.J.; Siddiqi, H.M.; Nicholson, T. Thermal, rheological, mechanical and morphological behavior of HDPE/chitosan blend. Carbohydr. Polym. 2011, 83, 414–421. [Google Scholar] [CrossRef]
- Lima, P.S.; Brito, R.S.; Santos, B.F.; Tavares, A.A.; Agrawal, P.; Andrade, D.L.; Wellen, R.M.; Canedo, E.L.; Silva, S.M. Rheological properties of HDPE/chitosan composites modified with PE-g-MA. J. Mater. Res. 2017, 32, 775–787. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Influence of impregnation of chitosan beads with cetyl trimethyl ammonium bromide on their structure and adsorption of congo red from aqueous solutions. Chem. Eng. J. 2009, 155, 254–259. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, E.; Pollet, E.; Ulrich, G.; de Jesús Sosa-Santillán, G.; Avérous, L. Original method for synthesis of chitosan-based antimicrobial agent by quaternary ammonium grafting. Carbohydr. Polym. 2017, 157, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Rúnarsson, Ö.V.; Holappa, J.; Malainer, C.; Steinsson, H.; Hjálmarsdóttir, M.; Nevalainen, T.; Másson, M. Antibacterial activity of N-quaternary chitosan derivatives: Synthesis, characterization and structure activity relationship (SAR) investigations. Eur. Polym. J. 2010, 46, 1251–1267. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Cárdenas, G.; Díaz, J.; Meléndrez, M.; Cruzat, C. Physicochemical properties of edible films from chitosan composites obtained by microwave heating. Polym. Bull. 2008, 61, 737–748. [Google Scholar] [CrossRef]
- Bettini, R.; Romani, A.A.; Morganti, M.M.; Borghetti, A.F. Physicochemical and cell adhesion properties of chitosan films prepared from sugar and phosphate-containing solutions. Eur. J. Pharm. Biopharm. 2008, 68, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Q.S.; Di, Y.B.; Wang, X.; Hong, W. Effect of Chitosan as Antimicrobial Agent on Flame Retardant Protein Viscose Fiber. Adv. Mater. Res. 2012, 427, 32–37. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Dhanikula, A.B.; Panchagnula, R. Development and characterization of biodegradable chitosan films for local delivery of paclitaxel. AAPS J. 2004, 6, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites; Rapra Technology Limited: Akron, OH, USA, 2004; Volume 1. [Google Scholar]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Samuels, R.J. Solid state characterization of the structure of chitosan films. J. Polym. Sci. Part B Polym. Phys. 1981, 19, 1081–1105. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Miya, M. Dependence on the preparation procedure of the polymorphism and crystallinity of chitosan membranes. Biosci. Biotechnol. Biochem. 1992, 56, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.W.; Penha, F.G.; Braga, R.M.; de Araújo Melo, D.M.; Pergher, S.B.; Petkowicz, D.I. Synthesis and characterization of organoclays using differents contents of cationic surfactant hexadecyltrimethylammonium bromide. Química Nova 2011, 34, 1152–1156. [Google Scholar] [CrossRef]
- Marras, S.; Tsimpliaraki, A.; Zuburtikudis, I.; Panayiotou, C. Thermal and colloidal behavior of amine-treated clays: The role of amphiphilic organic cation concentration. J. Colloid Interface Sci. 2007, 315, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Luo, J. Biopolymer/montmorillonite nanocomposite: Preparation, drug-controlled release property and cytotoxicity. Nanotechnology 2008, 19, 065707. [Google Scholar] [CrossRef] [PubMed]
- Mohanambe, L.; Vasudevan, S. Anionic clays containing anti-inflammatory drug molecules: Comparison of molecular dynamics simulation and measurements. J. Phys. Chem. B 2005, 109, 15651–15658. [Google Scholar] [CrossRef] [PubMed]
- Stelescu, D.M.; Airinei, A.; Homocianu, M.; Fifere, N.; Timpu, D.; Aflori, M. Structural characteristics of some high density polyethylene/EPDM blends. Polym. Test. 2013, 32, 187–196. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Wu, C. Thermal and Crystallization Properties of HDPE and HDPE/PP Blends Modified with DCP. Adv. Polym. Technol. 2014, 33. [Google Scholar] [CrossRef]
- Quiroz-Castillo, J.M.; Rodríguez-Félix, D.E.; Grijalva-Monteverde, H.; Lizárraga-Laborín, L.L.; Castillo-Ortega, M.M.; del Castillo-Castro, T.; Rodríguez-Félix, F.; Herrera-Franco, P.J. Preparation and characterization of films extruded of polyethylene/chitosan modified with poly (lactic acid). Materials 2014, 8, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, K.; Sailaja, R. Blends of LDPE/chitosan using epoxy-functionalized LDPE as compatibilizer. J. Appl. Polym. Sci. 2012, 124, 3264–3275. [Google Scholar] [CrossRef]
- Dean, K.; Sangwan, P.; Way, C.; Zhang, X.; Martino, V.P.; Xie, F.; Halley, P.J.; Pollet, E.; Avérous, L. Glycerol plasticised chitosan: A study of biodegradation via carbon dioxide evolution and nuclear magnetic resonance. Polym. Degrad. Stab. 2013, 98, 1236–1246. [Google Scholar] [CrossRef]
- Braskem. High Density Polyethylene JV-060U Technical Data Sheet; Revision 8; Braskem: São Paulo, Brazil, 2015. [Google Scholar]
- Sekiguchi, S. Molecular weight dependency of antimicrobial activity by chitooligosaccharides. In Food Hydrocolloides: Structures, Properties, and Functions; Nishinari, K., Doi, E., Eds.; Springer: Boston, MA, USA; New York, NY, USA, 1994; pp. 71–76. [Google Scholar]
- Gunawan, N.S.; Indraswati, N.; Ju, Y.-H.; Soetaredjo, F.E.; Ayucitra, A.; Ismadji, S. Bentonites modified with anionic and cationic surfactants for bleaching of crude palm oil. Appl. Clay Sci. 2010, 47, 462–464. [Google Scholar] [CrossRef]
- Leite, I.F. Experimental and Theoretical Study of the Intercalation Behavior of Organic Salts in Clays and its Effect on PET Nanocomposites Properties. Ph.D. Thesis (Materials Science), Federal University of Recife (UFPE), Recife, Brazil, 2010. [Google Scholar]
- Khonakdar, H.; Jafari, S.; Taheri, M.; Wagenknecht, U.; Jehnichen, D.; Häussler, L. Thermal and wide angle X-ray analysis of chemically and radiation-crosslinked low and high density polyethylenes. J. Appl. Polym. Sci. 2006, 100, 3264–3271. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. Polymer Handbook; Wiley: New York, NY, USA, 1989; Volume 7. [Google Scholar]
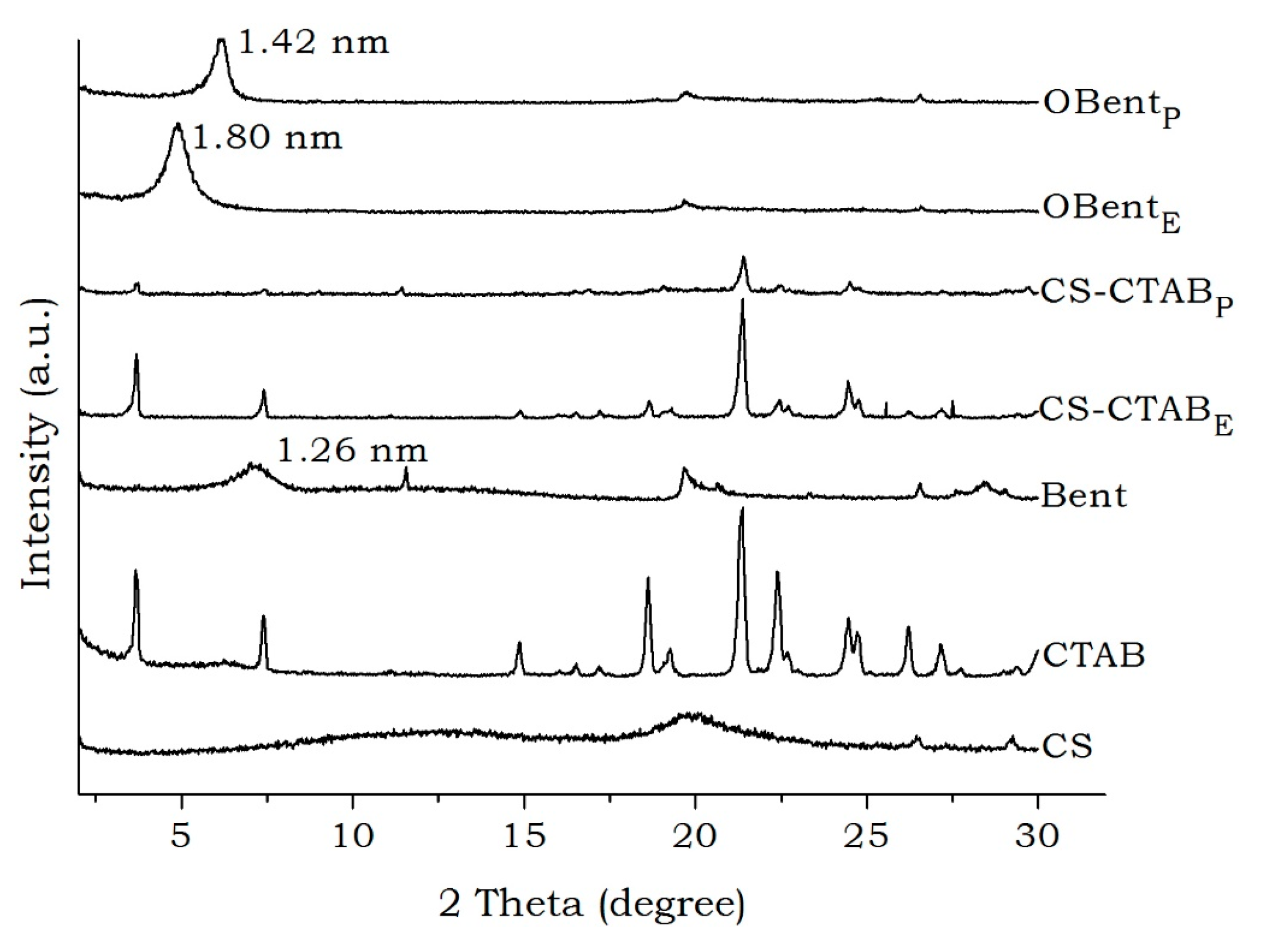

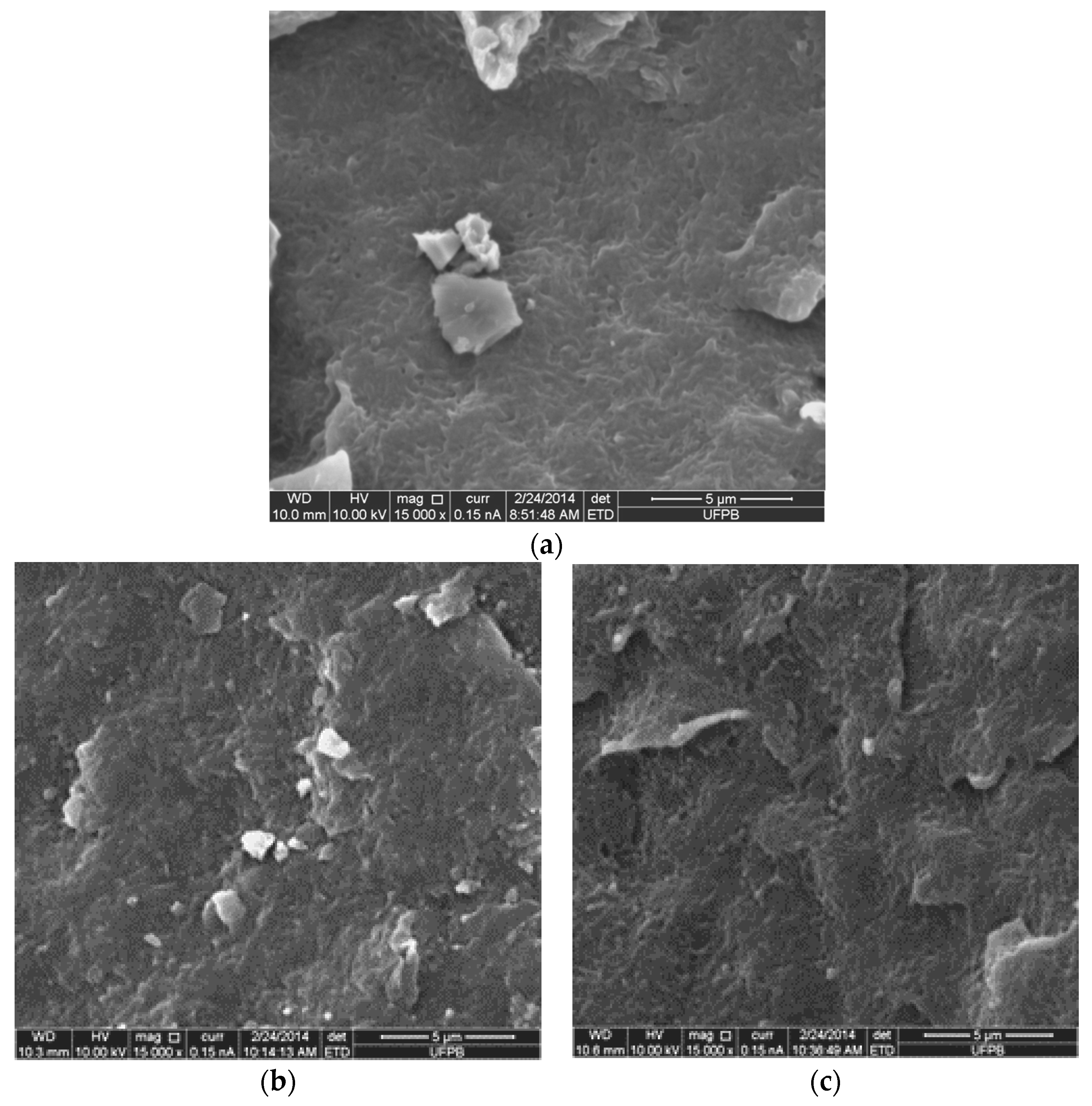
| Sample | Xc (%) |
|---|---|
| HDPE | 54 |
| HDPE/CS9 | 45 |
| HDPE/CS8 | 43 |
| HDPE/CS7 | 38 |
| HDPE/CS6 | 39 |
| HDPE/CS9/OBentE | 46 |
| HDPE/CS8/OBentE | 44 |
| HDPE/CS7/OBentE | 35 |
| HDPE/CS6/OBentE | 37 |
| HDPE/CS9/OBentP | 48 |
| HDPE/CS8/OBentP | 44 |
| HDPE/CS7/OBentP | 44 |
| HDPE/CS6/OBentP | 42 |
| Sample | Tc (°C) | ∆Hc (J/g) | Tm (°C) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|
| HDPE | 115 | 155 | 130 | 184 | 64 |
| HDPE/CS9 | 115 | 110 | 130 | 141 | 53 |
| HDPE/CS8 | 114 | 40 | 129 | 99 | 42 |
| HDPE/CS7 | 113 | 45 | 128 | 56 | 27 |
| HDPE/CS6 | 114 | 50 | 127 | 63 | 36 |
| HDPE/CS9/OBentE | 114 | 78 | 130 | 104 | 39 |
| HDPE/CS8/OBentE | 115 | 65 | 129 | 90 | 38 |
| HDPE/CS7/OBentE | 114 | 57 | 129 | 68 | 33 |
| HDPE/CS6/OBentE | 115 | 51 | 129 | 64 | 36 |
| HDPE/CS9/OBentP | 115 | 91 | 129 | 106 | 40 |
| HDPE/CS8/OBentP | 115 | 79 | 129 | 95 | 41 |
| HDPE/CS7/OBentP | 113 | 60 | 127 | 71 | 35 |
| HDPE/CS6/OBentP | 114 | 47 | 128 | 68 | 39 |
| Sample | Ti–Tf (°C) | ||
|---|---|---|---|
| I | II | III | |
| HDPE | 463–489 | ||
| CS | 62–98 | 263–309 | 496–592 |
| HDPE/CS9 | 40–78 | 263–311 | 459–490 |
| HDPE/CS8 | 38–77 | 268–312 | 458–489 |
| HDPE/CS7 | 36–78 | 265–310 | 451–486 |
| HDPE/CS6 | 50–93 | 267–309 | 406–488 |
| HDPE/CS9/OBentE | 37–71 | 270–307 | 450–486 |
| HDPE/CS8/OBentE | 37–68 | 260–308 | 450–486 |
| HDPE/CS7/OBentE | 35–77 | 269–311 | 452–486 |
| HDPE/CS6/OBentE | 42–86 | 269–311 | 454–486 |
| HDPE/CS9/OBentP | 39–68 | 268–312 | 460–490 |
| HDPE/CS8/OBentP | 37–73 | 267–314 | 458–489 |
| HDPE/CS7/OBentP | 38–77 | 266–310 | 451–485 |
| HDPE/CS6/OBentP | 37–79 | 265–311 | 452–485 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| HDPE | 16.38 ± 2.11 | 400.72 ± 52.49 | 67.14 ± 12.33 |
| HDPE/CS9 | 16.70 ± 0.58 | 443.50 ± 25.59 | 15.99 ± 0.65 |
| HDPE/CS8 | 18.35 ± 0.98 | 538.28 ± 21.47 | 12.01 ± 3.92 |
| HDPE/CS7 | 17.26 ± 0.45 | 537.47 ± 16.41 | 11.88 ± 0.88 |
| HDPE/CS6 | 17.52 ± 0.60 | 597.84 ± 22.78 | 9.17 ± 1.35 |
| HDPE/CS9/OBentE | 17.19 ± 0.66 | 467.53 ± 13.09 | 20.15 ± 5.66 |
| HDPE/CS8/OBentE | 16.94 ± 2.95 | 531.29 ± 64.01 | 8.04 ± 2.13 |
| HDPE/CS7/OBentE | 17.99 ± 1.22 | 612.76 ± 43.71 | 7.10 ± 0.93 |
| HDPE/CS6/OBentE | 15.41 ± 1.33 | 565.76 ± 40.92 | 5.44 ± 0.29 |
| HDPE/CS9/OBentP | 15.38 ± 0.36 | 408.43 ± 11.41 | 20.57 ± 5.14 |
| HDPE/CS8/OBentP | 18.98 ± 0.65 | 554.68 ± 15.02 | 15.00 ± 3.38 |
| HDPE/CS7/OBentP | 15.74 ± 0.45 | 546.68 ± 42.41 | 7.58 ± 0.89 |
| HDPE/CS6/OBentP | 15.01 ± 1.05 | 567.82 ± 39.13 | 5.96 ± 0.32 |
| Code | Composition | HDPE (g) | CS (g) | OBentE * (g) | OBentP * (g) |
|---|---|---|---|---|---|
| HDPE | 100 | 50 | 0 | 0 | 0 |
| HDPE/CS9 | 90/10 | 45 | 5 | 0 | 0 |
| HDPE/CS8 | 80/20 | 40 | 10 | 0 | 0 |
| HDPE/CS7 | 70/30 | 35 | 15 | 0 | 0 |
| HDPE/CS6 | 60/40 | 30 | 20 | 0 | 0 |
| HDPE/CS9/OBentE | 90/10/0.1 | 45 | 5 | 0.05 | 0 |
| HDPE/CS8/OBentE | 80/20/0.2 | 40 | 10 | 0.10 | 0 |
| HDPE/CS7/OBentE | 70/30/0.3 | 35 | 15 | 0.15 | 0 |
| HDPE/CS6/OBentE | 60/40/0.4 | 30 | 20 | 0.20 | 0 |
| HDPE/CS9/OBentP | 90/10/0.1 | 45 | 5 | 0 | 0.05 |
| HDPE/CS8/OBentP | 80/20/0.2 | 40 | 10 | 0 | 0.10 |
| HDPE/CS7/OBentP | 70/30/0.3 | 35 | 15 | 0 | 0.15 |
| HDPE/CS6/OBentP | 60/40/0.4 | 30 | 20 | 0 | 0.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Araújo, M.J.G.; Barbosa, R.C.; Fook, M.V.L.; Canedo, E.L.; Silva, S.M.L.; Medeiros, E.S.; Leite, I.F. HDPE/Chitosan Blends Modified with Organobentonite Synthesized with Quaternary Ammonium Salt Impregnated Chitosan. Materials 2018, 11, 291. https://doi.org/10.3390/ma11020291
De Araújo MJG, Barbosa RC, Fook MVL, Canedo EL, Silva SML, Medeiros ES, Leite IF. HDPE/Chitosan Blends Modified with Organobentonite Synthesized with Quaternary Ammonium Salt Impregnated Chitosan. Materials. 2018; 11(2):291. https://doi.org/10.3390/ma11020291
Chicago/Turabian StyleDe Araújo, Maria José G., Rossemberg C. Barbosa, Marcus Vinícius L. Fook, Eduardo L. Canedo, Suédina M. L. Silva, Eliton S. Medeiros, and Itamara F. Leite. 2018. "HDPE/Chitosan Blends Modified with Organobentonite Synthesized with Quaternary Ammonium Salt Impregnated Chitosan" Materials 11, no. 2: 291. https://doi.org/10.3390/ma11020291
APA StyleDe Araújo, M. J. G., Barbosa, R. C., Fook, M. V. L., Canedo, E. L., Silva, S. M. L., Medeiros, E. S., & Leite, I. F. (2018). HDPE/Chitosan Blends Modified with Organobentonite Synthesized with Quaternary Ammonium Salt Impregnated Chitosan. Materials, 11(2), 291. https://doi.org/10.3390/ma11020291