Mechanical Properties of U-Cu Intermetallic Compound Measured by Nanoindentation
Abstract
:1. Introduction
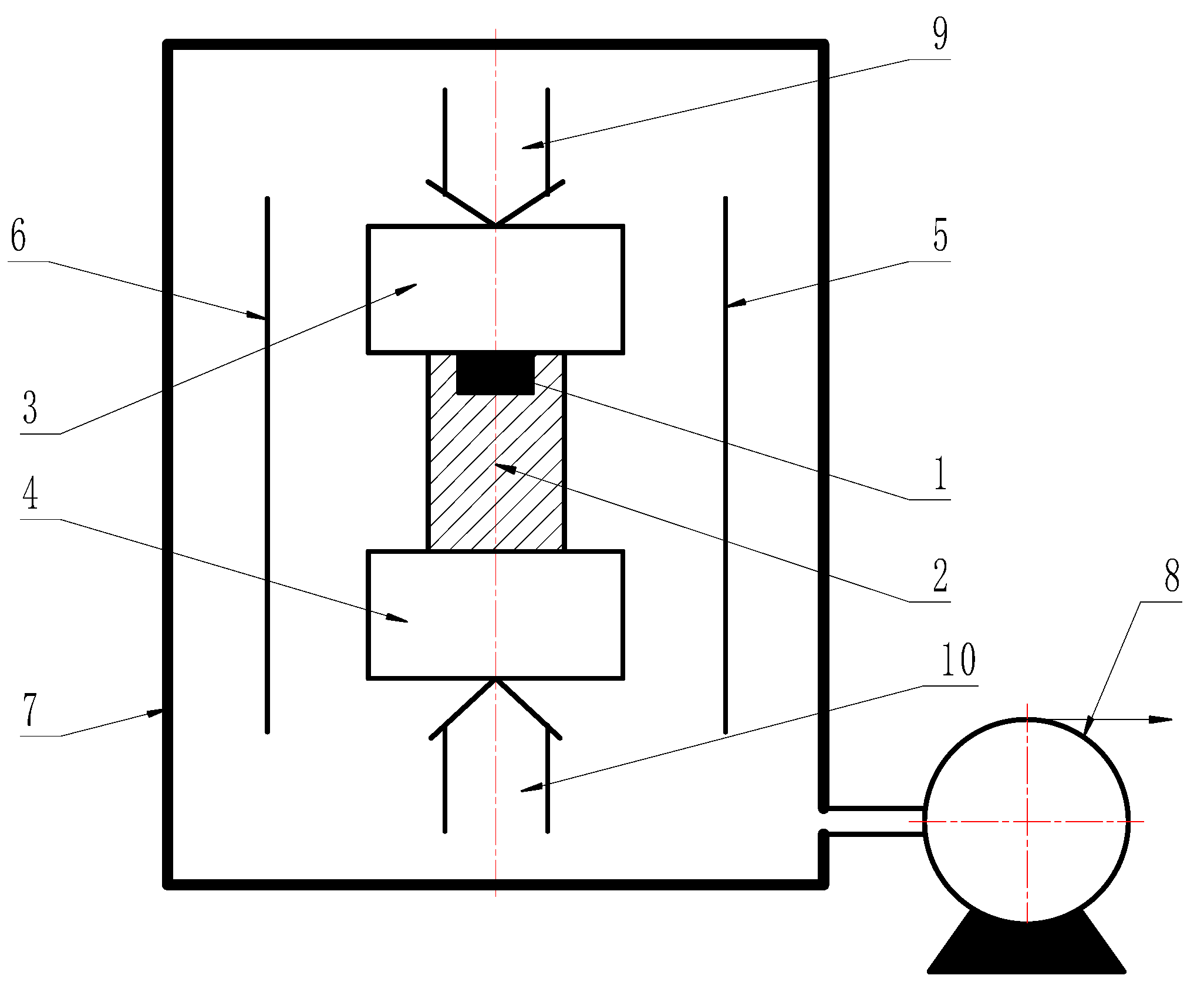
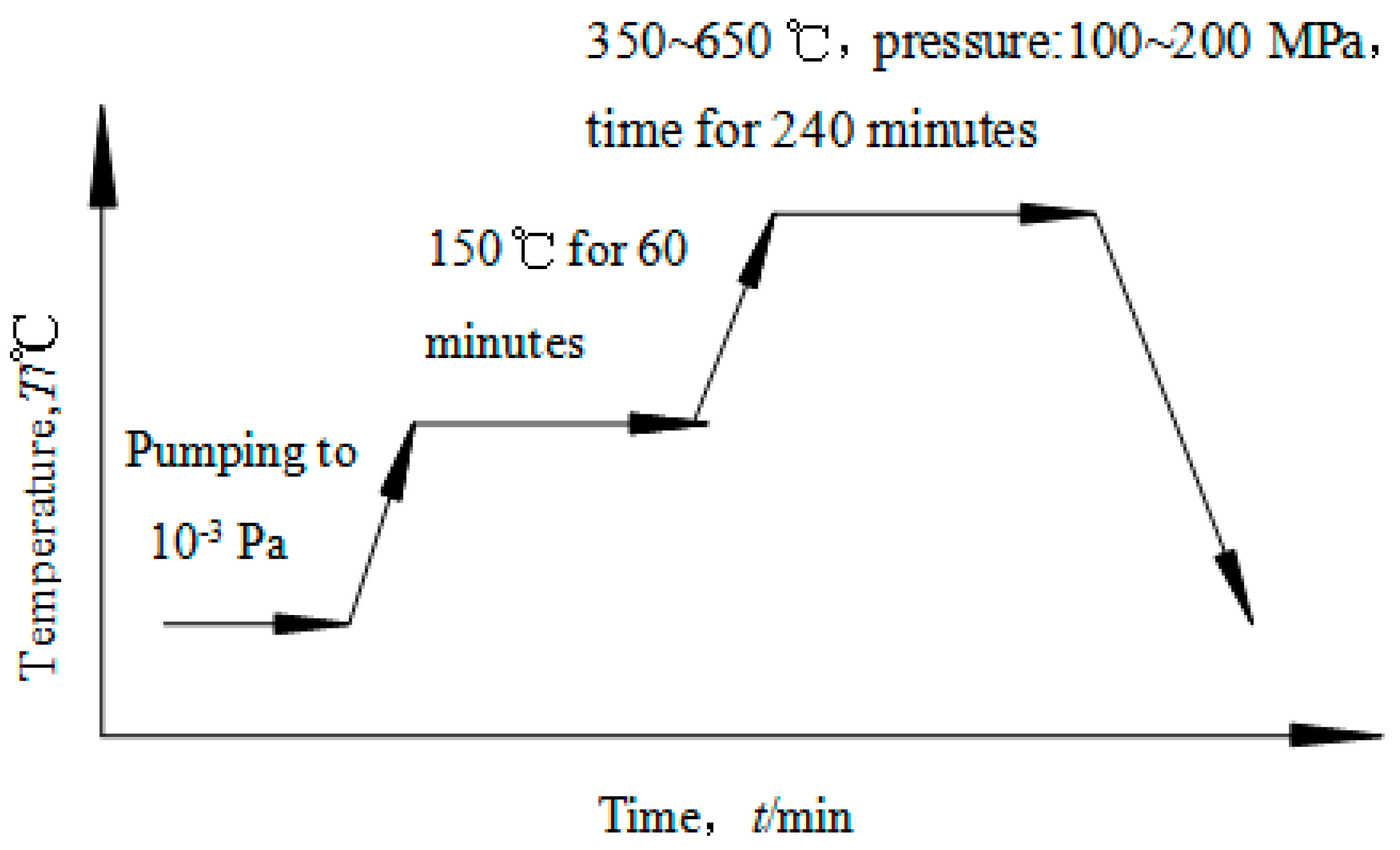
2. Experimental Procedure
3. Results and Discussion
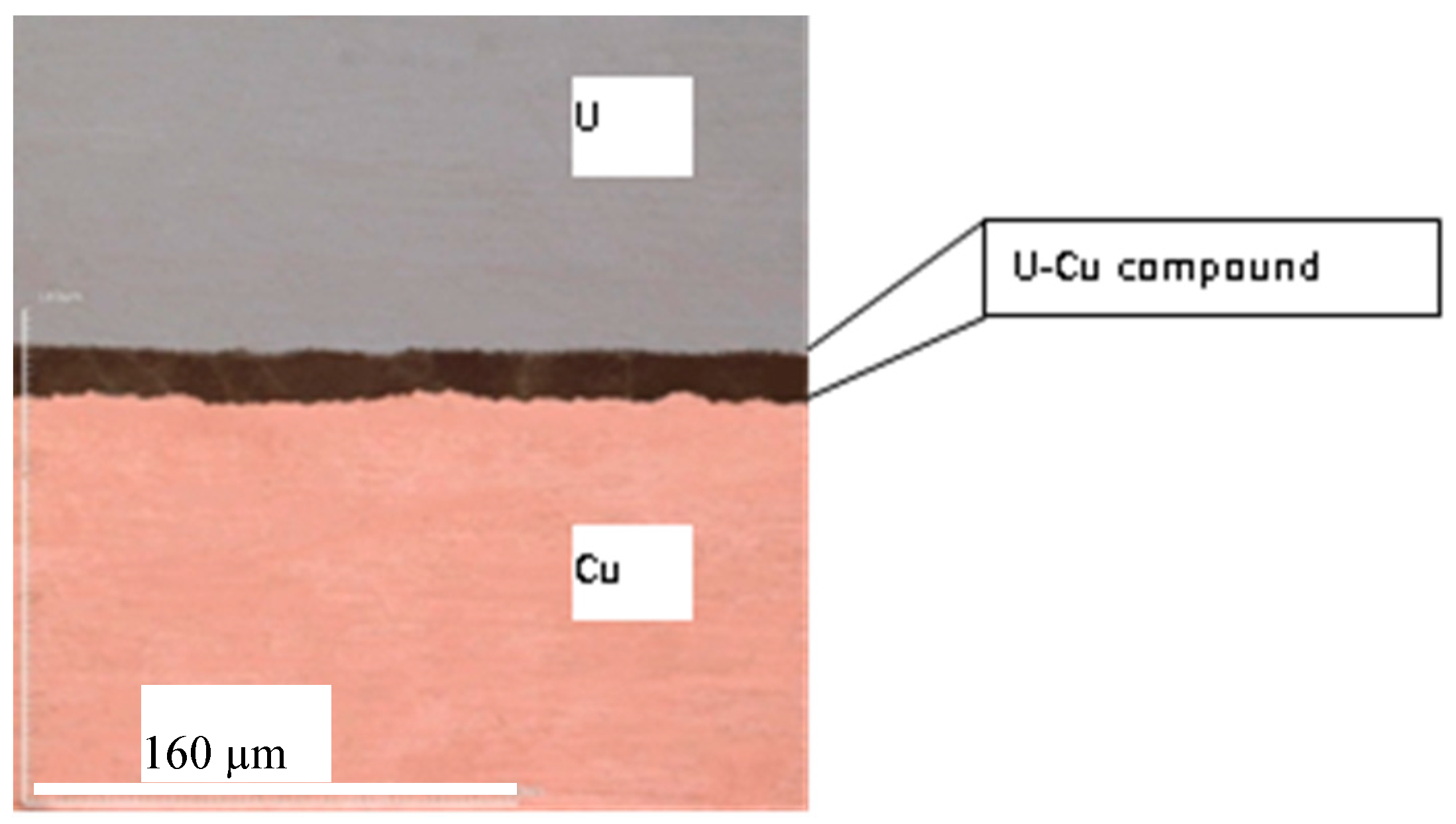
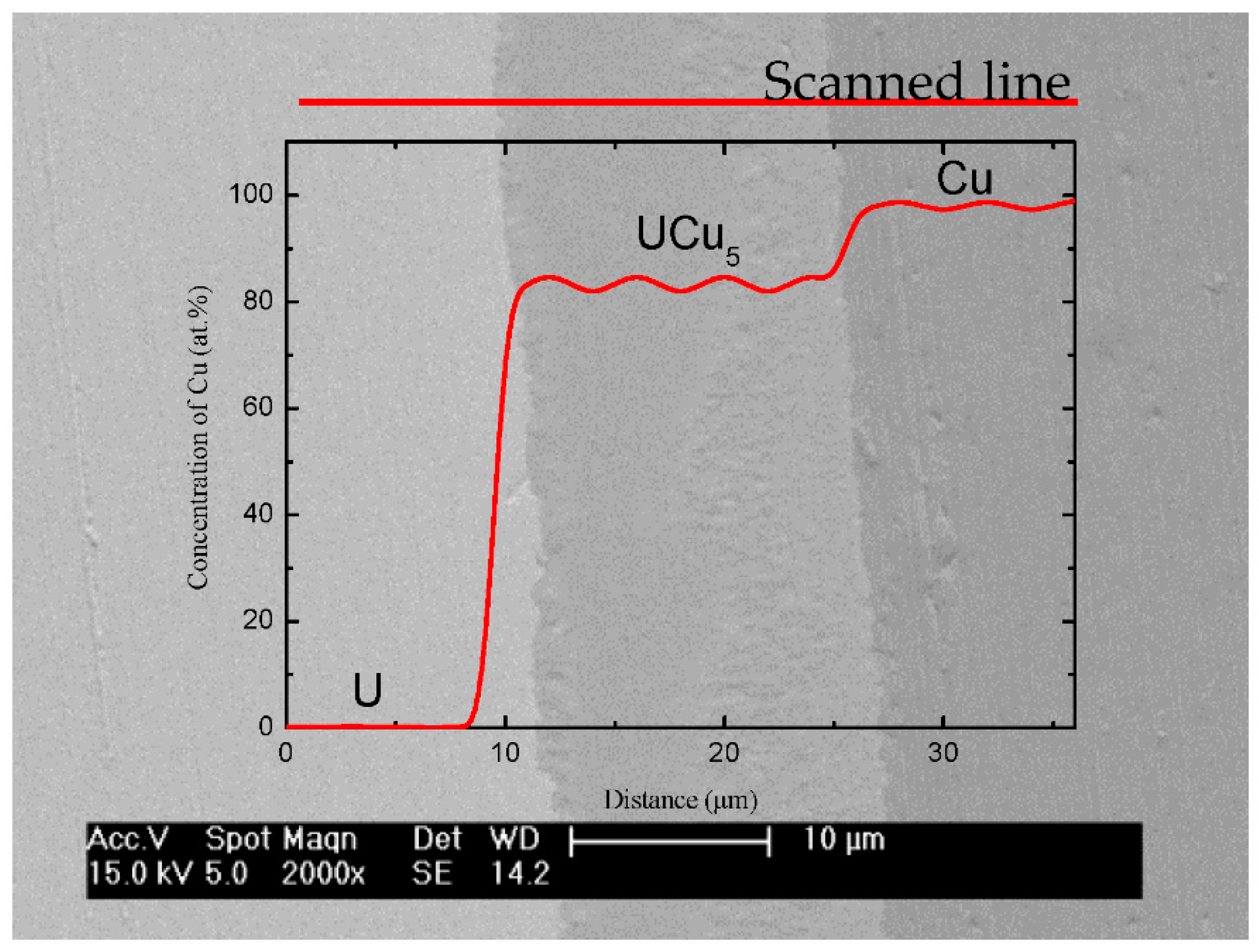
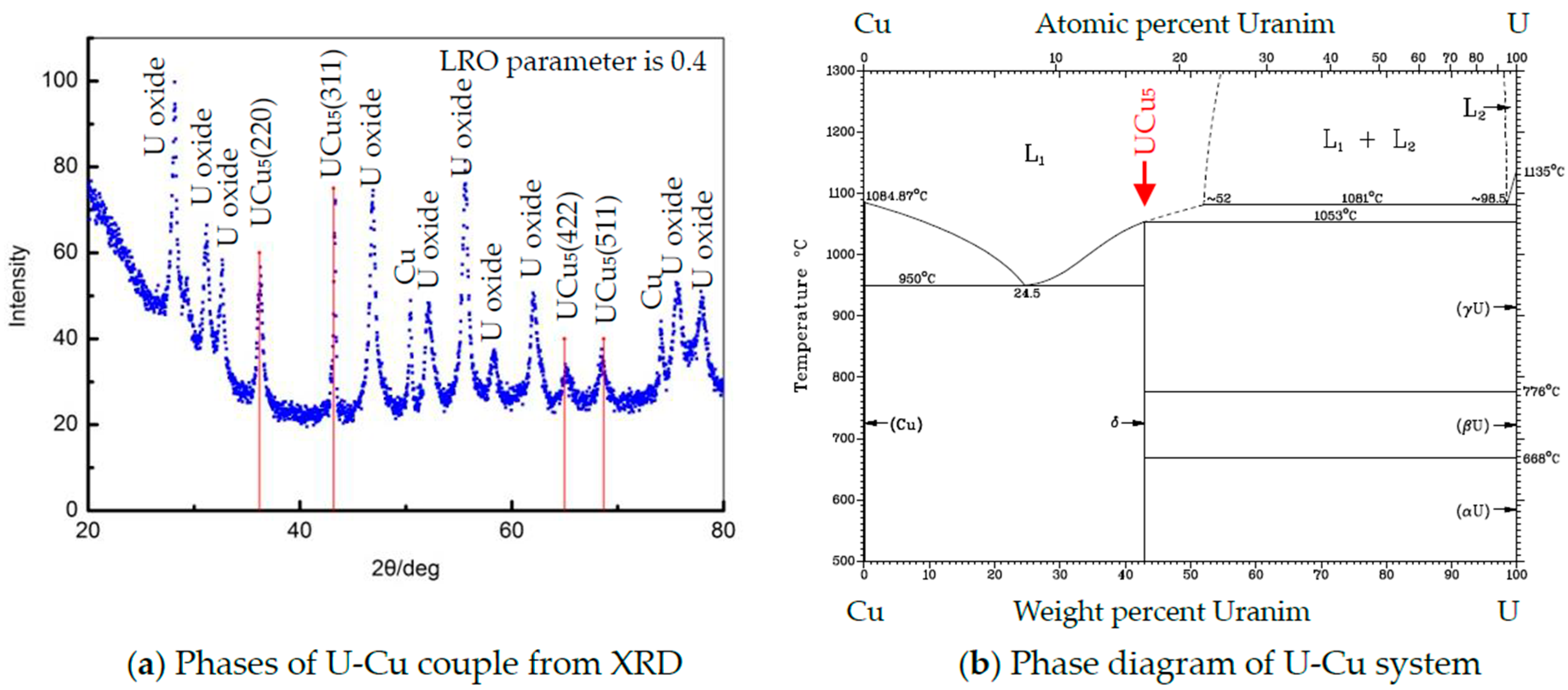
3.1. Structure and Morphology of U-Cu Intermetallic Compounds
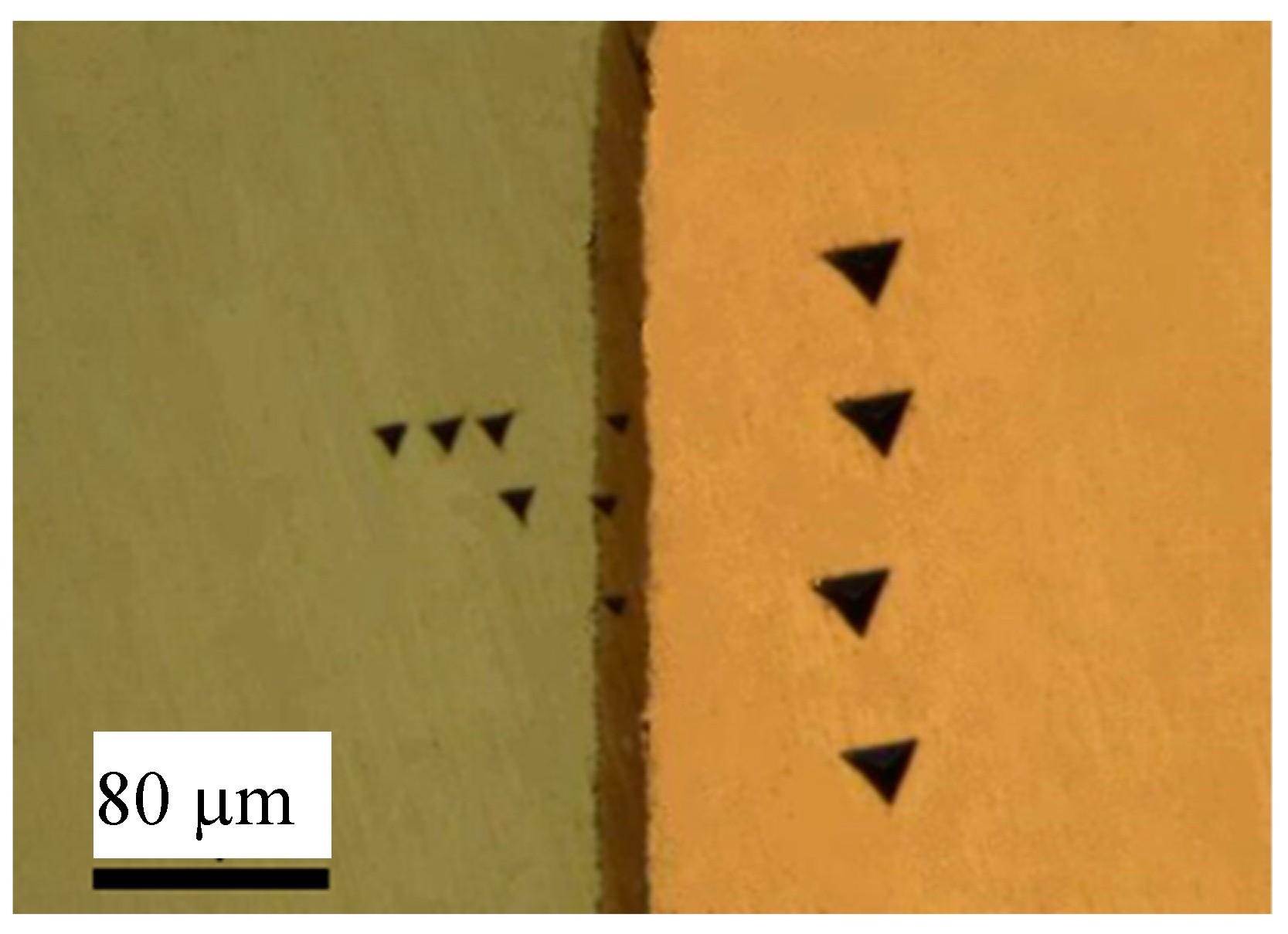
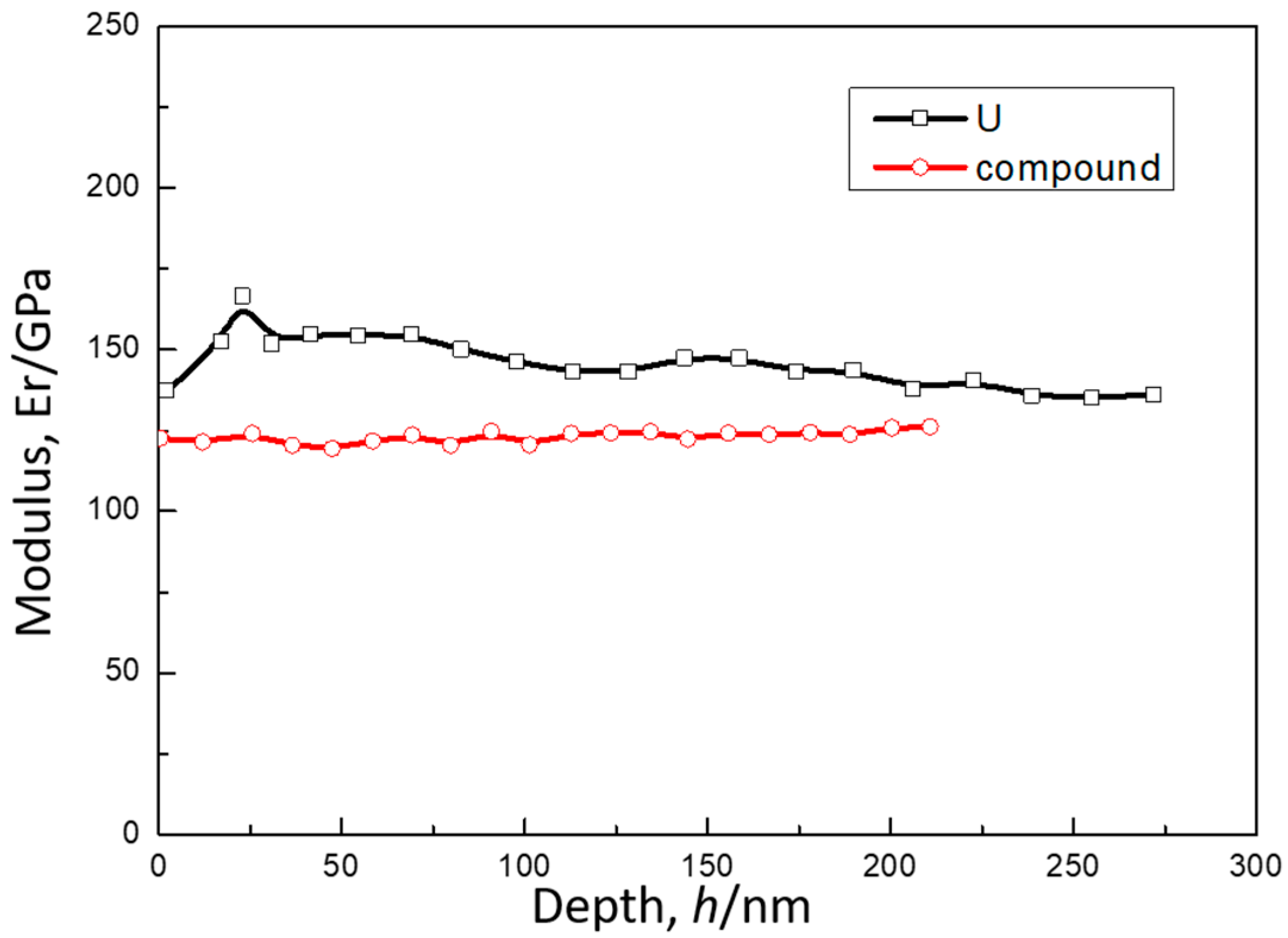
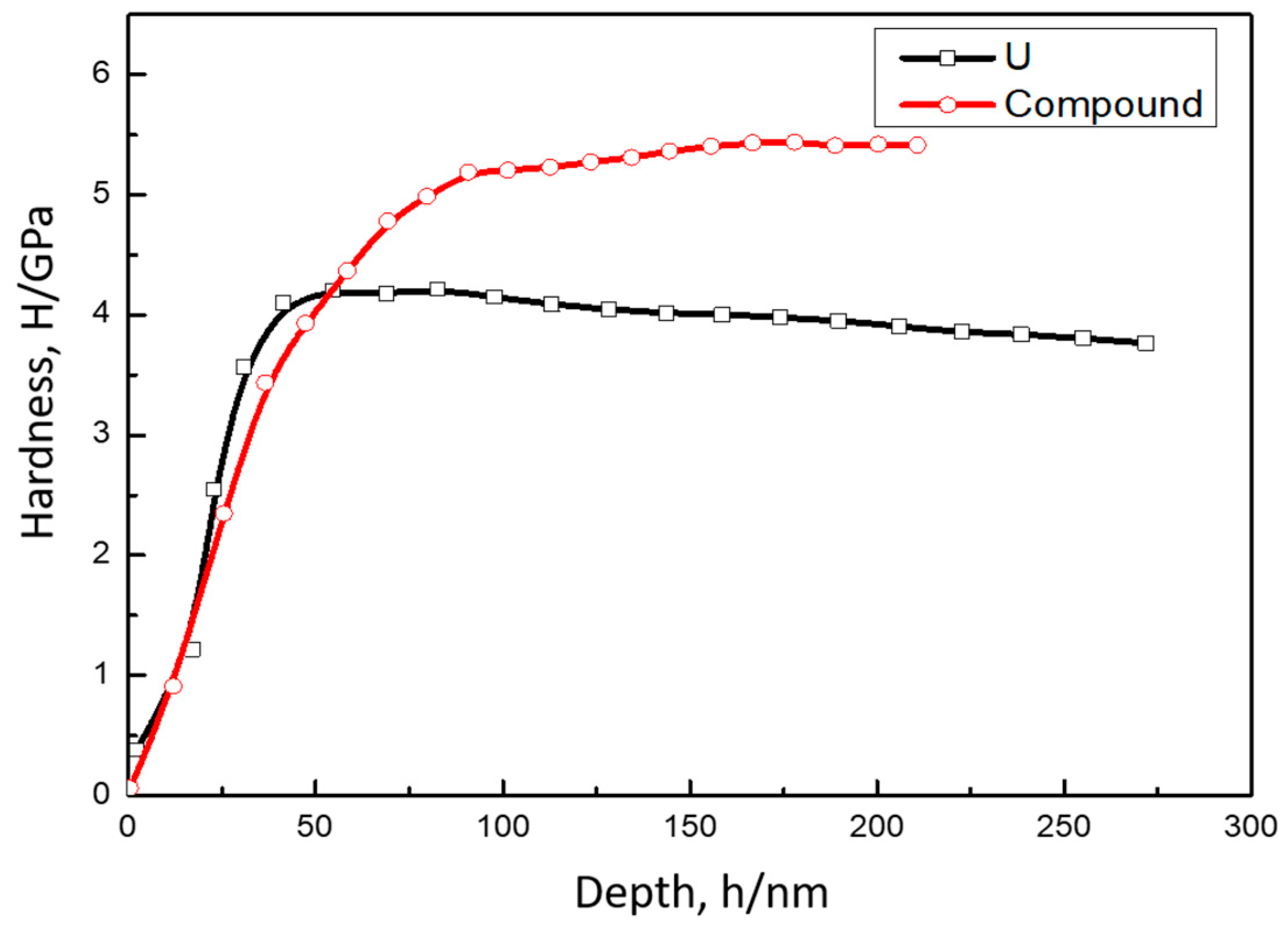
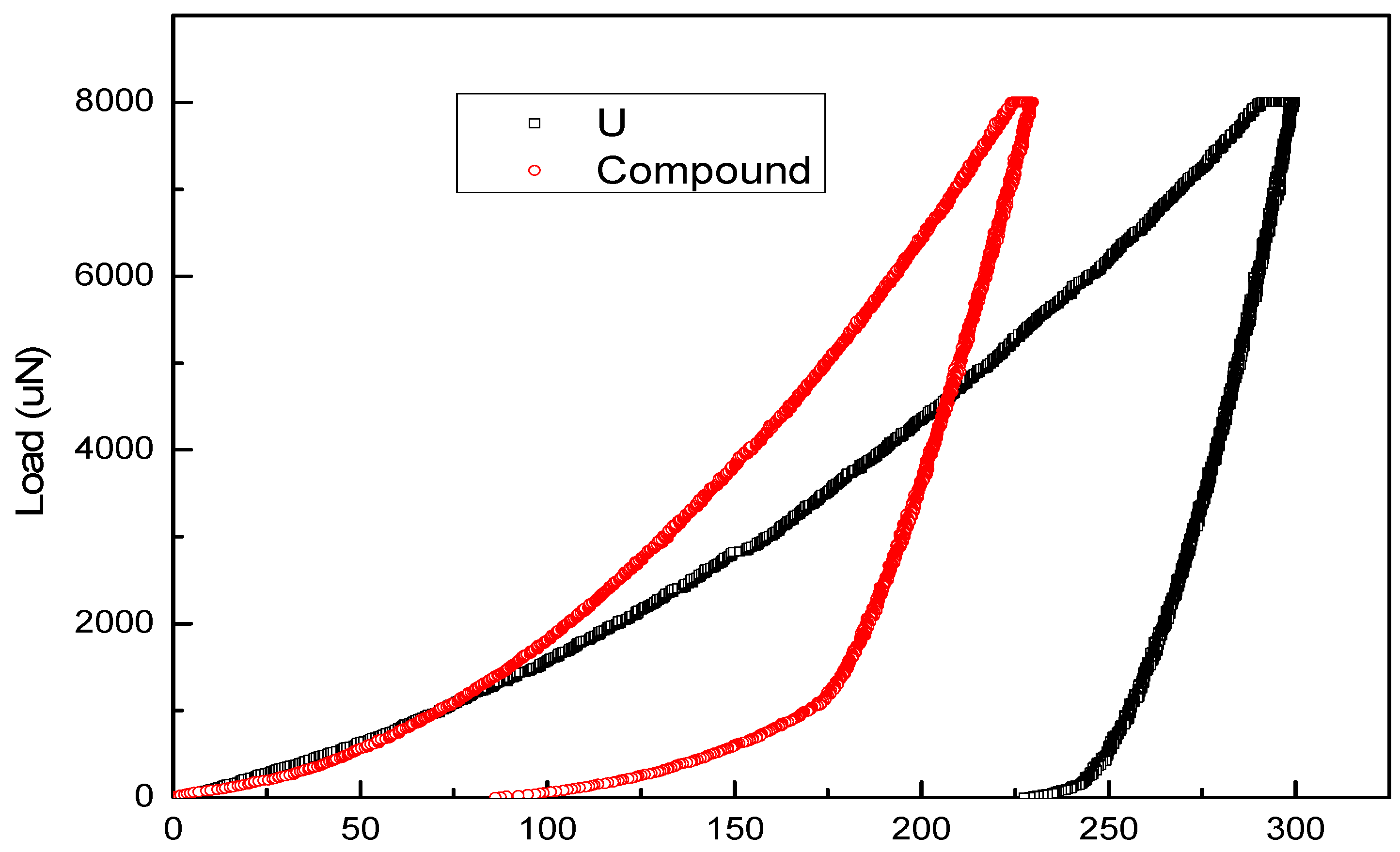
3.2. Mechanical Properties of U-Cu System at Interface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kniznik, L.; Alonso, P.R.; Gargano, P.H.; Rubiolo, G.H. Simulation of U-Al4 growth in an U-Al3/Al diffusion couple. J. Nucl. Mater. 2011, 414, 309–315. [Google Scholar] [CrossRef]
- Kniznik, L.; Alonso, P.R.; Gargano, P.H.; Rubiolo, G.H. First principles study of U-Al system ground state. Procedia Mater. Sci. 2012, 1, 514–519. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Kimmel, G.; Cotler, C.; Aizenshtein, M. Structure of U-Al4 prepared by solid state reaction. J. Alloys Compd. 2001, 329, 189–194. [Google Scholar] [CrossRef]
- Jun, H.; Kim, Y.; Lee, S. Anomalous multiple pop-in behavior in Cu-Sn-based intermetallic compounds during nanoindentation. Mater. Sci. Eng. A 2014, 612, 192–196. [Google Scholar] [CrossRef]
- Deng, X.; Chawla, N.; Chawla, K.K.; Koopman, M. Deformation behavior of (Cu, Ag)–Sn intermetallics by nanoindentation. Acta Mater. 2004, 52, 4291–4303. [Google Scholar] [CrossRef]
- Deng, X.; Koopman, M.; Chawla, N.; Chawla, K.K. Young’s modulus of (Cu, Ag)–Sn intermetallics measured by nanoindentation. Mater. Sci. Eng. A 2004, 364, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Lai, Y.; Jian, S.; Chen, J.; Chen, R. Nanoindentation identifications of mechanical properties of Cu6-Sn5, Cu3-Sn, and Ni3-Sn4 intermetallic compounds derived by diffusion couples. Mater. Sci. Eng. A 2008, 485, 305–310. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Dong, Z.; Huang, H.; Nishimura, T.; Nogita, K. Nanoindentation characterization of intermetallic compounds formed between Sn–Cu (–Ni) ball grid arrays and Cu substrates. Mater. Sci. Eng. B 2009, 164, 44–50. [Google Scholar] [CrossRef]
- Yamanaka, S.; Yamada, K.; Tsuzuki, T. Mechanical and thermal properties of uranium intermetallic compounds. J. Alloys Compd. 1998, 271–273, 549–556. [Google Scholar] [CrossRef]
- Nakamura, H.; Kitaoka, Y.; Asayama, K.; Shiga, M.; Nuki, Y. Nuclear magnetic relaxation in the ordered state of U-Cu5. Physica B 1996, 223–224, 53–55. [Google Scholar] [CrossRef]
- Degiorgi, L.; Ott, H.R.; Dressel, M. Optical probing of the antiferromagnetic phase transitions in the heavy-electron compounds U2-Zn17 and U-Cu5. Europhys. Lett. 1994, 26, 221–226. [Google Scholar] [CrossRef]
- Nakotte, H.; Buschow, K.H.J.; Brück, E.J.; Klaasse, C.P.; Proke, K.; De Boer, F.R.; Andreev, A.V.; Lacerda, A. Non-fermi-liquid scaling in U(Cu,Al)5 compounds. Physica B 1997, 230–232, 616–619. [Google Scholar] [CrossRef]
- Nakotte, H.; Buschow, K.H.J.; Klaasse, J.C.P.; Andreev, A.V.; Prokes, K. Possible heavy-fermion behaviour of new U(Cu, Al)5 compounds. J. Magn. Magn. Mater. 1995, 140–144, 1261–1262. [Google Scholar] [CrossRef]
- Baenziger, N.C.; Rundle, R.E.; Snow, A.I.; Wilson, A.S. Compounds of Uranium with the transition metals of the 1st long period. Acta Crystallogr. 1950, 3, 34–40. [Google Scholar] [CrossRef]
- Verbovytskyy, Y.; Goncalves, A.P. On the U-Cu-Al and U-Cu-Ga systems at 600 degrees. Intermetallics 2013, 33, 16–26. [Google Scholar] [CrossRef]
- Ott, H.R.; Rudigier, H.; Felder, E.; Fisk, Z.; Thompson, J.D. Formation and destruction of the heavy electron ground state in U compounds with AuBe5 crystal structure. Phys. Rev. B 1987, 35, 1452–1455. [Google Scholar] [CrossRef]
- Newell, R.; Park, Y.; Mehta, A.; Keiser, D., Jr.; Sohn, Y. Mechanical properties examined by nanoindentation for selected phases relevant to the development of monolithic uranium-molybdenum metallic fuels. J. Nucl. Mater. 2017, 487, 443–452. [Google Scholar] [CrossRef]
- Beeler, B.; Deo, C.; Baskes, M.; Okuniewski, M. First principles calculations of the structure and elastic constants of a, β, and γ uranium. J. Nucl. Mater. 2013, 433, 143–152. [Google Scholar] [CrossRef]
- Wheeler, D.W.; Morris, S.T. Micro-mechanical characterisation of uranium. J. Nucl. Mater. 2009, 385, 122–125. [Google Scholar] [CrossRef]
| UCu5 | |
|---|---|
| Space group | F-43m |
| Lattice parameters | a = b = c = 7.107 Å, (7.033–7.038 Å in reference [14]) α = β = γ = 90° |
| Atomic positions(Wyckoff Positions) | U: 4a Cu1: 4c Cu2: 16e |
| Density | 10.28 g/cm3 |
| Formula weight | 555.8 g/mol |
| Units per cell | 4 |
| Materials | Reduced Modulus/GPa | Young’s Modulus/GPa | Hardness/GPa | Poisson Ratio |
|---|---|---|---|---|
| U | 146 ± 5 | 160 ± 5 | 4.0 | 0.24 |
| U-Cu compound | 123 ± 8 | 121 ± 8 | 5.2 | 0.3 |
| Cu | 78 ± 10 | 88 ± 10 | 1.6 | 0.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Mo, C.; Liao, Y. Mechanical Properties of U-Cu Intermetallic Compound Measured by Nanoindentation. Materials 2018, 11, 2215. https://doi.org/10.3390/ma11112215
Li R, Mo C, Liao Y. Mechanical Properties of U-Cu Intermetallic Compound Measured by Nanoindentation. Materials. 2018; 11(11):2215. https://doi.org/10.3390/ma11112215
Chicago/Turabian StyleLi, Ruiwen, Chuan Mo, and Yichuan Liao. 2018. "Mechanical Properties of U-Cu Intermetallic Compound Measured by Nanoindentation" Materials 11, no. 11: 2215. https://doi.org/10.3390/ma11112215




