The Effect and Associate Mechanism of Nano SiO2 Particles on the Diffusion Behavior of Water in Insulating Oil
Abstract
:1. Introduction
2. Materials and Methods
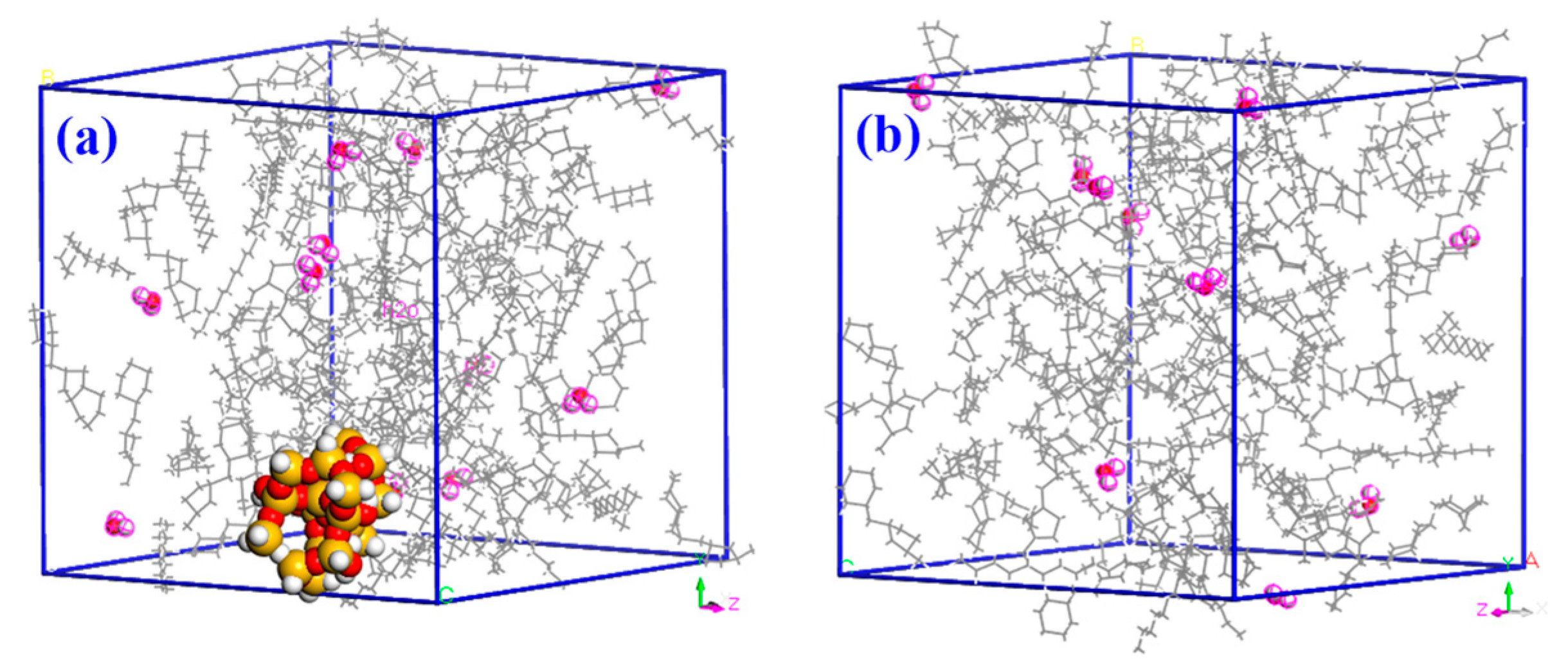
2.1. Model Building
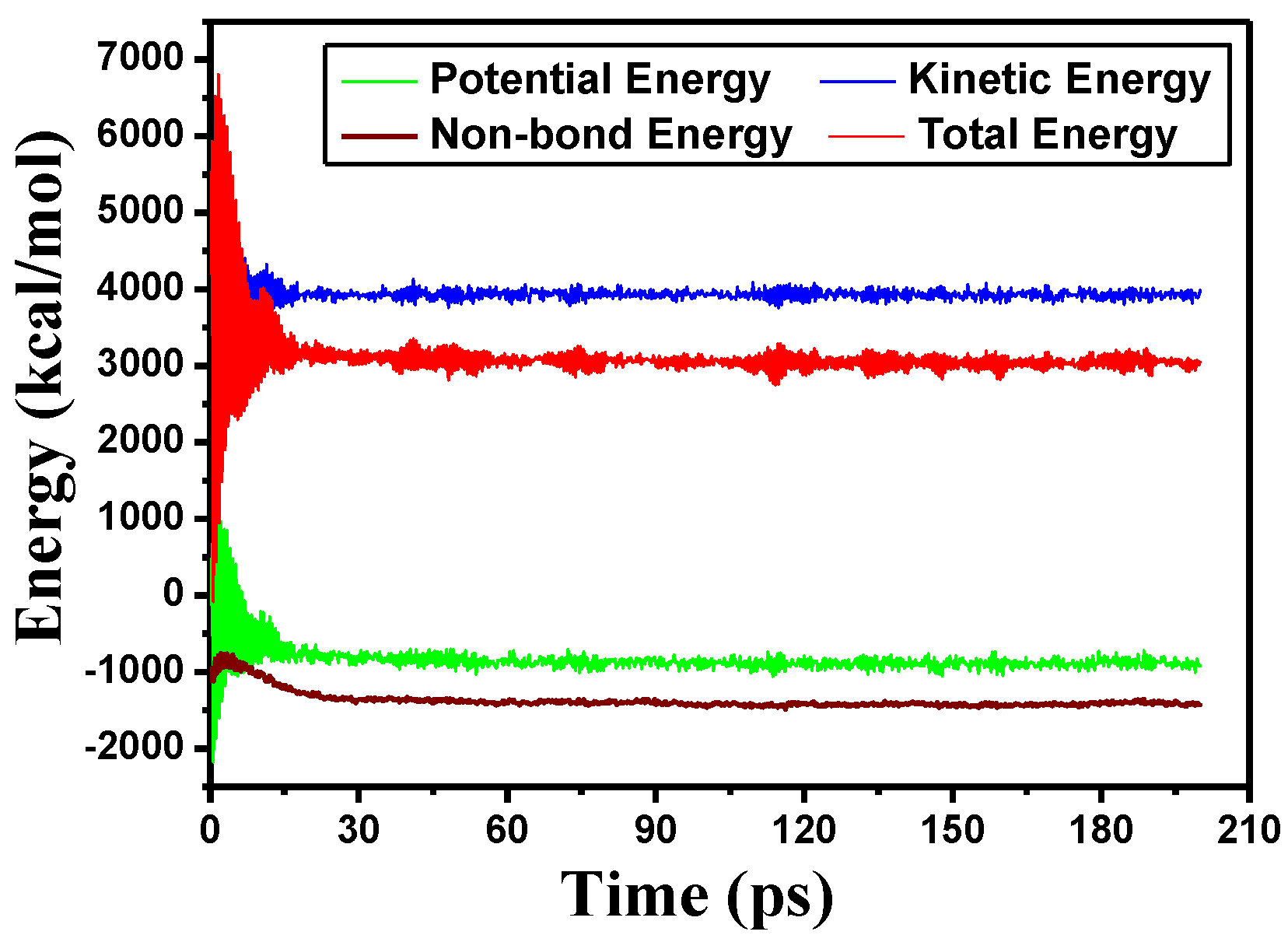
2.2. Simulation Details
3. Results
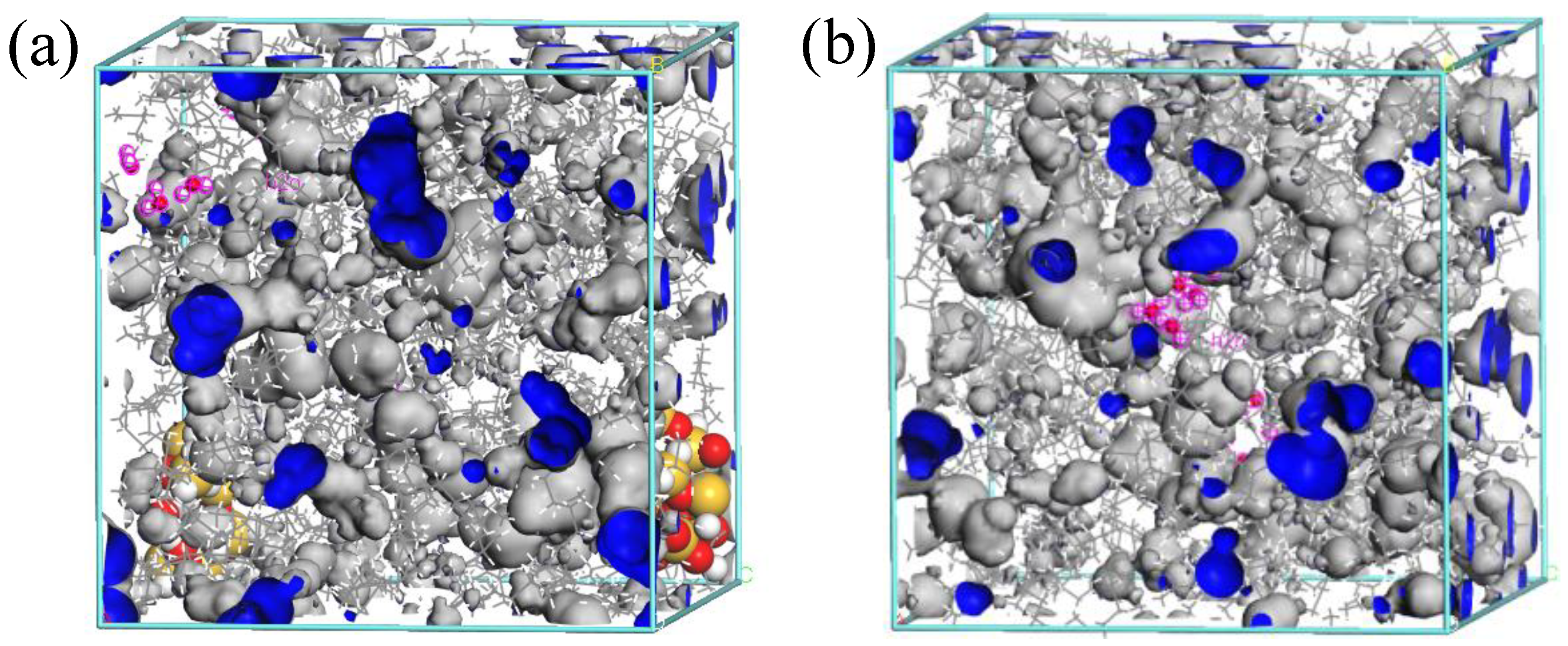
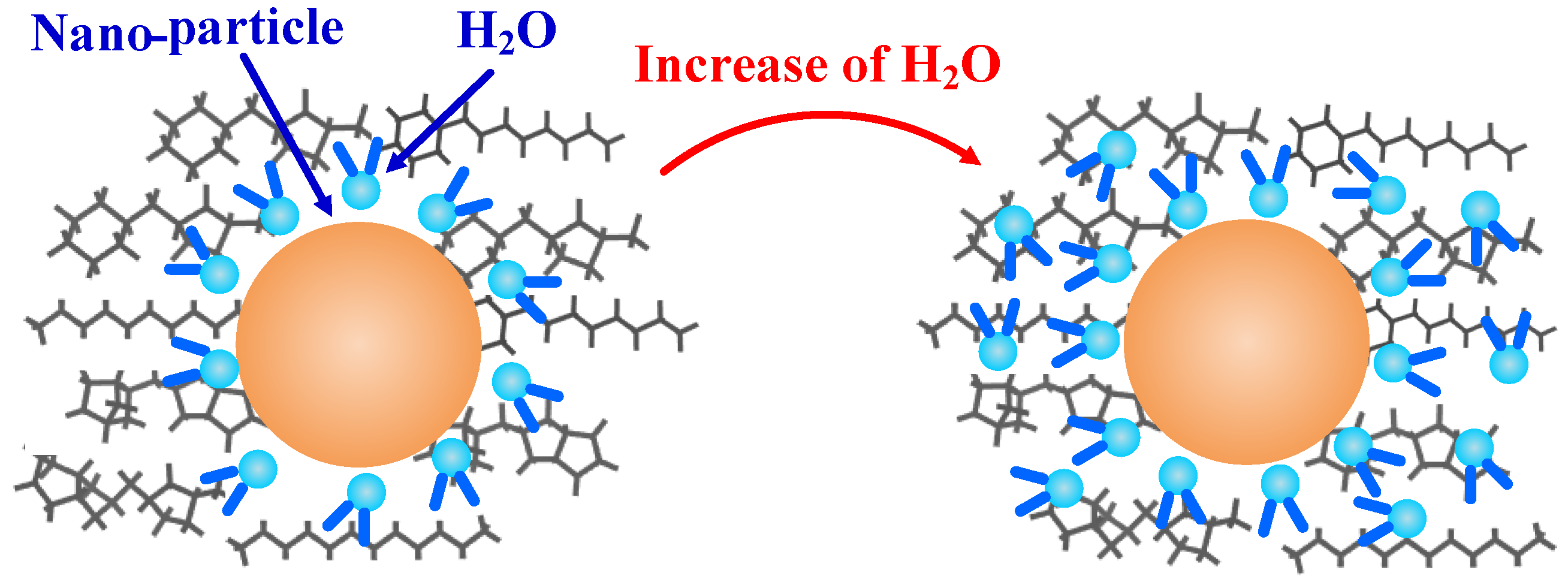
3.1. Free Volume
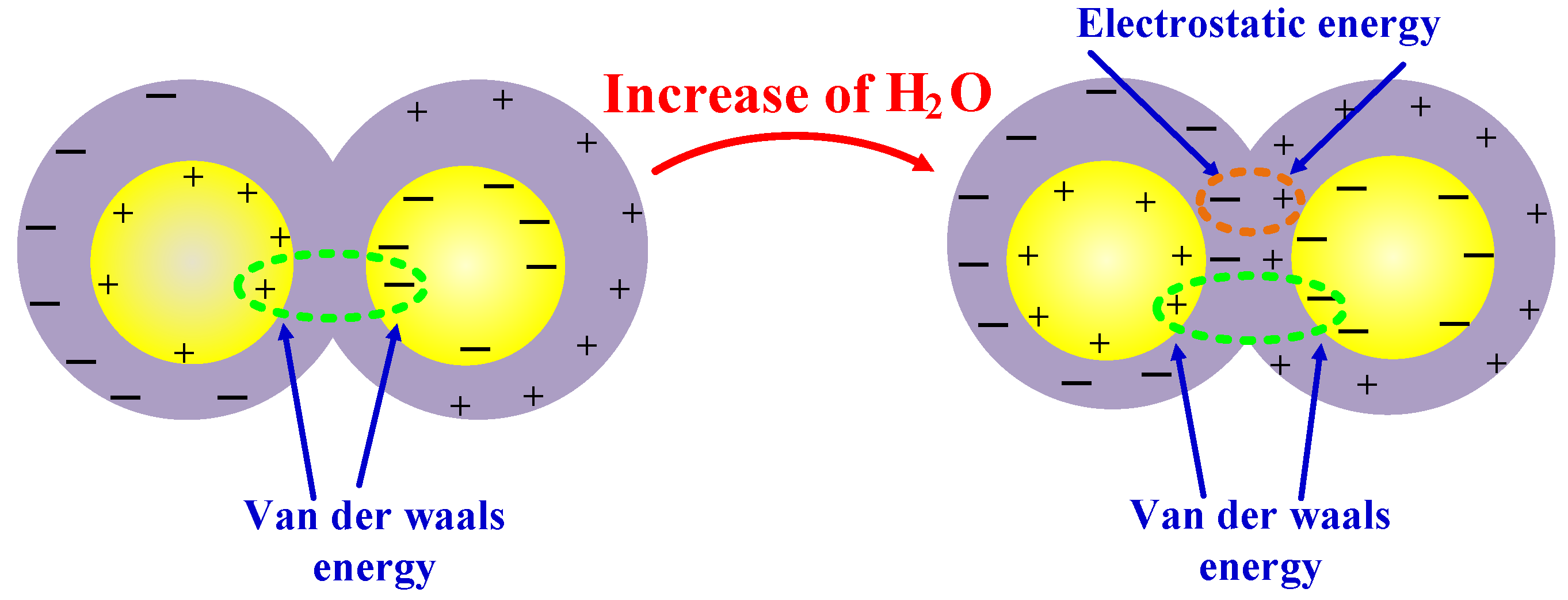
3.2. Interaction Energy
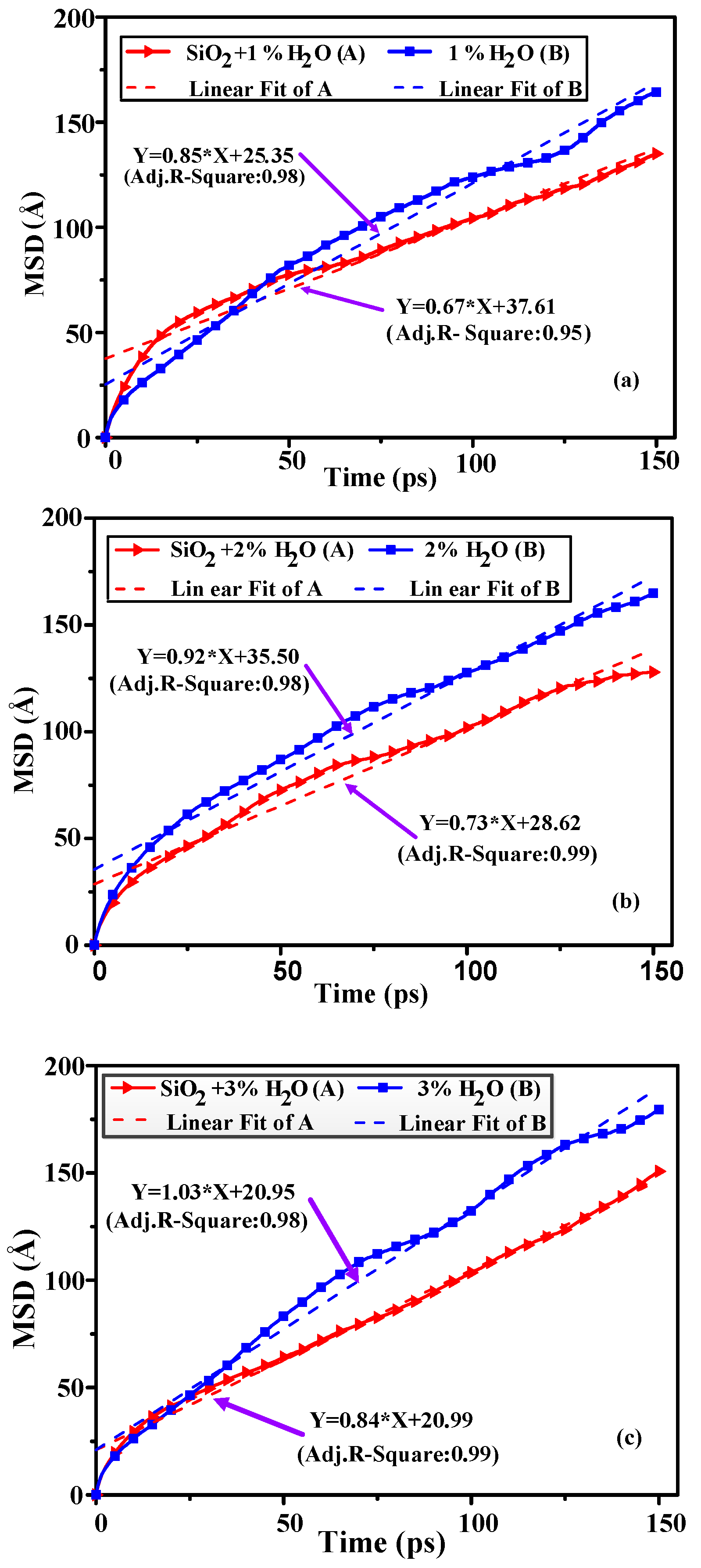
3.3. Mean Square Displacement
4. Conclusions
- (1)
- The free volume fractions in the models containing nano-SiO2 particles were reduced, and so the diffusion of water molecules was restricted. Thus, water molecules had a smaller diffusion coefficient in oils containing nano-particles, meaning less diffusion occurred.
- (2)
- The model containing nano-SiO2 particles showed greater interaction energy between the oil and water molecules, demonstrating that the addition of these particles increased the binding of water molecules by the oil.
- (3)
- The results prove that the addition of nano-SiO2 particles can effectively increase the binding of insulating oil to water molecules and reduce the diffusion of water molecules in insulating oil. This paper provides a theoretical basis for the modification of insulating oil with nano-SiO2 particles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Kawano, S.; Kida, T.; Miyawaki, K. Cyclodextrin polymers as highly effective adsorbents for removal and recovery of polychlorobiphenyl (PCB) contaminants in insulating oil. Environ. Sci. Technol. 2014, 48, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chao, T.; Chen, G.; Qu, Z.; Lu, C.; Xu, L. The influence and mechanism of nano Al2O3 to the thermal stability of cellulose insulation paper. Sci. Sin. 2015, 45, 1167. [Google Scholar]
- Mansour, D.E.A.; Atiya, E.G.; Khattab, R.M.; Azmy, A.M. Effect of titania nanoparticles on the dielectric properties of transformer oil-based nanofluids. In Proceedings of the 2012 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Montreal, QC, Canada, 14–17 October 2012. [Google Scholar]
- Xiao, B.W.; Chao, T.; Bo, H.; Jian, H.; George, C. Review of research progress on the electrical properties and modification of mineral insulating oils used in power transformers. Energies 2018, 11, 487. [Google Scholar] [CrossRef]
- Du, Y.F.; Lv, Y.Z.; Zhou, J.Q.; Li, X.X.; Li, C.R. Breakdown properties of transformer oil-based TiO2 nanofluid. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectic Phenomena, West Lafayette, IN, USA, 17–20 October 2010. [Google Scholar]
- Katiyar, A.; Dhar, P.; Nandi, T. Effects of nanostructure permittivity and dimensions on the increased dielectric strength of nano insulating oils. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 235–243. [Google Scholar] [CrossRef]
- Zmarz, Y.D.; Dobry, D. Analysis of properties of aged mineral oil doped with C60 fullerenes. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1119–1126. [Google Scholar] [CrossRef]
- Prasath, R.T.A.R.; Roy, N.K.; Mahato, S.N.; Thomas, P. Mineral oil based high permittivity CaCu3Ti4O12 (CCTO) nanofluids for power transformer application. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2344–2353. [Google Scholar] [CrossRef]
- Cavallini, A.; Karthik, R.; Negri, F. The effect of magnetite, graphene oxide and silicone oxide nanoparticles on dielectric withstand characteristics of mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2592–2600. [Google Scholar] [CrossRef]
- Yang, L.; Ming, M.; Jian, Z.D. Molecular dynamics simulation on impact of temperature on viscosity of nano-modified transformer oil. Insul. Mater. 2017, 7, 66–70. [Google Scholar]
- Rafiq, M.; Li, C.; Lv, Y. Breakdown characteristics of transformer oil based silica nanofluids. In Proceedings of the 19th International Multi-Topic Conference (INMIC), Islamabad, Pakistan, 5–6 December 2016. [Google Scholar]
- Zhu, J.Z.; Yi, J.; Yi, H.W. Study on characteristics of transformer oil modified by silica nanoparticles. Insul. Material. 2016, 49, 47–52. [Google Scholar]
- Zhou, Y.; Sui, S.Y.; Li, J. Statistical analysis of moisture’s effect on AC breakdown strength of TiO2 nanofluids. J. Mol. Liq. 2018, 249, 420–428. [Google Scholar] [CrossRef]
- Suleiman, A.A.; Muhamad, N.A.; Bashir, N. Effect of moisture on breakdown voltage and structure of palm based insulation oils. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2119–2126. [Google Scholar] [CrossRef]
- Lu, X.C.; Yu, J.; Alberto, S. Nanoparticles adsorbed at the water/oil Interface: Coverage and composition effects on structure and diffusion. Langmuir 2013, 29, 7221–7228. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Chen, W.; Ji, Z. Influence of moisture content on electrical properties of insulating oil. Insul. Materials. 2016, 7, 79–82. [Google Scholar]
- Xu, L.; Chao, T.; Qian, W.; Xiao, P.L.; Jian, H. Molecular simulation research on the micro effect mechanism of interfacial properties of nano SiO2/meta-aramid fiber. Int. J. Heat Mass Transf. 2017, 35, 123–129. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, Q.M.; Zhang, Y.; Yang, R.; Gao, S.G. Simulation of reactive molecular dynamics of transformer oil Pyrolysis at high temperature and the influence mechanism of acid in oil. High Volt. Eng. 2017, 43, 247–255. [Google Scholar]
- Meng, Z.Z.; Rui, J.L.; Xin, Z. Molecular dynamics simulation of diffusion behavior for hydronium ion in hydrous oil dielectric. High Volt. Eng. 2011, 37, 1930–1936. [Google Scholar]
- Theodorou, D.N.; Suter, U.W. Detailed molecular structure of a vinyl polymer glass. Macromolecules 1985, 18, 1467–1478. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, C.; Hao, J. Thermal stability and dielectric properties of nano-SiO2-doped cellulose. Appl. Phys. Lett. 2017, 111, 012902. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Ming, D. Molecular dynamics simulation of temperature impact on the viscosity of transformer oil-based nanofluids. In Proceedings of the International Conference on Condition Monitoring and Diagnosis (CMD), Xi’an, China, 25–28 September 2016. [Google Scholar]
- Balazs, A.C.; Zhou, Z.; Yeung, C. Behavior of amphiphilic comb copolymers in oil/water mixtures: A molecular dynamics study. Langmuir 1992, 8, 2295–2300. [Google Scholar] [CrossRef]
- Rui, J.L.; Chun, Y.G.; Xin, Z. Diffusion behavior of gas molecules in oil-paper insulation system based on molecular dynamic simulation. High Volt. Eng. 2012, 38, 2373–2382. [Google Scholar]
- Xiao, B.W.; Tang, C.; Wang, Q.; Li, X.; Hao, J. Selection of optimal polymerization degree and force field in the molecular dynamics simulation of insulating paper cellulose. Energies 2017, 10, 1377. [Google Scholar] [CrossRef]
- Dai, J.Z.; Dong, M.; Wen, F.X. The Molecular dynamic simulation investigation of the dispersion stability of nano-modified transformer oil. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Ann Arbor, MI, USA, 18–21 October 2015. [Google Scholar]
- Song, Y.; Luo, M.; Dai, L.L. Understanding nanoparticle diffusion and exploring interfacial nanorheology using molecular dynamics simulations. Langmuir 2010, 26, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Karaborni, S.; Os, N.M.V.; Esselink, K. Molecular dynamics simulations of oil solubilization in surfactant solutions. Langmuir 1993, 9, 1175–1178. [Google Scholar] [CrossRef]
- Fox, T.G.F., Jr.; Flory, P.J. Second-Order transition temperatures and related properties of polystyrene I influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Hanus, J.; Mazeau, K. The xyloglucan-cellulose assembly at the atomic scale. Biopolymers 2006, 82, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, W.; Yu, L. Molecular dynamics simulation of corrosive particle diffusion in benzimidazole inhibitor films. Corros. Sci. 2011, 53, 1331–1336. [Google Scholar] [CrossRef]

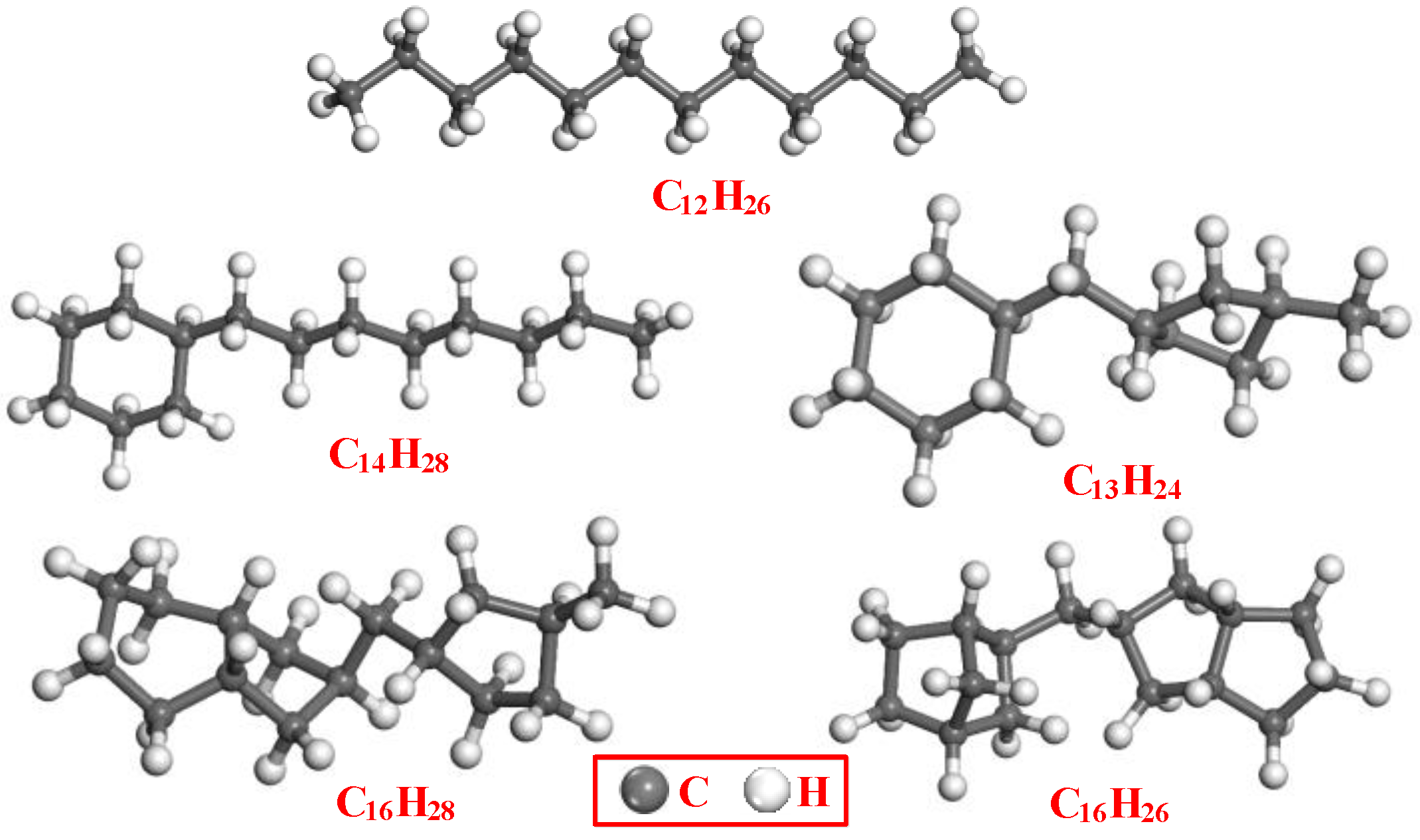
| Composition | Chain Hydrocarbon | Cycloparaffins | Total | |||
|---|---|---|---|---|---|---|
| Monocyclic | Dicyclic | Tricyclic | Tetracyclic | |||
| ωB (%) | 11.6 | 15.5 | 28.5 | 23.3 | 9.7 | 88.6 |
| Nano-SiO2 Particles | Without Nano-SiO2 Particles | |||||
|---|---|---|---|---|---|---|
| Moisture | 1% | 2% | 3% | 1% | 2% | 3% |
| Occupied volume | 35,308 | 35,840 | 34,914 | 34,327 | 34,719 | 33,692 |
| Free volume | 3485 | 3550 | 5902 | 3640 | 4107 | 5808 |
| FFV | 0.089 | 0.090 | 0.145 | 0.096 | 0.103 | 0.147 |
| Nano-SiO2 Particles | Without Nano-SiO2 Particles | |||||
|---|---|---|---|---|---|---|
| Moisture | 1% | 2% | 3% | 1% | 2% | 3% |
| Interaction energy | −16.86 | −30.50 | −32.98 | −15.80 | −29.89 | −30.62 |
| van der Waals energy | −15.59 | −28.24 | −20.75 | −14.91 | −27.32 | −18.75 |
| Electrostatic energy | 0.59 | −0.38 | −8.09 | −0.578 | −1.037 | −10.22 |
| Nano-SiO2 Particles | Without Nano-SiO2 Particles | |||||
|---|---|---|---|---|---|---|
| Moisture | 1% | 2% | 3% | 1% | 2% | 3% |
| D | 0.11 | 0.12 | 0.14 | 0.14 | 0.15 | 0.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Tang, C.; Wang, Q.; Zhang, S.; Yang, Y. The Effect and Associate Mechanism of Nano SiO2 Particles on the Diffusion Behavior of Water in Insulating Oil. Materials 2018, 11, 2373. https://doi.org/10.3390/ma11122373
Tian W, Tang C, Wang Q, Zhang S, Yang Y. The Effect and Associate Mechanism of Nano SiO2 Particles on the Diffusion Behavior of Water in Insulating Oil. Materials. 2018; 11(12):2373. https://doi.org/10.3390/ma11122373
Chicago/Turabian StyleTian, Wenxin, Chao Tang, Qian Wang, Shiling Zhang, and Yali Yang. 2018. "The Effect and Associate Mechanism of Nano SiO2 Particles on the Diffusion Behavior of Water in Insulating Oil" Materials 11, no. 12: 2373. https://doi.org/10.3390/ma11122373
APA StyleTian, W., Tang, C., Wang, Q., Zhang, S., & Yang, Y. (2018). The Effect and Associate Mechanism of Nano SiO2 Particles on the Diffusion Behavior of Water in Insulating Oil. Materials, 11(12), 2373. https://doi.org/10.3390/ma11122373





