Effect of Ratio in Ammonium Nitrate on the Structural, Microstructural, Magnetic, and AC Conductivity Properties of BaFe12O19
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of BaFe12O19 via the Salt Melt Method
2.2. Characterizations of BaFe12O19
3. Results and Discussion
3.1. Structural Analysis
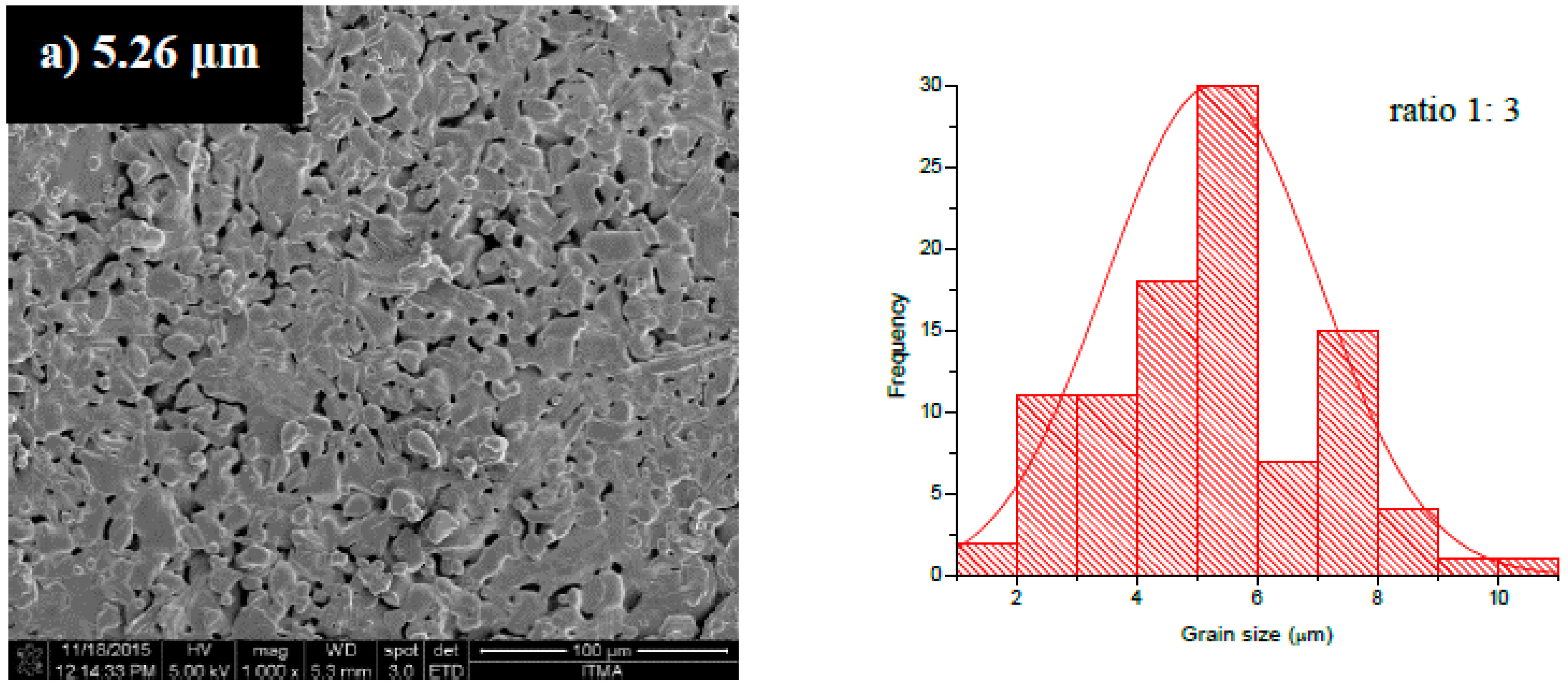
3.2. Microstructural Analysis
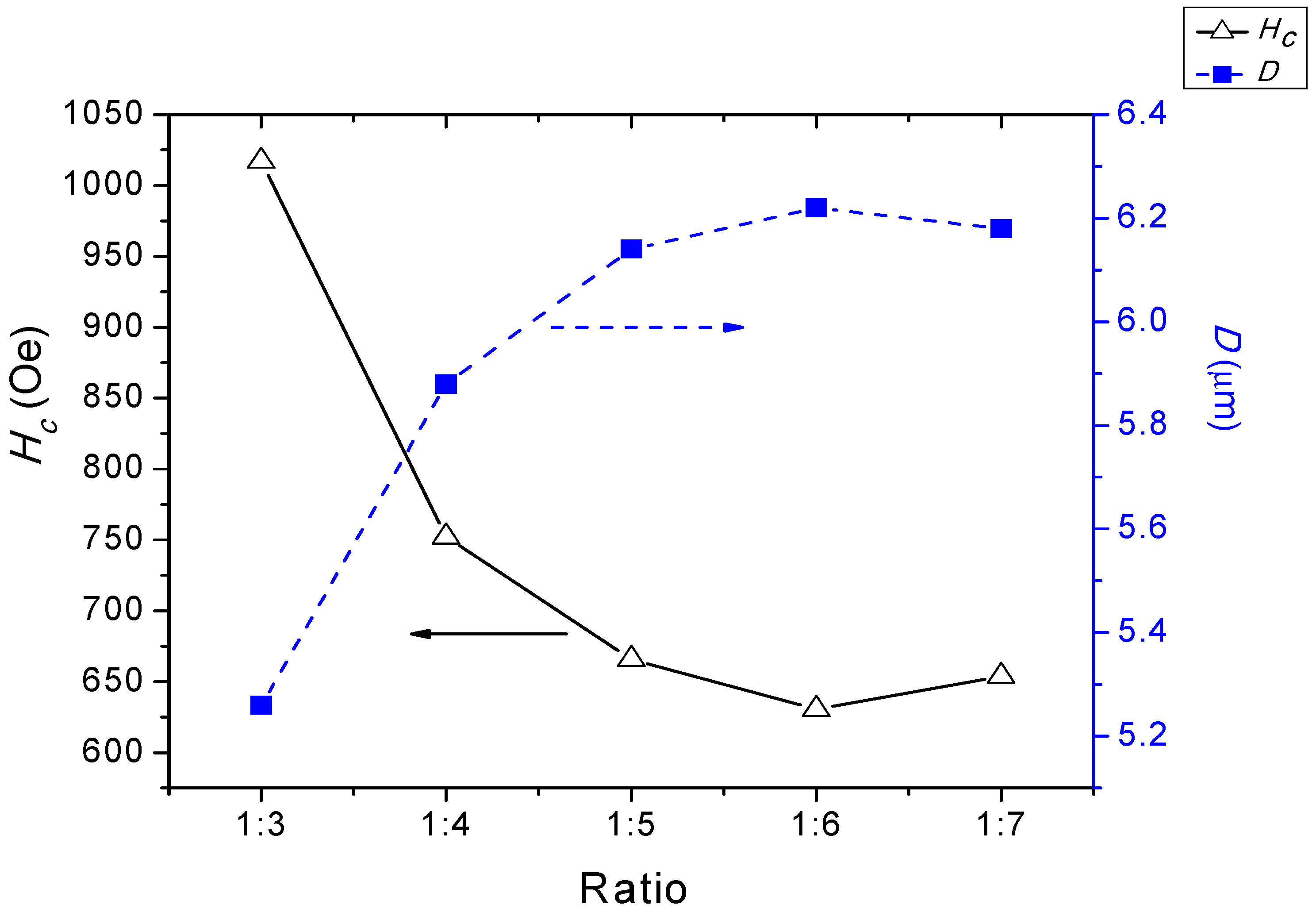
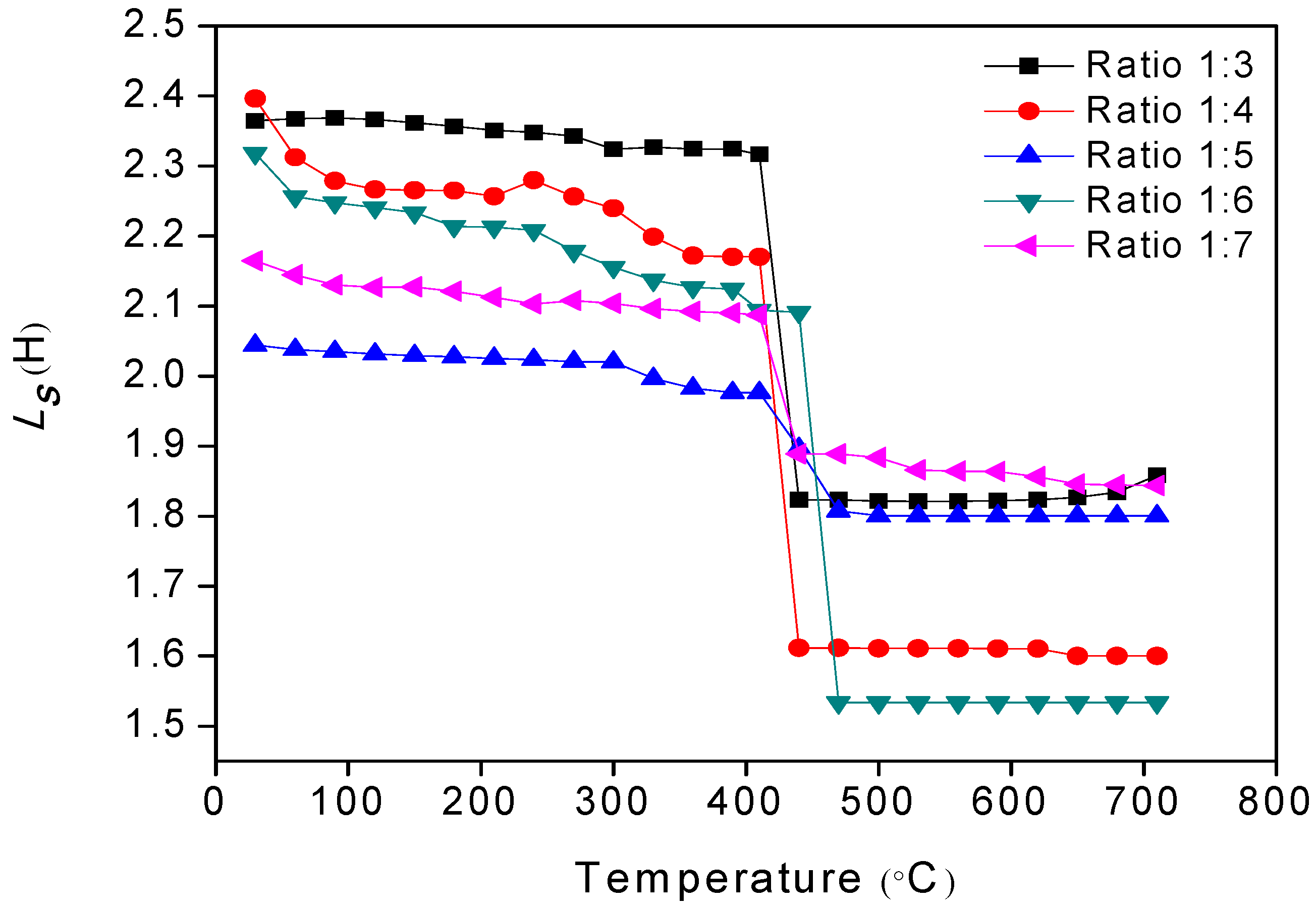
3.3. Magnetic Hysteresis
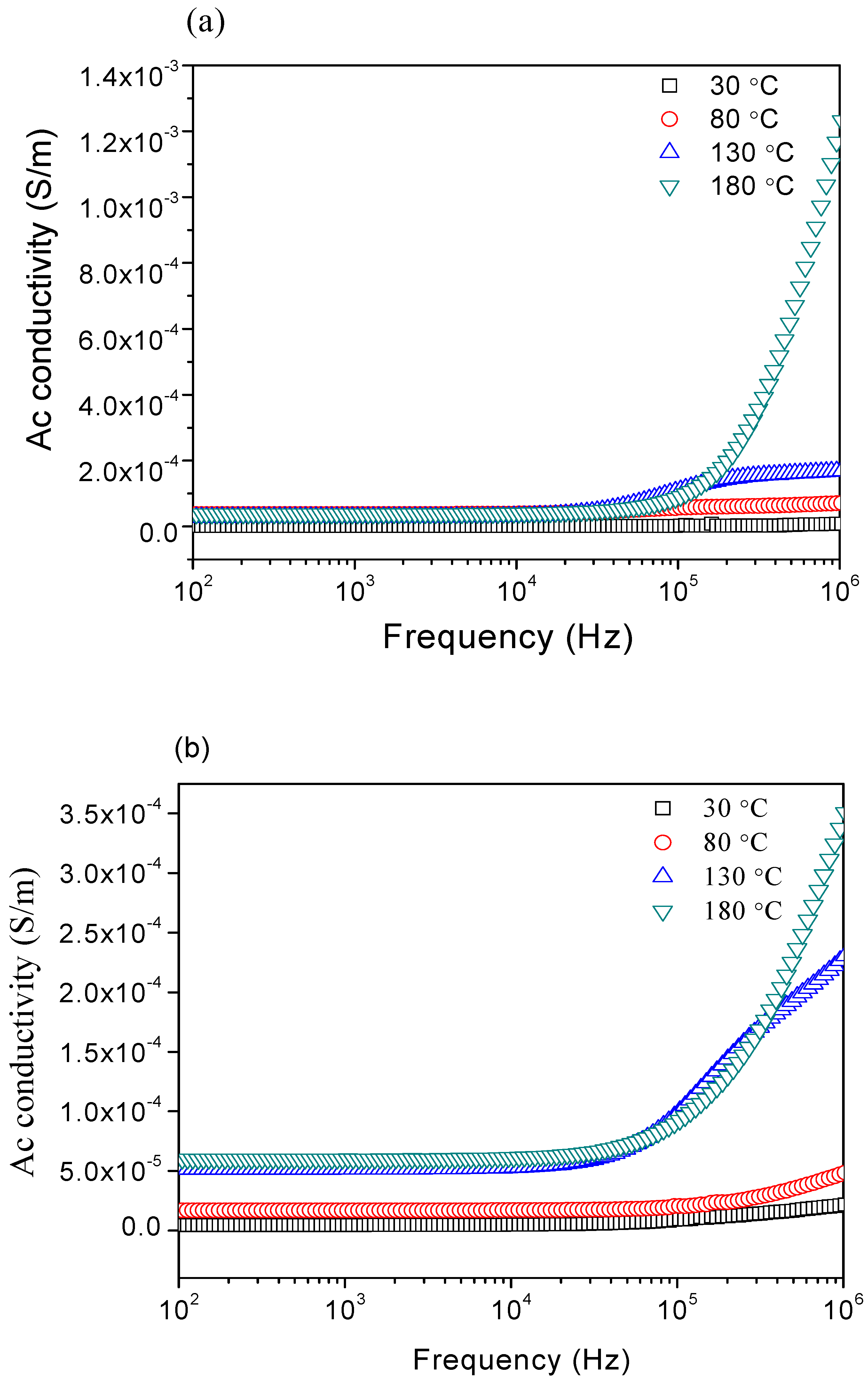
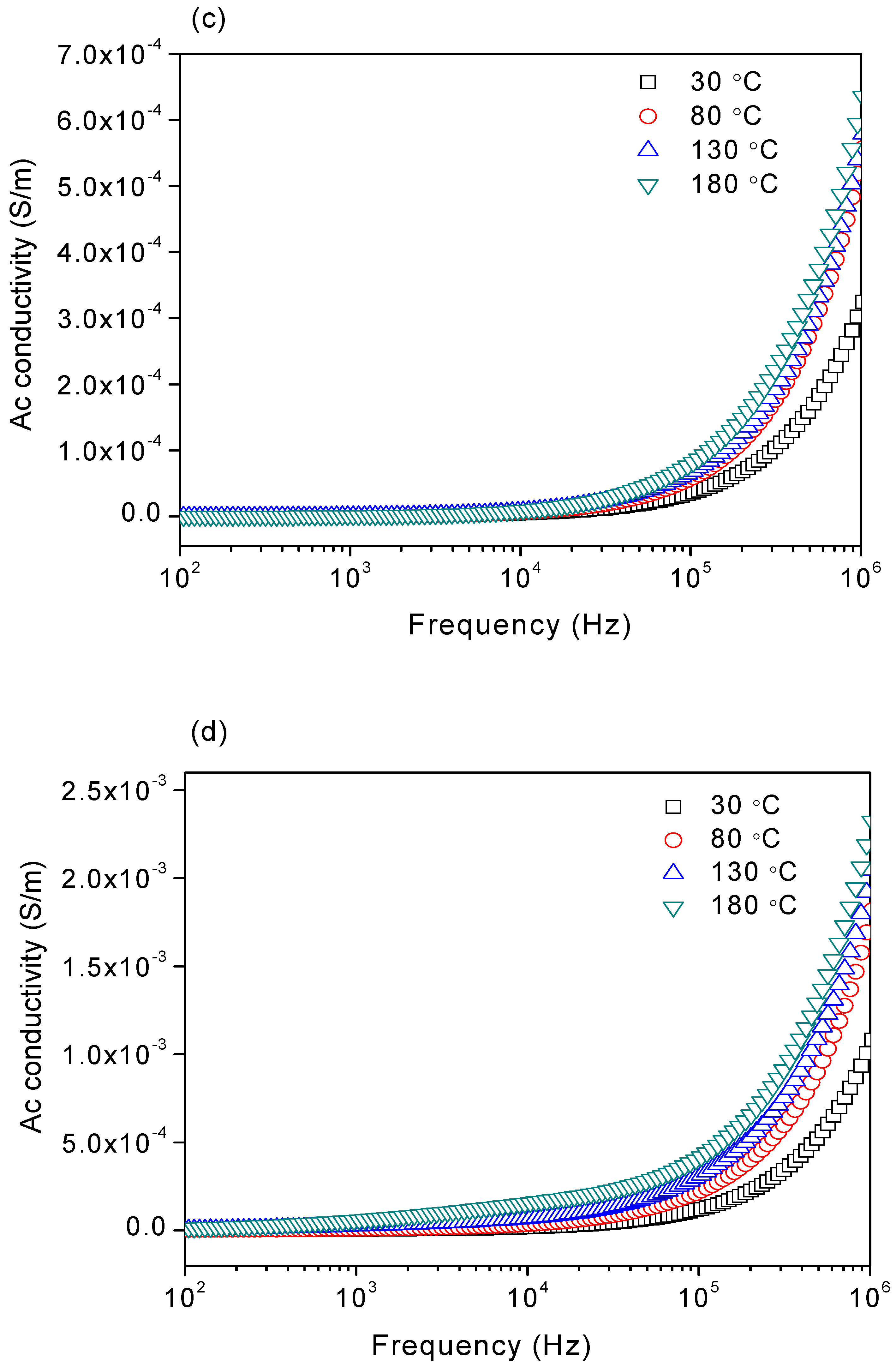
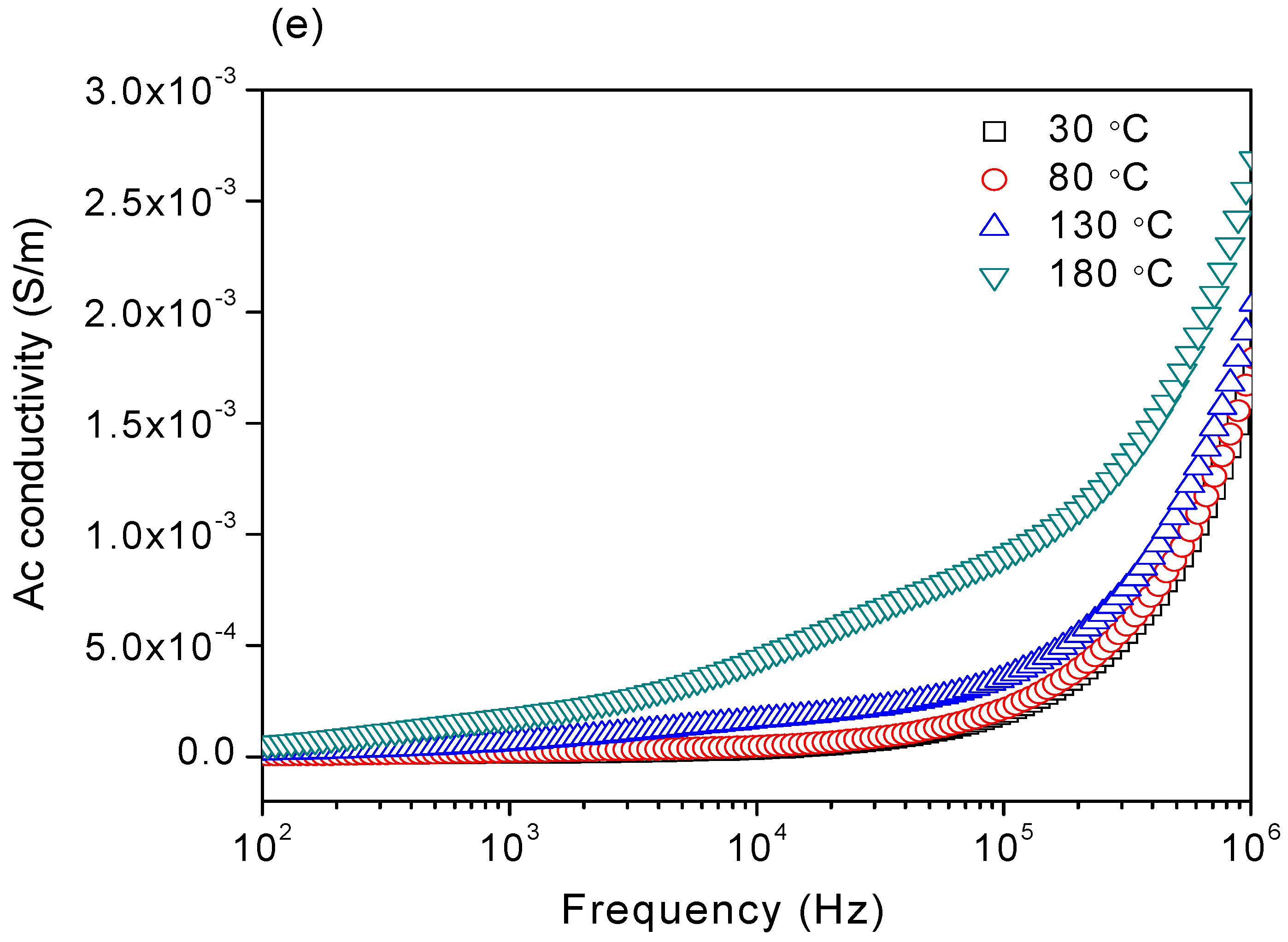
3.4. AC Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Trukhanov, S.V.; Balagurov, A.M.; Kostishyn, V.G.; Trukhanov, A.V.; Panina, L.V.; Trukhanova, E.L. Features of crystal structure and dual ferroic properties of BaFe12−xMexO19 (Me = In3+ and Ga3+; x = 0.1–1.2). J. Magn. Magn. Mater. 2018, 464, 139–147. [Google Scholar]
- Summergard, R.N.; Banks, E. New hexagonal ferrimagnetic oxides. J. Phys. Chem. Solids 1957, 2, 312–317. [Google Scholar] [CrossRef]
- Meng, Y.Y.; He, M.H.; Zeng, Q.; Jiao, D.L.; Shukla, S.; Ramanujan, R.V.; Liu, Z.W. Synthesis of barium ferrite ultrafine powders by a sol–gel combustion method using glycine gels. J. Alloys Compd. 2014, 583, 220–225. [Google Scholar] [CrossRef]
- Campbell, P. Permanent Magnet Materials and Their Application; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Martirosyan, K.S.; Galstyan, E.; Hossain, S.M.; Wang, Y.J.; Litvinov, D. Barium hexaferrite nanoparticles: Synthesis and magnetic properties. Mater. Sci. Eng. B 2011, 176, 8–13. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Trukhanov, A.V.; Turchenko, V.A.; Tishkevich, D.I.; Trukhanova, E.L.; Yakovenko, O.S.; Matzui, L.Y. Investigation into the structural features and microwave absorption of doped barium hexaferrites. Dalton Trans. 2017, 46, 9010–9021. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Chen, N.; Pan, X.; Shen, H.; Gu, M. Preparation and microwave absorption properties of barium ferrite nanorods. Mater. Lett. 2008, 62, 840–842. [Google Scholar] [CrossRef]
- Syazwan, M.M.; Azis, R.S.; Hashim, M.; Ismayadi, I.; Kanagesan, S.; Hapishah, A.N. Co–Ti- and Mn–Ti-substituted barium ferrite for electromagnetic property tuning and enhanced microwave absorption synthesized via mechanical alloying. J. Aus. Ceram. Soc. 2017, 53, 465–474. [Google Scholar] [CrossRef]
- Rafiq, M.A.; Waqar, M.; Mirza, T.A.; Farooq, A.; Zulfiqar, A. Effect of Ni2+ Substitution on the Structural, Magnetic, and Dielectric Properties of Barium Hexagonal Ferrites (BaFe12O19). J. Electron. Mater. 2017, 46, 241–246. [Google Scholar] [CrossRef]
- Kubo, O.; Ido, T.; Yokoyama, H. Properties of Ba Ferrite Particles for Perpendicular Magnetic Recording Media. IEEE Trans. Magn. 1982, 18, 1122–1124. [Google Scholar] [CrossRef]
- Karmakar, M.; Mondal, B.; Pal, M.; Mukherjee, K. Acetone and ethanol sensing of barium hexaferrite particles: A case study considering the possibilities of non-conventional hexaferrite sensor. Sens. Actuators B Chem. 2014, 190, 627–633. [Google Scholar] [CrossRef]
- Iulian, P.; Florin, T. Influence of partial substitution of Fe3+ with W3+ on the microstructure, humidity sensitivity, magnetic and electrical properties of barium hexaferrite. Superlattices Microstruct. 2014, 70, 46–53. [Google Scholar]
- Florin, T.; Iulian, P.; Paul, D.P.; Sorin, T. Influence of thermal treatment on the structure, humidity sensitivity, electrical and magnetic properties of barium–tungsten ferrite. Compos. Part B 2013, 51, 106–111. [Google Scholar]
- Tudorache, F.; Brinza, F.; Popa, P.D.; Petrila, I.; Grigoras, M.; Tascu, S. Comparison between Powders of Strontium Hexaferrite Processed by Dynamic Gas Heat Treatment and Re-Calcination. Acta Phys. Pol. A 2012, 121, 92–94. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Prakash, A.; Mayo, J.T.; Colvin, V.L. Magnetic separations: From steel plants to biotechnology. Chem. Eng. Sci. 2009, 64, 2510–2521. [Google Scholar] [CrossRef]
- Saberifar, S.; Jafari, F.; Kardi, H.; Jafarzadeh, M.A.; Mousavi, S.A. Recycling Evaluation of Mill Scale in Electric Arc Furnace. J. Adv. Mater. Process. 2014, 2, 73–78. [Google Scholar]
- El-Hussiny, N.A.; Mohamed, F.M.; Shalabi, M.E.H. Recycling of mill scale in sintering process. Sci. Sinter. 2011, 43, 21–31. [Google Scholar] [CrossRef]
- Azis, R.A.; Hashim, M.; Saiden, N.M.; Daud, N.; Shahrani, N.M. Study the Iron Environments of the Steel Waste Product and its Possible Potential Applications in Ferrites. Adv. Mater. Res. 2015, 1109, 295–299. [Google Scholar] [CrossRef]
- Daud, N.; Azis, R.A.; Hashim, M.; Matori, K.A.; Jumiah, H.; Saiden, N.M.; Shahrani, M.; Mariana, N. Preparation and Characterization of Sr1−xNdxFe12O19 Derived from Steel-Waste Product via Mechanical Alloying. Mater. Sci. Forum 2016, 846, 403–409. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Trukhanov, S.V.; Panina, L.V.; Kostishyn, V.G.; Panina, L.V.; Salem, M.M.; Kazakevich, I.S.; Turchenko, V.A.; Kochervinskii, V.V.; Krivchenya, D.A. Multiferroic properties and structural features of M-type Al-substituted barium hexaferrites. Phys. Solid State 2017, 59, 737–745. [Google Scholar] [CrossRef]
- Dong, L.; Han, Z.; Zhang, Y.; Wu, Z.; Zhang, X. Synthesis of hexagonal barium ferrite nanoparticle by sol-gel method. Rare Met. 2006, 25, 605–608. [Google Scholar] [CrossRef]
- Ataie, A.; Piramoon, M.R.; Harris, I.R.; Ponton, C.B. Effect of hydrothermal synthesis environment on the particle morphology, chemistry and magnetic properties of barium hexaferrite. J. Mater. Sci. 1995, 30, 5600–5606. [Google Scholar] [CrossRef]
- Huang, L.Z.; Hashim, M.; Ismail, I.; Kanagesan, S.; Shafie, M.S.; Idris, F.M.; Ibrahim, I.R. Development of Magnetic B-H Hysteresis Loops Through Stages of Microstructure Evolution of Bulk BaFe12O19. J. Supercond. Nov. Magn. 2015, 28, 3075–3086. [Google Scholar] [CrossRef]
- Azis, R.A.; Hashim, M.; Zakaria, A.; Hassan, J.; Daud, N.; Shahrani, N.M.; Siang, P.C. Magnetic properties and microstructures of cobalt substituted barium hexaferrites derived from steel waste product via mechanical alloying technique. Mater. Sci. Forum 2016, 846, 388–394. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishyn, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Structure and magnetic properties of BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J. Alloy Compd. 2016, 689, 383–393. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Trukhanov, S.V.; Panina, L.V.; Kostishyn, V.G.; Kazakevich, I.S.; Trukhanov, A.V.; Trukhanova, E.L.; Natarov, V.O.; Turchenko, V.A.; Salem, M.M.; et al. Evolution of structure and magnetic properties for BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J. Magn. Magn. Mater. 2016, 426, 487–496. [Google Scholar] [CrossRef]
- Ibrahim, I.R.; Hashim, M.; Nazlan, R.; Ismail, I.; Ab Rahman, W.N.; Abdullah, N.H.; Idris, F.M.; Shafie, M.S.; Zulkimi, M.M. Grouping trends of magnetic permeability components in their parallel evolution with microstructure in Ni0.3Zn0.7Fe2O4. J. Magn. Magn. Mater. 2014, 355, 265–275. [Google Scholar] [CrossRef]
- Kimura, T. Molten Salt Synthesis of Ceramic Powders. In Advances in Ceramics—Synthesis and Characterization, Processing and Specific Applications; INTECH Open Access Publisher: New York, NY, USA, 2003; pp. 75–100. [Google Scholar]
- Elwell, D.; Scheel, H.J. Crystal Growth from High-Temperature Solutions; Academic Press: London, UK, 1975. [Google Scholar]
- Nitta, K.; Inazawa, S.; Sakai, S.; Fukunaga, A.; Itani, E.; Numata, K.; Hagiwara, R.; Nohira, T. Development of molten salt electrolyte battery. SEI Tech. Rev. 2013, 76, 33–39. [Google Scholar]
- Inman, D.; White, S.H. The production of refractory metals by the electrolysis of molten salts; design factors and limitations. J. Appl. Electrochem. 1978, 8, 375–390. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Direct electrochemical reduction of titanium in molten calcium chloride. Nature 2000, 407, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Nohira, T.; Yasuda, K.; Ito, Y. Pinpoint and bulk electrochemical reduction of insulating silicon dioxide to silicon. Nat. Mater. 2003, 2, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Topal, U. Ammonium nitrate melt technique for the synthesis of oxide compounds. J. Phys. Conf. Ser. 2009, 153, 1–5. [Google Scholar] [CrossRef]
- Akdogan, E.K.; Brennan, R.E.; Allahverdi, M.; Safari, A. Effects of Molten Salt Synthesis (MSS) Parameters on the Morphology of Sr3Ti2O7 and SrTiO3 Seed Crystals. J. Electroceram. 2006, 16, 159–165. [Google Scholar] [CrossRef]
- Che Muda, N.N.; Azis, R.S.; Hashim, M.; Hassan, J.; Sulaiman, S.; Daud, N.; Mohd Sharani, N.M.; Azizan, A.H.; Musa, M.A. Synthesis and Characterization of Barium Hexaferrites Derived from Steel Waste by Ammonium Nitrate Salt Melt Synthesis. J. Solid State Sci. Technol. Lett. 2015, 16, 73–77. [Google Scholar]
- Azizan, A.H.; Azis, R.S.; Hassan, J.; Hashim, M.; Mohd Shahrani, N.M.; Daud, N.; Sulaiman, S.; Che Muda, N.N.; Musa, M.A. Structural and Magnetic Properties of Aluminum Substituted Yttrium Iron Garnet Via Sol-Gel Synthesis. J. Solid State Sci. Technol. Lett. 2015, 16, 58–61. [Google Scholar]
- Smit, J.; Wijn, H.P.J. Ferrites; John Wiley: New York, NY, USA, 1959. [Google Scholar]
- Jonscher, A.K. The “universal” dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Jonscher, A.K. A new understanding of the dielectric relaxation of solids. J. Mater. Sci. 1981, 16, 2037–2060. [Google Scholar] [CrossRef]
- Topal, U.; Husnu, O.; Lev, D. Finding Optimal Fe/Ba Ratio to Obtain Single Phase BaFe12O19 Prepared by Ammonium Nitrate Melt Technique. J. Alloys Compd. 2007, 428, 17–21. [Google Scholar] [CrossRef]
- Shafie, M.S.E.; Hashim, M.; Ismail, I.; Kanagesan, S.; Fadzidah, M.I.; Idza, I.R.; Hajalilou, A.; Sabbaghizadeh, R. Magnetic M-H Loops Family Characteristics in the Microstructure Evolution of BaFe12O19. J. Mater. Sci. Mater. Electron. 2014, 25, 3787–3794. [Google Scholar] [CrossRef]
- Sort, J.; Nogués, J.; Suriñach, S.; Muñoz Domínguez, J.S.; Baró, M.D. Coercivity Enhancement in Ball-Milled and Heat-Treated Sr-Ferrite with Iron Sulphide. J. Metastable Nanocryst. Mater. 2003, 6, 599–606. [Google Scholar] [CrossRef]
- Dursun, S.; Ramazan, T.; Numan, A.; Sedat, A. Comparison of the Structural and Magnetic Properties of Submicron Barium Hexaferrite Powders Prepared by Molten Salt and Solid State Calcination Routes. Ceram. Int. 2012, 38, 3801–3806. [Google Scholar] [CrossRef]
- Li, W.; Qiao, X.; Li, M.; Liu, T.; Peng, H.X. La and Co substituted M-type barium ferrites processed by sol–gel combustion synthesis. Mater. Res. Bull. 2013, 48, 4449–4453. [Google Scholar] [CrossRef]
- Singh, V.P.; Kumar, G.; Dhiman, P.; Kotnala, R.K.; Shah, J.; Batoo, K.M.; Singh, M. Structural, Dielectric and Magnetic Properties of Nanocrystalline BaFe12O19 Hexaferrite Processed via Sol-gel Technique. Adv. Mater. Lett. 2014, 5, 447–452. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Rao, P.K.; Syed, I.A. Sintering temperature effect on density, structural and morphological properties of Mg- and Sr-doped ceria. J. Taibah Univ. Sci. 2016, 10, 381–385. [Google Scholar] [CrossRef]
- Tchouank, T.C.T.; Jyoti, S.; Mohammed, J.; Sachin, K.; Srivastava, A.K. Effect of temperature on the magnetic properties of nano-sized M-type barium hexagonal ferrites. AIP Conf. Proc. 2017, 1860, 02008. [Google Scholar]
- Huang, J.; Hanrui, Z.; Wenlan, L. Synthesis and Characterization of Nano Crystalline BaFe12O19 Powders by Low Temperature Combustion. Mater. Res. Bull. 2003, 38, 149–159. [Google Scholar] [CrossRef]
- Vinaykumar, R.; Mazumder, R.; Bera, J. Characterization of SrCo1.5Ti1.5Fe9O19 Hexagonal Ferrite Synthesized by Sol-Gel Combustion and Solid State Route. J. Magn. Magn. Mater. 2017, 429, 359–366. [Google Scholar] [CrossRef]
- Mali, A.; Ataie, A. Structural characterization of nano-crystalline BaFe12O19 powders synthesized by sol–gel combustion route. Scr. Mater. 2005, 53, 1065–1070. [Google Scholar] [CrossRef]
- Mandizadeh, S.; Soofivand, F.; Salavati-Niasari, M.; Bagheri, S. Auto-Combustion Preparation and Characterization of BaFe12O19 Nanostructures by Using Maleic Acid as Fuel. J. Ind. Eng. Chem. 2015, 26, 167–172. [Google Scholar] [CrossRef]
- Baykal, A.; Auwal, I.A.; Güner, S.; Sözeri, H. Magnetic and Optical Properties of Zn2+ Ion Substituted Barium Hexaferrites. J. Magn. Magn. Mater. 2017, 430, 29–35. [Google Scholar] [CrossRef]
- Brightlin, B.C.; Balamurugan, S. The effect of post annealing treatment on the citrate sol–gel derived nanocrystalline BaFe12O19 powder: Structural, morphological, optical and magnetic properties. Appl. Nanosci. 2016, 6, 1199–1210. [Google Scholar] [CrossRef]
- Jiang, J.; Ai, L.H. Facile synthesis, characterization and properties of Ba-hexaferrite/ZnO hybrid structures. Phys. B 2010, 405, 2640–2642. [Google Scholar] [CrossRef]
- Sathyamoorthy, R.; Chandramohan, S.; Sudhagar, P.; Kanjilal, D.; Kabiraj, D.; Asokan, K. Structural and photoluminescence properties of swift heavy ion irradiated CdS thin films. Sol. Energy Mater. Sol. Cells 2006, 90, 2297–2304. [Google Scholar] [CrossRef]
- Mishra, S.K.; Pathak, L.C.; Rao, V. Synthesis of submicron Ba-hexaferrite powder by a self-propagating chemical decomposition process. Mater. Lett. 1997, 32, 137–141. [Google Scholar] [CrossRef]
- Rodziah, N.; Hashim, M.; Idza, I.R.; Ismayadi, I.; Hapishah, A.N.; Khamirul, M.A. Dependence of developing magnetic hysteresis characteristics on stages of evolving microstructure in polycrystalline yttrium iron garnet. Appl. Surf. Sci. 2012, 258, 2679–2685. [Google Scholar] [CrossRef]
- Zhi, H.L.; Soo, K.C.; Ismayadi, I.; Kim, S.T.; Liew, J.Y.C. Structural transformations of mechanically induced top-down approach BaFe12O19 nanoparticles synthesized from high crystallinity bulk materials. J. Magn. Magn. Mater. 2017, 429, 192–202. [Google Scholar]
- Tomohisa, Y.; Yasunori, T.; Takao, S.; Hirotaro, M.; Tsukasa, C.; Shunsaku, K.; Yuji, W. Barium ferrite powders prepared by microwave-induced hydrothermal reaction and magnetic property. J. Magn. Magn. Mater. 2009, 321, 8–11. [Google Scholar]
- Shafqat, M.B.; Arif, O.; Atiq, S.; Saleem, M.; Ramay, S.M.; Mahmood, A.; Naseem, S. Influence of sintering temperature on structural, morphological and magnetic properties of barium hexaferrite nanoparticles. Mod. Phys. Lett. B 2016, 30, 1650254. [Google Scholar] [CrossRef]
- Dho, J.; Lee, E.K.; Park, J.Y.; Hur, N.H. Effects of the grain boundary on the coercivity of barium ferrite BaFe12O19. J. Magn. Magn. Mater. 2005, 285, 164–168. [Google Scholar] [CrossRef]
- Durmus, Z.; Sozeri, H.; Toprak, M.S.; Baykal, A. The Effect of Condensation on the Morphology and Magnetic Properties of Modified Barium Hexaferrite (BaFe12O19). Nano-Micro Lett. 2011, 3, 108–114. [Google Scholar] [CrossRef]
- Kanagesan, S.; Jesurani, S.; Sivakumar, M.; Thirupathi, C.; Kalaivani, T. Effect of Microwave Calcinations on Barium Hexaferrite Synthesized via Sol-Gel Combustion. J. Sci. Res. 2011, 3, 451–456. [Google Scholar] [CrossRef]
- Mallick, K.K.; Philip, S.; Roger, J.G. Magnetic Properties of Cobalt Substituted M-Type Barium Hexaferrite Prepared by Co-Precipitation. J. Magn. Magn. Mater. 2007, 312, 418–429. [Google Scholar] [CrossRef]
- Gilleo, M.A. Superexchange Interaction Energy for Fe3+-O2−-Fe3+ Linkages. Phys. Rev. 1958, 109, 777–781. [Google Scholar] [CrossRef]
- Dimri, M.C.; Subhash, C.K.; Dube, D.C. Electrical and Magnetic Properties of Barium Hexaferrite Nanoparticles Prepared by Citrate Precursor Method. Ceram. Int. 2004, 30, 1623–1626. [Google Scholar] [CrossRef]
- Goldman, A. Modern Ferrite Technology; Springer Publication: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Mazen, S.A.; Abu-Elsaad, N.I. Structural, magnetic and electrical properties of the lithium ferrite obtained by ball milling and heat treatment. Appl. Nanosci. 2015, 5, 105–114. [Google Scholar] [CrossRef]
- Pubby, K.; Narang, S.B.; Chawla, S.K.; Mudsainiyan, R.K. Effect of temperature on dielectric and electrical properties of Co–Zr doped barium hexaferrites prepared by sol–gel method. J. Mater. Sci. Mater. Electron. 2016, 27, 11220–11230. [Google Scholar] [CrossRef]
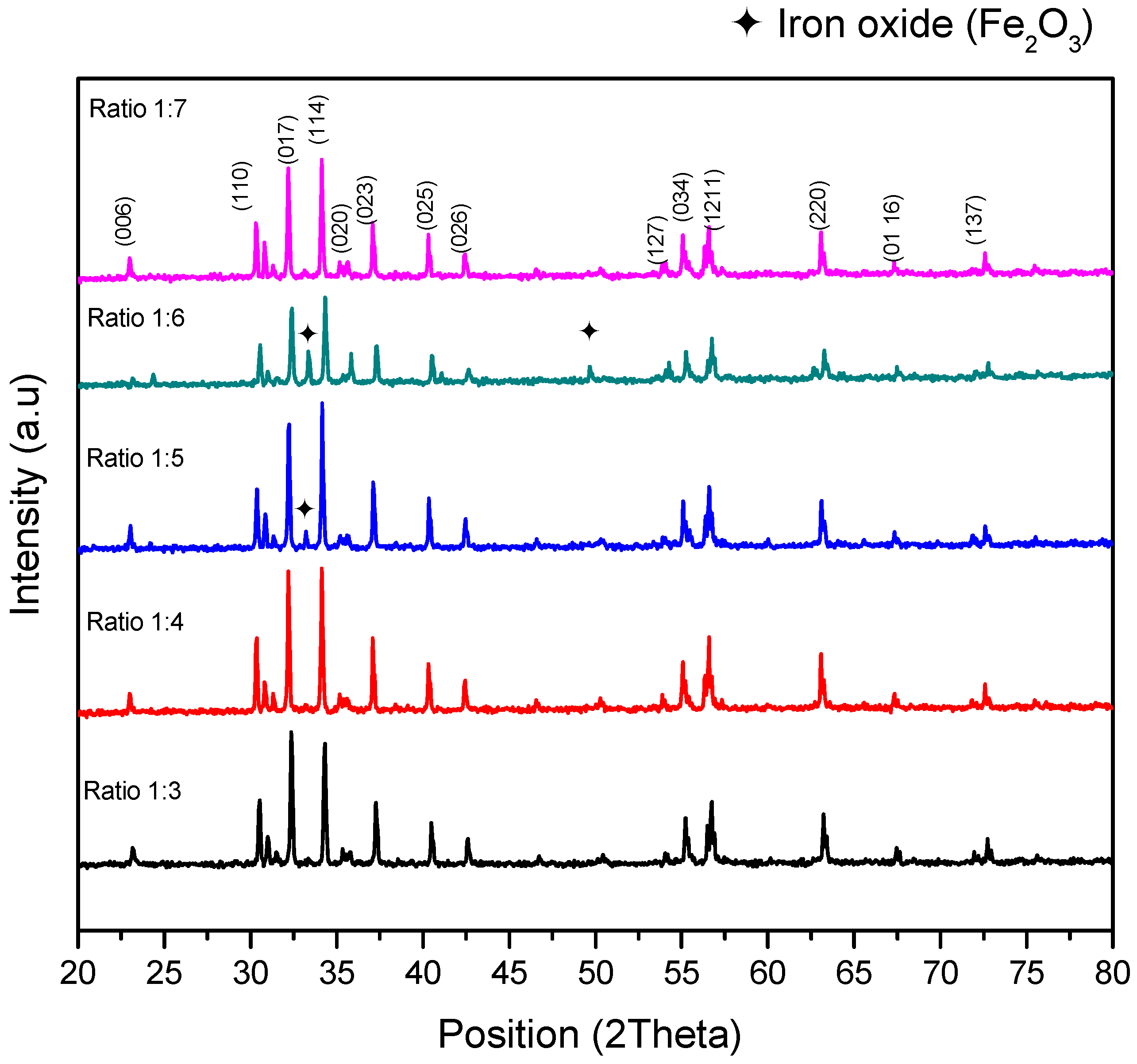
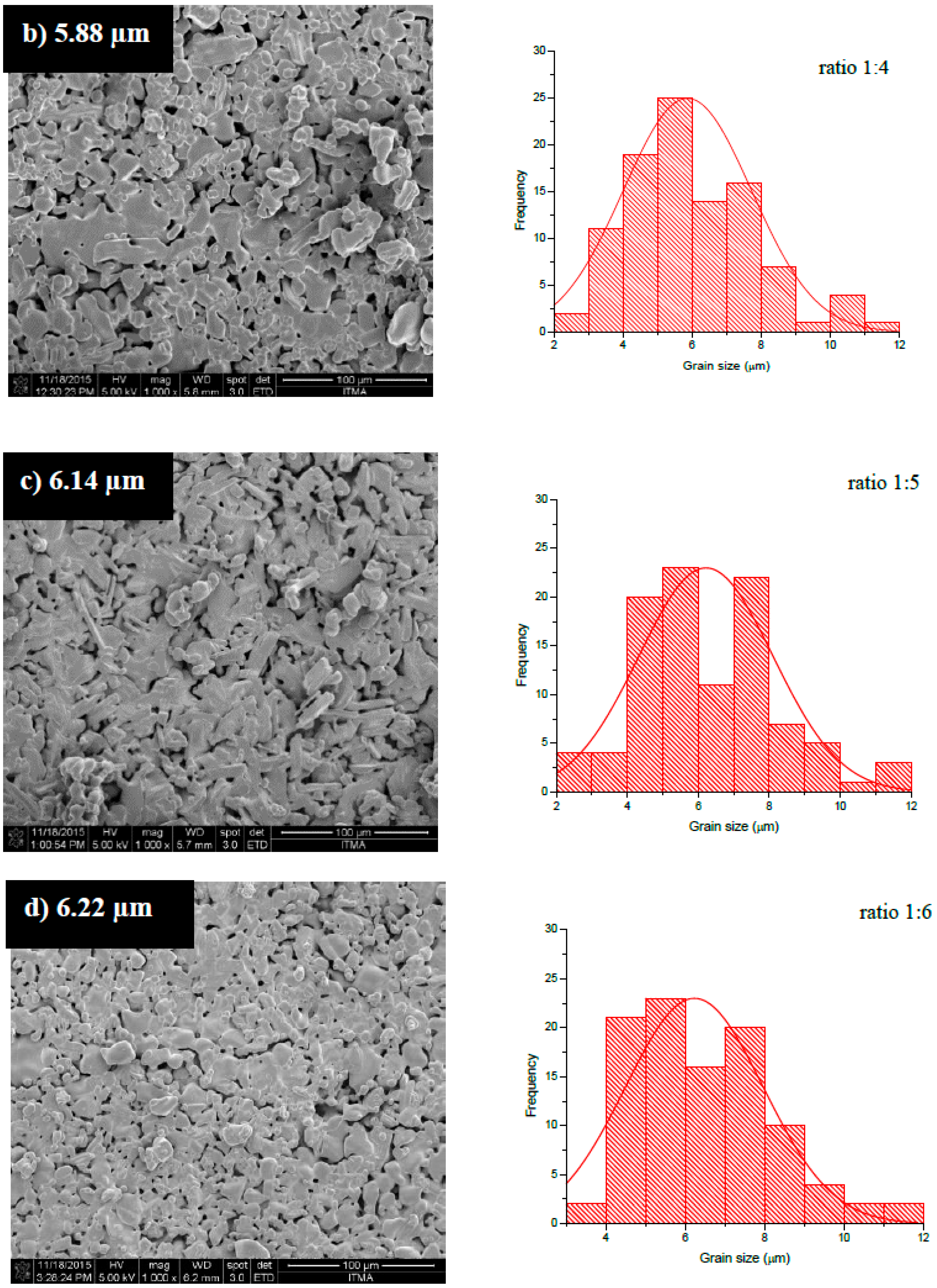
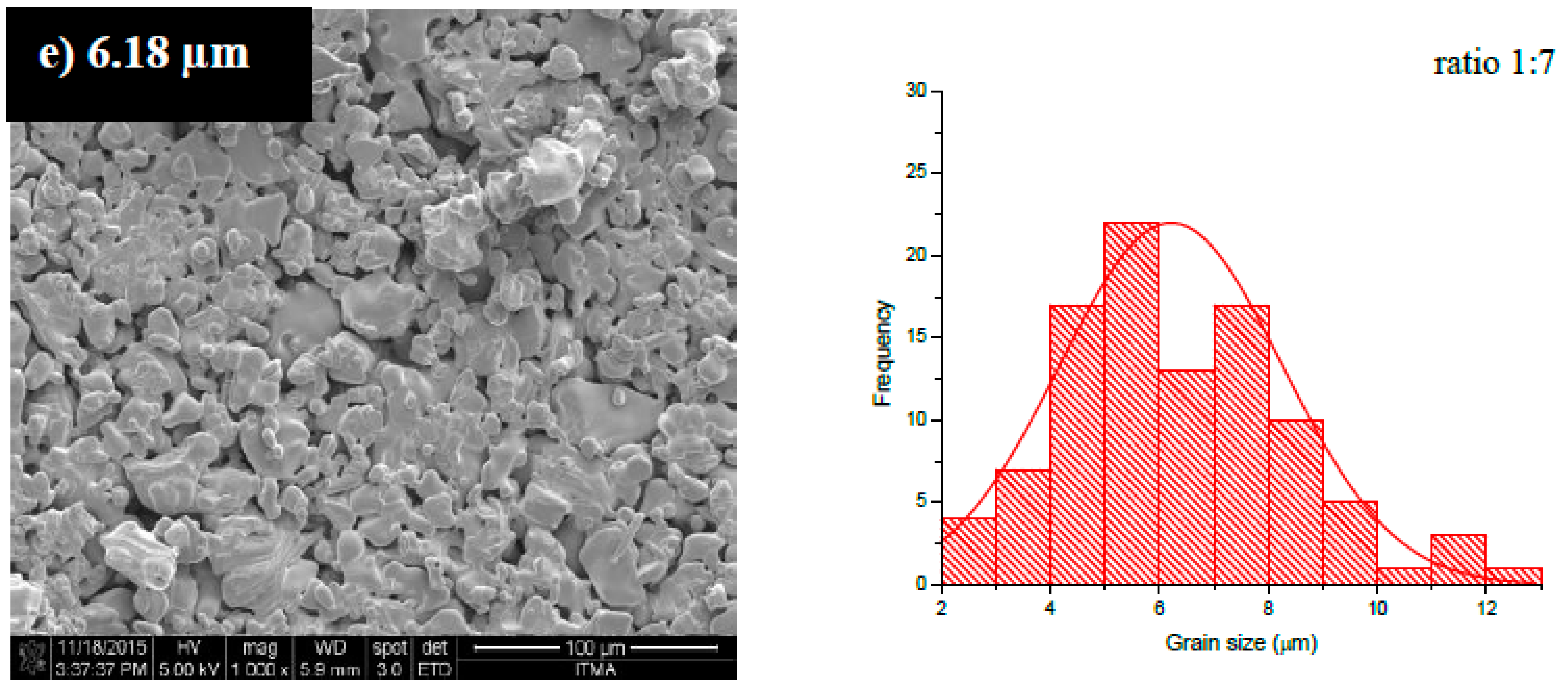


| Sample | Peak (hkl) | Position 2θ(o) | d-Spacing | Phase | Reference Code | Space Group |
|---|---|---|---|---|---|---|
| 1:3 | 114 | 34.304 | 2.62631 | Barium hexaferrites | 98-001-9939 | P63/mmc |
| 1:4 | 114 | 34.137 | 2.62631 | Barium hexaferrites | 98-001-9939 | P63/mmc |
| 1:5 | 114 | 34.160 | 2.62631 | Barium hexaferrites | 98-001-9939 | P63/mmc |
| 104 | 33.331 | 2.68455 | Hematite | 98-004-6404 | R-3 c | |
| 1:6 | 114 | 34.335 | 2.62631 | Barium hexaferrites | 98-001-9939 | P63/mmc |
| 104 | 33.337 | 2.68455 | Hematite | 98-004-6404 | R-3 c | |
| 1:7 | 114 | 34.130 | 2.62631 | Barium hexaferrites | 98-001-9939 | P63/mmc |
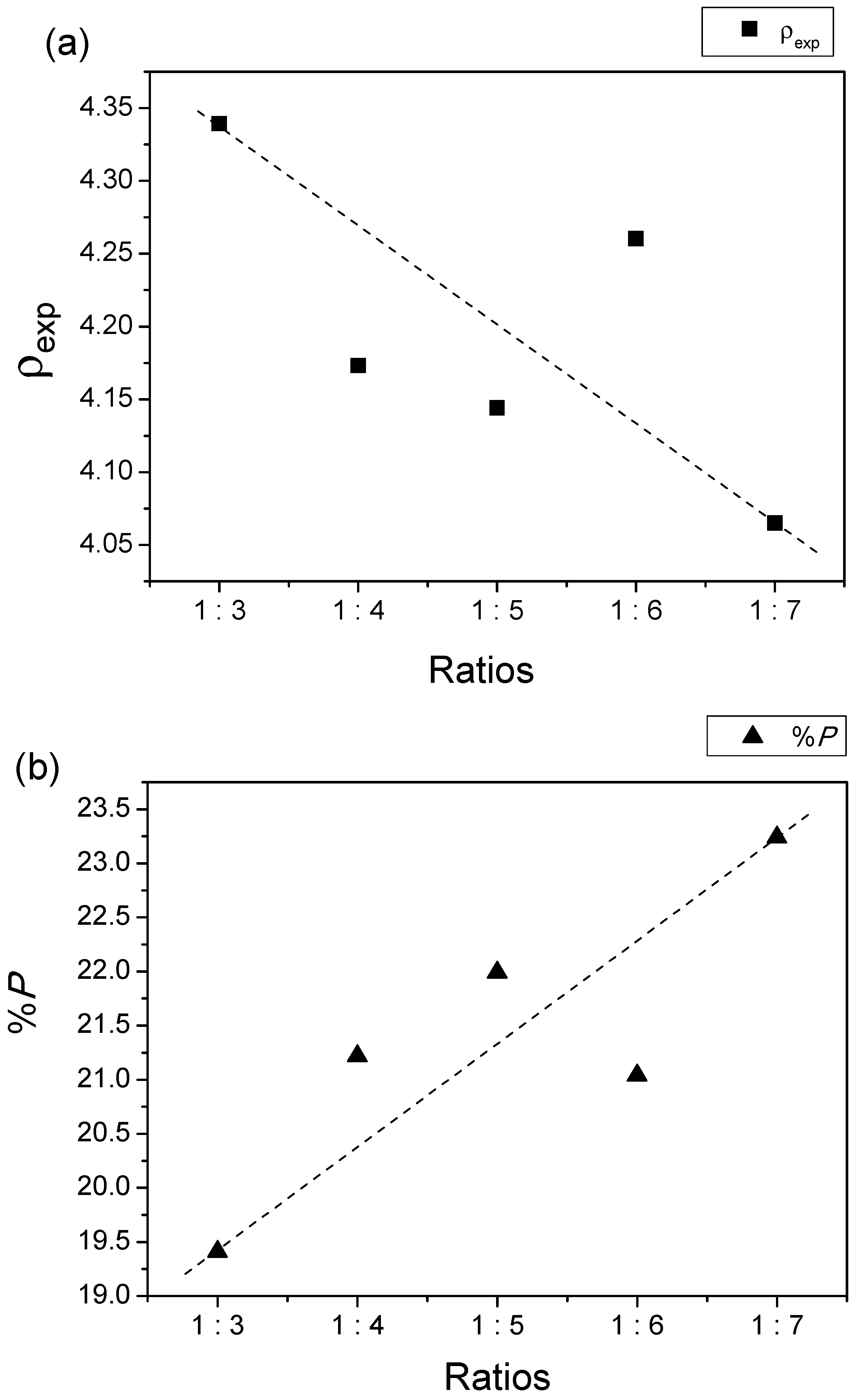
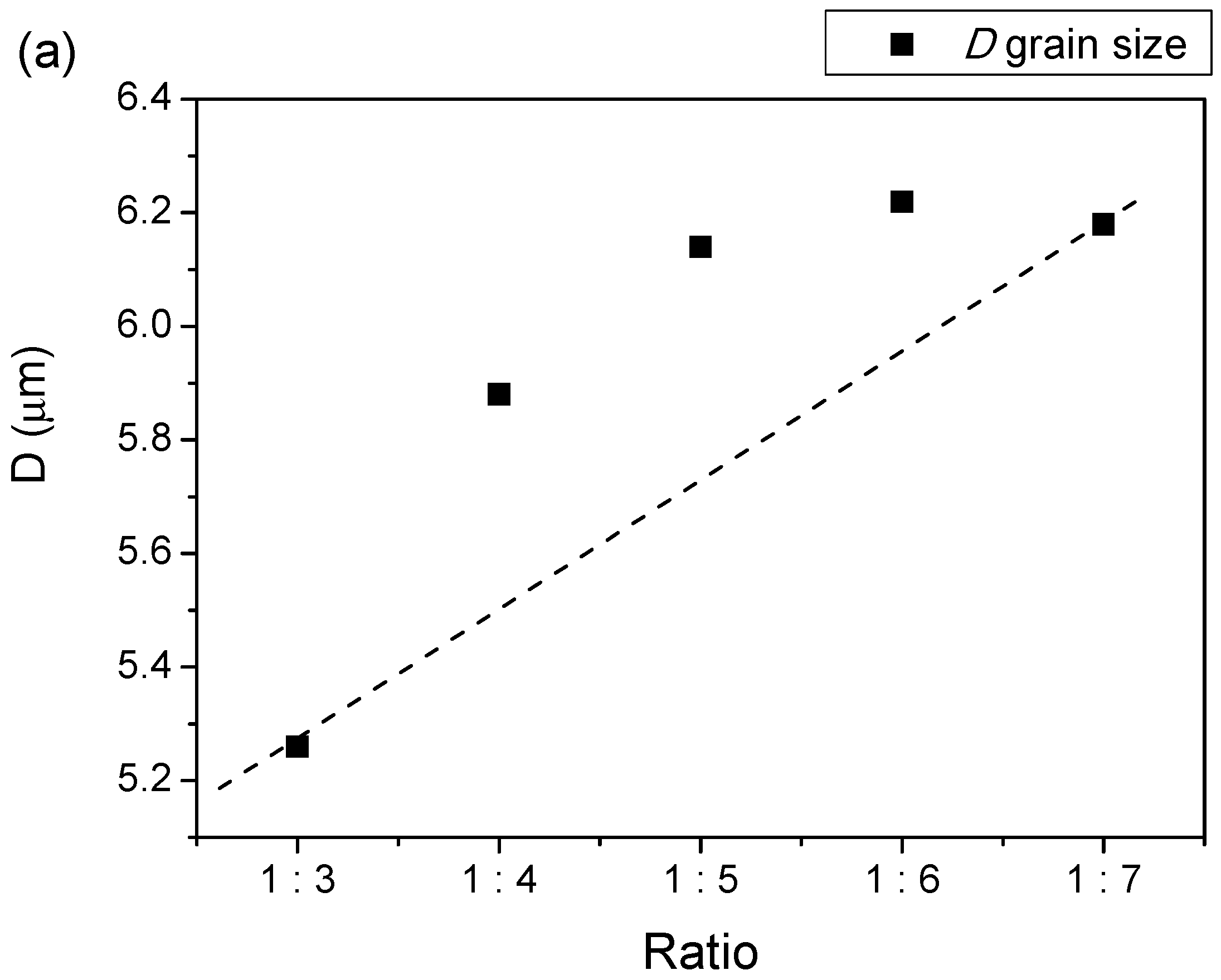
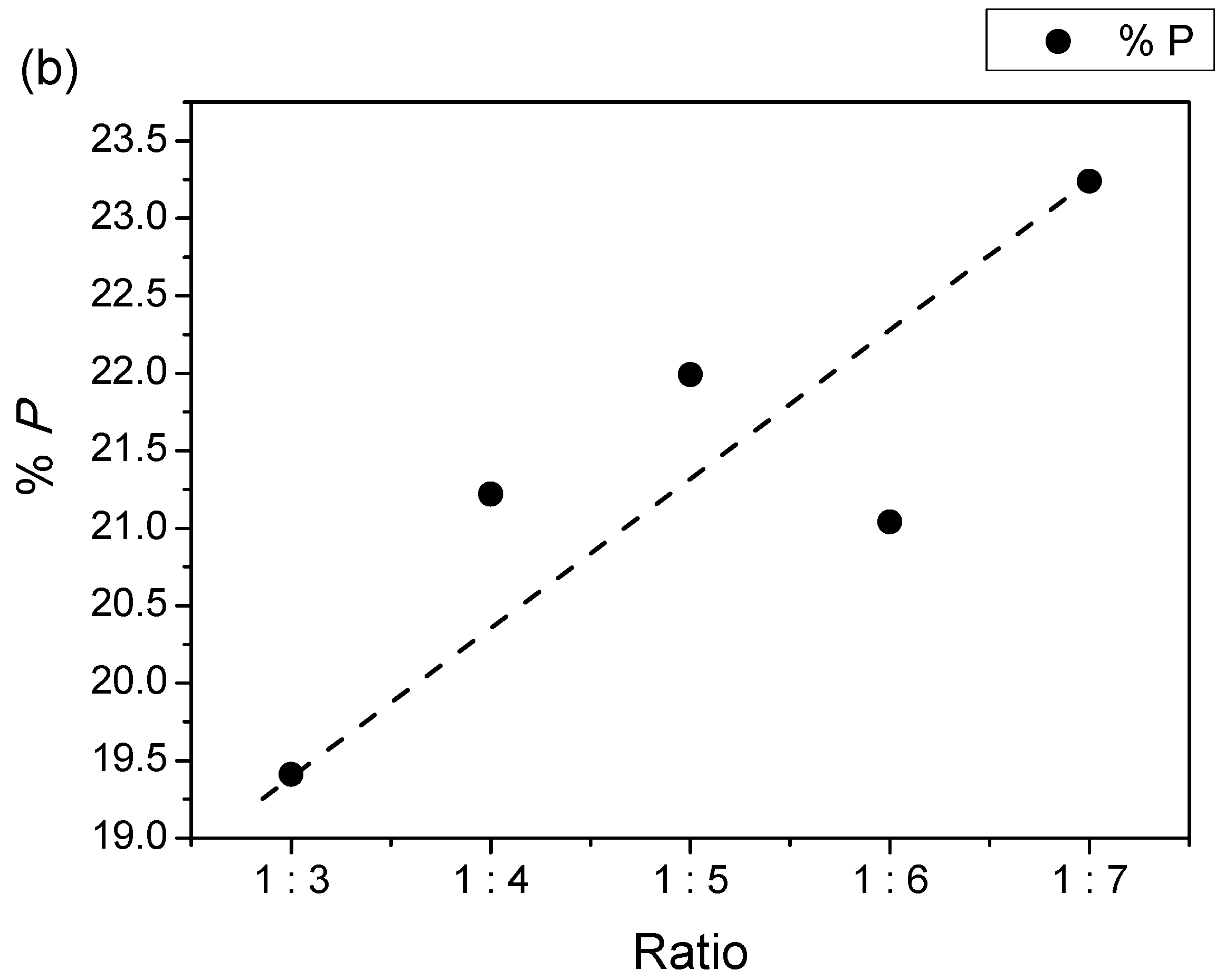
| Samples | Lattice Parameter | c/a | V (Å)3 | ρx (g/cm3) | ρb (g/cm3) | %P | |
|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | ||||||
| 1:3 | 5.8563 | 23.0784 | 3.9408 | 685.4412 | 5.3847 | 4.3393 | 19.41 |
| 1:4 | 5.8883 | 23.2048 | 3.9408 | 696.7477 | 5.2974 | 4.1730 | 21.22 |
| 1:5 | 5.8829 | 23.1819 | 3.9406 | 694.7841 | 5.3123 | 4.1440 | 21.99 |
| 1:6 | 5.8509 | 23.0733 | 3.9435 | 684.0265 | 5.3959 | 4.2603 | 21.04 |
| 1:7 | 5.8891 | 23.2048 | 3.9403 | 696.9371 | 5.2959 | 4.0650 | 23.24 |
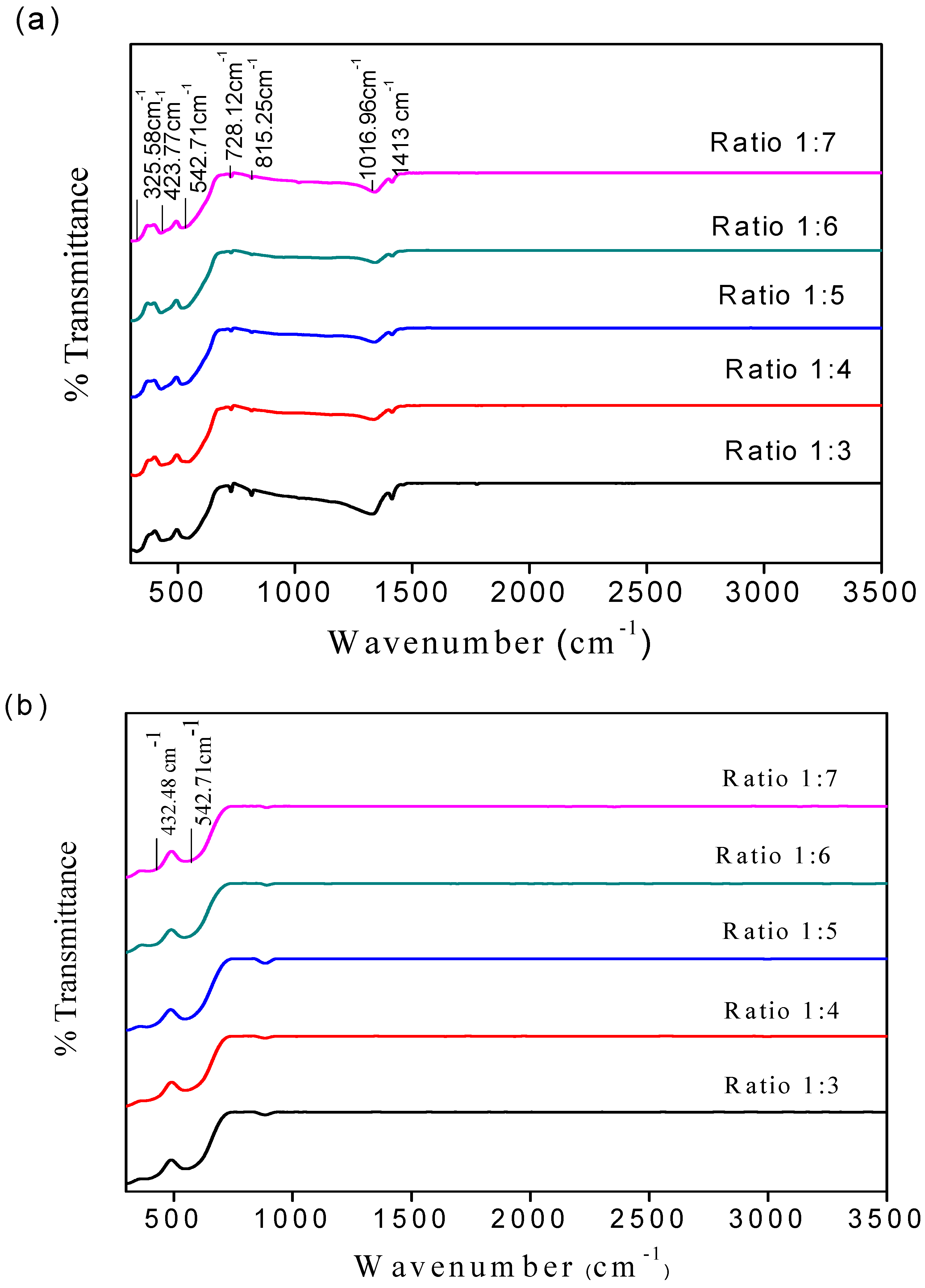
| Samples | Wave Number (cm−1) | |
|---|---|---|
| 1:3 | 432.48 | 542.71 |
| 1:4 | 420.14 | 548.82 |
| 1:5 | 419.62 | 563.90 |
| 1:6 | 419.13 | 569.63 |
| 1:7 | 417.62 | 569.93 |
| Samples | Ms (emu/g) | Hc (Oe) | Mr (emu/g) | Mr/Ms |
|---|---|---|---|---|
| 1:3 | 90.87 | 1317.0 | 43.83 | 0.49 |
| 1:4 | 56.18 | 752.02 | 32.57 | 0.59 |
| 1:5 | 61.01 | 665.67 | 23.42 | 0.40 |
| 1:6 | 42.89 | 630.51 | 13.58 | 0.32 |
| 1:7 | 58.00 | 653.85 | 26.12 | 0.46 |
| Sample | σdc (Ω−1m−1) | |||
|---|---|---|---|---|
| 30 °C | 80 °C | 130 °C | 180 °C | |
| 1:3 | 1.58 × 10−6 | 3.78 × 10−5 | 2.85 × 10−5 | 3.86 × 10−5 |
| 1:4 | 4.53 ×10−6 | 1.69 × 10−5 | 5.13 × 10−5 | 6.01 × 10−5 |
| 1:5 | 7.39 × 10−7 | 8.29 × 10−7 | 9.85 × 10−7 | 1.31 × 10−6 |
| 1:6 | 3.71 × 10−6 | 9.72 × 10−6 | 2.53 × 10−5 | 5.96 × 10−5 |
| 1:7 | 1.35 × 10−5 | 2.41 × 10−5 | 6.40 × 10−5 | 1.83 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azis, R.S.; Che Muda, N.N.; Hassan, J.; Shaari, A.H.; Ibrahim, I.R.; Mustaffa, M.S.; Sulaiman, S.; Matori, K.A.; Fen, Y.W. Effect of Ratio in Ammonium Nitrate on the Structural, Microstructural, Magnetic, and AC Conductivity Properties of BaFe12O19. Materials 2018, 11, 2190. https://doi.org/10.3390/ma11112190
Azis RS, Che Muda NN, Hassan J, Shaari AH, Ibrahim IR, Mustaffa MS, Sulaiman S, Matori KA, Fen YW. Effect of Ratio in Ammonium Nitrate on the Structural, Microstructural, Magnetic, and AC Conductivity Properties of BaFe12O19. Materials. 2018; 11(11):2190. https://doi.org/10.3390/ma11112190
Chicago/Turabian StyleAzis, Raba’ah Syahidah, Nor Nadhirah Che Muda, Jumiah Hassan, Abdul Halim Shaari, Idza Riati Ibrahim, Muhammad Syazwan Mustaffa, Sakinah Sulaiman, Khamirul Amin Matori, and Yap Wing Fen. 2018. "Effect of Ratio in Ammonium Nitrate on the Structural, Microstructural, Magnetic, and AC Conductivity Properties of BaFe12O19" Materials 11, no. 11: 2190. https://doi.org/10.3390/ma11112190
APA StyleAzis, R. S., Che Muda, N. N., Hassan, J., Shaari, A. H., Ibrahim, I. R., Mustaffa, M. S., Sulaiman, S., Matori, K. A., & Fen, Y. W. (2018). Effect of Ratio in Ammonium Nitrate on the Structural, Microstructural, Magnetic, and AC Conductivity Properties of BaFe12O19. Materials, 11(11), 2190. https://doi.org/10.3390/ma11112190






