White-Light Emitting Di-Ureasil Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Characterization
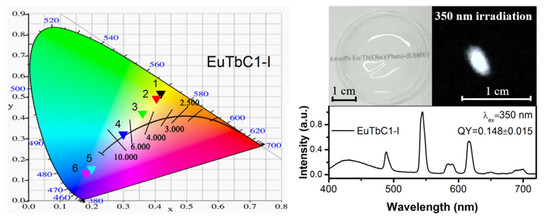
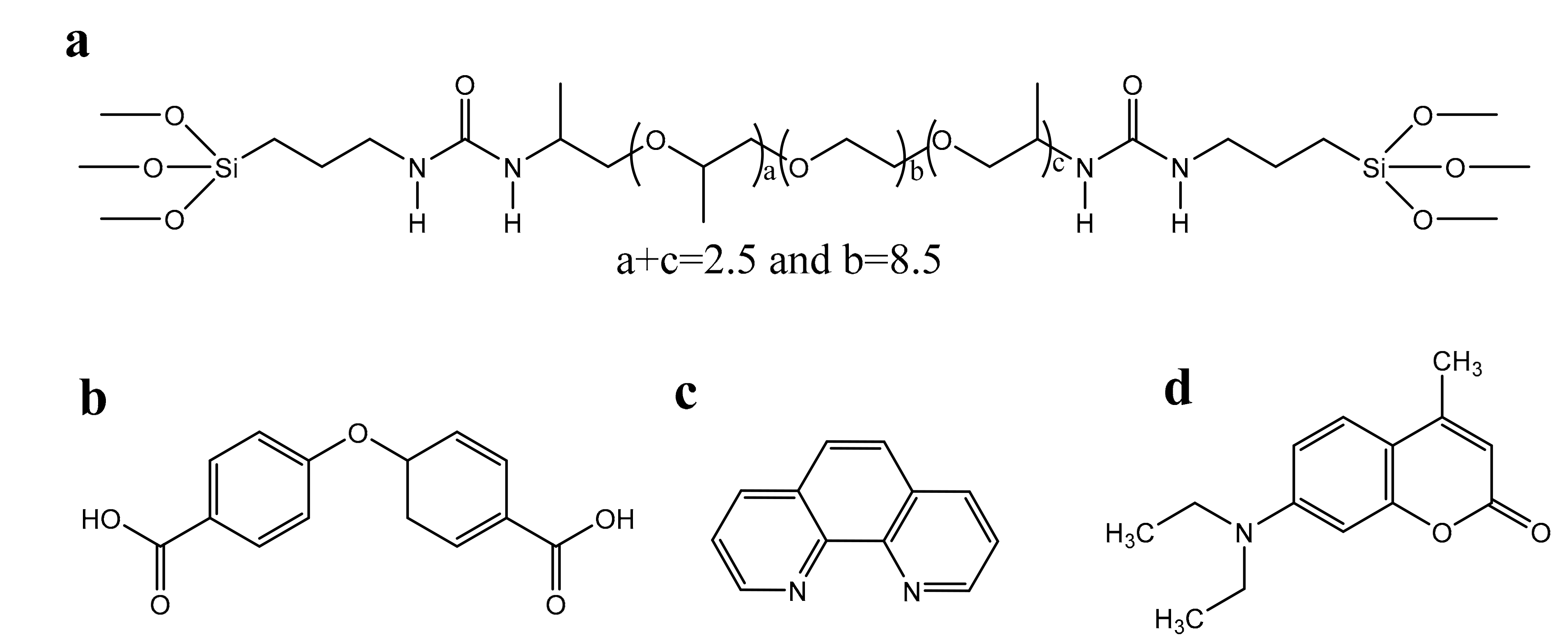
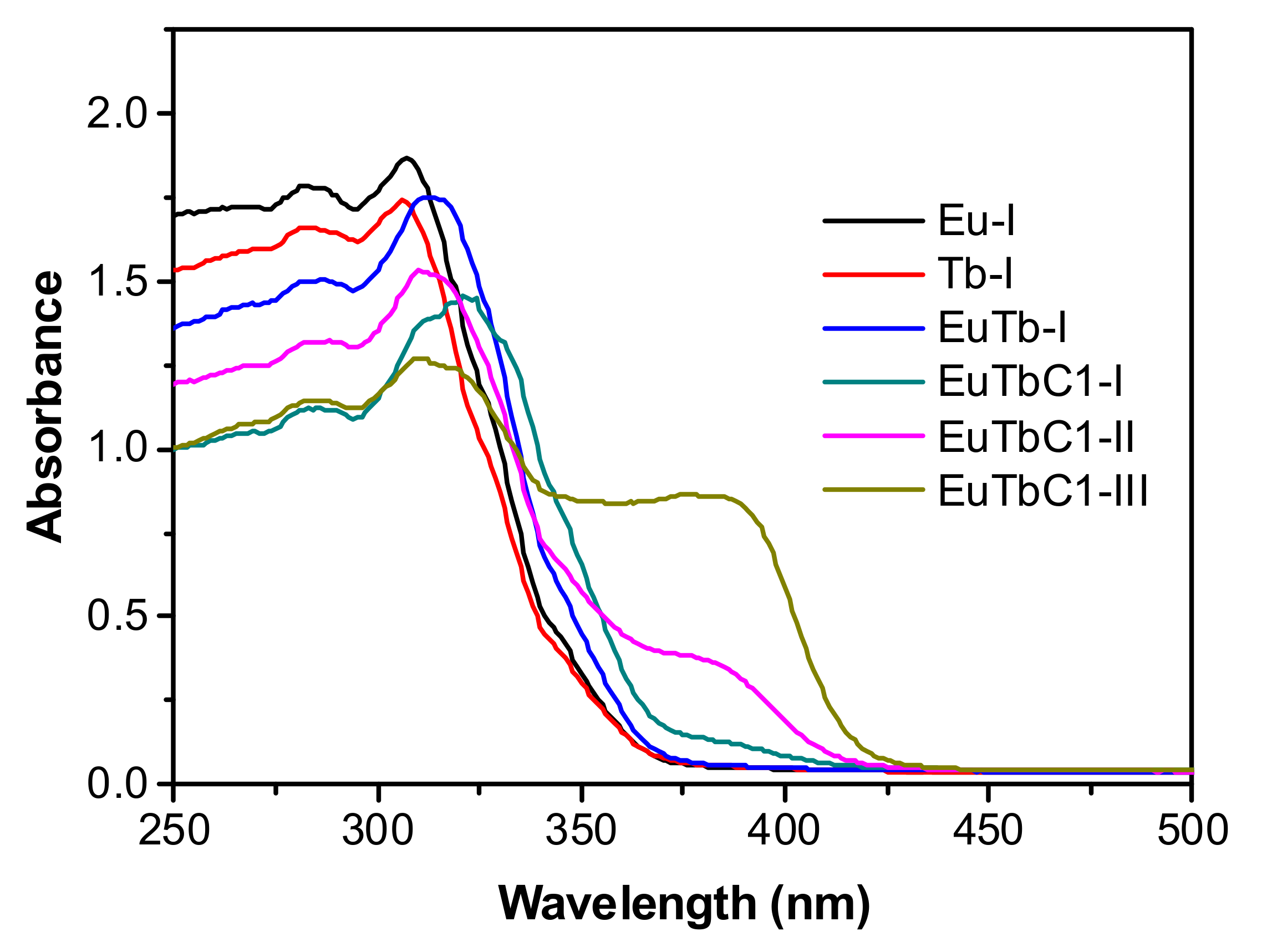
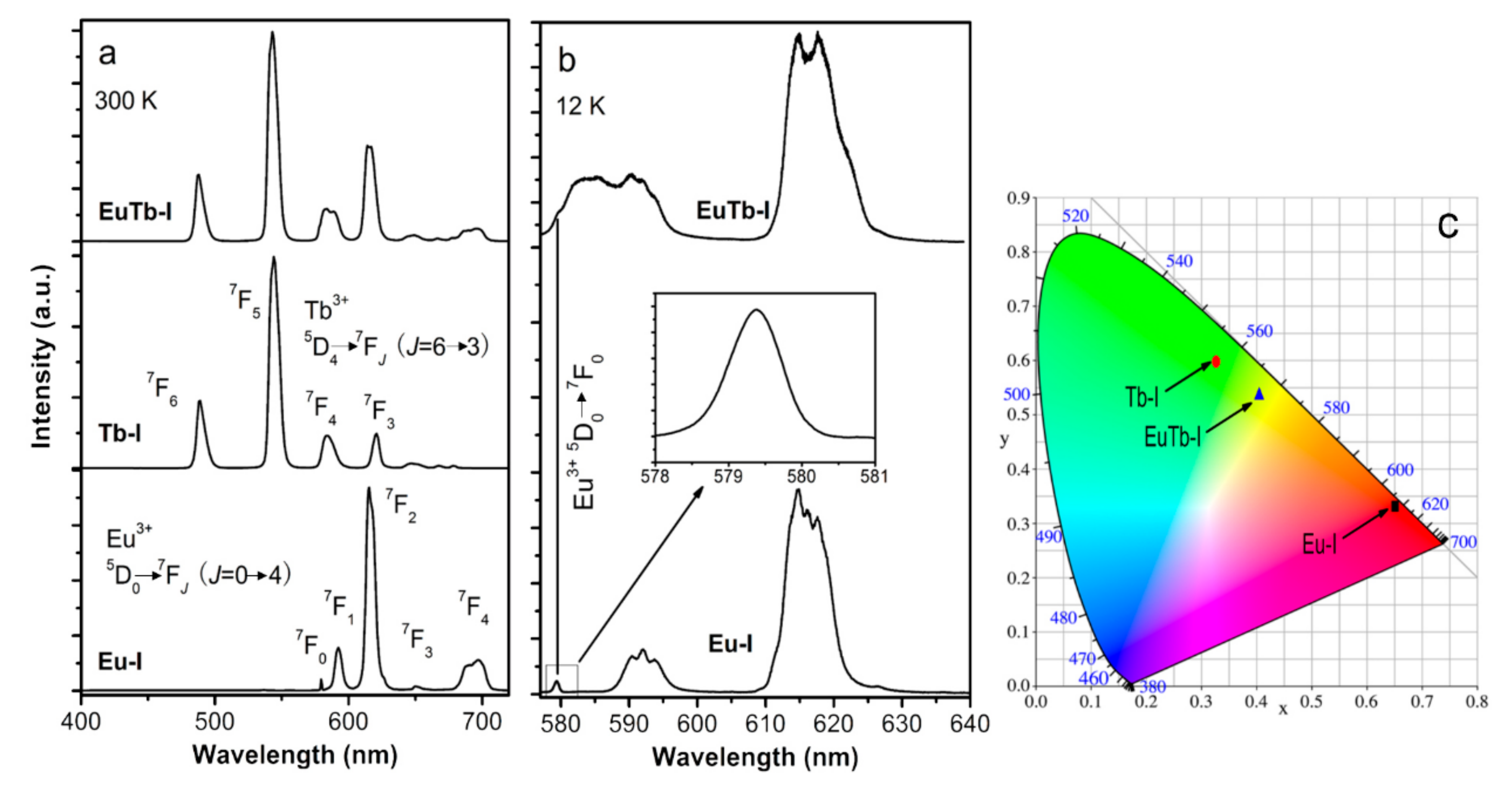
3. Results and Discussion
3.1. Structural Studies
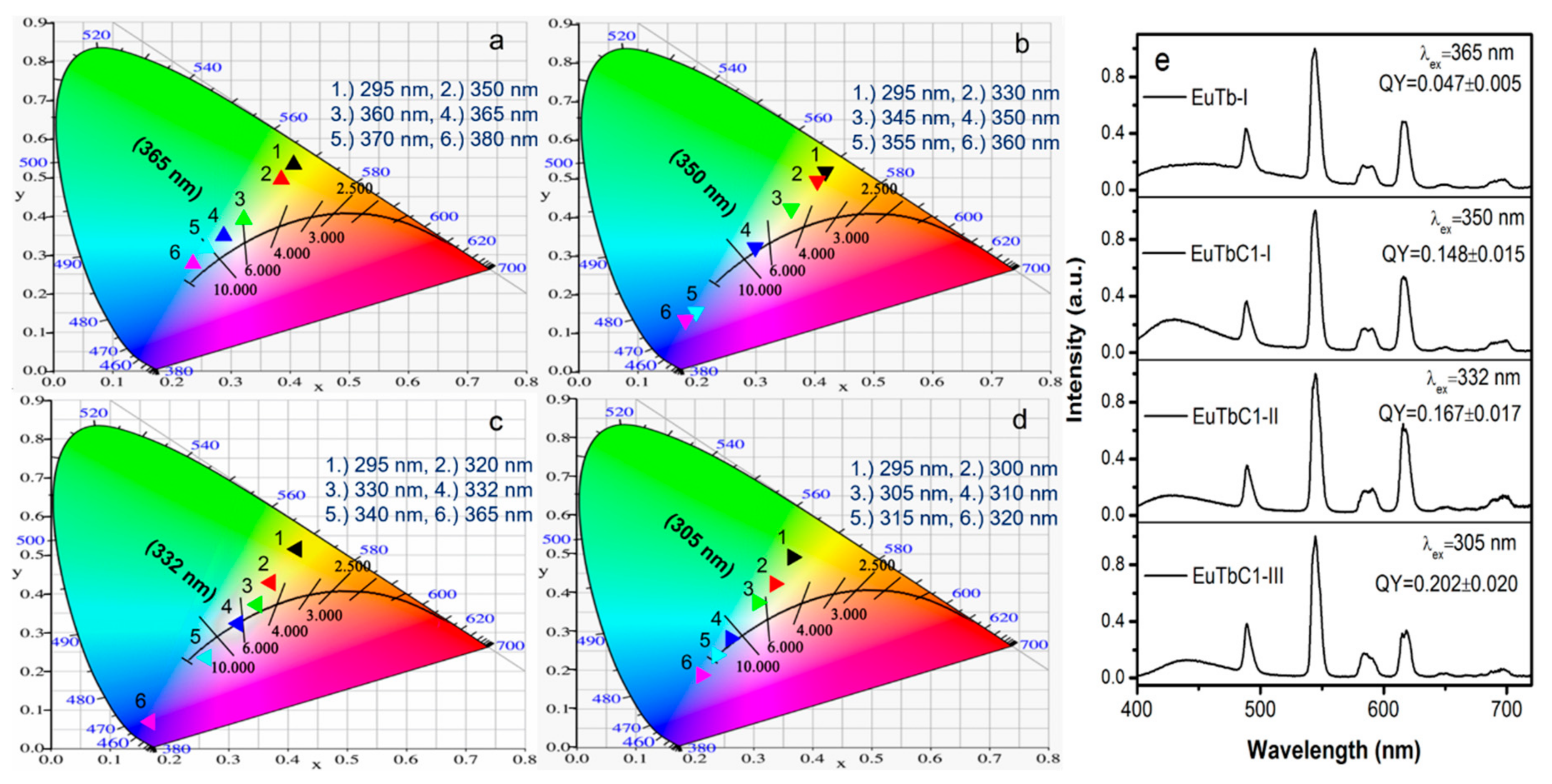
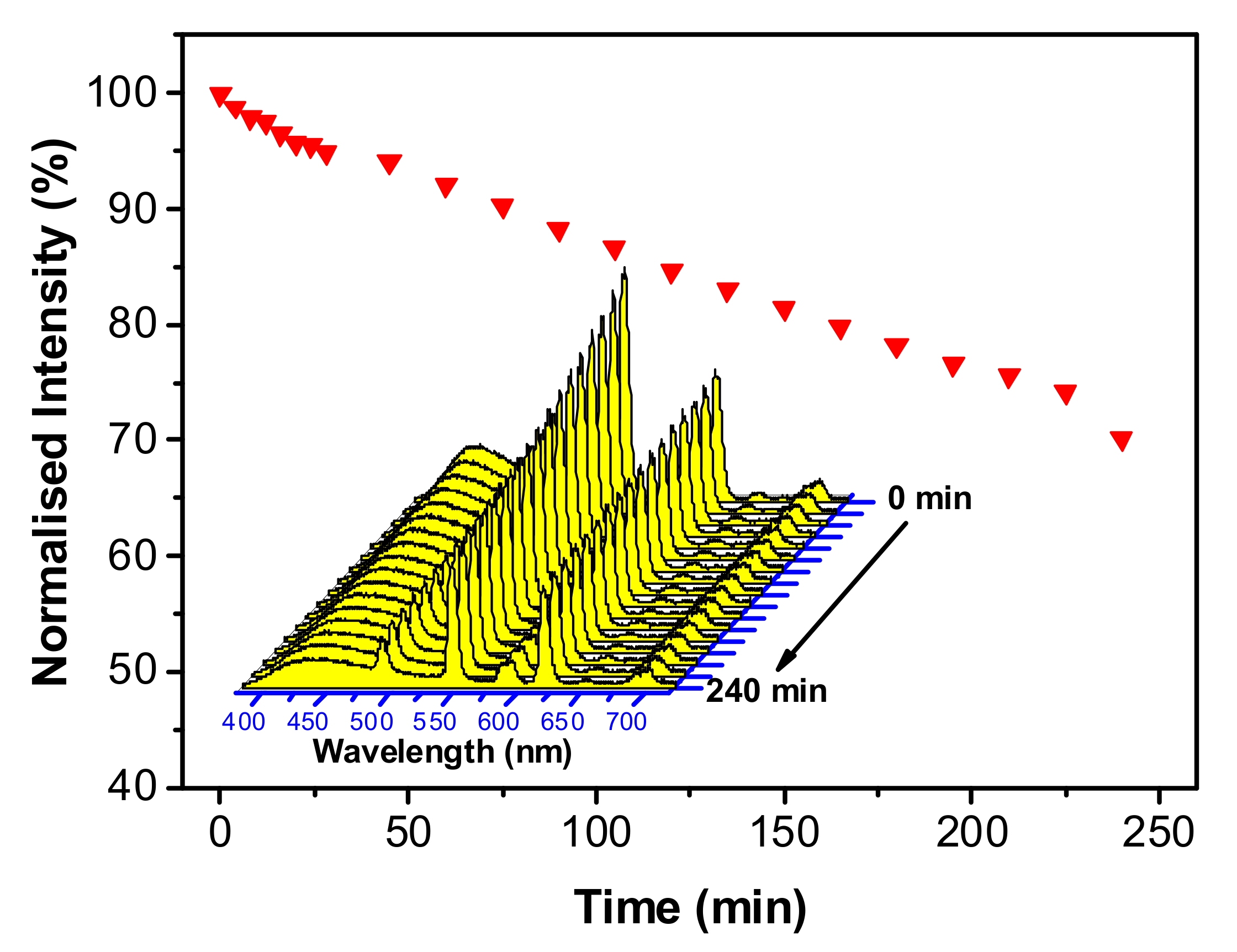
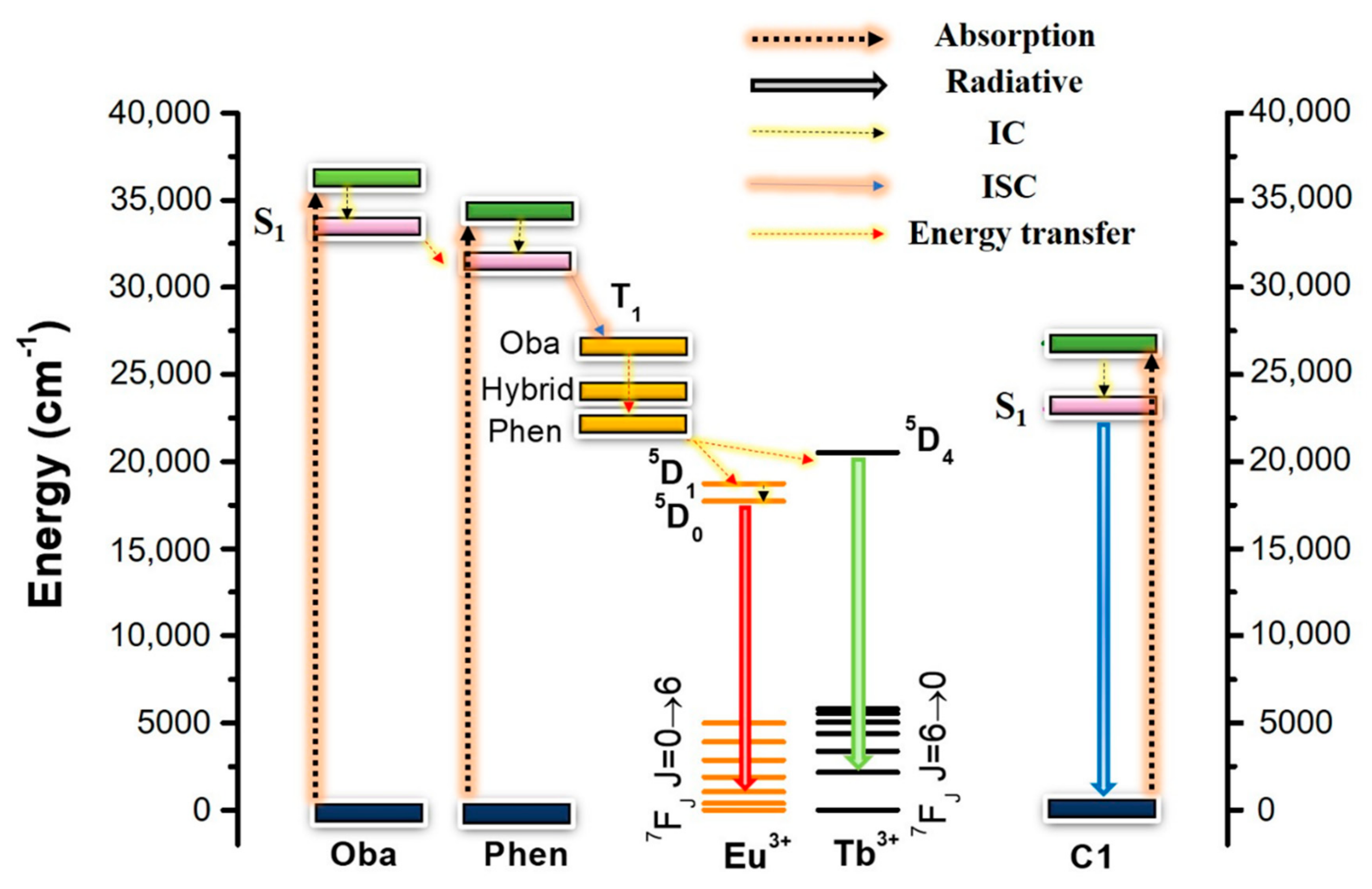
3.2. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Schubert, E.F.; Kim, J.K. Solid-state light sources getting smart. Science 2005, 308, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.D.; Ferreira, R.A.S.; De Zea Bermudez, V.; Ribeiro, S.J.L. Lanthanide-containing light-emitting organic–inorganic hybrids: A bet on the future. Adv. Mater. 2009, 21, 509–534. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Julián-López, B.; Planelles-Aragó, J.; Cordoncillo, E.; Viana, B.; Sanchez, C. Photonic and nanobiophotonic properties of luminescent lanthanide-doped hybrid organic–inorganic materials. J. Mater. Chem. 2008, 18, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Sutar, P.; Suresh, V.M.; Maji, T.K. Tunable emission in lanthanide coordination polymer gels based on a rationally designed blue emissive gelator. Chem. Commun. 2015, 51, 9876–9879. [Google Scholar] [CrossRef] [PubMed]
- Laishram, R.; Bhowmik, S.; Maitra, U. White light emitting soft materials from off-the-shelf ingredients. J. Mater. Chem. C 2015, 3, 5885–5889. [Google Scholar] [CrossRef]
- Yang, J.; Yan, Y.; Hui, Y.; Huang, J. White emission thin films based on rationally designed supramolecular coordination polymers. J. Mater. Chem. C 2017, 5, 5083–5089. [Google Scholar] [CrossRef]
- Chen, W.; Fan, R.; Zhang, H.; Dong, Y.; Wang, P.; Yang, Y. Tunable white-light emission PMMA-supported film materials containing lanthanide coordination polymers: Preparation, characterization, and properties. Dalton Trans. 2017, 46, 4265–4277. [Google Scholar] [CrossRef] [PubMed]
- Narayana, Y.S.L.V.; Basak, S.; Baumgarten, M.; Müllen, K.; Chandrasekar, R. White-emitting conjugated polymer/inorganic hybrid spheres: Phenylethynyl and 2,6-bis(pyrazolyl)pyridine copolymer coordinated to Eu(tta)3. Adv. Funct. Mater. 2013, 23, 5875–5880. [Google Scholar] [CrossRef]
- Balamurugan, A.; Reddy, M.L.P.; Jayakannan, M. Single polymer photosensitizer for Tb3+ and Eu3+ ions: An approach for white light emission based on carboxylic-functionalized poly(m-phenylenevinylene)s. J. Phys. Chem. B 2009, 113, 14128–14138. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Yan, B. Novel core–shell structure microspheres based on lanthanide complexes for white-light emission and fluorescence sensing. Dalton Trans. 2016, 45, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Du, S. Tunable luminescence and white light emission of mixed lanthanide–organic frameworks based on polycarboxylate ligands. J. Mater. Chem. C 2016, 4, 3364–3374. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakanishi, T. Luminescent lanthanide coordination polymers for photonic applications. RSC Adv. 2015, 5, 338–353. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2011, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kim, Y.J.; Ha, S.W.; Lee, J.K. White light-emitting diodes using thermally and photochemically stable fluorescent silica nanoparticles as color-converters. J. Mater. Chem. C 2013, 1, 5879–5884. [Google Scholar] [CrossRef]
- SeethaLekshmi, S.; Ramya, A.R.; Reddy, M.L.P.; Varughese, S. Lanthanide complex-derived white-light emitting solids: A survey on design strategies. J. Photochem. Photobiol. C 2017, 33, 109–131. [Google Scholar] [CrossRef]
- Huo, R.; Li, X.; Ma, D. Lanthanide coordination frameworks constructed from 1,3-benzenedicarboxylate, oxalate and 1,10-phenanthroline: Crystal structure, multicolor luminescence and high-efficiency white light emission. CrystEngComm 2015, 17, 3838–3844. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, X.; Zhou, L.; Lin, P.; Li, R.; Ma, E.; Guo, X.; Du, S. Full-colour fluorescent materials based on mixed-lanthanide(iii) metal–organic complexes with high-efficiency white light emission. J. Mater. Chem. C 2013, 1, 888–891. [Google Scholar] [CrossRef]
- Ramya, A.R.; Varughese, S.; Reddy, M.L.P. Tunable white-light emission from a mixed lanthanide (Eu3+ Gd3+ Tb3+) coordination polymers derived from 4-(dipyridin-2-yl)aminobenzoate. Dalton Trans. 2014, 43, 10940–10946. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Zhang, J.H.; Sun, Z.M. Tunable emission based on lanthanide(iii) metal–organic frameworks: An alternative approach to white light. J. Mater. Chem. 2012, 22, 8868–8873. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Zhang, Y.H.; Huo, R.; Ma, D. White light emission by a lanthanide doped Sm(III) framework constructed from 4-sulfobenzoate and 1H-imidazo[4,5-f][1,10]-phenanthroline. Dalton Trans. 2014, 43, 5974–5977. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.L.; Ji, C.; Zang, S.Q. Syntheses, structures, tunable emission and white light emitting Eu3+ and Tb3+ doped lanthanide metal–organic framework materials. Dalton Trans. 2013, 42, 10579–10586. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Cha, Y.E.; Jin, L.P. Highly thermostable one-dimensional lanthanide(III) coordination polymers constructed from benzimidazole-5,6-dicarboxylic acid and 1,10-phenanthroline: Synthesis, structure, and tunable white-light emission. Cryst. Growth Des. 2012, 12, 5227–5232. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, P.; Li, H.; Zou, X.; Hou, G.; Li, G. Towards full-color-tunable emission of two component Eu(III)-doped Gd(III) coordination frameworks by the variation of excitation light. Dalton Trans. 2014, 43, 12574–12581. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, Q.; Yang, X.; Cui, Y.; Yang, Y.; Wu, C.; Chen, B.; Qian, G. Color tunable and white light emitting Tb3+ and Eu3+ doped lanthanide metal–organic framework materials. J. Mater. Chem. 2012, 22, 3210–3214. [Google Scholar] [CrossRef]
- Liu, J.; Sun, W.; Liu, Z. White-light emitting materials with tunable luminescence based on steady Eu(III) doping of Tb(III) metal–organic frameworks. RSC Adv. 2016, 6, 25689–25694. [Google Scholar] [CrossRef]
- Liu, Z.F.; Wu, M.F.; Wang, S.H.; Zheng, F.K.; Wang, G.E.; Chen, J.; Xiao, Y.; Wu, A.Q.; Guo, G.C.; Huang, J.S. Eu3+-Doped Tb3+ metal–organic frameworks emitting tunable three primary colors towards white light. J. Mater. Chem. C 2013, 1, 4634–4639. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Zhang, Y.-H. White light emission of an Eu(III)-doped Gd(III) complex with 3-sulfobenzoate and 1H-imidazo[4,5-f][1,10]-phenanthroline. Dalton Trans. 2013, 42, 10409–10412. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Guo, D.; He, C.; Zhang, X.; Zhao, X.; Duan, C. A color-tunable europium complex emitting three primary colors and white light. Angew. Chem. Int. Ed. 2009, 48, 6132–6135. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.H.; Sazanovich, I.V.; Weinstein, J.A.; Ward, M.D. Controllable three-component luminescence from a 1,8-naphthalimide/Eu(III) complex: White light emission from a single molecule. Chem. Commun. 2012, 48, 2749–2751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Wu, K.; Jiang, J.J.; Hsu, C.W.; Pan, M.; Lehn, J.M.; Su, C.Y. Pure white-light and yellow-to-blue emission tuning in single crystals of Dy(III) metal–organic frameworks. Chem. Commun. 2014, 50, 7702–7704. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Chorazy, S.; Nakabayashi, K.; Sieklucka, B.; Ohkoshi, S. Achieving white light emission and increased magnetic anisotropy by transition metal substitution in functional materials based on dinuclear DyIII(4-pyridone)[MIII(CN)6]3− (M=Co, Rh) molecules. J. Mater. Chem. C 2018, 6, 473–481. [Google Scholar] [CrossRef]
- Zhang, X.P.; Wang, D.G.; Su, Y.; Tian, H.R.; Lin, J.J.; Feng, Y.L.; Cheng, J.W. Luminescent 2D bismuth–cadmium–organic frameworks with tunable and white light emission by doping different lantahnide ions. Dalton Trans. 2014, 42, 10384–10387. [Google Scholar] [CrossRef] [PubMed]
- Dar, W.A.; Ahmed, Z.; Iftikhar, K. Cool white light emission from the yellow and blue emission bands of the Dy(III) complex under UV-excitation. J. Photochem. Photobiol. A 2018, 356, 502–511. [Google Scholar] [CrossRef]
- Kerbellec, N.; Kustaryono, D.; Haquin, V.; Etienne, M.; Daiguebonne, C.; Guillou, O. An unprecedented family of lanthanide-containing coordination polymers with highly tunable emission properties. Inorg. Chem. 2009, 48, 2837–2843. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, G.; Cui, Y.; Yang, Y.; Qian, G. Encapsulation of coumarin dye within lanthanide MOFs as highly efficient white-light-emitting phosphors for white LEDs. CrystEngComm 2016, 18, 8366–8371. [Google Scholar] [CrossRef]
- Wen, Y.; Sheng, T.; Zhu, X.; Zhuo, C.; Su, S.; Li, H.; Hu, S.; Zhu, Q.L.; Wu, X. Introduction of red-green-blue fluorescent dyes into a metal–organic framework for tunable white light emission. Adv. Mater. 2017, 29, 1700778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lin, B.; Hu, X.; Wei, Y.; Zhang, C.; An, B.; Wang, C.; Lin, W. Warm-white-light-emitting diode based on a dye-loaded metal-organic framework for fast white-light communication. ACS Appl. Mater. Interfaces 2017, 9, 35253–35259. [Google Scholar] [CrossRef] [PubMed]
- De Zea Bermudez, V.; Carlos, L.D.; Alcácer, L. Sol–gel derived urea cross-linked organically modified silicates. 1. room temperature mid-infrared spectra. Chem. Mater. 1999, 11, 569–580. [Google Scholar] [CrossRef]
- Carlos, L.D.; De Zea Bermudez, V.; Ferreira, R.A.S.; Marques, L.; Assunção, M. Sol–gel derived urea cross-linked organically modified silicates. 2. blue-light emission. Chem. Mater. 1999, 11, 581–588. [Google Scholar] [CrossRef]
- Ma, D.; Li, X.; Huo, R. A high-efficiency white light-emitting lanthanide–organic framework assembled from 4,4′-oxybis(benzoic acid), 1,10-phenanthroline and oxalate. J. Mater. Chem. C 2014, 2, 9073–9076. [Google Scholar] [CrossRef]
- Fang, M.; Fu, L.S.; Correia, S.F.H.; Ferreira, R.A.S.; Carlos, L.D. Highly efficient luminescent polycarboxylate lanthanide complexes incorporated into di-ureasils by an in-situ sol–gel Process. Polymers 2018, 10, 434. [Google Scholar] [CrossRef]
- Diraison, M.; Millie, P.; Pommeret, S.; Gustavsson, T.; Mialocq, J.C. Solvation structure of coumarin 1 in acetonitrile: Role of the electrostaticsolute-solvent potential. Chem. Phys. Lett. 1998, 282, 152–158. [Google Scholar] [CrossRef]
- Gupta, M.; Maity, D.K.; Singh, M.K.; Nayak, S.K.; Ray, A.K. Supramolecular interaction of coumarin 1 dye with cucurbit[7]uril as host: Combined experimental and theoretical study. J. Phys. Chem. B 2012, 116, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, S.; Pleil, M.W.; Borst, W.L. Study of energy transfer in a solution of coumarin 460 and rhodamine 6G by time-resolved laser-induced fluorescence spectroscopy. J. Lumin. 1987, 39, 105–110. [Google Scholar] [CrossRef]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
- Fu, L.S.; Ferreira, R.A.S.; Fernandes, M.; Nunes, S.C.; De Zea Bermudez, V.; Hungerford, G.; Rocha, J.; Carlos, L.D. Photoluminescence and quantum yields of organic/inorganic hybrids prepared through formic acid solvolysis. Opt. Mater. 2008, 30, 1058–1064. [Google Scholar] [CrossRef]
- Borie, B. X-ray diffraction in crystals, imperfect crystals, and amorphous bodies. J. Am. Chem. Soc. 1965, 87, 140–141. [Google Scholar] [CrossRef]
- Skrovanek, D.J.; Howe, S.E.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymers: Infrared temperature studies of an amorphous polyamide. Macromolecules 1985, 18, 1676–1683. [Google Scholar] [CrossRef]
- Coleman, M.M.; Lee, K.H.; Skrovanek, D.J.; Painter, P.C. Hydrogen bonding in polymers. 4. infrared temperature studies of a simple polyurethane. Macromolecules 1986, 19, 2149–2157. [Google Scholar] [CrossRef]
- Fu, L.S.; Ferreira, R.A.S.; Silva, N.J.O.; Carlos, L.D.; De Zea Bermudez, V.; Rocha, J. Photoluminescence and quantum yields of urea and urethane cross-linked nanohybrids derived from carboxylic acid solvolysis. Chem. Mater. 2004, 16, 1507–1516. [Google Scholar] [CrossRef]
- Dingemans, G.; van Helvoirt, C.A.A.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of low temperature SiO2. ECS Trans. 2011, 35, 191–204. [Google Scholar]
- Lima, P.P.; Almeida Paz, F.A.; Ferreira, R.A.S.; De Zea Bermudez, V.; Carlos, L.D. Ligand-assisted rational design and supramolecular tectonics toward highly luminescent Eu3+ containing organic–inorganic hybrids. Chem. Mater. 2009, 21, 5099–5111. [Google Scholar] [CrossRef]
- Fernandes, M.; De Zea Bermudez, V.; Ferreira, R.A.S.; Carlos, L.D.; Martins, N.V. Incorporation of the Eu(tta)3(H2O)2 complex into a co-condensed d-U(600)/d-U(900) matrix. J. Lumin. 2008, 128, 205–212. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, N.; Miyagawa, S.; Date, K.; Aroonjaeng, W.; Aono, M.; Watanabe, Y. Optical properties of dye-doped deoxyribonucleic acid films. J. Mater. Sci. 2009, 44, 4999–5003. [Google Scholar] [CrossRef]
- Irfanullah, M.; Iftikhar, K. The correlation between f-f absorption and sensitized visible light emission of luminescent Pr(III) complexes: Role of solvents and ancillary ligands on sensitivity. J. Lumin. 2011, 21, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.P.; Ferreira, R.A.S.; Júnior, S.A.; Malta, O.L.; Carlos, L.D. Terbium(III)-containing organic–inorganic hybrids synthesized through hydrochloric acid catalysis. J. Photochem. Photobiol. A Chem. 2009, 201, 214–221. [Google Scholar] [CrossRef]
- Correia, S.F.H.; Lima, P.P.; Pecoraro, E.; Ribeiro, S.J.L.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. Scale up the collection area of luminescent solar concentrators towards metre-length flexible waveguiding photovoltaics. Prog. Photovolt. Res. Appl. 2016, 24, 1178–1193. [Google Scholar] [CrossRef]
- Nolasco, M.M.; Vaz, P.M.; Freitas, V.T.; Lima, P.P.; André, P.S.; Ferreira, R.A.S.; Vaz, P.D.; Ribeiro-Claro, P.; Carlos, L.D. Engineering highly efficient Eu(III)-based tri-ureasil hybrids toward luminescent solar concentrators. J. Mater. Chem. A 2013, 1, 7339–7350. [Google Scholar] [CrossRef]
- Ferreira, R.A.S.; Carlos, L.D.; De Zea Bermudez, V. Excitation energy dependence of luminescent sol–gel organically modified silicates. Thin Solid Films 1999, 343, 476–480. [Google Scholar] [CrossRef]
- Carlos, L.D.; Ferreira, R.A.S.; Pereira, R.N.; Assunção, M.; De Zea Bermudez, V. White-light emission of amine-functionalized organic/inorganic hybrids: Emitting centers and recombination mechanisms. J. Phys. Chem. B 2004, 108, 14924–14932. [Google Scholar] [CrossRef]
- Carnall, W.T.; Goodman, G.L.; Rajnak, K.; Rana, R.S. A systematic analysis of the spectra of the lanthanides doped into single crystal LaF3. J. Chem. Phys. 1989, 90, 3443–3457. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; van der Tol, E.B.; Verhoeven, J.W. New sensitizer-modified calix[4]arenes enabling near-UV excitation of complexed luminescent lanthanide ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Bala, M.; Kumar, S.; Taxak, V.B.; Boora, P.; Khatkar, S.P. Optical features of efficient europium(III) complexes with β-diketonato and auxiliary ligands and mechanistic investigation of energy transfer process. J. Fluoresc. 2016, 26, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.P.; Ferreira, R.A.S.; Freire, R.O.; Almeida Paz, F.A.; Fu, L.S.; Alves, S.; Carlos, L.D.; Malta, O.L. Spectroscopic study of a UV-photostable organic–inorganic-hybrids incorporating an Eu3+ β-diketonate complex. Chem. Phys. Chem. 2006, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Fu, L.; Ferreira, R.A.S.; Carlos, L.D. White-Light Emitting Di-Ureasil Hybrids. Materials 2018, 11, 2246. https://doi.org/10.3390/ma11112246
Fang M, Fu L, Ferreira RAS, Carlos LD. White-Light Emitting Di-Ureasil Hybrids. Materials. 2018; 11(11):2246. https://doi.org/10.3390/ma11112246
Chicago/Turabian StyleFang, Ming, Lianshe Fu, Rute A. S. Ferreira, and Luís D. Carlos. 2018. "White-Light Emitting Di-Ureasil Hybrids" Materials 11, no. 11: 2246. https://doi.org/10.3390/ma11112246
APA StyleFang, M., Fu, L., Ferreira, R. A. S., & Carlos, L. D. (2018). White-Light Emitting Di-Ureasil Hybrids. Materials, 11(11), 2246. https://doi.org/10.3390/ma11112246