Hollow Fiber Porous Nanocomposite Membranes Produced via Continuous Extrusion: Morphology and Gas Transport Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
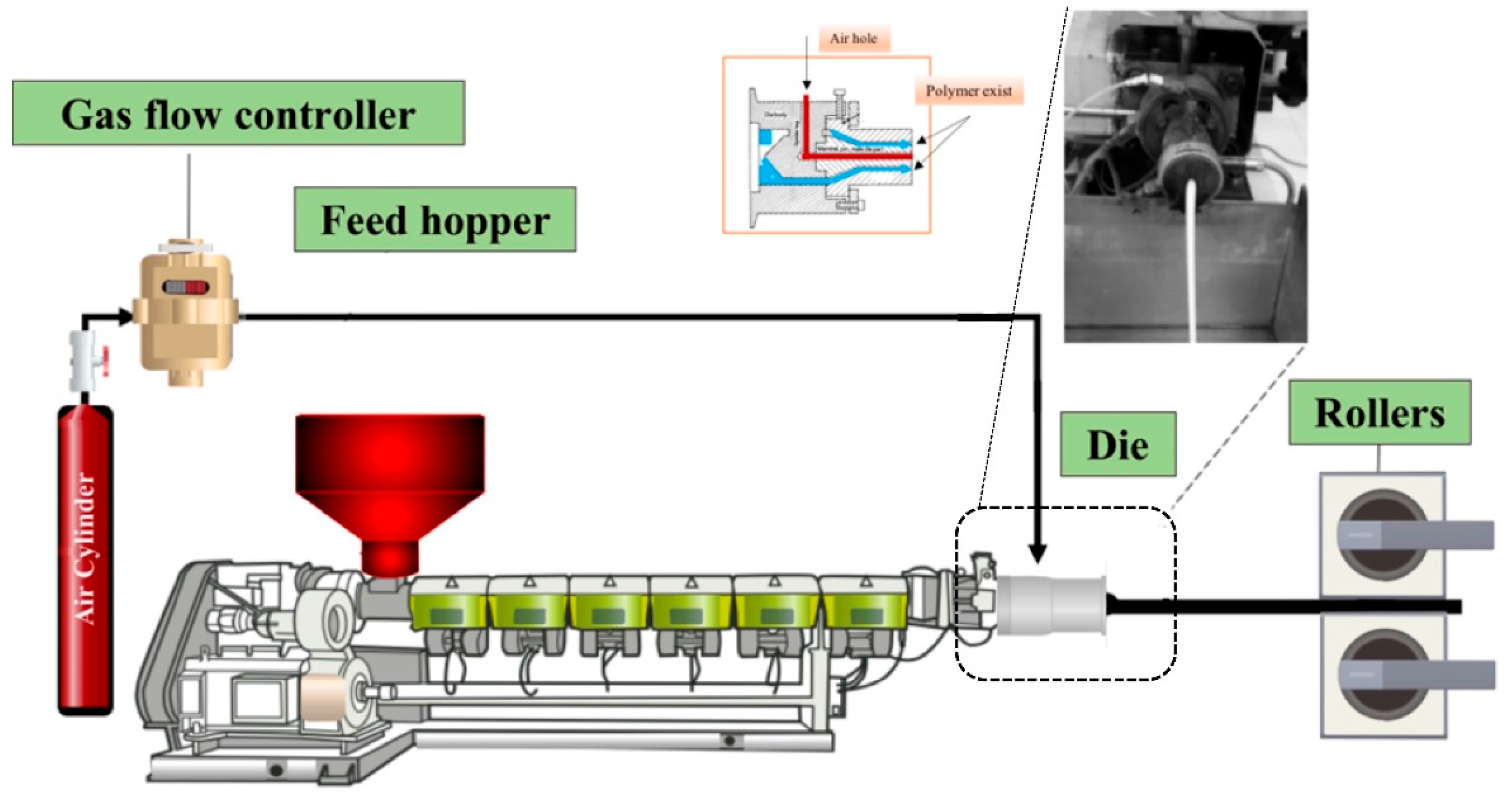
2.2. Sample Preparation
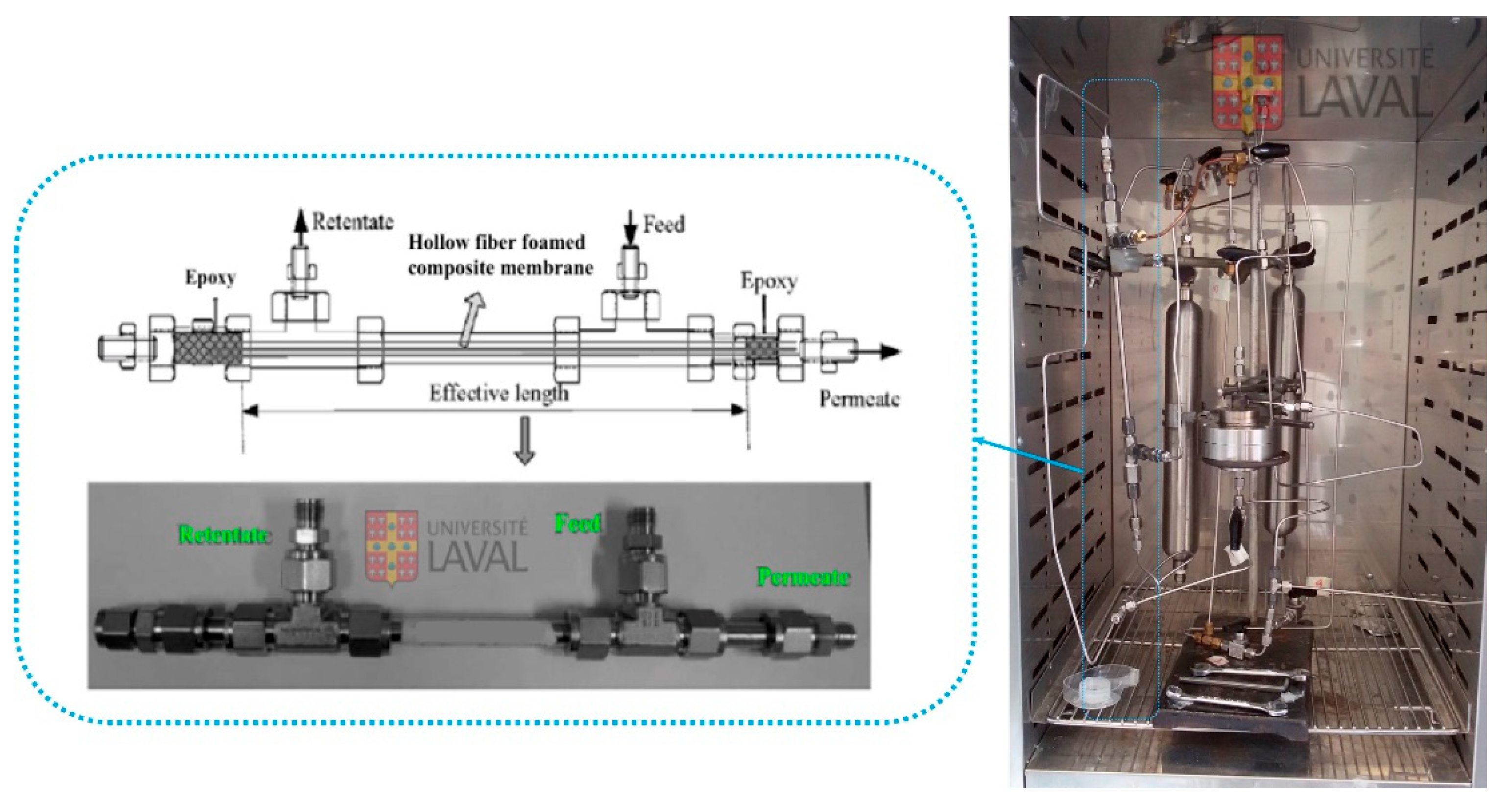
2.3. Characterization
2.4. Gas Permeation
3. Results and Discussion
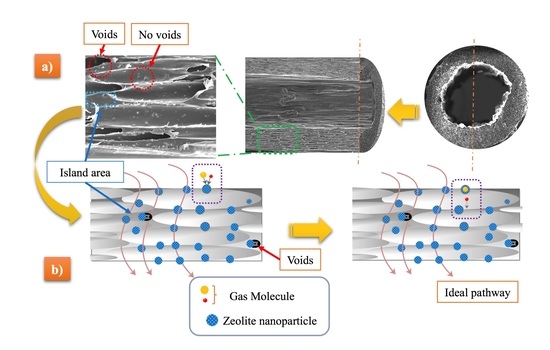
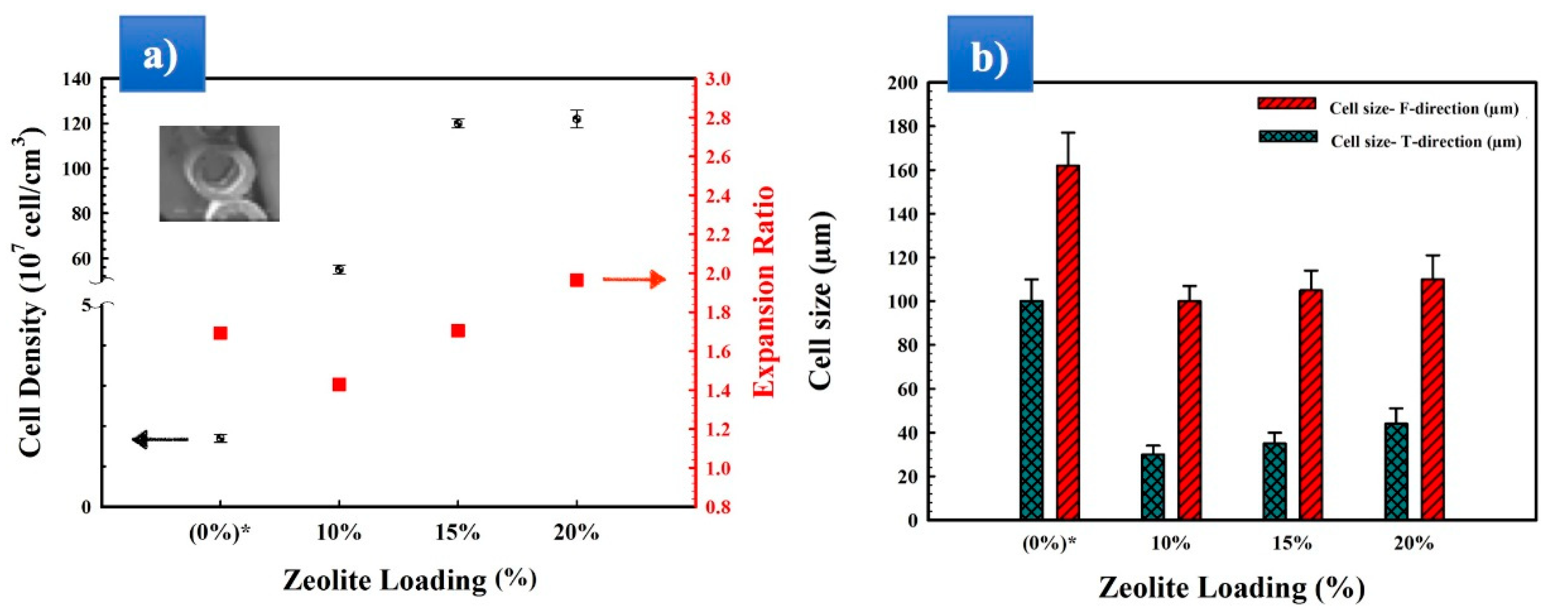
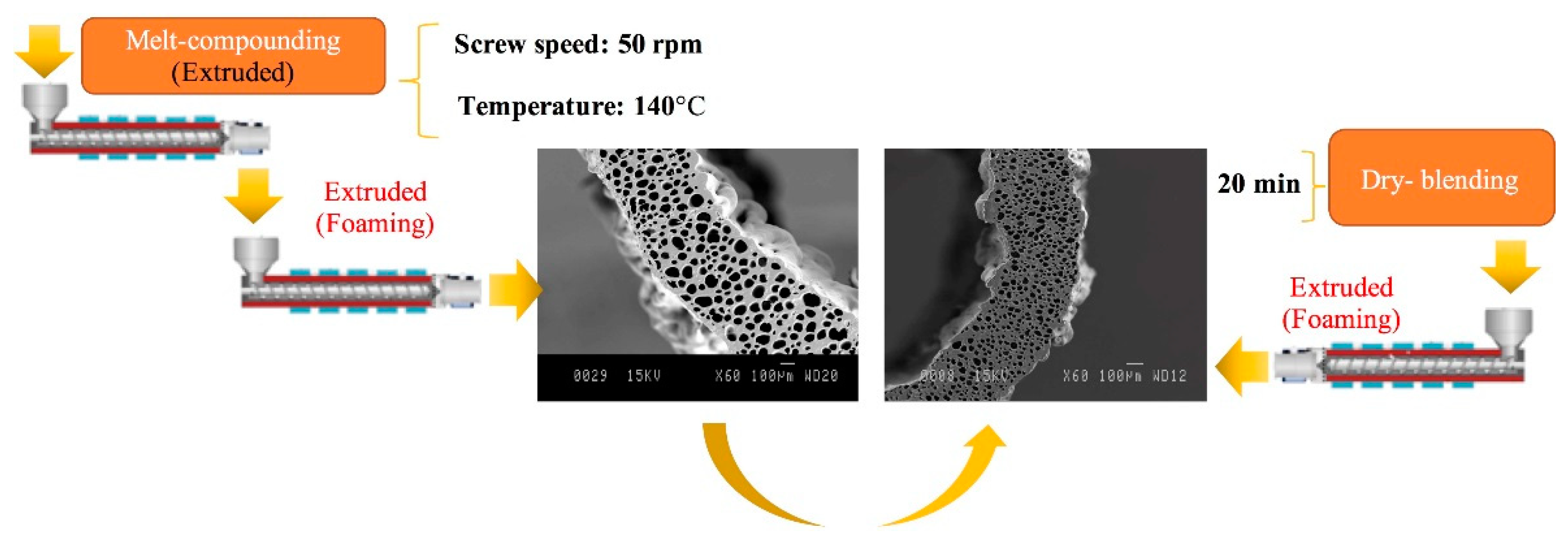
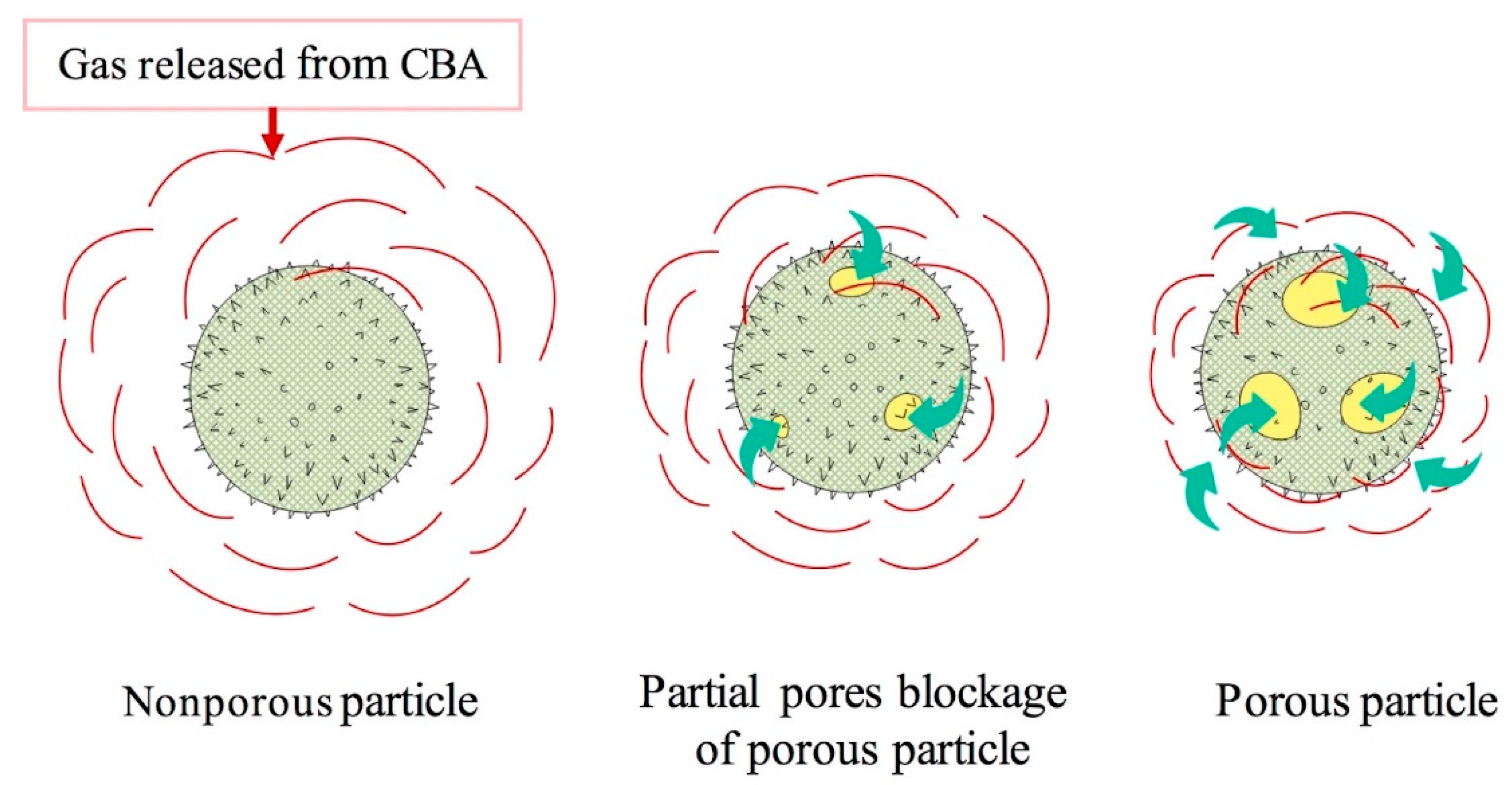
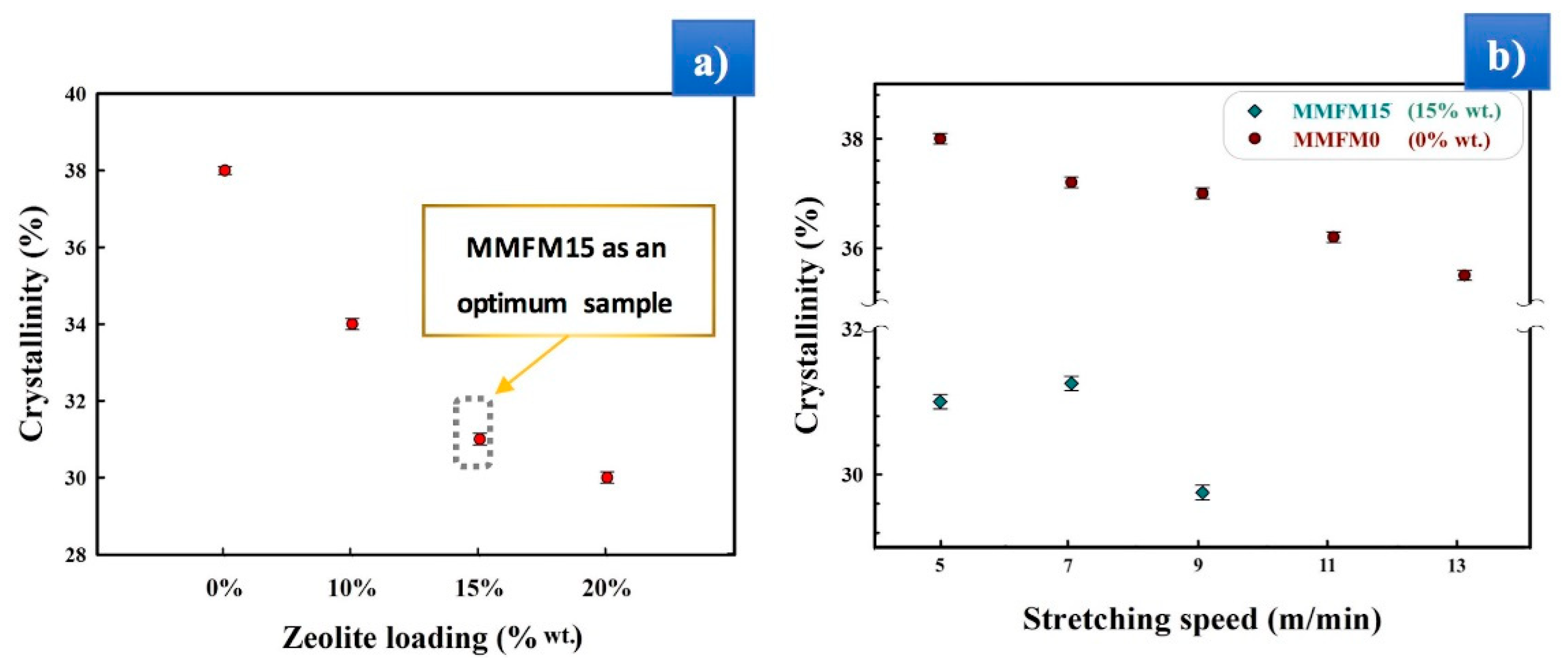
3.1. Preparation and Optimization of MMFMs
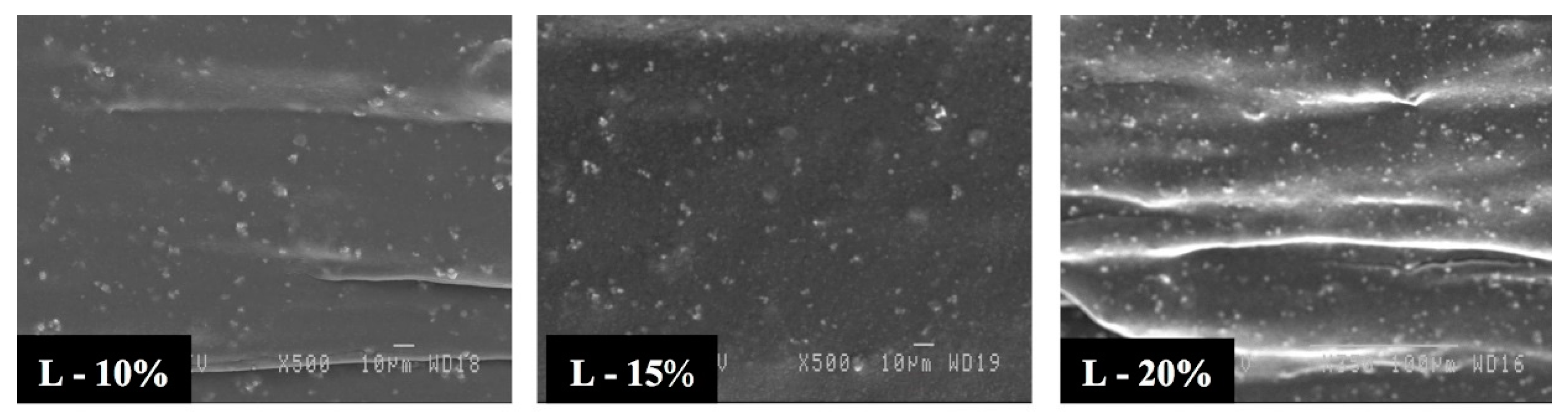
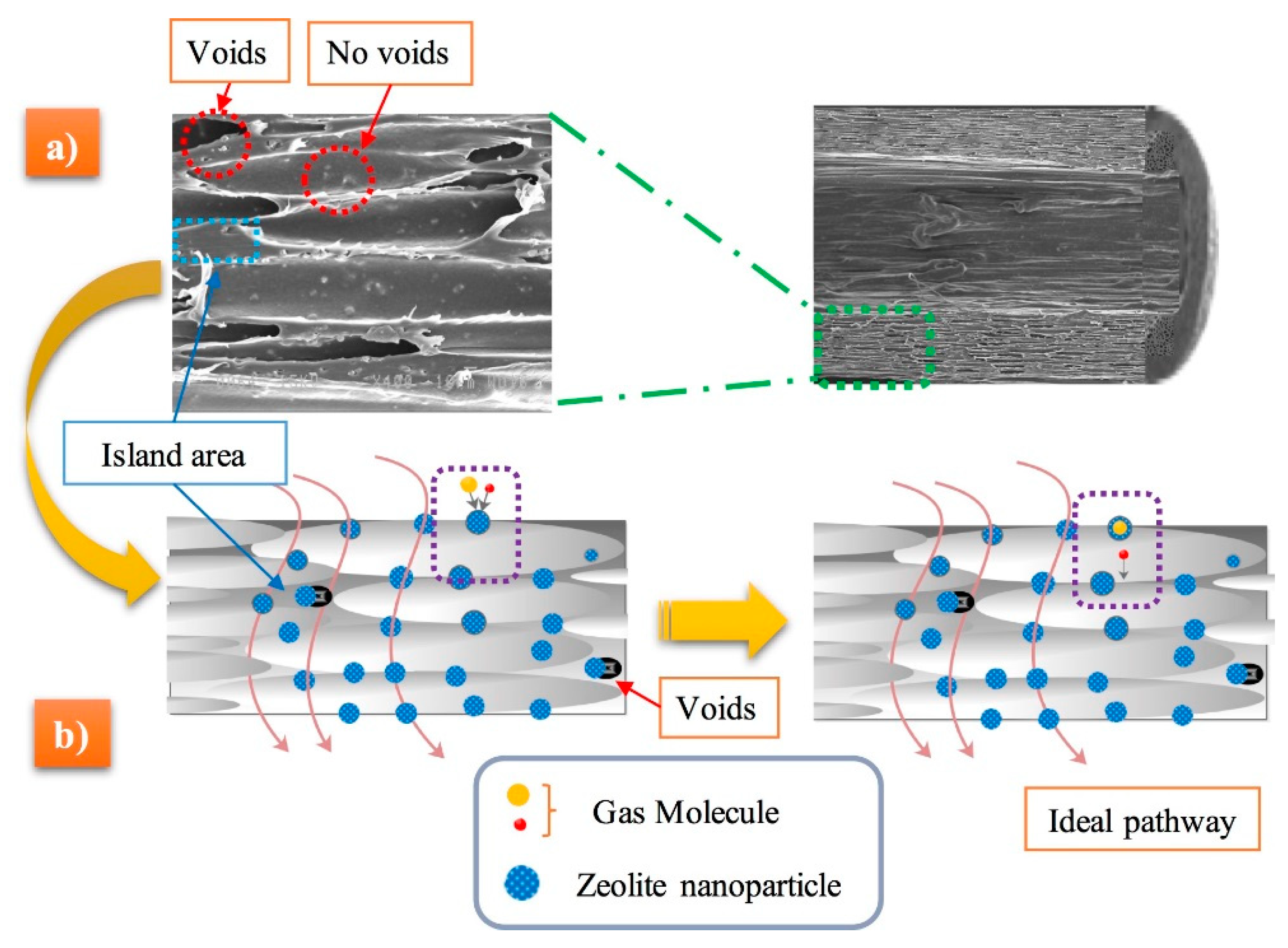
3.2. Morphology
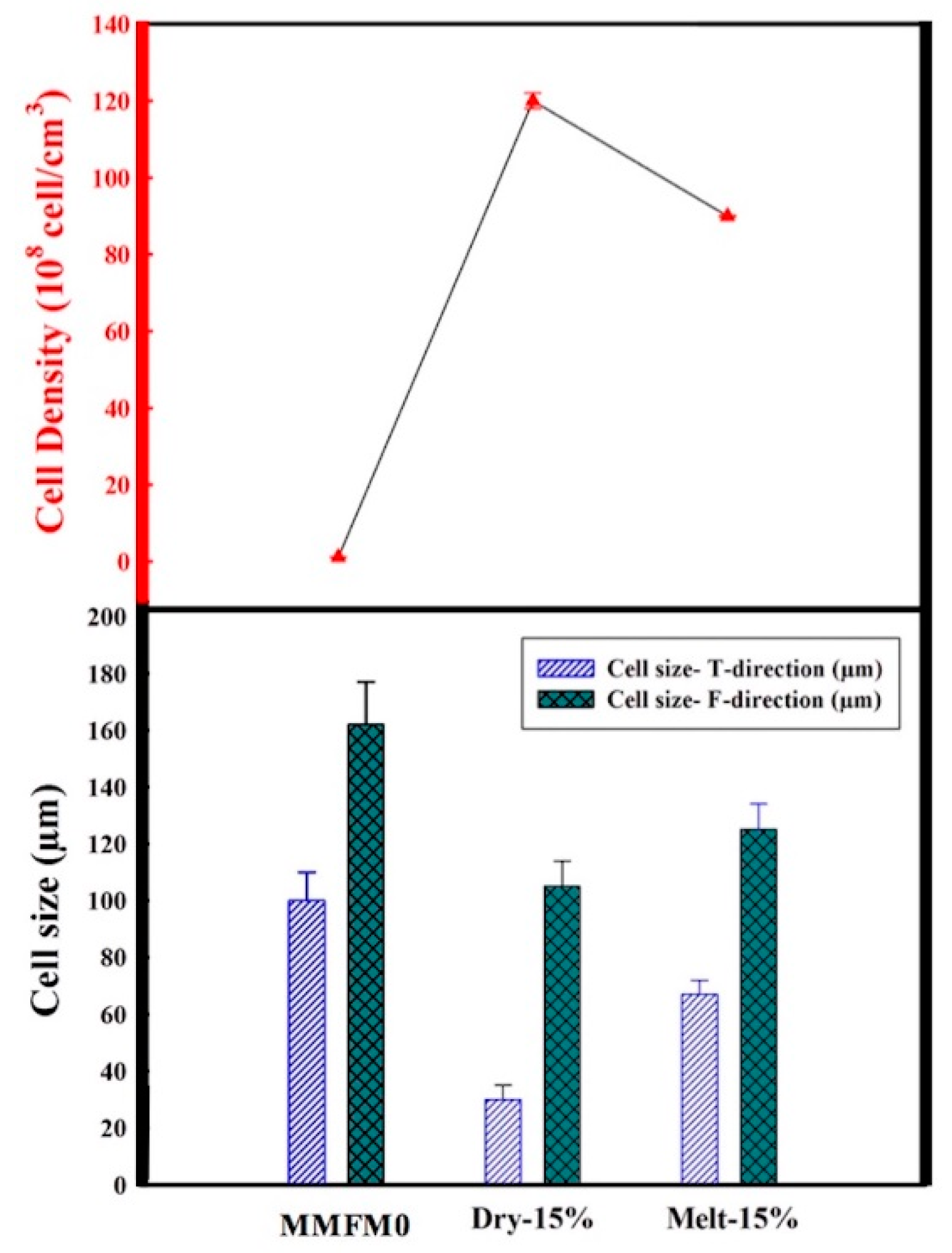
3.3. Effect of Blending Method on the Cellular Structure
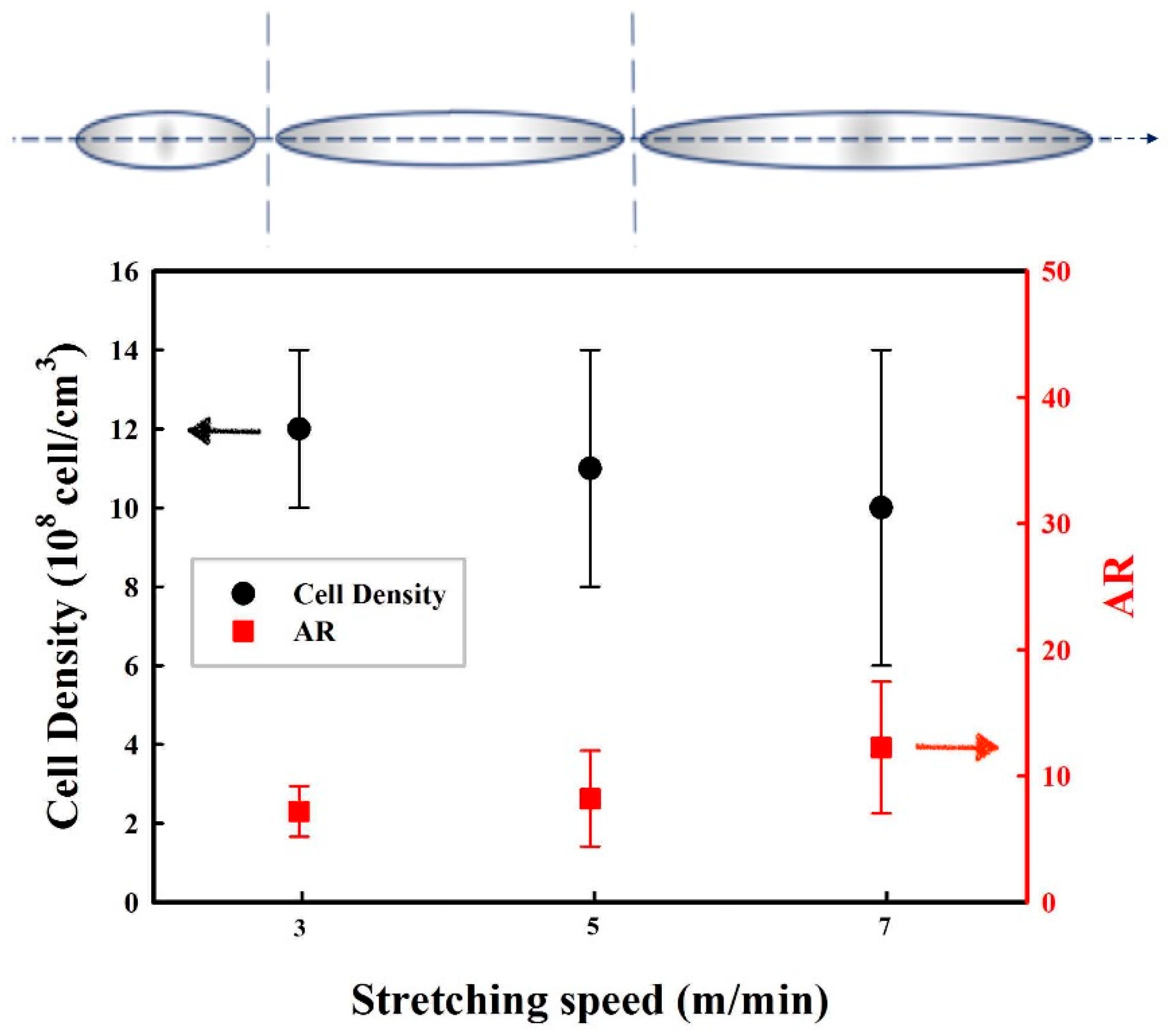
3.4. Effect of the Stretching Rate on the Cellular Structure
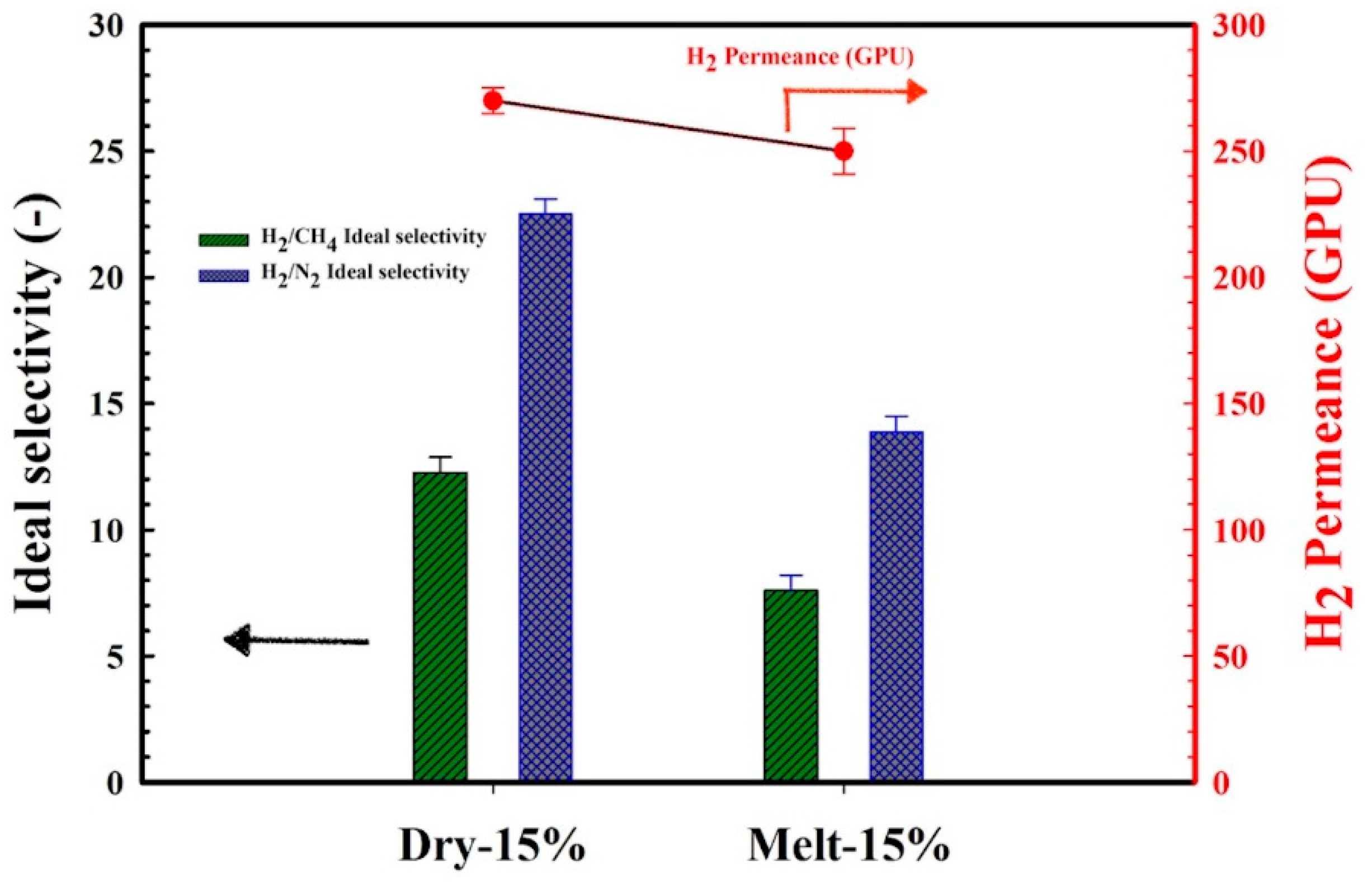
3.5. Gas Permeation Performances
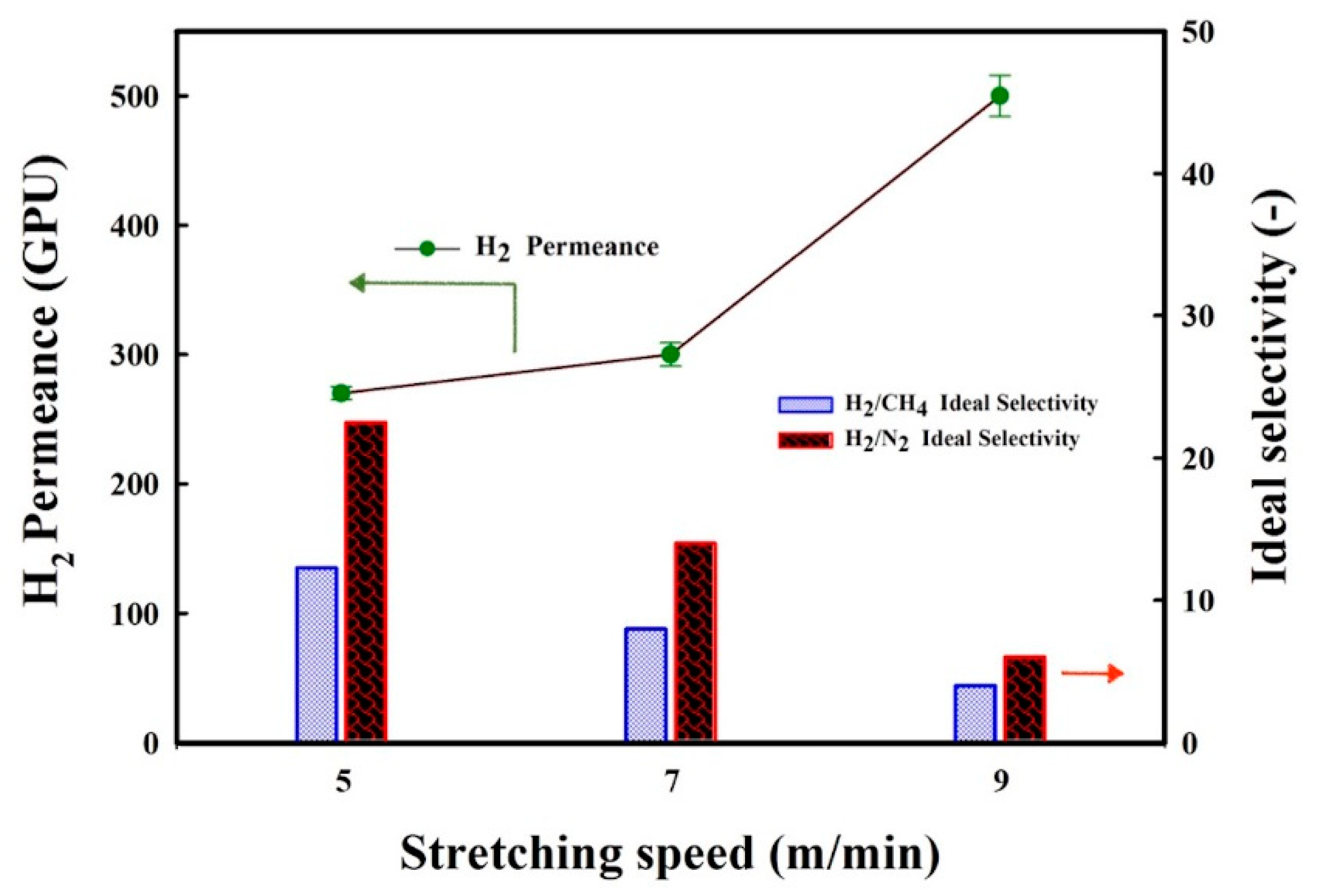
3.6. Influence of Stretching Speed on the Membranes’ Gas Transport
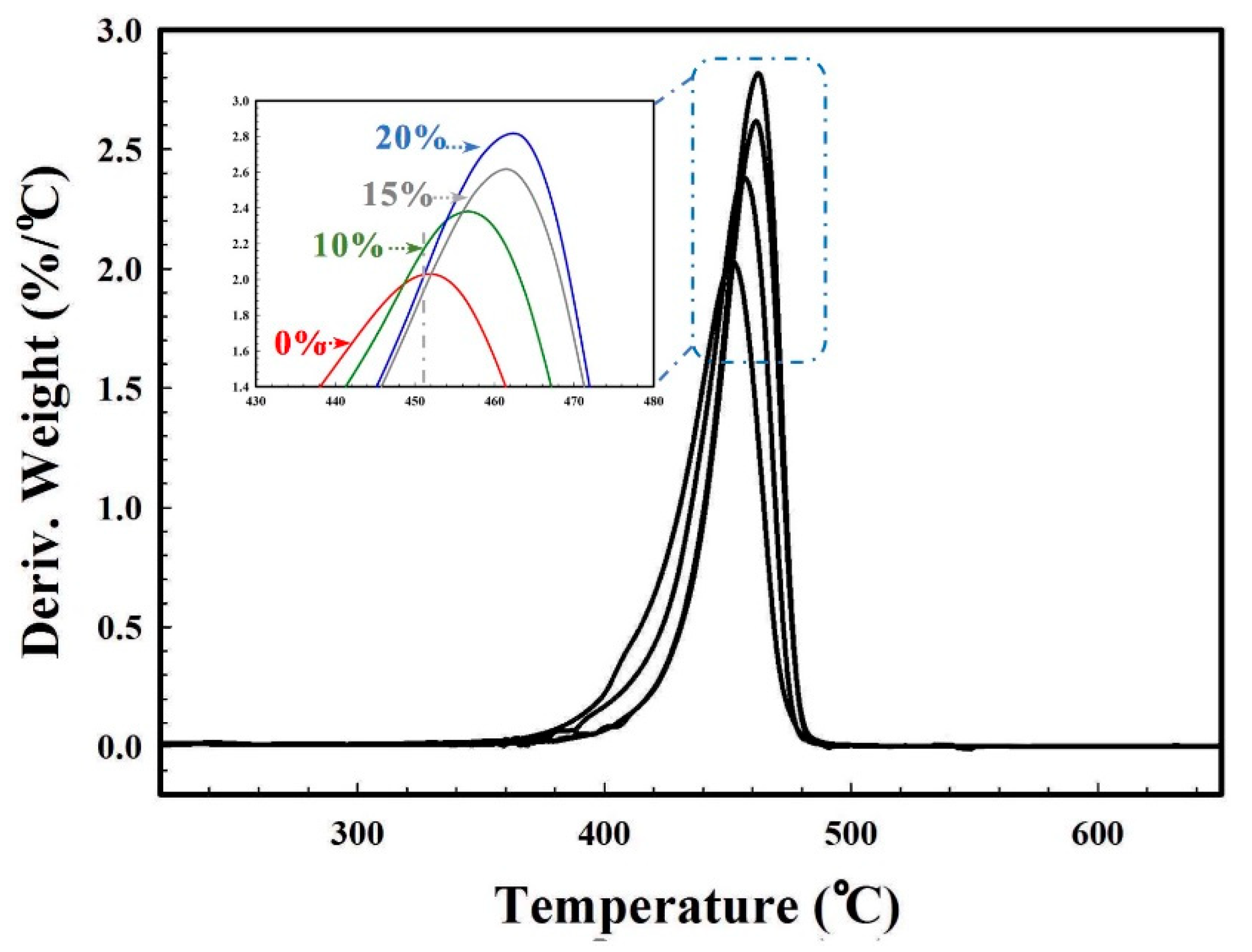
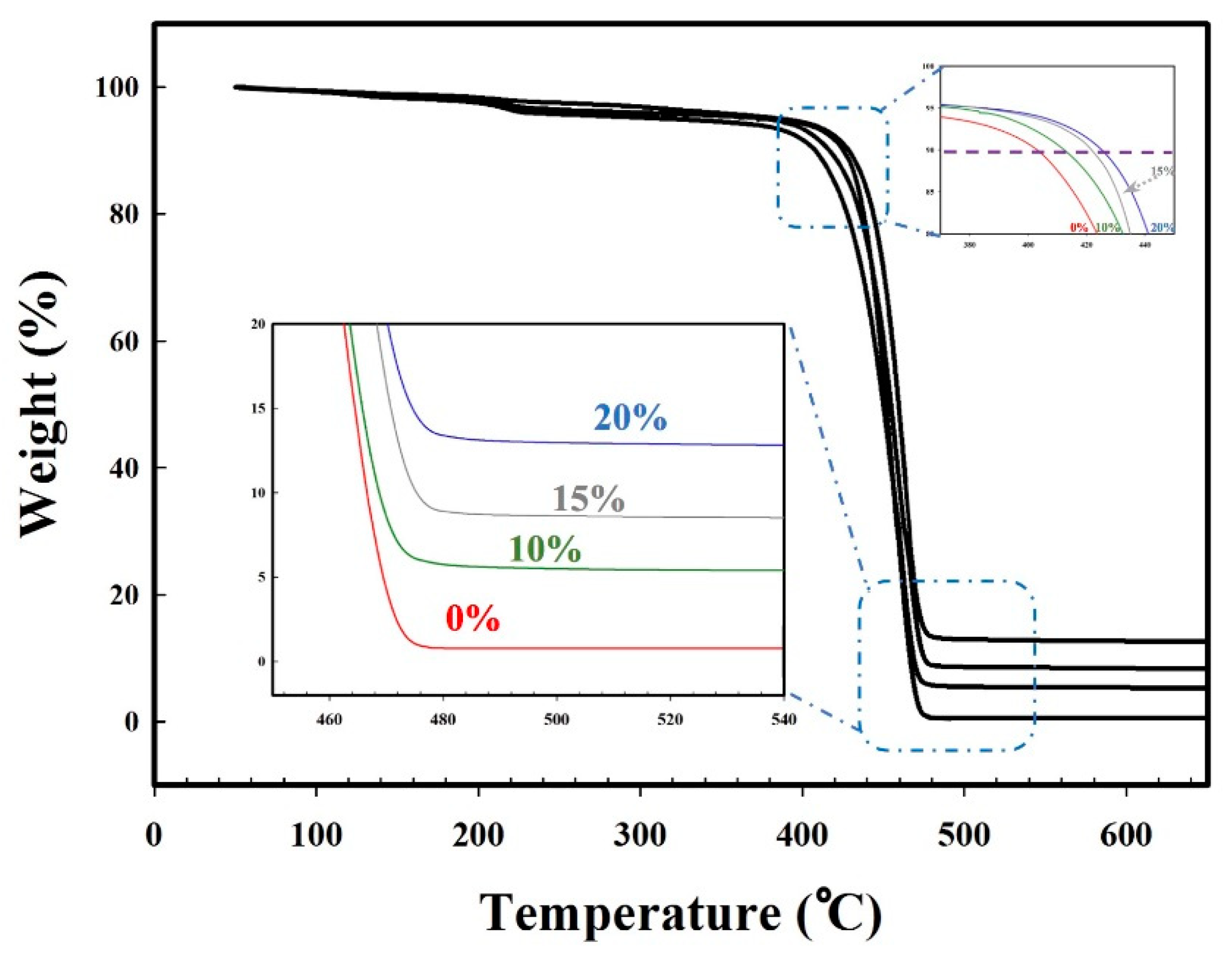
3.7. Mechanical and Thermal Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandey, P.; Chauhan, R.S. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Nijmeijer, K.; Ribeiro, C.P., Jr.; Freeman, B.D.; Wessling, M. On the effects of plasticization in CO2/light gas separation using polymeric solubility selective membranes. J. Membr. Sci. 2011, 367, 33–44. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Ge, B.; Xu, Y.; Zhao, H.; Sun, H.; Guo, Y.; Wang, W. High Performance Gas Separation Mixed Matrix Membrane Fabricated by Incorporation of Functionalized Submicrometer-Sized Metal-Organic Framework. Materials 2018, 11, 1421. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Vinh-Thang, H.; Rodrigue, D.; Kaliaguine, S. Effect of macrovoids in nano-silica/polyimide mixed matrix membranes for high flux CO2/CH4 gas separation. RSC Adv. 2014, 4, 12235–12244. [Google Scholar] [CrossRef]
- Chen, X.Y.; Razzaz, Z.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on silica nanoparticles and microcellular polymers for CO2/CH4 separation. J. Cell Plast. 2018, 54, 309–331. [Google Scholar] [CrossRef]
- Razzaz, Z.; Mohebbi, A.; Rodrigue, D. Effect of processing conditions on the cellular morphology of polyethylene hollow fiber foams for membrane applications. Cell. Polym. 2018. accepted. [Google Scholar] [CrossRef]
- Husain, S.; Koros, W.J. Mixed matrix hollow fiber membranes made with modified HSSZ-13 zeolite in polyetherimide polymer matrix for gas separation. J. Membr. Sci. 2007, 288, 195–207. [Google Scholar] [CrossRef]
- Narkis, M.; Joseph, E. Tensile Properties of Rigid Polymeric Foams Produced by Salt Extraction. J. Cell Plast. 1978, 14, 45–49. [Google Scholar] [CrossRef]
- Verdolotti, L.; Colini, S.; Porta, G.; Iannace, S. Effects of the addition of LiCl, LiClO4, and LiCF3SO3 salts on the chemical structure, density, electrical, and mechanical properties of rigid polyurethane foam composite. Polym. Eng. Sci. 2011, 51, 1137–1144. [Google Scholar] [CrossRef]
- Mosadegh-Sedghi, S.; Brisson, J.; Rodrigue, D.; Iliuta, M.C. Morphological, chemical and thermal stability of microporous LDPE hollow fiber membranes in contact with single and mixed amine based CO2 absorbents. Sep. Purif. Technol. 2012, 96, 117–123. [Google Scholar] [CrossRef]
- Wei, X.; Haire, C. Biaxially Oriented Microporous Membrane. WO Patent 2007098339, 30 August 2007. [Google Scholar]
- Razzaz, Z.; Mohebbi, A.; Rodrigue, D. Gas transport properties of cellular hollow fiber membranes based on LLDPE/LDPE blends. Cell. Polym. 2018. accepted. [Google Scholar]
- Mohebbi, A.; Mighri, F.; Ajji, A.; Rodrigue, D. Effect of Processing Conditions on the Cellular Morphology of Polypropylene Foamed Films for Piezoelectric Applications. Cell. Polym. 2017, 36, 13. [Google Scholar] [CrossRef]
- Yousefian, H.; Rodrigue, D. Effect of nanocrystalline cellulose on morphological, thermal, and mechanical properties of nylon 6 composites. Polym. Compos. 2016, 37, 1473–1479. [Google Scholar] [CrossRef]
- Guan, R.; Wang, B.; Lu, D.; Fang, Q.; Xiang, B. Microcellular thin PET sheet foam preparation by compression molding. J. Appl. Polym. Sci. 2004, 93, 1698–1704. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim. Acta 2007, 454, 90–98. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, T.G. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 1999, 20, 1783–1790. [Google Scholar] [CrossRef]
- Klempner, D.; Sendijareviʹc, V.; Aseeva, R.M. Handbook of Polymeric Foams and Foam Technology; Hanser Publishers: Munich, Germany, 2004. [Google Scholar]
- Lee, S.T. Foam Extrusion Overview; Foam Extrusion; Technomic Publishing Co.: Lancaster, PA, USA, 2000. [Google Scholar]
- Saiz-Arroyo, C.; Rodríguez-Pérez, M.Á.; Velasco, J.I.; de Saja, J.A. Influence of foaming process on the structure–properties relationship of foamed LDPE/silica nanocomposites. Compos. Part B-Eng. 2013, 48, 40–50. [Google Scholar] [CrossRef]
- Fan, W.; Snyder, M.A.; Kumar, S.; Lee, P.S.; Yoo, W.C.; McCormick, A.V.; Penn, R.L.; Stein, A.; Tsapatsis, M. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat. Mater. 2008, 7, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.J.; Shao, H.J.; Wu, B.; Zhang, K.Z.; Luo, D.J.; Qin, S.H.; Hao, Z. Effect of Spin-Draw Rate and Stretching Ratio on Polypropylene Hollow Fiber Membrane Made by Melt-Spinning and Stretching Method. Int. Polym. Proc. 2018, 33, 13–19. [Google Scholar] [CrossRef]
- Cao, J.-H.; Zhu, B.-K.; Lu, H.; Xu, Y.-Y. Study on polypropylene hollow fiber based recirculated membrane bioreactor for treatment of municipal wastewater. Desalination 2005, 183, 431–438. [Google Scholar] [CrossRef]
- Razzaz, Z.; Mohammad, A.W.; Mahmoudi, E. Foaming Prevention in Absorption Columns through Removal of Contaminants from Amine-Based Solutions Using a Solvent Resistant Nanofiltration (SRNF) Membrane. Ind. Eng. Chem. Res. 2015, 54, 12135–12142. [Google Scholar] [CrossRef]
- Covarrubias, C.; Quijada, R. Preparation of aluminophosphate/polyethylene nanocomposite membranes and their gas permeation properties. J. Membr. Sci. 2010, 358, 33–42. [Google Scholar] [CrossRef]
- Mohebbi, A.; Mighri, F.; Ajji, A.; Rodrigue, D. Current issues and challenges in polypropylene foaming: A Review. Cell. Polym. 2015, 34, 299–337. [Google Scholar] [CrossRef]
- Doroudiani, S.; Park, C.B.; Kortschot, M.T. Effect of the crystallinity and morphology on the microcellular foam structure of semicrystalline polymers. Polym. Eng. Sci. 1996, 36, 2645–2662. [Google Scholar] [CrossRef]
- Compañ, V.; Del Castillo, L.F.; Hernández, S.I.; Mar López-González, M.; Riande, E. On the Crystallinity Effect on the Gas Sorption in Semicrystalline Linear Low Density Polyethylene (LLDPE). J. Polym. Sci. B Polym. Phys. 2007, 45, 1798–1807. [Google Scholar] [CrossRef]
- Chen, L.; Rende, D.; Schadler, L.S.; Ozisik, R. Polymer nanocomposite foams. J. Mater. Chem. A 2012, 3837–3850. [Google Scholar] [CrossRef]
- Zepeda Sahagun, C.; Gonzalez-Nunez, R.; Rodrigue, D. Morphology of Extruded PP/HDPE Foam Blends. J. Cell. Plast. 2006, 42, 469–485. [Google Scholar] [CrossRef]
- Mohebbi, A. Optimization of polypropylene cellular films for piezoelectric applications. Ph.D. Thesis, Université Laval, Quebec, Canada, December 2016. [Google Scholar]
- Lee, S.; Park, C.B. Foam Extrusion Principles and Practicetem; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Zhai, W.; Kuboki, T.; Wang, L.; Park, C.B.; Lee, E.K.; Naguib, H.E. Cell structure evolution and the crystallization behavior of polypropylene/clay nanocomposites foams blown in continuous extrusion. Ind. Eng. Chem. Res. 2010, 49, 9834–9845. [Google Scholar] [CrossRef]
- Laguna-Gutierrez, E.; Saiz-Arroyo, C.; Velasco, J.I.; Rodriguez-Perez, M.A. Low density polyethylene/silica nanocomposite foams. Relationship between chemical composition, particle dispersion, cellular structure and physical properties. Eur. Polym. J. 2016, 173–185. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Qiu, J.; Jiang, Z.; Xing, H.; Li, M.; Tang, T. Insight into the influence of OA-Fe3O4 nanoparticles on the morphology and scCO2 batch-foaming behavior of cocontinuous LLDPE/PS immiscible blends at semi-solid state. Polymer 2017, 129, 169–178. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.; Kulprathipanja, S. Novel Ag+-zeolite/polymer mixed matrix membranes with a high CO2/CH4 selectivity. AIChE J. 2007, 53, 610–616. [Google Scholar] [CrossRef]
- Gosselin, R.; Rodrigue, D. Cell morphology analysis of high density polymer foams. Polym. Test. 2005, 24, 1027–1035. [Google Scholar] [CrossRef]
- Antunes, M.; Gedler, G.; Velasco, J.I. Multifunctional nanocomposite foams based on polypropylene with carbon nanofillers. J. Cell. Plast. 2013, 49, 259–279. [Google Scholar] [CrossRef]
- Adams, R.T.; Lee, J.S.; Bae, T.H.; Ward, J.K.; Johnson, J.R.; Jones, C.W.; Nair, S.; Koros, W.J. CO2-CH4 permeation in high zeolite 4A loading mixed matrix membranes. J. Membr. Sci. 2011, 367, 197–203. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Membrane Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Funk, C.V.; Lloyd, D.R. Zeolite-filled microporous mixed matrix (ZeoTIPS) membranes: Prediction of gas separation performance. J. Membr. Sci. 2008, 313, 224–231. [Google Scholar] [CrossRef]
- Biswas, J.; Kim, H.; Choe, S.; Kundu, P.P.; Park, Y.-H.; Lee, D.S. Linear low density polyethylene (LLDPE)/zeolite microporous composite film. Macromol. Res. 2003, 11, 357–367. [Google Scholar] [CrossRef]
- Kim, H.; Biswas, J.; Choe, S. Effects of stearic acid coating on zeolite in LDPE, LLDPE, and HDPE composites. Polymer 2006, 47, 3981–3992. [Google Scholar] [CrossRef]
- Chen, L.; Sheth, H.; Kim, R. Gas absorption with filled polymer systems. Polym. Eng. Sci. 2001, 41, 990–997. [Google Scholar] [CrossRef]
- Yang, J.; Huang, L.; Zhang, Y.; Chen, F.; Fan, P.; Zhong, M.; Yeh, S. ordered mesoporous silica particles in polymethyl methacrylate supercritical carbon dioxide microcellular foaming. Ind. Eng. Chem. Res. 2013, 52, 14169–14178. [Google Scholar] [CrossRef]
- Soles, E.; Smith, J.M.; Parrish, W.R. Gas transport through polyethylene membranes. AIChE J. 1982, 28, 474–479. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J. Flow of gases through polyethylene. J. Polym. Sci. 1961, 50, 413–439. [Google Scholar] [CrossRef]
- Checchetto, R.; Miotello, A.; Nicolais, L.; Carotenuto, G. Gas transport through nanocomposite membrane composed by polyethylene with dispersed graphite nanoplatelets. J. Membr. Sci. 2014, 463, 196–204. [Google Scholar] [CrossRef]
- Bounos, G.; Andrikopoulos, K.S.; Moschopoulou, H.; Lainioti, G.C.; Roilo, D.; Checchetto, R.; Ioannides, T.; Kallitsis, J.K.; Voyiatzis, G.A. Enhancing water vapor permeability in mixed matrix polypropylene membranes through carbon nanotubes dispersion. J. Membr. Sci. 2017, 524, 576–584. [Google Scholar] [CrossRef]
- Graunke, T.; Schmitt, K.; Wöllenstein, J. Organic Membranes for Selectivity Enhancement of Metal Oxide Gas Sensors. J. Sens. 2016, 22. [Google Scholar] [CrossRef]
- Barber, P.; Balasubramanian, S.; Anguchamy, Y.; Gong, S.; Wibowo, A.; Gao, H.; Ploehn, H.; Zur Loye, H.C. Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage. Materials 2009, 2, 1697–1733. [Google Scholar] [CrossRef]
- Biswas, J.; Kim, H.; Shim, S.E.; Kim, G.J.; Choe, S. Comparative study of zeolite-filled LLDPE and HDPE composite films. Ind. Eng. Chem. 2004, 10, 582–591. [Google Scholar]
- Dutta, R.C.; Bhatia, S.K. Transport diffusion of light gases in polyethylene using atomistic simulations. Langmuir 2017, 33, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, H.; Rodrigue, D. Nano-crystalline cellulose, chemical blowing agent, and mold temperature effect on morphological, physical/mechanical properties of polypropylene. J. Appl. Polym. Sci. 2015, 132, 2–10. [Google Scholar] [CrossRef]






| Unfoamed (Solid) | Foamed | |||||
|---|---|---|---|---|---|---|
| Sample | Zeolite Concentration (wt %) | Uniaxial Stretching Speed (m/min) | Sample | CBA (wt %) | Uniaxial Stretching Speed (m/min) | Zeolite Concentration (wt %) |
| L-0 | 0 | 5, 7, 9, 11, 13 | MMFM0 | 1.75 | 5, 7, 9, 11, 13 | 0 |
| L-10 | 10 | 5, 7, 9, 11, 13 | MMFM10 | 2.5 | 5, 7, 9 | 10 |
| L-15 | 15 | 5, 7, 9, 11, 13 | MMFM15 | 2.5 | 5, 7, 9 | 15 |
| L-20 | 20 | 5, 7, 9, 11, 13 | MMFM20 | 2.5 | 5, 7, 9 | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaz, Z.; Rodrigue, D. Hollow Fiber Porous Nanocomposite Membranes Produced via Continuous Extrusion: Morphology and Gas Transport Properties. Materials 2018, 11, 2311. https://doi.org/10.3390/ma11112311
Razzaz Z, Rodrigue D. Hollow Fiber Porous Nanocomposite Membranes Produced via Continuous Extrusion: Morphology and Gas Transport Properties. Materials. 2018; 11(11):2311. https://doi.org/10.3390/ma11112311
Chicago/Turabian StyleRazzaz, Zahir, and Denis Rodrigue. 2018. "Hollow Fiber Porous Nanocomposite Membranes Produced via Continuous Extrusion: Morphology and Gas Transport Properties" Materials 11, no. 11: 2311. https://doi.org/10.3390/ma11112311
APA StyleRazzaz, Z., & Rodrigue, D. (2018). Hollow Fiber Porous Nanocomposite Membranes Produced via Continuous Extrusion: Morphology and Gas Transport Properties. Materials, 11(11), 2311. https://doi.org/10.3390/ma11112311