Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices
Abstract
1. Introduction
2. Materials and Methods
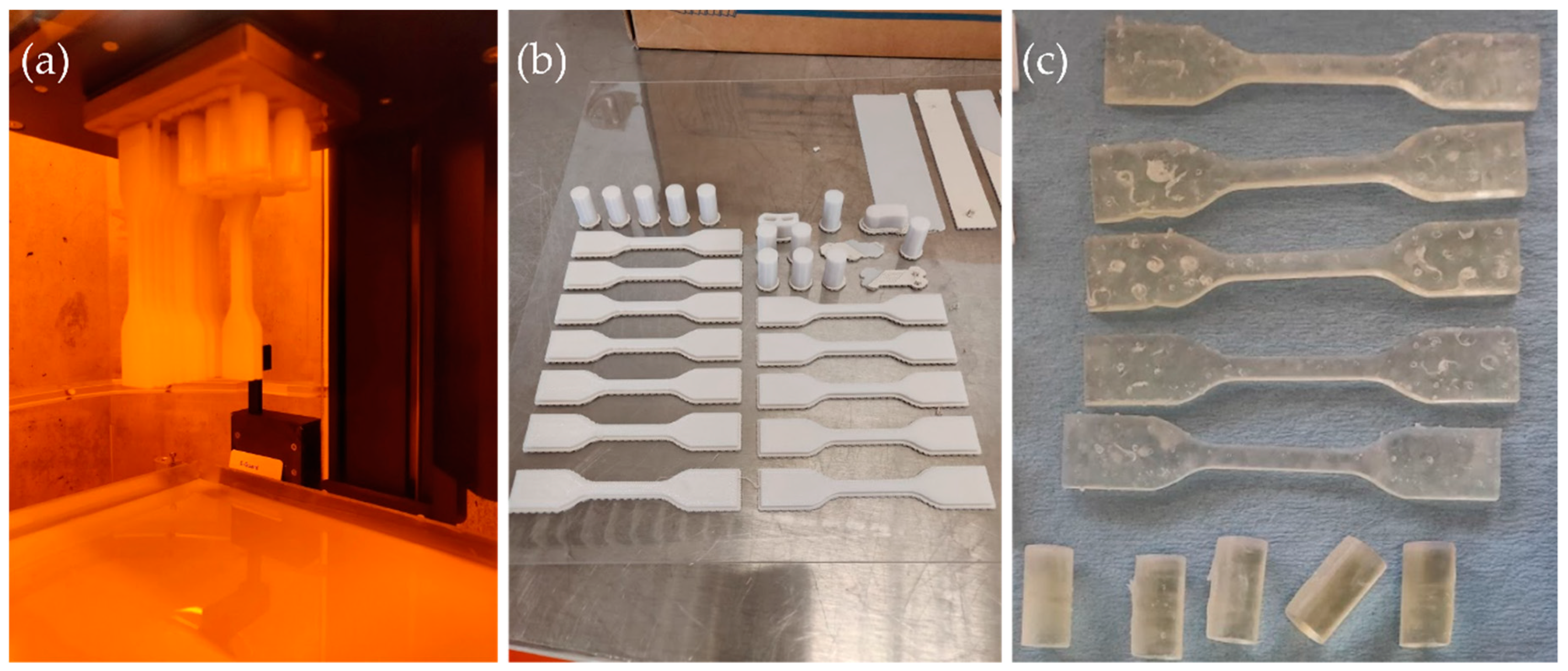
2.1. Additive Manufacturing Technologies and Materials
2.2. Sterilization Methods
2.3. Mechanical Testing
2.4. Geometrical and Surface Characterization
2.5. Microbiological Screening of the Process
3. Results
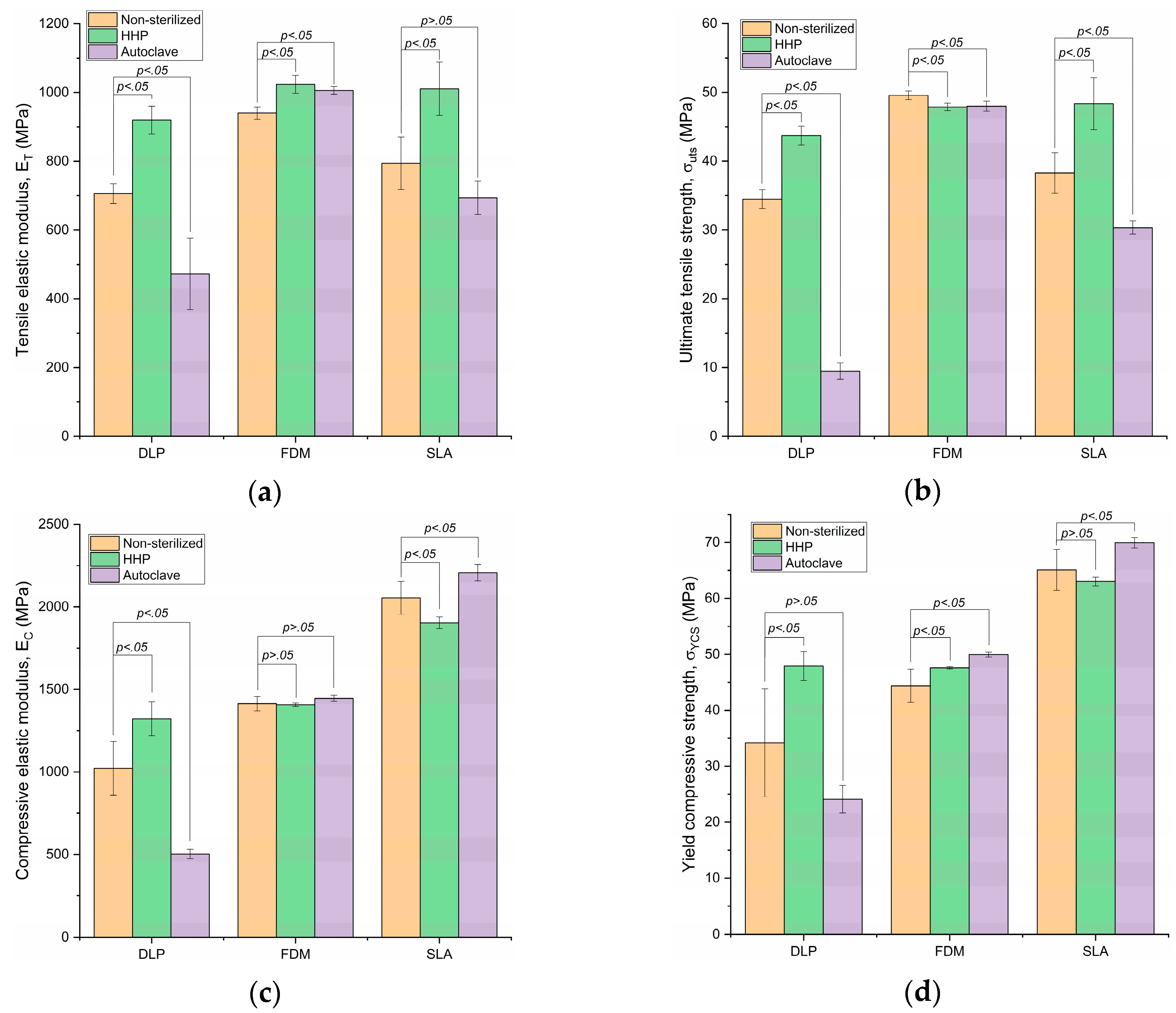
3.1. Tensile Elastic Modulus
3.2. Ultimate Tensile Strength
3.3. Compressive Elastic Modulus
3.4. Yield Compressive Strength
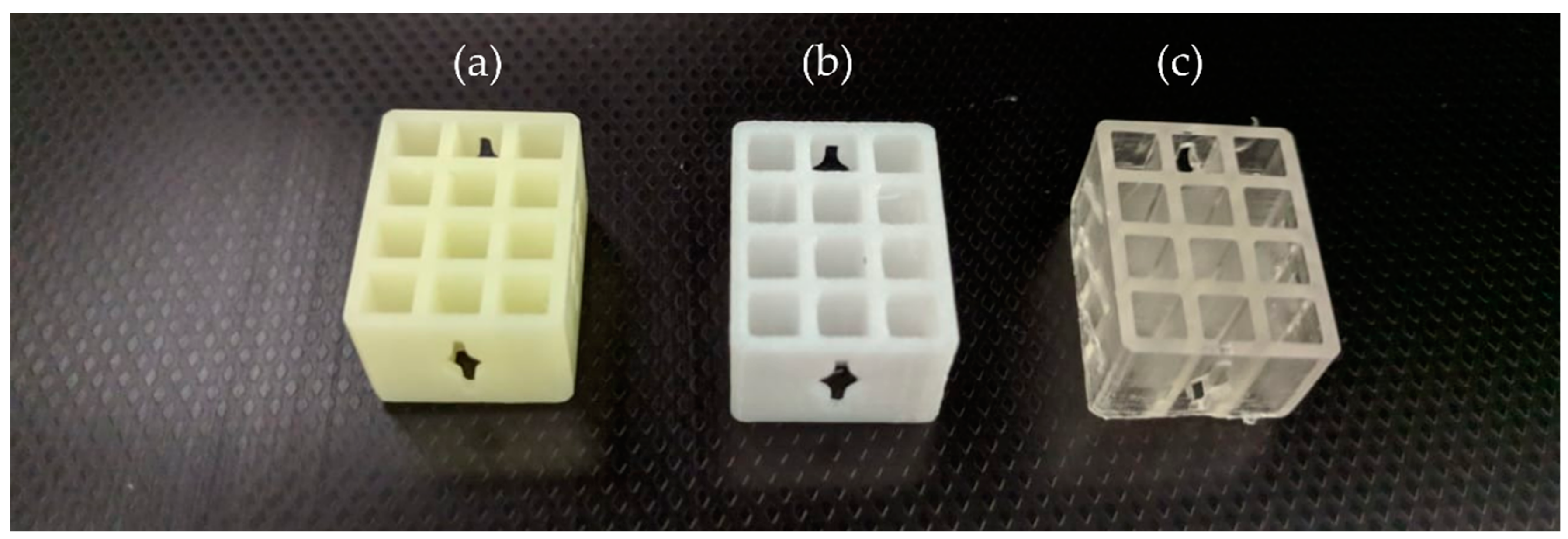
3.5. Geometrical and Surface Characterizations
4. Discussion
5. Conclusions
- Hydrostatic high-pressure processing, a method widely used for food preservation, shows the potential to be employed for the sterilization of biomedical devices. It is necessary to evaluate the sanitization capacity of the proposed technology.
- The potential of the technology for cleansing the surface of biomedical devices without employing heat could help overcome the limitation for processing polymer-based medical devices without suffering losses in the mechanical properties, typically associated with heat-based treatments. Additionally, this work has shown that employing a high-pressure sterilization procedure for materials processed under an additive manufacturing process could increase the mechanical properties of some materials. Further research should be conducted to investigate the effects that this sterilization technique has on the microstructure of the polymers.
- Furthermore, it has been proven that PC-ISO processed by FDM technology can withstand both sterilization methods and has a stable mechanical behavior.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, G.; Cui, X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol. Lett. 2016, 38, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive Manufacturing of Biomaterials. Prog. Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Radenkovic, D.; Solouk, A.; Seifalian, A. Personalized development of human organs using 3D printing technology. Med. Hypotheses 2016, 87, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—Regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, E. Chemical disinfection of medical and surgical materials. In Disinfection, Sterilization, and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1968; ISBN 0683307401. [Google Scholar]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: What Clinicians Need to Know. Clin. Infect. Dis. 2004, 39, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Sehulster, L.; Chinn, R.Y.W.; Arduino, M.J.; Carpenter, J.; Donlan, R.; Ashford, D.; Besser, R.; Fields, B.; McNeil, M.; Whitney, C.; et al. Guidelines for Environmental Infection Control in Health-Care Facilities: Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. 2003, 52, 1–44. [Google Scholar]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed]
- FDA Classify Your Medical Device. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/ (accessed on 11 November 2018).
- FDA. Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile Guidance for Industry and Food and Drug Administration Staff; FDA: Silver Spring, MD, USA, 2016.
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Martínez-López, J.I.; Mojica, M.; Rodríguez, C.A.; Siller, H.R. Xurography as a Rapid Fabrication Alternative for Point-of-Care Devices: Assessment of Passive Micromixers. Sensors 2016, 16, 705. [Google Scholar] [CrossRef]
- Martínez-López, J.I.; Betancourt, H.A.; García-López, E.; Rodriguez, C.A.; Siller, H.R. Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation. Micromachines 2017, 8, 144. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Yordanov, D.G.; Angelova, G.V. High pressure processing for foods preserving. Biotechnol. Biotechnol. Equip. 2010, 24, 1940–1945. [Google Scholar] [CrossRef]
- Sugita, N.; Ishii, K.; Sui, J.; Terashima, M. Multi-grooved cutting tool to reduce cutting force and temperature during bone machining. CIRP Ann. Manuf. Technol. 2014, 63, 101–104. [Google Scholar] [CrossRef]
- ABE, F. Exploration of the Effects of High Hydrostatic Pressure on Microbial Growth, Physiology and Survival: Perspectives from Piezophysiology. Biosci. Biotechnol. Biochem. 2007, 71, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Farkas, D.F. A Short History of Research and Development Efforts Leading to the Commercialization of High-Pressure Processing of Food. In High Pressure Processing of Food; Springer: New York, NY, USA, 2016; pp. 19–36. ISBN 1493932330. [Google Scholar]
- Castro, S.M.; Saraiva, J.A. High-Pressure Processing of Fruits and Fruit Products. Emerg. Technol. Food Process. 2014, 65–76. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Bourell, D.; Espalin, D.; Arcaute, K.; Rodriguez, D.; Medina, F.; Posner, M.; Wicker, R. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010, 16, 164–173. [Google Scholar] [CrossRef]
- Domanski, J.; Skalski, K.; Grygoruk, R.; Mróz, A. Rapid prototyping in the intervertebral implant design process. Rapid Prototyp. J. 2015, 21, 735–746. [Google Scholar] [CrossRef]
- Puppi, D.; Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive manufacturing of wet-spun polymeric scaffolds for bone tissue engineering. Biomed. Microdevices 2012, 14, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and characterization of a coronary polylactic acid stent prototype generated by selective laser melting. J. Mater. Sci. Mater. Med. 2013, 24, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Béduer, A.; Piacentini, N.; Aeberli, L.; Da Silva, A.; Verheyen, C.A.; Bonini, F.; Rochat, A.; Filippova, A.; Serex, L.; Renaud, P.; et al. Additive manufacturing of hierarchical injectable scaffolds for tissue engineering. Acta Biomater. 2018, 76, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Obaton, A.F.; Fain, J.; Djemaï, M.; Meinel, D.; Léonard, F.; Mahé, E.; Lécuelle, B.; Fouchet, J.J.; Bruno, G. In vivo XCT bone characterization of lattice structured implants fabricated by additive manufacturing. Heliyon 2017, 3, e00374. [Google Scholar] [CrossRef] [PubMed]
- Neches, R.Y.; Flynn, K.J.; Zaman, L.; Tung, E.; Pudlo, N. On the intrinsic sterility of 3D printing. PeerJ 2016, 4, e2661. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.M. 3D Printed Antibacterial Prostheses. Appl. Sci. 2018, 8, 1651. [Google Scholar] [CrossRef]
- ASTM International. ASTM D638-14, Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. ASTM D695-15, Standard Test Method for Compressive Properties of Rigid Plastics; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Schroettner, H.; Schmied, M.; Scherer, S. Comparison of 3D surface reconstruction data from certified depth standards obtained by SEM and an infinite focus measurement machine (IFM). Microchim. Acta 2006, 155, 279–284. [Google Scholar] [CrossRef]
- NOM-092-SSA1-1994: Bienes y Servicios. Método para la cuenta de bacterias aerobias en placa. Available online: http://www.salud.gob.mx/unidades/cdi/nom/092ssa14.html (accessed on 11 November 2018).
- NOM-111-SSA1-1994, Bienes y Servicios. Método para la cuenta de moho y levaduras en alimentos. Available online: http://www.salud.gob.mx/unidades/cdi/nom/111ssa14.html (accessed on 11 November 2018).


| Property | AM | Non-Sterilized | HHP | Autoclave | ||
|---|---|---|---|---|---|---|
| Mean ± SD (MPa) | Mean ± SD (MPa) 1 | Change 1 | Mean ± SD (MPa) | Change 2 | ||
| DLP | 705.8 ± 28.37 | 919.58 ± 40.79 p = 3.2 × 10−4 | 30.29% | 472.65 ± 103.94 p = 1.4 × 10−4 | −33.03% | |
| FDM | 940.6 ± 17.76 | 1023.6 ± 25.88 p = 1.1 × 10−5 | 8.82% | 1006.02 ± 11.18 p = 8.7 × 10−5 | 6.95% | |
| SLA | 794.31 ± 76.17 | 1011.05 ± 77.23 p = 1.1 × 10−3 | 27.29% | 693.86 ± 48.26 p = 14.93 × 10−2 | −12.65% | |
| DLP | 34.48 ± 1.38 | 43.71 ± 1.38 p = 1.0 × 10−7 | 26.79% | 9.48 ± 1.19 p = 4.1 × 10−13 | −72.49% | |
| FDM | 49.58 ± 0.64 | 47.88 ± 0.56 p = 2.3 × 10−3 | −3.41% | 48 ± 0.73 p = 2.8 × 10−3 | −3.18% | |
| SLA | 38.28 ± 2.96 | 48.37 ± 3.77 p = 7.0 × 10−4 | 26.35% | 30.35 ± 0.96 p = 4.4 × 10−3 | −20.72% | |
| DLP | 1022.15 ± 162.69 | 1322.25 ± 101.61 p = 2.9 × 10−3 | 29.36% | 502.53 ± 28.66 p = 1.7 × 10−5 | −50.84% | |
| FDM | 1413.98 ± 43.09 | 1407.11 ± 10.08 p = 1.0 × 100 | −0.49% | 1446.27 ± 18.95 p = 27.4 × 10−2 | 2.28% | |
| SLA | 2054.57 ± 99.18 | 1903.29 ± 35.37 p = 1.1 × 10−2 | −7.36% | 2207.35 ± 50.4 p = 1.0 × 10−3 | 7.44% | |
| σYCS | DLP | 34.19 ± 9.63 | 47.91 ± 2.56 p = 9.6 × 10−3 | 40.14% | 24.1 ± 2.46 p = 6.0 × 10−2 | −29.51% |
| FDM | 44.39 ± 2.98 | 47.6 ± 0.2 p = 4.1 × 10−2 | 7.23% | 49.95 ± 0.42 p = 8.2 × 10−4 | 12.54% | |
| SLA | 65.09 ± 3.68 | 63.03 ± 0.8 p = 53.4 × 10−2 | −3.15% | 69.95 ± 0.93 p = 1.5 × 10−2 | 7.47% | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares-Alvelais, J.A.R.; Figueroa-Cavazos, J.O.; Chuck-Hernandez, C.; Siller, H.R.; Rodríguez, C.A.; Martínez-López, J.I. Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices. Materials 2018, 11, 2540. https://doi.org/10.3390/ma11122540
Linares-Alvelais JAR, Figueroa-Cavazos JO, Chuck-Hernandez C, Siller HR, Rodríguez CA, Martínez-López JI. Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices. Materials. 2018; 11(12):2540. https://doi.org/10.3390/ma11122540
Chicago/Turabian StyleLinares-Alvelais, José A. Robles, J. Obedt Figueroa-Cavazos, C. Chuck-Hernandez, Hector R. Siller, Ciro A. Rodríguez, and J. Israel Martínez-López. 2018. "Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices" Materials 11, no. 12: 2540. https://doi.org/10.3390/ma11122540
APA StyleLinares-Alvelais, J. A. R., Figueroa-Cavazos, J. O., Chuck-Hernandez, C., Siller, H. R., Rodríguez, C. A., & Martínez-López, J. I. (2018). Hydrostatic High-Pressure Post-Processing of Specimens Fabricated by DLP, SLA, and FDM: An Alternative for the Sterilization of Polymer-Based Biomedical Devices. Materials, 11(12), 2540. https://doi.org/10.3390/ma11122540









