Preparation and Surface Properties Study of Novel Fluorine-Containing Methacrylate Polymers for Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials
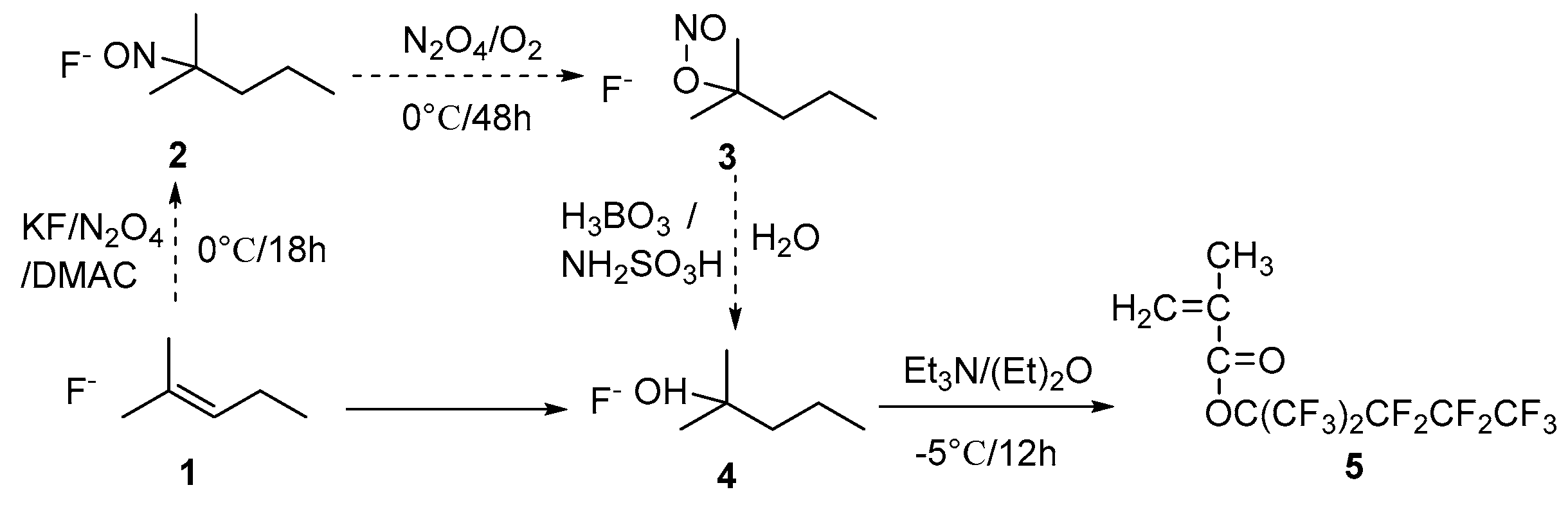
2.2. Synthesis of Monomer 5
2.2.1. Synthesis of Perfluoro-2-methyl-2-pentanol (4)
2.2.2. Synthesis of 1,1,1,3,3,4,4,5,5,5-decafluoro-2-(trifluoromethyl)pentan-2-methacrylate (5)
2.3. Synthesis of Homopolymer (6)
2.4. Synthesis of Copolymers
2.5. Preparation of Polymer Film
2.6. Characterization
3. Results and Discussion
3.1. Synthesis Analysis
3.2. GPC, 1H-NMR, FT-IR, Elemental Analysis Results of Polymers
3.3. Thermal Properties Analysis
3.4. Surface Properties Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sherman, P.O.; Smith, S. Organic Solvent Soluble Perfluorocarbon Copolymers. US3341497A, 12 September 1967. [Google Scholar]
- Bongiovanni, R.; Malucelli, G.; Lombardi, V. Surface properties of methacrylic copolymers containing a perfluoropolyether structure. Polymer 2001, 42, 2299–2305. [Google Scholar] [CrossRef]
- Iezzi, R.A.; Gaboury, S.; Wood, K. Acrylic-fluoropolymer mixtures and their use in coatings. Prog. Org. Coat. 2000, 40, 55–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, H. Function Material of Fluoride; Chemical Industry Press: Beijing, China, 2008; pp. 287–291. (in Chinese) [Google Scholar]
- Baker, B.E.; Zipfel, R.J. Tetrafluorethylene Polymerization Process. WO97/08214A1, 6 March 1997. [Google Scholar]
- Dichiarante, V.; Milani, R.; Metrangolo, P. Natural surfactants towards a more sustainable fluorine chemistry. Green Chem. 2018, 20, 13–27. [Google Scholar] [CrossRef]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C. Biological monitoring of polyfluoroalkyl substances: A review. Enviro. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef]
- George, G.; Michael, A.; Jay, F.; Richard, M. Degradable, amorphous, fluorochemical acrylate polymers. US6649719B2, 18 November 2003. [Google Scholar]
- Honda, K.; Morita, M.; Otsuka, H.; Takahara, A. Molecular aggregation structure and surface properties of poly(fluoroalkyl acrylate) thin films. Macromolecules 2005, 38, 5699–5705. [Google Scholar] [CrossRef]
- Fielding, H.C. Organofluorine surfactants and textile chemicals. In Organofluorine Chemicals and Their Industrial Applications; Banks, R.E., Ed.; Ellis Horwood Ltd.: Chichester, UK, 1979; pp. 214–234. [Google Scholar]
- Guo, J.; Resnick, P.; Efimenko, K.; Genzer, J.; De Simone, J.M. Alternative fluoropolymers to avoid the challenges associated with perfluorooctanoic acid. Ind. Eng. Chem. Res. 2008, 47, 502–508. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Zhan, X.; Chen, F. Structure and surface properties of polyacrylates with short fluorocarbon side chain: Role of the main chain and spacer group. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2584–2593. [Google Scholar] [CrossRef]
- Jin, C.; Yan, P.; Wang, C.; Xiao, J. Effect of counterions on fluorinated surfactants 1. Surface activity and micellization. Acta Chim. Sinica 2005, 63, 279–282. [Google Scholar]
- Zhou, H.; Gao, A.; Xing, H.; Gou, Z.; Xiao, J. Abnormal surface-active behavior of perfluorooctanoates induced by salt. Acta Chim. Sinica 2011, 69, 1035–1040. [Google Scholar]
- Xing, H.; Lin, C.; Xiao, J. Interactions between β-cyclodextrin and the equimolar mixture of sodium tetradecyl sulfate and sodium perfluorooctanoate. Acta Chim. Sinica 2008, 66, 1382–1384. [Google Scholar]
- Wang, C.; Chen, X.; Zhu, Z.; Xiao, J. Interactions between cationic hydrogenated/fluorinated surfactants and neutral polymers. Acta Chim. Sinica 2009, 67, 1425–1429. [Google Scholar]
- Sha, M.; Zhang, D.; Pan, R.; Xing, P.; Jiang, B. Synthesis and surface properties study of novel fluorine-containing homopolymer and copolymers for coating applications. Appl. Surf. Sci. 2015, 349, 496–502. [Google Scholar] [CrossRef]
- Sha, M.; Xing, P.; Jiang, B. Strategies for synthesizing non-bioaccumulable alternatives to PFOA and PFOS. Chin. Chem. Lett. 2015, 26, 491–498. [Google Scholar] [CrossRef]
- Sha, M.; Pan, R.; Xing, P.; Jiang, B. Synthesis and surface activity study of branched fluorinated cationic (FCS), gemini (FGS) and amphoteric (FAS) surfactants with CF3CF2CF2C(CF3)2 group. J. Fluorine Chem. 2015, 169, 61–65. [Google Scholar] [CrossRef]
- Sha, M.; Pan, R.; Zhan, L.; Xing, P.; Jiang, B. Synthesis and surface activity study of a novel branched fluorinated anion surfactant with CF3CF2CF2C(CF3)2 group. Chin. J. Org. Chem. 2014, 32, 995–998. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, R.; Yang, B.; Li, T.; Tryk, D.; Fujishima, A.; Hashimoto, K.; Zhu, D. Binary cooperative complementary nanoscale interfacial materials. Pure Appl. Chem. 2000, 72, 73–81. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Huang, Y.; Meng, W.; Qing, F. Synthesis of fluorinated hyperbranched polymers capable as highly hydrophobic and oleophobic coating materials. Eur. Polym. J. 2010, 46, 506–518. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X. Fabrication and characterization of stable superhydrophobic fluorinated-polyacrylate/silica hybrid coating. Appl. Surf. Sci. 2014, 298, 214–220. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, L.; Li, W.; Jiang, G.; Liu, H.; Chen, H. Anchoring CeO2 nanoparticles on monodispersed SiO2 spheres to construct hydrophobic polymer coating with enhanced UV absorption ability. Chem. Eng. J. 2017, 321, 268–276. [Google Scholar] [CrossRef]
- Han, J.; Xu, X.; Cho, K. Diverse access to artificial superhydrophobic surfaces using block copolymers. Langmuir 2005, 21, 6662–6665. [Google Scholar] [CrossRef] [PubMed]
- Aruna, S.; Binsy, P.; Richard, E.; Basu, B.J. Properties of phase separation method synthesized superhydrophobic polystyrene films. Appl. Surf. Sci. 2012, 258, 3202–3207. [Google Scholar]
- Fan, Y.; Li, C.; Chen, Z.; Chen, H. Study on fabrication of the superhydrophobic sol-gel films based on copper wafer and its anti-corrosive properties. Appl. Surf. Sci. 2012, 258, 6531–6536. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M. Application of fluorinated compounds to cotton fabrics via sol-gel. Appl. Surf. Sci. 2013, 275, 201–207. [Google Scholar] [CrossRef]
- Huang, X.; Liao, W.; Ye, L.; Zhang, N.; Lan, S.; Fan, H.; Qu, J. Fabrication of hydrophobic composite films by sol-gel process between POSS-containing fluorinated polyacrylate latexes and colloidal silica particles. Microporous Mesoporous Mater. 2017, 243, 311–318. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Yu, X.; Liu, H.; Fu, Y.; Wang, Z.; Jiang, L.; Li, X. Polyelectrolyte multilayer as matrix for electrochemical deposition of gold clusters: Toward super-hydrophobic surface. J. Am. Chem. Soc. 2004, 126, 3064–3065. [Google Scholar] [CrossRef] [PubMed]
- Milionis, A.; Dang, K.; Prato, M.; Loth, E.; Bayer, I. Liquid repellent nanocomposites obtained from one-step water-based spray. J. Mater. Chem. A 2015, 3, 12880–12889. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.H.; Kim, K.B.; Lee, J.K. Fabrication of hierarchical structures on a polymer surface to mimic natural superhydrophobic surfaces. Adv. Mater. 2007, 19, 2330–2335. [Google Scholar] [CrossRef]
- Peng, C.-W.; Chang, K.-C.; Weng, C.-J.; Lai, M.-C.; Hsu, C.-H.; Hsu, S.-C.; Li, S.-Y.; Wei, Y.; Yeh, J.-M. UV-curable nanocasting technique to prepare bio-mimetic super-hydrophobic non-fluorinated polymeric surfaces for advanced anticorrosive coatings. Polym. Chem. 2013, 4, 926–932. [Google Scholar] [CrossRef]
- Scherer Jr, K.V.; Terranova, T.F. F-2-Methyl-2-pentanol. An easily prepared perfluorinated tertiary alcohol. J. Org. Chem. 1981, 11, 2379–2381. [Google Scholar] [CrossRef]
- Teng, H.; Wan, Z.; Koike, Y.; Okamoto, Y. Thermal and optical properties of highly fluorinated copolymers of methacrylates. Polym. Adv. Technol. 2013, 24, 520–523. [Google Scholar] [CrossRef]
- Wang, J.C. Tg design and selection of acrylic resin. Chin. Coat. 2008, 10, 52–56. (in Chinese). [Google Scholar]
- Li, J.; Zhang, X.; Liu, Z.; Li, W.; Dai, J. Studies on waterborne polyurethanes based on new medium length fluorinated diols. J. Fluor. Chem. 2015, 175, 12–17. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Tan, J. Synthesis and properties of fluorine-containing polyurethane based on long chain fluorinated polyacrylate. J. Mater. Chem. A 2016, 53, 41–48. [Google Scholar] [CrossRef]
- Deng, J.; Zheng, Z.; Ding, X. Study on preparation and the water and oil repellent properties of fluorinated acrylate polymer with short fluorocarbon chain. Chin. Plast. Ind. 2015, 10, 100–118. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Gao, Y.; He, C.; Huang, Y. Novel water and oil repellent POSS-based organic/inorganic nanomaterial: Preparation, characterization and application to cotton fabrics. Polymer 2010, 51, 5997–6004. [Google Scholar] [CrossRef]
- Li, L.; Huang, X. Studies on micro-phase separation of thermoplastie polyurethane by atomie force mieroseope (AFM). J. Donghua Univ. Nat. Sci. 2004, 30, 9–13. (in Chinese). [Google Scholar]
- Xu, W.; An, Q.; Hao, L.; Zhang, D.; Zhang, M. Synthesis and characterization of self-crosslinking fluorinated polyacrylate soap-free latices with core–shell structure. Appl. Surf. Sci. 2013, 268, 373–380. [Google Scholar] [CrossRef]
- Qiang, F.; Hu, L.-L.; Gong, L.-X.; Zhao, L.; Li, S.-N.; Tang, L.-C. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbon/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, L.; Wu, D. Fabrication of superhydrophobic surfaces from microstructured ZnO-based surfaces via a wet-chemical route. Langmuir 2005, 21, 2665–2667. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.; Wu, D.; van Benthem, R. Superhydrophobic films from raspberry-like particles. Nano Lett. 2005, 5, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Suh, K.Y.; Lee, H.H. A geometry controllable approach for the fabrication of biomimetic hierarchical structure and its superhydrophobicity with near-zero sliding angle. Nanotechnology 2008, 19, 275305. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, L.; Zhai, J.; Jiang, L.; Zhu, D. Reversible wettability of a chemical vapor deposition prepared ZnO film between superhydrophobicity and superhydrophilicity. Langmuir 2004, 20, 5659–5661. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Cebeci, F.C.; Cohen, R.E.; Rubner, M.F. Stable superhydrophobic coatings from polyelectrolyte multilayers. Nano Lett. 2004, 4, 1349–1353. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Coulson, S.R.; Woodward, I.S.; Badyal, J.P.S. Ultralow surface energy plasma polymer films. Chem. Mater. 2000, 12, 2031–2038. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and ultralyophobic surfaces: Some comments and examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Kim, T.; Tahk, D.; Lee, H.H. Wettability-controllable super water-and moderately oil-repellent surface fabricated by wet chemical etching. Langmuir 2009, 25, 6576–6579. [Google Scholar] [CrossRef] [PubMed]









| Polymer | 5:7 b | Yield c (%) | Mn d (×104) | Mw/Mnd | Tge (°C) | Tdf (°C) | θwater g (°) | θBioctyl g (°) | 5:7 h (%) | F i (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 0 | 79 | – | – | 59.47 | 248.27 | 113.84 | 65.55 | – | 58.07 |
| Poly(5-co-BMA) | 1:1 | 59 | 1.07 | 1.22 | 52.76 | 238.97 | 102.37 | 46.23 | 80.4 | 42.92 |
| 1:2 | 63 | 1.41 | 1.43 | 51.27 | 236.73 | 100.03 | 44.52 | 27.6 | 27.73 | |
| 1:6 | 87 | 2.85 | 5.81 | 46.21 | 245.21 | 99.74 | 43.27 | 22.0 | 22.96 | |
| 1:8 | 76 | 2.16 | 1.83 | 42.82 | 243.73 | 97.41 | 42.68 | 14.4 | 17.40 | |
| 1:15 | 71 | 4.50 | 4.12 | 41.64 | 234.02 | 95.32 | 40.47 | 6.8 | 9.41 | |
| Poly(5-co-MMA) | 1:1 | 66 | 1.02 | 1.55 | 49.40 | 232.34 | 85.06 | 52.70 | 87.8 | – |
| 1:2 | 74 | 1.02 | 1.31 | 56.02 | 253.24 | 60.74 | 48.62 | 60.8 | – | |
| 1:6 | 81 | 1.56 | 1.43 | 68.65 | 249.97 | 32.49 | 45.37 | 36.2 | – | |
| 1:8 | 81 | 1.87 | 1.60 | 84.80 | 244.18 | 30.57 | 42.35 | 29.6 | – | |
| 1:15 | 68 | 1.67 | 1.62 | 103.80 | 241.27 | 18.58 | 36.53 | 15.2 | – | |
| Poly(5-co-EMA) | 1:1 | 59 | 1.36 | 1.25 | 58.13 | 240.72 | 106.83 | 54.90 | 83.6 | – |
| 1:2 | 77 | 1.45 | 1.74 | 53.71 | 241.09 | 101.64 | 50.53 | 30.8 | – | |
| 1:6 | 67 | 2.14 | 1.69 | 53.34 | 236.24 | 99.93 | 45.03 | 22.0 | – | |
| 1:8 | 59 | 3.13 | 1.91 | 52.15 | 241.08 | 95.64 | 42.10 | 10.4 | – | |
| 1:15 | 57.9 | 3.70 | 1.951 | 53.10 | 249.92 | 97.86 | 39.64 | 8.2 | – | |
| Poly(5-co-HMA) | 1:1 | 68 | 1.90 | 1.66 | 11.28 | 229.64 | 103.37 | 51.78 | 74.8 | – |
| 1:2 | 69 | 1.27 | 1.74 | 12.12 | 231.20 | 96.46 | 49.13 | 27.6 | – | |
| 1:6 | 79 | 2.72 | 8.83 | 1.76 | 239.61 | 97.39 | 36.96 | 13.6 | – | |
| 1:8 | 78 | 2.71 | 5.11 | −0.61 | 242.75 | 95.87 | 35.05 | 8.8 | – | |
| 1:15 | 88 | 2.05 | 3.13 | −5.06 | 246.26 | 98.12 | 34.75 | 4.0 | – | |
| Poly(5-co-OMA) | 1:1 | 58 | 1.04 | 1.44 | 3.43 | 234.90 | 97.20 | 41.81 | 74.8 | – |
| 1:2 | 64 | 1.37 | 2.07 | 5.12 | 217.04 | 103.28 | 30.31 | 21.1 | – | |
| 1:6 | 67 | 2.49 | 4.13 | 6.94 | 242.10 | 109.28 | 27.04 | 12.8 | – | |
| 1:8 | 61 | 1.94 | 2.92 | 6.89 | 240.13 | 109.47 | 22.20 | 8.0 | – | |
| 1:15 | 94 | 1.81 | 2.12 | 9.58 | 248.13 | 109.20 | 18.26 | 3.2 | – | |
| Poly(5-co-LMA) | 1:1 | 59 | 1.10 | 1.53 | −1.60 | 220.47 | 102.59 | 37.66 | 49.6 | – |
| 1:2 | 65 | 1.57 | 1.70 | −25.08 | 234.45 | 99.10 | 33.51 | 20.8 | – | |
| 1:6 | 67 | 2.24 | 2.20 | −48.8 | 234.39 | 95.09 | 26.48 | 14.8 | – | |
| 1:8 | 67 | 2.26 | 2.15 | −51.17 | 234.12 | 92.02 | 26.04 | 9.6 | – | |
| 1:15 | 88 | 2.31 | 2.24 | −44.76 | 248.65 | 113.23 | 19.51 | 2.4 | – | |
| Poly(5-co-SMA) | 1:1 | 67 | 1.63 | 1.81 | 14.36 | 283.71 | 107.18 | 37.06 | 34.4 | – |
| 1:2 | 74 | 2.23 | 2.37 | 25.61 | 283.35 | 106.99 | 36.39 | 19.6 | – | |
| 1:6 | 91 | 5.13 | 5.51 | 29.76 | 344.39 | 104.42 | 30.64 | 10.0 | – | |
| 1:8 | 97 | 4.13 | 3.78 | 30.69 | 349.30 | 99.68 | 19.34 | 5.2 | – | |
| 1:15 | 80 | 2.45 | 2.41 | 33.48 | 354.89 | 95.98 | 5.26 | 0.8 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Xing, P.; Pan, R.; Lin, X.; Sha, M.; Jiang, B. Preparation and Surface Properties Study of Novel Fluorine-Containing Methacrylate Polymers for Coating. Materials 2018, 11, 2258. https://doi.org/10.3390/ma11112258
Zhang D, Xing P, Pan R, Lin X, Sha M, Jiang B. Preparation and Surface Properties Study of Novel Fluorine-Containing Methacrylate Polymers for Coating. Materials. 2018; 11(11):2258. https://doi.org/10.3390/ma11112258
Chicago/Turabian StyleZhang, Ding, Ping Xing, Renming Pan, Xiangyang Lin, Min Sha, and Biao Jiang. 2018. "Preparation and Surface Properties Study of Novel Fluorine-Containing Methacrylate Polymers for Coating" Materials 11, no. 11: 2258. https://doi.org/10.3390/ma11112258





