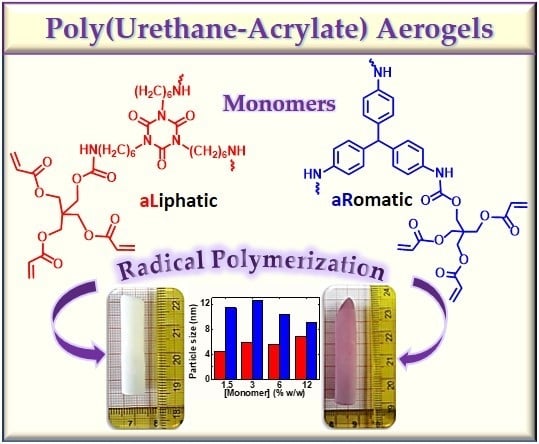
Poly(Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers
Abstract
:1. Introduction


2. Materials and Methods
2.1. Preparation of Poly(urethane acrylate) (aL-PUAc and aR-PUAc) Aerogels
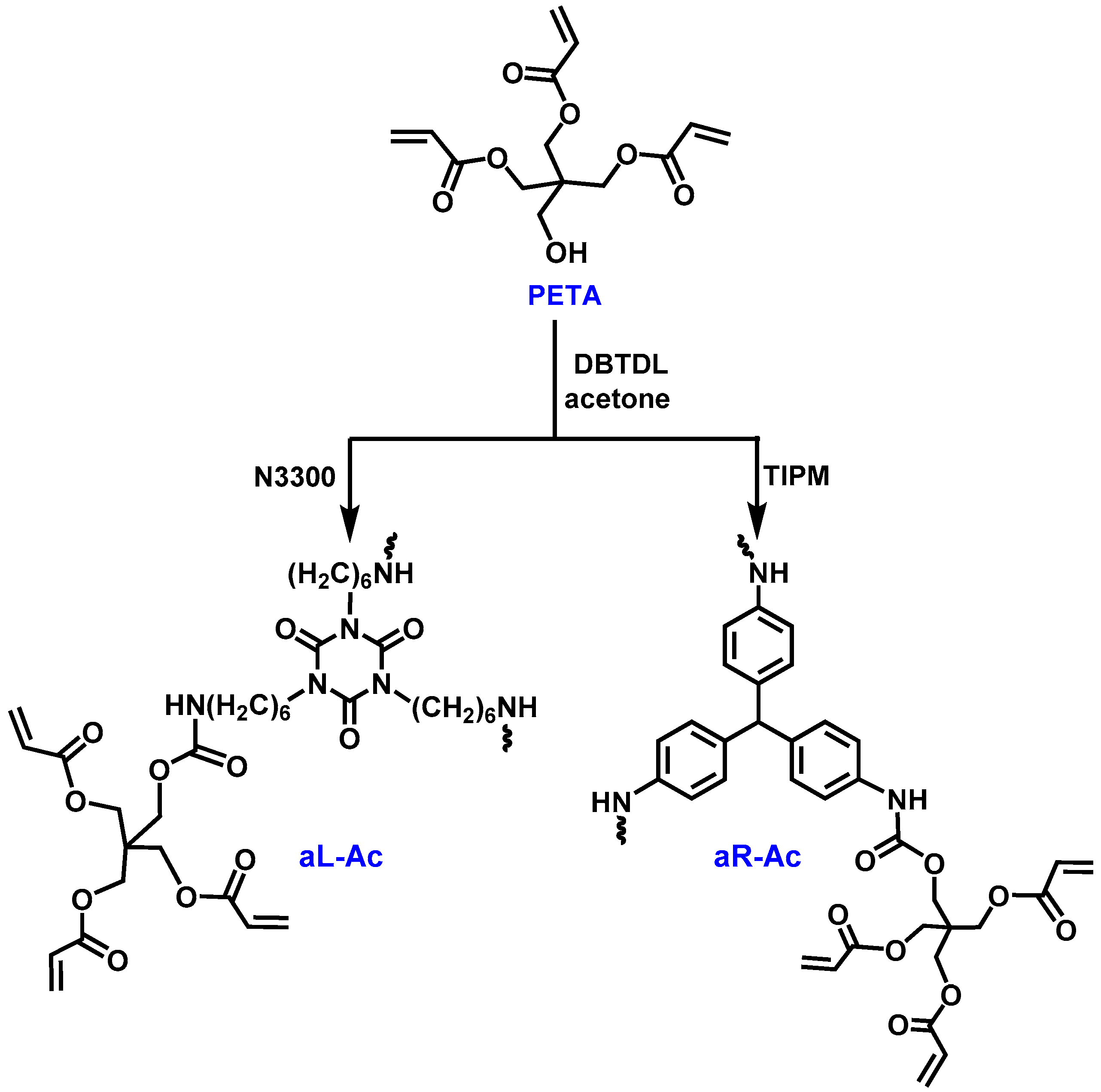
2.1.1. Synthesis of Dendritic Monomers
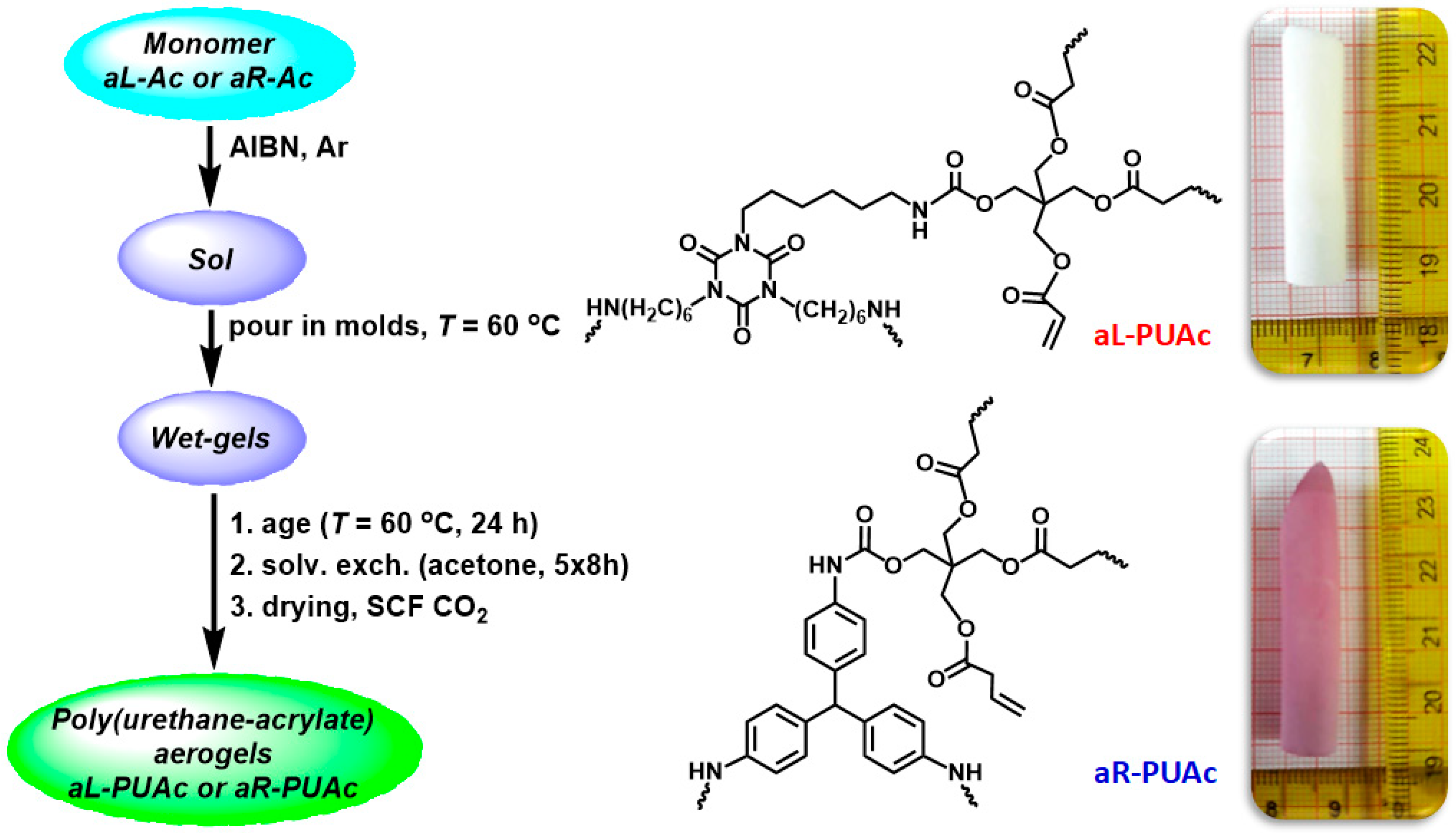
2.1.2. Polymerization Reactions
2.1.3. Pyrolysis of aR-PUAc Aerogels
3. Results and Discussion
3.1. Synthesis and Characterization of Dendritic Monomers aL-Ac and aR-Ac
3.2. Synthesis of aL-PUAc and aR-PUAc Aerogels via Free Radical Polymerization
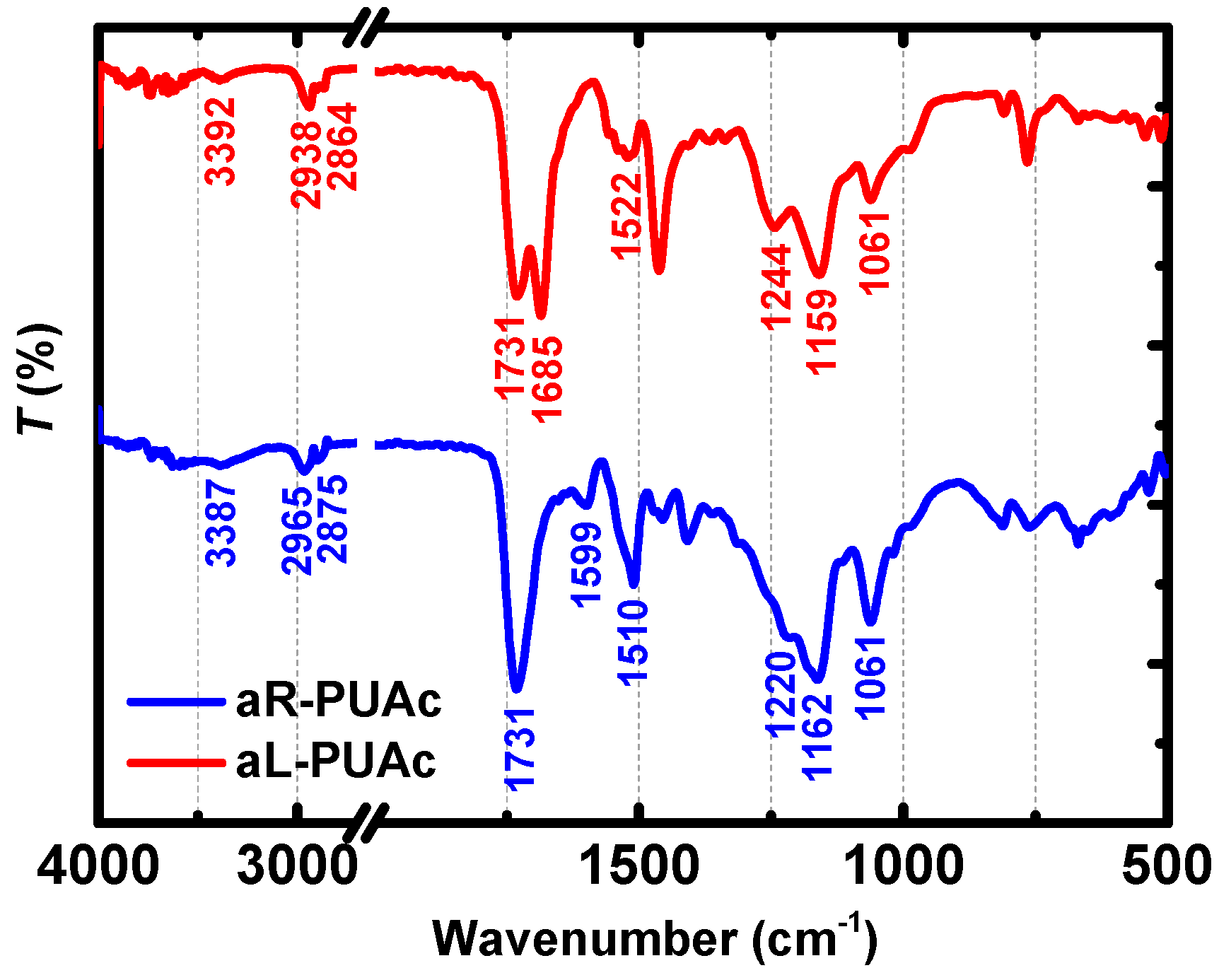
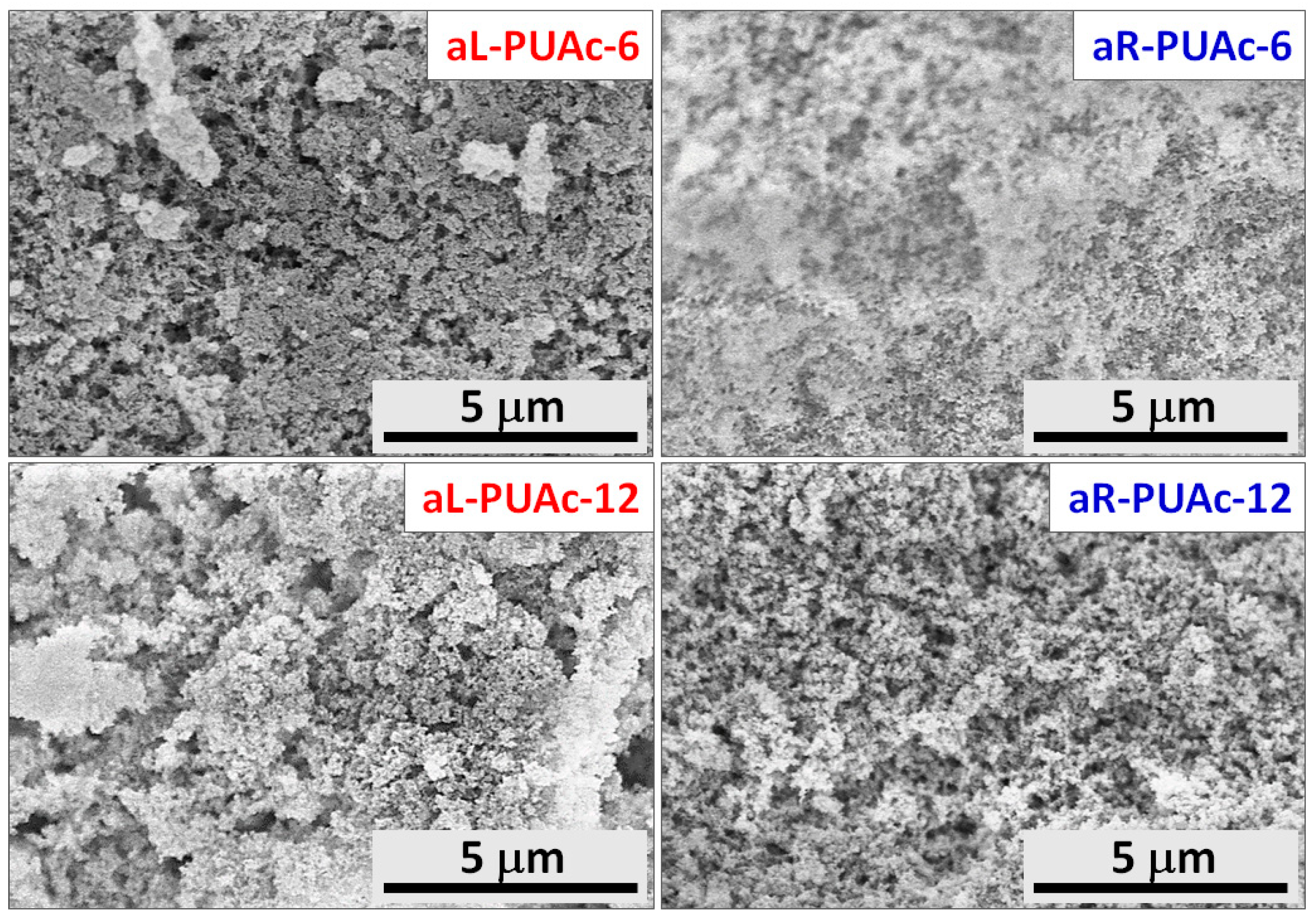
3.3. Characterization of Aerogels aL-PUAc and aR-PUAc
3.4. Pyrolysis of aR-PUAc Aerogels
3.5. Comparison of aL-PUAc and aR-PUAc Aerogels with Relevant Literature Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer Science & Business Media: Berlin, Germany, 2011; ISBN 978-1-4419-7589-8. [Google Scholar]
- Kistler, S.S. Coherent Expanded-Aerogels. J. Phys. Chem. 1931, 36, 52–64. [Google Scholar] [CrossRef]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click Synthesis of Monolithic Silicon Carbide Aerogels from Polyacrylonitrile-Coated 3D Silica Networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Kistler, S.S. Method of Making Aerogels. U.S. Patent 2249767A, 22 July 1941. [Google Scholar]
- Tsou, P. Silica aerogel captures cosmic dust intact. J. Non-Cryst. Solids 1995, 186, 415–427. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Alexa, L.C.; Huber, G.M.; Lolos, G.J.; Farzanpay, F.; Garibaldi, F.; Jodice, M.; Leone, A.; Perrino, R.; Papandreou, Z.; Humphrey, D.L.; et al. Empirical tests and model of a silica aerogel Cherenkov detector for CEBAF. Nucl. Instrum. Methods Phys. Res. A 1995, 365, 299–307. [Google Scholar] [CrossRef]
- Cantin, M.; Casse, M.; Koch, L.; Jouan, R.; Mestreau, P.; Roussel, D.; Bonnin, F.; Moutel, J.; Teichner, S.J. Silica aerogels used as Cherenkov radiators. Nucl. Instrum. Methods 1974, 118, 177–182. [Google Scholar] [CrossRef]
- Da Cunha, J.P.; Neves, F.; Lopes, M.I. On the reconstruction of Cherenkov rings from aerogel radiators. Nucl. Instrum. Methods Phys. Res. A 2000, 452, 401–421. [Google Scholar] [CrossRef] [Green Version]
- Ward, D.A.; Ko, E.I. Preparing Catalytic Materials by the Sol-Gel Method. Ind. Eng. Chem. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]
- Schneider, M.; Baiker, A. Aerogels in Catalysis. Catal. Rev. 1995, 37, 515–556. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-González, L.; Izquierdo-Barba, I.; González-Calbet, J.M. Revisiting silica based ordered mesoporous materials: Medical applications. J. Mater. Chem. 2006, 16, 26–31. [Google Scholar] [CrossRef]
- Bang, A.; Sadekar, A.G.; Buback, C.; Curtin, B.; Acar, S.; Kolasinac, D.; Yin, W.; Rubenstein, D.A.; Lu, H.; Leventis, N.; et al. Evaluation of Dysprosia Aerogels as Drug Delivery Systems: A Comparative Study with Random and Ordered Mesoporous Silicas. ACS Appl. Mater. Interfaces 2014, 6, 4891–4902. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, J.L.; Arachchige, I.U.; Brock, S.L. Porous Semiconductor Chalcogenide Aerogels. Science 2005, 307, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Rewatkar, P.M.; Taghvaee, T.; Saeed, A.M.; Donthula, S.; Mandal, C.; Chandrasekaran, N.; Leventis, T.; Shruthi, T.K.; Sotiriou-Leventis, C.; Leventis, N. Sturdy, Monolithic SiC and Si3N4 Aerogels from Compressed Polymer-Cross-Linked Silica Xerogel Powders. Chem. Mater. 2018, 30, 1635–1647. [Google Scholar] [CrossRef]
- Leventis, N.; Vassilaras, P.; Fabrizio, E.F.; Dass, A. Polymer nanoencapsulated rare earth aerogels: Chemically complex but stoichiometrically similar core–shell superstructures with skeletal properties of pure compounds. J. Mater. Chem. 2007, 17, 1502–1508. [Google Scholar] [CrossRef]
- Schäfer, H.; Milow, B.; Ratke, L. Synthesis of inorganic aerogels via rapid gelation using chloride precursors. RSC Adv. 2013, 3, 15263–15272. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in Chemical Engineering: Strategies Toward Tailor-Made Aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels—Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Kanellou, A.; Anyfantis, G.C.; Chriti, D.; Raptopoulos, G.; Pitsikalis, M.; Paraskevopoulou, P. Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties. Molecules 2018, 23, 1007. [Google Scholar] [CrossRef] [PubMed]
- Donthula, S.; Mandal, C.; Leventis, T.; Schisler, J.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Shape Memory Superelastic Poly(isocyanurate-urethane) Aerogels (PIR-PUR) for Deployable Panels and Biomimetic Applications. Chem. Mater. 2017, 29, 4461–4477. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Agnello, M.; McCorkle, L.; Vivod, S.L.; Wilmoth, N. Moisture-Resistant Polyimide Aerogels Containing Propylene Oxide Links in the Backbone. ACS Appl. Mater. Interfaces 2016, 8, 29073–29079. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Churu, G.; Mang, J.T. Multifunctional Polyurea Aerogels from Isocyanates and Water. A Structure−Property Case Study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Chriti, D.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P. Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution. Gels 2018, 4, 66. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Sotiriou-Leventis, C.; Lu, H. One-Pot Synthesis of Interpenetrating Inorganic/Organic Networks of CuO/Resorcinol-Formaldehyde Aerogels: Nanostructured Energetic Materials. J. Am. Chem. Soc. 2009, 131, 4576–4577. [Google Scholar] [CrossRef] [PubMed]
- Mohite, D.P.; Larimore, Z.J.; Lu, H.; Mang, J.T.; Sotiriou-Leventis, C.; Leventis, N. Monolithic Hierarchical Fractal Assemblies of Silica Nanoparticles Cross-Linked with Polynorbornene via ROMP: A Structure–Property Correlation from Molecular to Bulk through Nano. Chem. Mater. 2012, 24, 3434–3448. [Google Scholar] [CrossRef]
- Leventis, N. Three-Dimensional Core-Shell Superstructures: Mechanically Strong Aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, P.; Gurikov, P.; Raptopoulos, G.; Chriti, D.; Papastergiou, M.; Kypritidou, Z.; Skounakis, V.; Argyraki, A. Strategies toward catalytic biopolymers: Incorporation of tungsten in alginate aerogels. Polyhedron 2018, 154, 209–216. [Google Scholar] [CrossRef]
- Chen, H.-B.; Schiraldi, D.A. Flammability of Polymer/Clay Aerogel Composites: An Overview. Polym. Rev. 2018, 1–24. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Tersac, G. Chemistry and technology of polyols for polyurethanes. Milhail Ionescu. Rapra Technology, Shrewsbury, UK. Polym. Int. 2007, 56, 820. [Google Scholar] [CrossRef]
- Vermette, P. Biomedical Applications of Polyurethanes, 1st ed.; CRC Press: Georgetown/Austin, TX, USA, 2001; ISBN 978-1-58706-023-6. [Google Scholar]
- Biesmans, G.; Mertens, A.; Duffours, L.; Woignier, T.; Phalippou, J. Polyurethane based organic aerogels and their transformation into carbon aerogels. J. Non-Cryst. Solids 1998, 225, 64–68. [Google Scholar] [CrossRef]
- Bang, A.; Buback, C.; Sotiriou-Leventis, C.; Leventis, N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chem. Mater. 2014, 26, 6979–6993. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal Multiscale Nanoporous Polyurethanes: Flexible to Extremely Rigid Aerogels from Multifunctional Small Molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Bang, A. Flexible to Rigid Nanoporous Polyurethane-Acrylate (PUAC) Type Materials for Structural and Thermal Insulation Applications. U.S. Patent 0137376 A1, 18 May 2017. [Google Scholar]
- Oertel, G. Polyurethane Handbook: Chemistry, Raw Materials, Processing, Application, Properties; Carl Hanser Verlag: Munich, Germany, 1994; ISBN 978-3-446-17198-5. [Google Scholar]
- Britain, J.W.; Gemeinhardt, P.G. Catalysis of the isocyanate-hydroxyl reaction. J. Appl. Polym. Sci. 1960, 4, 207–211. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Macroporous Electrically Conducting Carbon Networks by Pyrolysis of Isocyanate-Cross-Linked Resorcinol-Formaldehyde Aerogels. Chem. Mater. 2008, 20, 6985–6997. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Saeed, A.M.; Donthula, S.; Majedi Far, H.; Rewatkar, P.M.; Kaiser, H.; Robertson, J.D.; Lu, H.; Churu, G. Nanoporous Polyurea from a Triisocyanate and Boric Acid: A Paradigm of a General Reaction Pathway for Isocyanates and Mineral Acids. Chem. Mater. 2016, 28, 67–78. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; Xu, L.; Sotiriou-Leventis, C.; Leventis, N. Robust monolithic multiscale nanoporous polyimides and conversion to isomorphic carbons. RSC Adv. 2013, 3, 26459–26469. [Google Scholar] [CrossRef]
- Leventis, N.; Chidambareswarapattar, C.; Mohite, D.P.; Larimore, Z.J.; Lu, H.; Sotiriou-Leventis, C. Multifunctional porous aramids (aerogels) by efficient reaction of carboxylic acids and isocyanates. J. Mater. Chem. 2011, 21, 11981–11986. [Google Scholar] [CrossRef]
- Wu, M.K. The Roughness of Aerosol Particles: Surface Fractal Dimension Measured Using Nitrogen Adsorption. Aerosol Sci. Technol. 1996, 25, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Donthula, S.; Mandal, C.; Schisler, J.; Leventis, T.; Meador, M.A.B.; Sotiriou-Leventis, C.; Leventis, N. Nanostructure-Dependent Marcus-Type Correlation of the Shape Recovery Rate and the Young’s Modulus in Shape Memory Polymer Aerogels. ACS Appl. Mater. Interfaces 2018, 10, 23321–23334. [Google Scholar] [CrossRef] [PubMed]
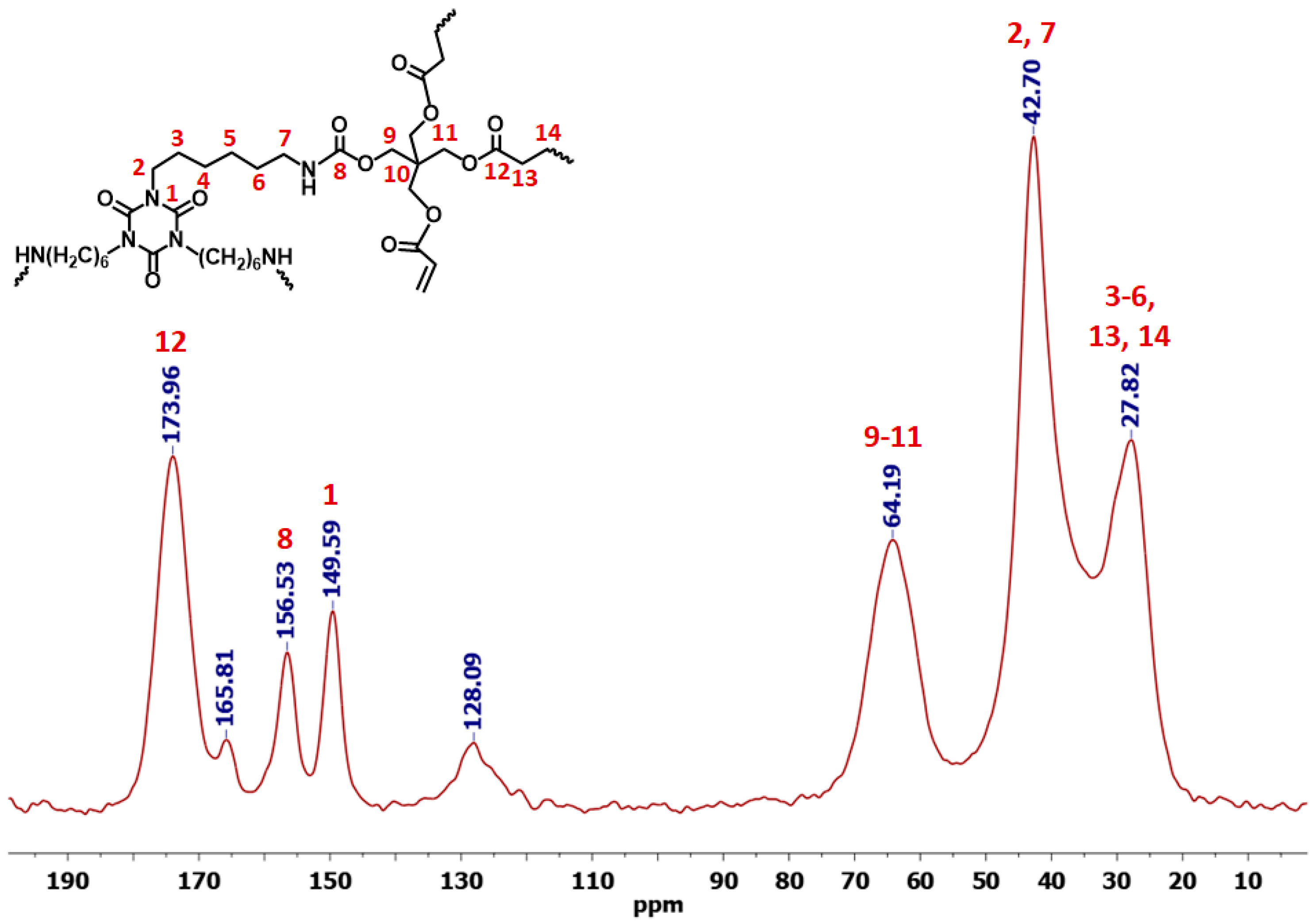
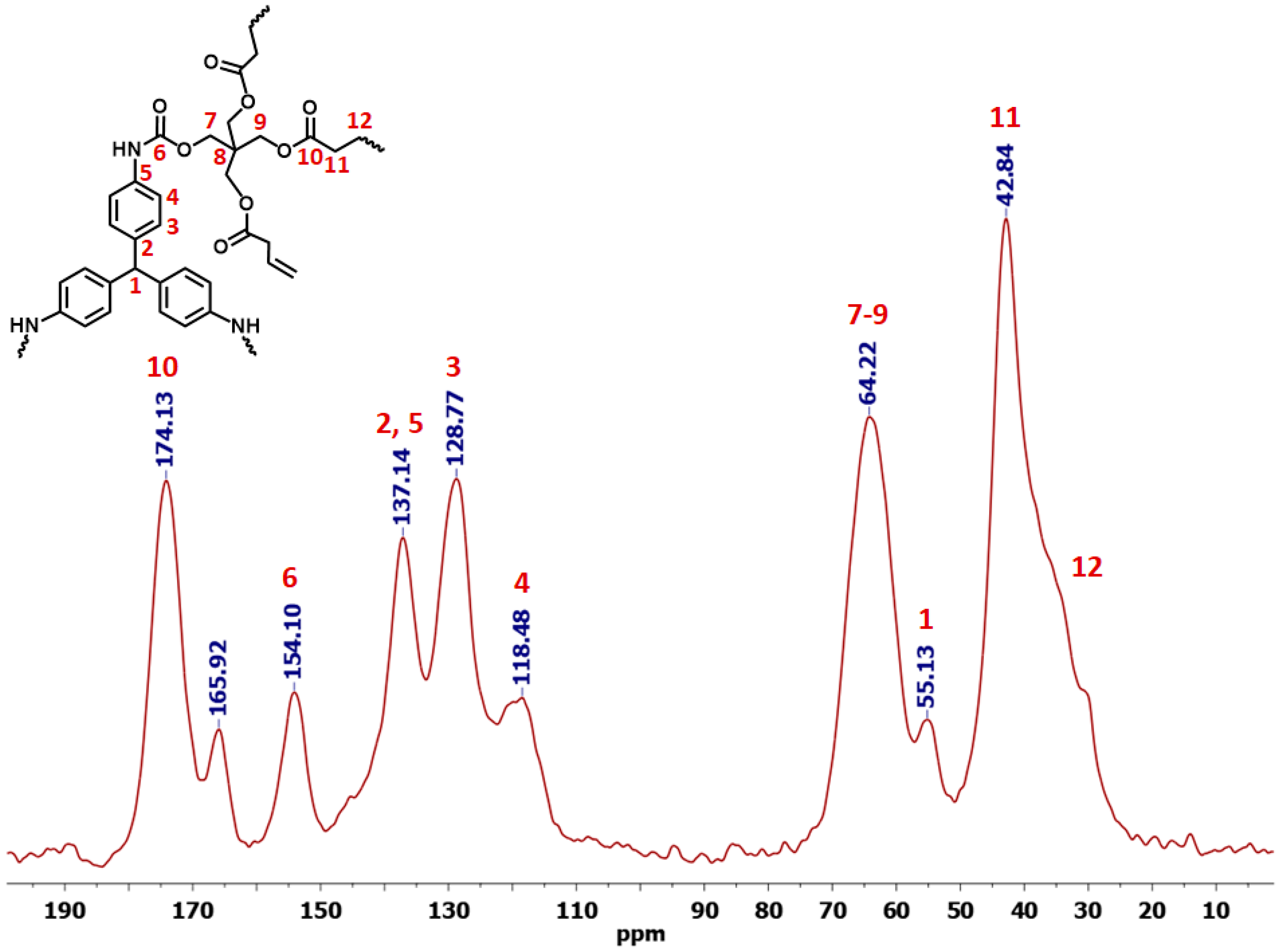

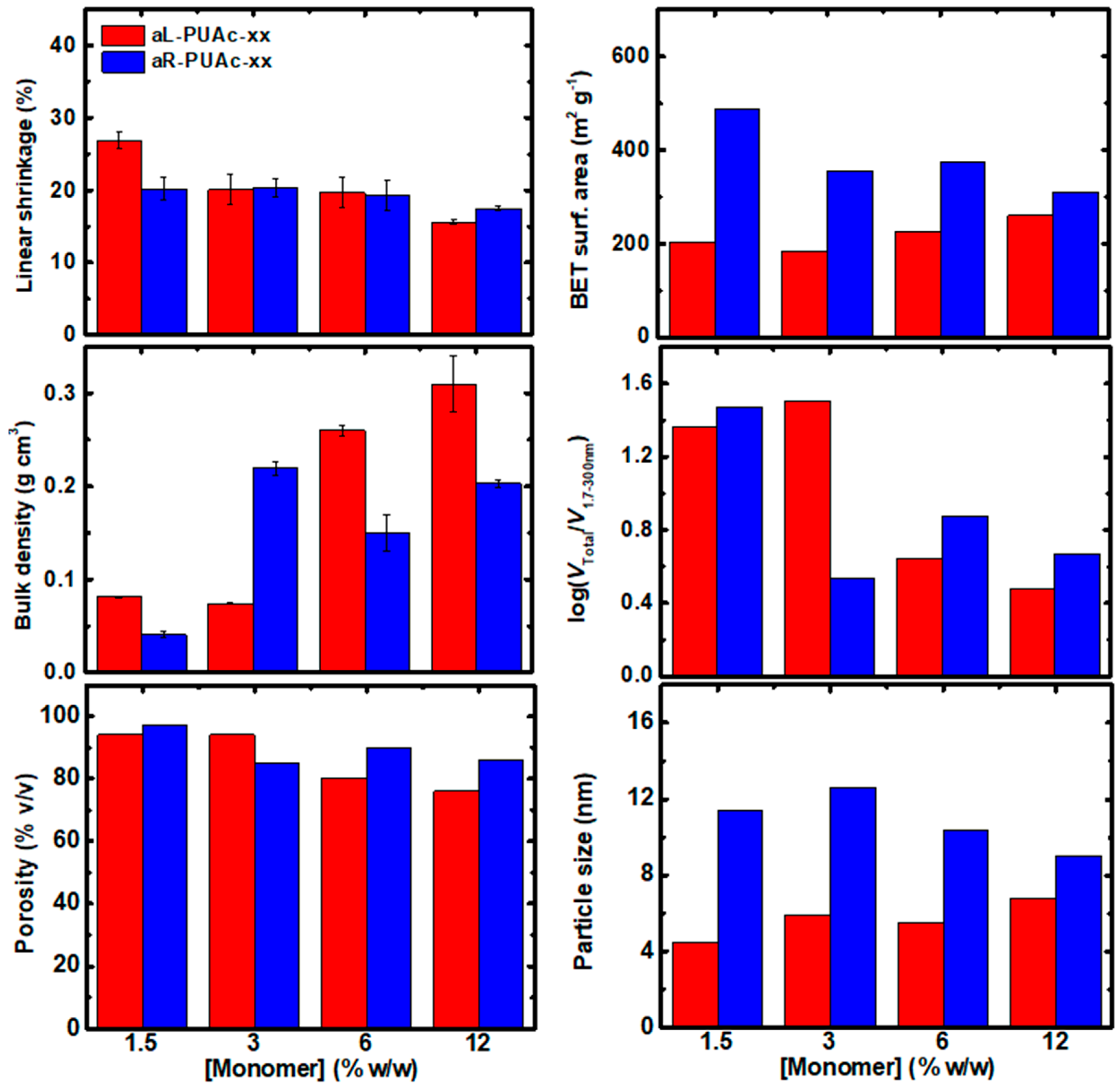
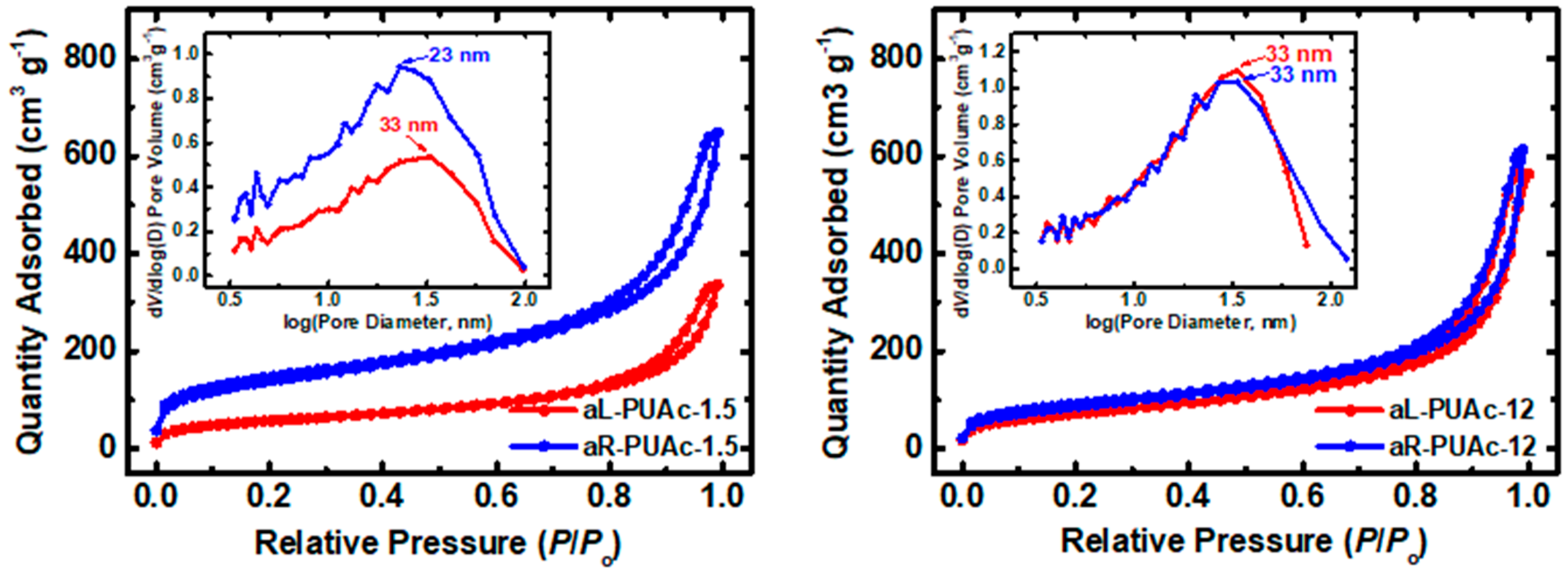

| Sample | Linear Shrinkage 1 (%) | Bulk Density ρb (g cm−3) | Skeletal Density ρs (g cm−3) | Porosity 2 Π (% v/v) | BET Surf. Area σ (m2 g−1) [Micropore Surf. Area] 3 | VTotal4 (V1.7–300 nm) 5 (cm3 g−1) | Av. Pore Diameter 6 (nm) | Particle Size 7 (nm) | Fractal Dimension 8 Ds |
|---|---|---|---|---|---|---|---|---|---|
| aL-PUAc-1.5 | 27 ± 1 | 0.081 ± 0.001 | 1.29 ± 0.01 | 94 | 203 | 11.6 (0.5) | 10.2 (228) | 11.4 | 2.59 |
| aL- PUAc-3 | 20 ± 2 | 0.074 ± 0.001 | 1.29 ± 0.02 | 94 | 185 | 12.7 (0.4) | 10.2 (276) | 12.6 | 2.58 |
| aL-PUAc-6 | 20 ± 2 | 0.260 ± 0.006 | 1.275 ± 0.005 | 80 | 225 | 3.1 (0.7) | 12.9 (55) | 10.4 | 2.55 |
| aL-PUAc-12 | 16 ± 2 | 0.31 ± 0.03 | 1.288 ± 0.001 | 76 | 260 | 2.4 (0.8) | 13.4 (38) | 9.0 | 2.54 |
| aR-PUAc-1.5 | 20 ± 2 | 0.041 ± 0.003 | 1.40 ± 0.01 | 97 | 488 [38] | 23.7 (0.8) | 8.2 (193) | 4.5 | 2.65 |
| aR-PUAc-3 | 20 ± 1 | 0.219 ± 0.008 | 1.43 ± 0.02 | 85 | 356 [13] | 3.9 (1.1) | 13.4 (42) | 5.9 | 2.60 |
| aR-PUAc-6 | 19 ± 2 | 0.15 ± 0.02 | 1.454 ± 0.004 | 90 | 374 [18] | 6.0 (0.8) | 9.8 (64) | 5.5 | 2.61 |
| aR-PUAc-12 | 17.5 ± 0.3 | 0.203 ± 0.004 | 1.414 ± 0.006 | 86 | 311 [2] | 4.2 (0.9) | 12.2 (54) | 6.8 | 2.58 |
| Sample | Skeletal Density ρs (g cm−3) | BET Surf. Area σ (m2 g−1) [Micropore Surf. Area] 2 | Quantity of CO2 Adsorbed (mmoL g−1) |
|---|---|---|---|
| aR-PUAc-1.5 | 1.40 ± 0.01 | 488 [38] | 0.63 |
| aR-PUAc-1.5-C | 2.0 ± 0.1 | 171 [154] | 1.09 |
| aR-PUAc-6 | 1.454 ± 0.004 | 374 [18] | 0.97 |
| aR-PUAc-6-C | 2.15 ± 0.03 | 639 [360] | 2.50 |
| aR-PUAc-12 | 1.414 ± 0.006 | 311 [2] | 0.84 |
| aR-PUAc-12-C | 2.3 ± 0.1 | 739 [429] | 0.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papastergiou, M.; Kanellou, A.; Chriti, D.; Raptopoulos, G.; Paraskevopoulou, P. Poly(Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers. Materials 2018, 11, 2249. https://doi.org/10.3390/ma11112249
Papastergiou M, Kanellou A, Chriti D, Raptopoulos G, Paraskevopoulou P. Poly(Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers. Materials. 2018; 11(11):2249. https://doi.org/10.3390/ma11112249
Chicago/Turabian StylePapastergiou, Maria, Aspasia Kanellou, Despoina Chriti, Grigorios Raptopoulos, and Patrina Paraskevopoulou. 2018. "Poly(Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers" Materials 11, no. 11: 2249. https://doi.org/10.3390/ma11112249
APA StylePapastergiou, M., Kanellou, A., Chriti, D., Raptopoulos, G., & Paraskevopoulou, P. (2018). Poly(Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers. Materials, 11(11), 2249. https://doi.org/10.3390/ma11112249