Preparation of LiFePO4/C Cathode Materials via a Green Synthesis Route for Lithium-Ion Battery Applications
Abstract
:Highlights:
- ✔
- LiFePO4/C is synthesized by using a green route where almost no wastewater and air polluted gases are discharged into the environment.
- ✔
- The reaction principles for the synthesis of LiFePO4/C are analyzed.
- ✔
- LiFePO4/C exhibits uniform nano-structure and carbon layer.
- ✔
- LiFePO4/C shows excellent rate capability and cycling capability.
1. Introduction
2. Experimental Section
2.1. Preparation of LiFePO4/C Composite
2.2. Characterization of LiFePO4/C Composite
2.3. Electrochemical Measurements
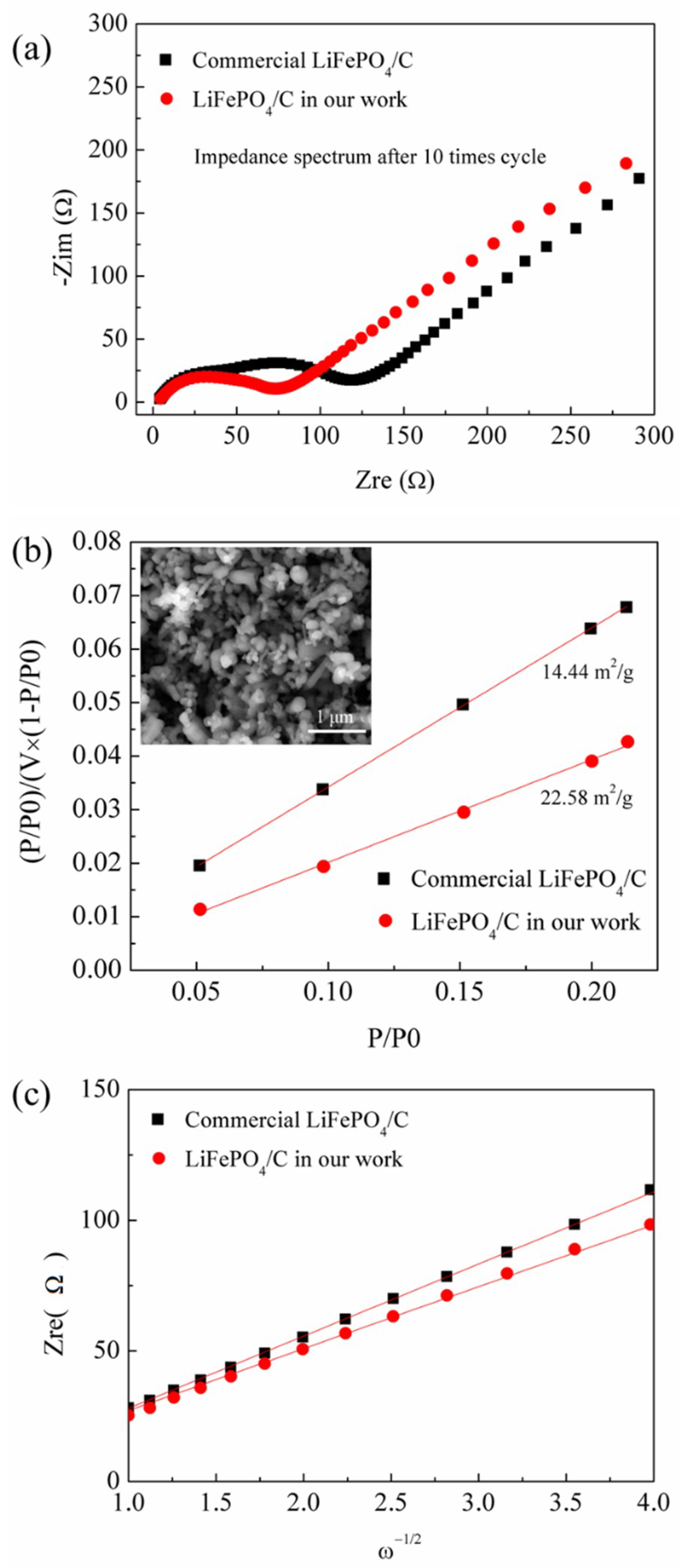
3. Results and Discussions
4. Conclusions
Prime Novelty Statement
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, L.X.; Wang, Z.H.; Zhang, W.X.; Hu, X.L.; Chen, J.T.; Huang, Y.H.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Gong, C.L.; Xue, Z.G.; Wen, S.; Ye, Y.S.; Xie, X.L. Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J. Power Sources 2016, 318, 93–112. [Google Scholar] [CrossRef]
- Wang, Y.; He, P.; Zhou, H. Olivine LiFePO4: development and future. Energy Environ. Sci. 2011, 4, 805–817. [Google Scholar] [CrossRef]
- Liu, J.; Banis, M.N.; Sun, Q.; Lushington, A.; Li, R.; Sham, T.-K.; Sun, X.L. Rational design of atomic-layer-deposited LiFePO4 as a high-performance cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 6472–6477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X. Olivine LiFePO4: the remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Zaghib, K.; Guerfi, A.; Hovington, P.; Vijh, A.; Trudeau, M.; Mauger, A.; Goodenough, J.B.; Julien, C.M. Review and analysis of nanostructured olivine-based lithium recheargeable batteries: Status and trends. J. Power Sources 2013, 232, 357–369. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Yang, W.Y.; Zhuang, Z.Y.; Chen, X.; Zou, M.Z.; Zhao, G.Y.; Feng, Q.; Li, J.X.; Lin, Y.B.; Huang, Z.G. A simple and novel Si surface modification on LiFePO4@C electrode and its suppression of degradation of lithium ion batteries. Appl. Surf. Sci. 2015, 359, 875–882. [Google Scholar] [CrossRef]
- Zhang, K.; Lee, J.T.; Li, P.; Kang, B.; Kim, J.H.; Yi, G.R.; Park, J.H. Conformal coating strategy comprising N-doped carbon and conventional graphene for achieving ultrahigh power and cyclability of LiFePO4. Nano Lett. 2015, 15, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Tang, Y.; Wang, D.; Li, X.; Hu, Y.; Li, R.; Liang, G.; Sham, T.K.; Sun, X. LiFePO4–graphene as a superior cathode material for rechargeable lithium batteries: impact of stacked graphene and unfolded graphene. Energy Environ. Sci. 2013, 6, 1521–1528. [Google Scholar] [CrossRef]
- Xu, D.; Chu, X.; He, Y.B.; Ding, Z.; Li, B.; Han, W.; Du, H.; Kang, F. Enhanced performance of interconnected LiFePO4/C microspheres with excellent multiple conductive network and subtle mesoporous structure. Electrochim. Acta 2015, 152, 398–407. [Google Scholar] [CrossRef]
- Gibot, P.; Casas-cabanas, M.; Laffont, L.; Levasseur, S.; Carlach, P.; Hamelet, S.; Tarascon, J.M.; Masquelier, C. Room-temperature single-phase Li insertion/extraction in nanoscale LixFePO4. Nat. Mater. 2008, 7, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.D.; Lubke, M.; Wu, O.Y.; Makwana, N.M.; Smales, G.J.; Islam, H.U.; Dedigama, R.Y.; Gruar, R.I.; Tighe, C.J.; Scanlon, D.O.; et al. Pilot-scale continuous synthesis of a vanadium-doped LiFePO4/C nanocomposite high-rate cathodes for lithium-ion batteries. J. Power Sources 2016, 302, 410–418. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Yang, F. Developments of lithium-ion batteries and challenges of LiFePO4 as one promising cathode material. J. Mater. Sci. 2009, 44, 2435–2443. [Google Scholar] [CrossRef]
- Singh, M.; Singh, B.; Willert-Porada, M. Reaction mechanism and morphology of the LiFePO4 materials synthesized by chemical solution deposition and solid-state reaction. J. Electroanal. Chem. 2017, 790, 11–19. [Google Scholar] [CrossRef]
- Cheng, W.H.; Wang, L.; Sun, Z.P.; Wang, Z.J.; Zhang, Q.B.; Lv, D.D.; Ren, W.; Bian, L.; Xu, J.B.; Chang, A.M. Preparation and characterization of LiFePO4·xLi3V2(PO4)3 composites by two-step solid-state reaction method for lithium-ion batteries. Mater. Lett. 2017, 198, 172–418. [Google Scholar] [CrossRef]
- Tang, H.; Si, Y.; Chang, K.; Fu, X.; Li, B.; Shangguan, E.; Chang, Z.; Yuan, X.Z.; Wang, H. Carbon gel assisted low temperature liquid-phase synthesis of C-LiFePO4/graphene layers with high rate and cycle performances. J. Power Sources 2015, 295, 131–138. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Xin, P.Y.; Yao, Q. Electrochemical performance of LiFePO4/C synthesized by sol-gel method as cathode for aqueous lithium ion batteries. J. Alloys Compd. 2018, 741, 404–408. [Google Scholar] [CrossRef]
- Wu, G.; Liu, N.; Gao, X.G.; Tian, X.H.; Zhu, Y.B.; Zhou, K.Y.; Zhu, Q.Y. A hydrothermally synthesized LiFePO4/C composite with superior low-temperature performance and cycle life. Appl. Surf. Sci. 2018, 435, 1329–1336. [Google Scholar] [CrossRef]
- Yen, H.; Rohan, R.; Chiou, C.Y.; Hsieh, C.J.; Bolloju, S.; Li, C.C.; Yang, Y.F.; Ong, C.W.; Lee, J.T. Hierarchy concomitant in situ stable iron(II)−carbon source manipulation using ferrocenecarboxylic acid for hydrothermal synthesis of LiFePO4 as high-capacity battery cathode. Electrochim. Acta 2017, 253, 227–238. [Google Scholar] [CrossRef]
- Guan, X.M.; Li, G.L.; Li, C.Y.; Ren, R.M. Synthesis of porous nano/micro structured LiFePO4/C cathode materials for lithium-ion batteries by spray-drying method. Trans. Nonferr. Met. Soc. China 2017, 27, 141–147. [Google Scholar] [CrossRef]
- Kashi, R.; Khosravi, M.; Mollazadeh, M. Effect of carbon precursor on electrochemical performance of LiFePO4-C nano composite synthesized by ultrasonic spray pyrolysis as cathode active material for Li ion battery. Mater. Chem. Phys. 2018, 203, 319–332. [Google Scholar] [CrossRef]
- Liu, H.W.; Yang, H.M.; Li, J.L. A novel method for preparing LiFePO4 nanorods as a cathode material for lithium-ion power batteries. Electrochim. Acta 2010, 55, 1626–1629. [Google Scholar] [CrossRef]
- Chen, J.J.; Whittingham, M.S. Hydrothermal synthesis of lithium iron phosphate. Electrochem. Commun. 2006, 8, 855–858. [Google Scholar] [CrossRef]
- Ahn, C.W.; Choi, J.J.; Ryu, J.; Hahn, B.D.; Kim, J.W.; Yoon, W.H.; Choi, J.H.; Park, D.S. Microstructure and electrochemical properties of graphite and C-coated LiFePO4 films fabricated by aerosol deposition method for Li ion battery. Carbon 2015, 82, 135–142. [Google Scholar] [CrossRef]
- Belharouak, I.; Johnson, C.; Amine, K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem. Commun. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Lou, X.M.; Zhang, Y.X. Synthesis of LiFePO4/C cathode materials with both high-rate capability and high tap density for lithium-ion batteries. J. Mater. Chem. 2011, 21, 4156–4160. [Google Scholar] [CrossRef]
- Lu, C.Y.; Rooney, D.W.; Jiang, X.; Sun, W.; Wang, Z.H.; Wang, J.J.; Sun, K.N. Achieving high specific capacity of lithium-ion battery cathodes by modification with “N–Oc” radicals and oxygen-containing functional groups. J. Mater. Chem. A 2017, 5, 24636–24644. [Google Scholar] [CrossRef]
- Tian, R.Y.; Liu, H.Q.; Jiang, Y.; Chen, J.K.; Tan, X.H.; Liu, G.Y.; Zhang, L.N.; Gu, X.H.; Guo, Y.J.; Wang, H.F.; et al. Drastically enhanced high-Rate performance of carbon-coated LiFePO4 nanorods using a green chemical vapor deposition (CVD) method for lithium ion battery: a selective carbon coating process. ACS Appl. Mater. Interfaces 2015, 7, 11377–11386. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, C.P.; Rui, X.H.; Cheng, T.; Chen, C.H. V2O3 modified LiFePO4/C composite with improved electrochemical performance. J. Power Sources 2011, 196, 5623–5630. [Google Scholar] [CrossRef]
- Ma, J.; Li, B.H.; Du, H.D.; Xu, C.J.; Kang, F.Y. Inorganic-based sol–gel synthesis of nano-structured LiFePO4/C composite materials for lithium ion batteries. J. Solid State Electrochem. 2012, 16, 1353–1362. [Google Scholar] [CrossRef]
- Gupta, H.; Kataria, S.; Balo, L.; Singh, V.K.; Singh, S.K.; Tripathi, A.K.; Verma, Y.L.; Singh, R.K. Electrochemical study of Ionic Liquid based polymer electrolyte with graphene oxide coated LiFePO4 cathode for Li battery. Solid State Ion. 2018, 320, 186–192. [Google Scholar] [CrossRef]
- Chen, J.S.; Cheah, Y.L.; Chen, Y.T.; Japaprakash, N.; Madhavi, S.; Yang, Y.H.; Lou, X.W. SnO2 nanoparticles with controlled carbon nanocoating as high-capacity anode materials for lithium-ion batteries. J. Phys. Chem. C 2009, 113, 20504–20508. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Zhang, Y.X. Synthesis of micro-nano hierarchical structured LiFePO4/C composite with both superior high-rate performance and high tap density. Nanoscale 2011, 3, 4434–4439. [Google Scholar] [CrossRef] [PubMed]
- Mazora, H.; Golodnitsky, D.; Burstein, L.; Gladkich, A.; Peled, E. Electrophoretic deposition of lithium iron phosphate cathode for thin-film 3D-microbatteries. J. Power Sources 2012, 198, 264–272. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Xiang, K.Q.; Zhou, W.; Zhu, Y.R.; Bai, N.B.; Chen, H. LiFePO4/C ultra-thin nano-flakes with ultra-high rate capability and ultra-long cycling life for lithium ion batteries. J. Alloys Compd. 2018, 749, 1063–1070. [Google Scholar] [CrossRef]
- Xiong, W.; Hu, Q.H.; Liu, S.T. A novel and accurate analytical method based on X-ray photoelectron spectroscopy for the quantitative detection of the lithium content in LiFePO4. Anal. Methods 2014, 6, 5708–5711. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, J.; Liu, G.Z.; Wang, L. Lithium-ions diffusion kinetic in LiFePO4/carbon nanoparticles synthesized by microwave plasma chemical vapor deposition for lithium-ion batteries. Appl. Surf. Sci. 2018, 433, 35–44. [Google Scholar] [CrossRef]
- Salah, A.A.; Mauger, A.; Zaghib, K.; Goodenough, J.B.; Ravet, N.; Gauthier, M.; Gendron, F.; Julien, C.M. Reduction Fe3+ of impurities in LiFePO4 from pyrolysis of organic precursor used for carbon deposition. J. Electrochem. Soc. 2006, 153, 1692–1701. [Google Scholar] [CrossRef]
- Shiraishi, K.; Dokko, K.; Kanamura, K. Formation of impurities on phospho-olivine LiFePO4 during hydrothermal synthesis. J. Power Sources 2005, 146, 555–558. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.Z.; Jin, J.; Liu, J.; Li, Y.; Wang, H.E.; Chen, L.H.; Wang, B.J.; Su, B.L. Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability. Sci. Rep. 2016, 6, 25942. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.Q.; Cao, X.X.; Wang, Y.P.; Hua, Y.; Pan, A.Q.; Cao, G.Z. Uniform 8LiFePO4·Li3V2(PO4)3/C nanoflakes for high-performance Li-ion batteries. Nano Energy 2016, 22, 48–58. [Google Scholar] [CrossRef]
- Huynh, L.T.N.; Tran, T.T.D.; Nguyen, H.H.A.; Nguyen, T.T.T.; Tran, V.M.; Grag, A.; Le, M.L.P. Carbon-coated LiFePO4–carbon nanotube electrodes for high-rate Li-ion battery. J. Solid State Electrochem. 2018, 22, 2247–2254. [Google Scholar] [CrossRef]
- Tang, K.; Yu, X.Q.; Sun, J.P.; Li, H.; Huang, X.J. Kinetic analysis on LiFePO4 thin films by CV, GITT, and EIS. Electrochim. Acta 2011, 56, 4869–4875. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion diffusion kinetics in LiFePO4 thin film prepared by radio frequency magnetron sputtering. Electrochim. Acta 2009, 54, 4631–4637. [Google Scholar] [CrossRef]
- Wu, X.L.; Guo, Y.G.; Su, J.; Xiong, J.W.; Zhang, Y.L.; Wan, L.J. Carbon-nanotube-decorated nano-LiFePO4@C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv. Energy Mater. 2013, 3, 1155–1160. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Feng, W.L.; Li, J.; Shen, T.D. Ultralong cycling stability of carbon-nanotube/LiFePO4 nanocomposites as electrode materials for lithium-ion batteries. Electrochim. Acta 2017, 232, 323–331. [Google Scholar] [CrossRef]
- Schmidt, J.; Chrobak, T.; Ender, M.; Illig, J.; Kiotz, D.; Ivers-Tiffée, E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J. Power Sources 2011, 196, 5342–5348. [Google Scholar] [CrossRef]
- Gong, C.; Xue, Z.; Wang, X.; Zhou, X.; Xie, X.; Mai, Y. Poly(ethylene glycol) grafted multi-walled carbon nanotubes/LiFePO4 composite cathodes for lithium ion batteries. J. Power Sources 2014, 246, 260–268. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, H.; Chen, Y.; Wang, W.; Ye, Y.; Zheng, C.; Deng, P.; Shi, Z. A three-dimensional LiFePO4/carbon nanotubes/graphene composite as a cathode material for lithium-ion batteries with superior high-rate performance. J. Alloys Compd. 2015, 626, 280–286. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Chen, J.; Li, Z.; Ding, Q.; An, X.; Pan, Y.; Zheng, Z.; Yang, M.; Fu, D. Preparation of LiFePO4/C Cathode Materials via a Green Synthesis Route for Lithium-Ion Battery Applications. Materials 2018, 11, 2251. https://doi.org/10.3390/ma11112251
Liu R, Chen J, Li Z, Ding Q, An X, Pan Y, Zheng Z, Yang M, Fu D. Preparation of LiFePO4/C Cathode Materials via a Green Synthesis Route for Lithium-Ion Battery Applications. Materials. 2018; 11(11):2251. https://doi.org/10.3390/ma11112251
Chicago/Turabian StyleLiu, Rongyue, Jianjun Chen, Zhiwen Li, Qing Ding, Xiaoshuai An, Yi Pan, Zhu Zheng, Minwei Yang, and Dongju Fu. 2018. "Preparation of LiFePO4/C Cathode Materials via a Green Synthesis Route for Lithium-Ion Battery Applications" Materials 11, no. 11: 2251. https://doi.org/10.3390/ma11112251
APA StyleLiu, R., Chen, J., Li, Z., Ding, Q., An, X., Pan, Y., Zheng, Z., Yang, M., & Fu, D. (2018). Preparation of LiFePO4/C Cathode Materials via a Green Synthesis Route for Lithium-Ion Battery Applications. Materials, 11(11), 2251. https://doi.org/10.3390/ma11112251



