CO2 Uptake of Carbonation-Cured Cement Blended with Ground Volcanic Ash
Abstract
1. Introduction
2. Experimental Program
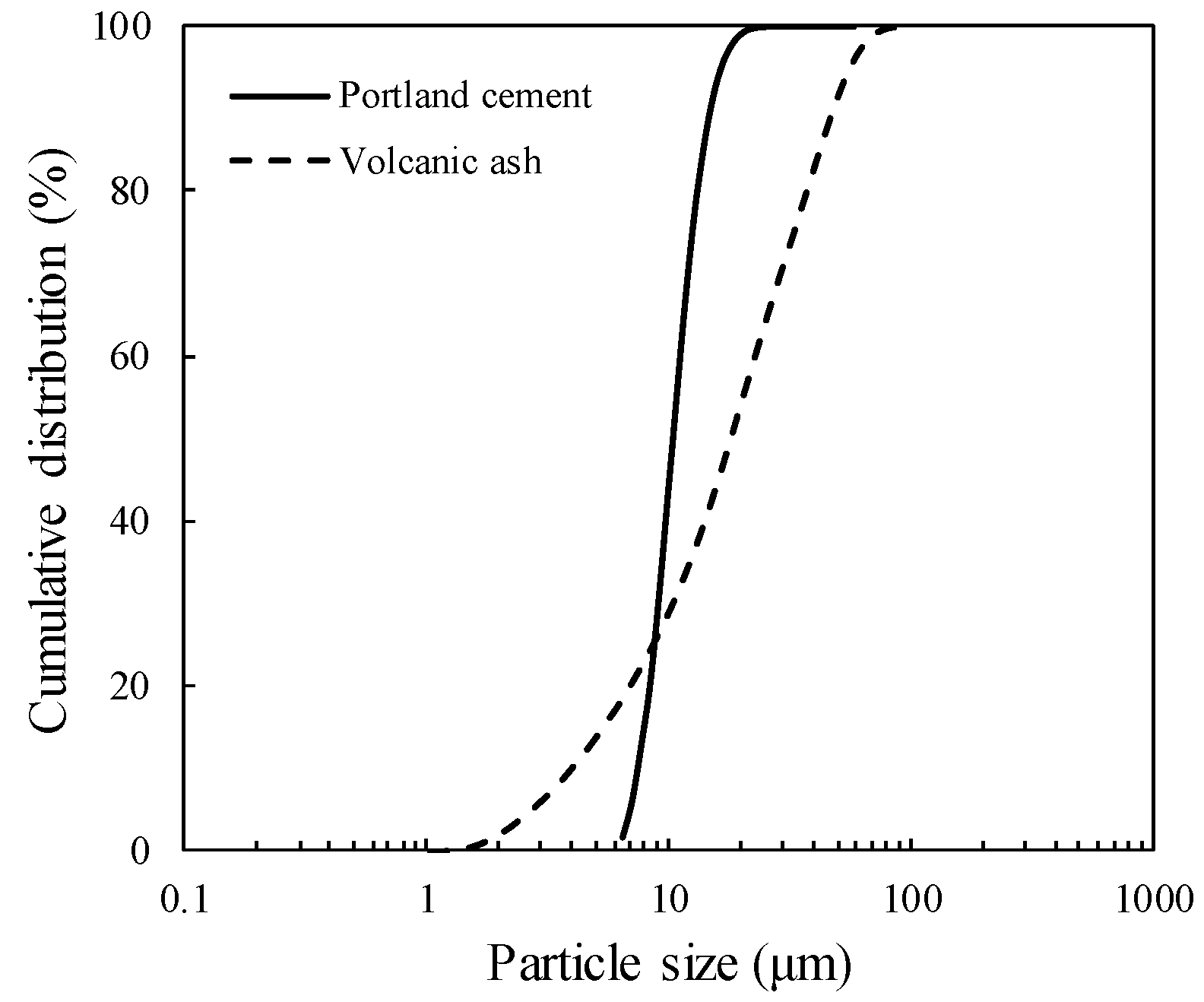
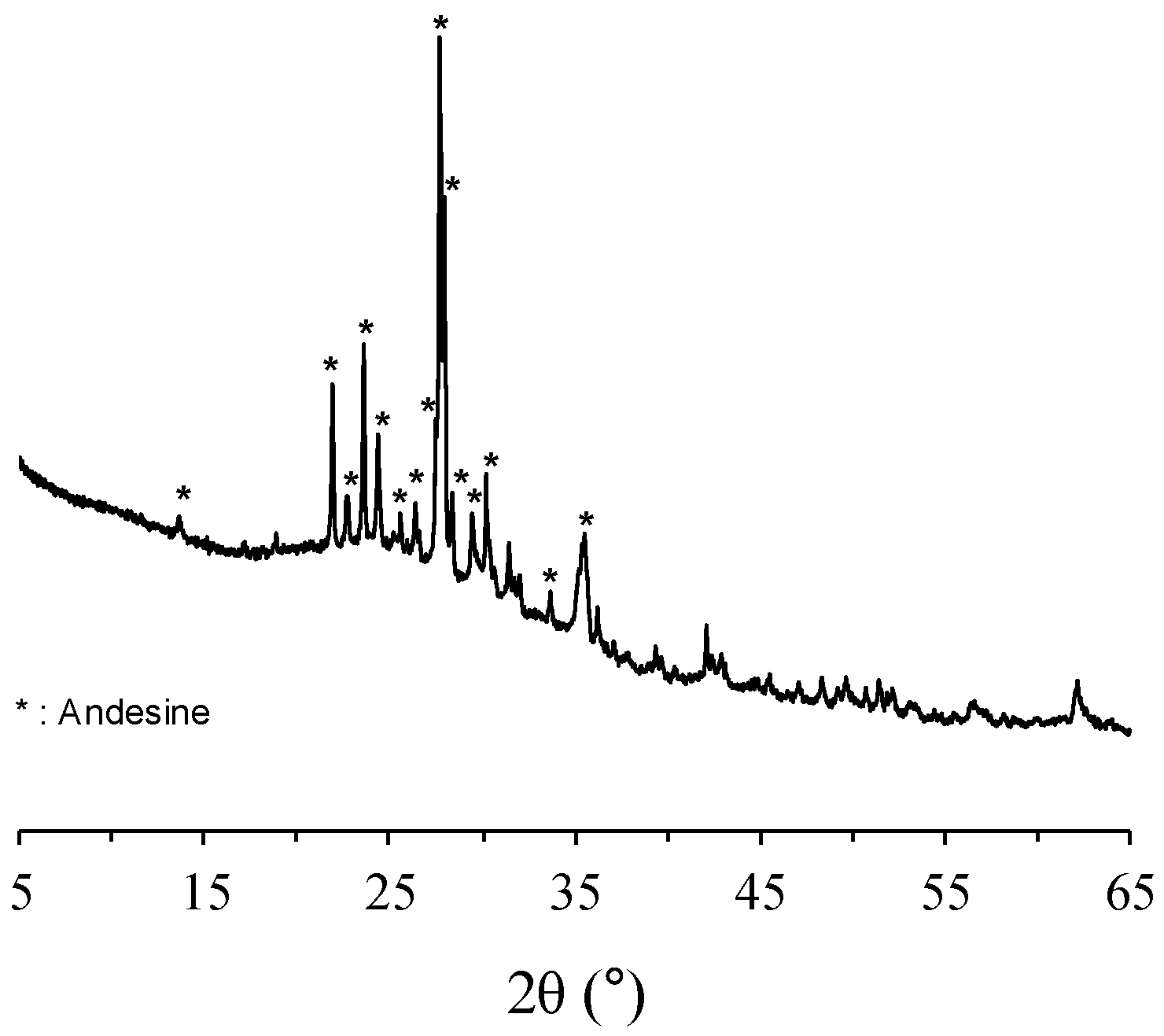
2.1. Materials and Sample Preparation
2.2. Test Methods
3. Results
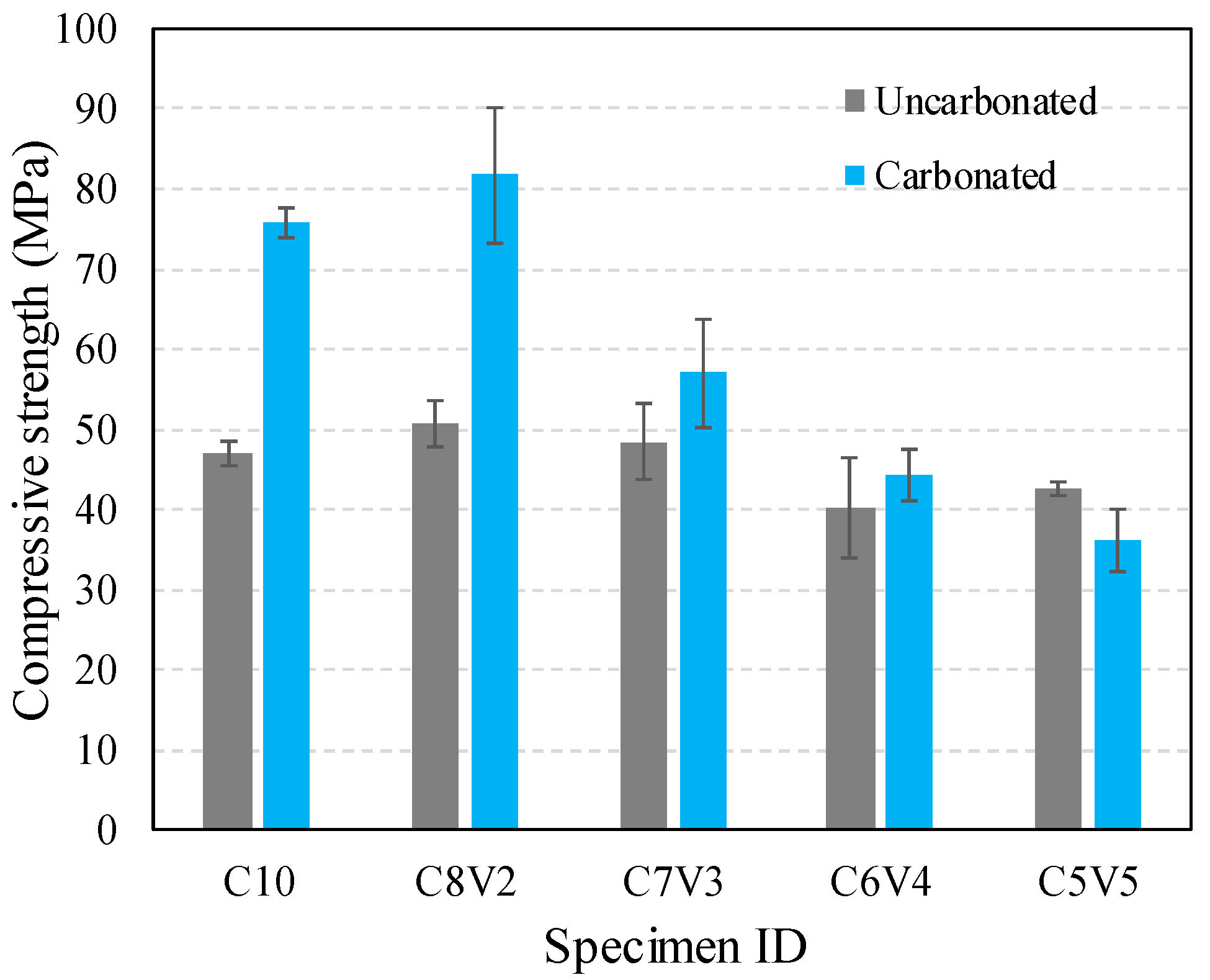
3.1. Compressive Strength
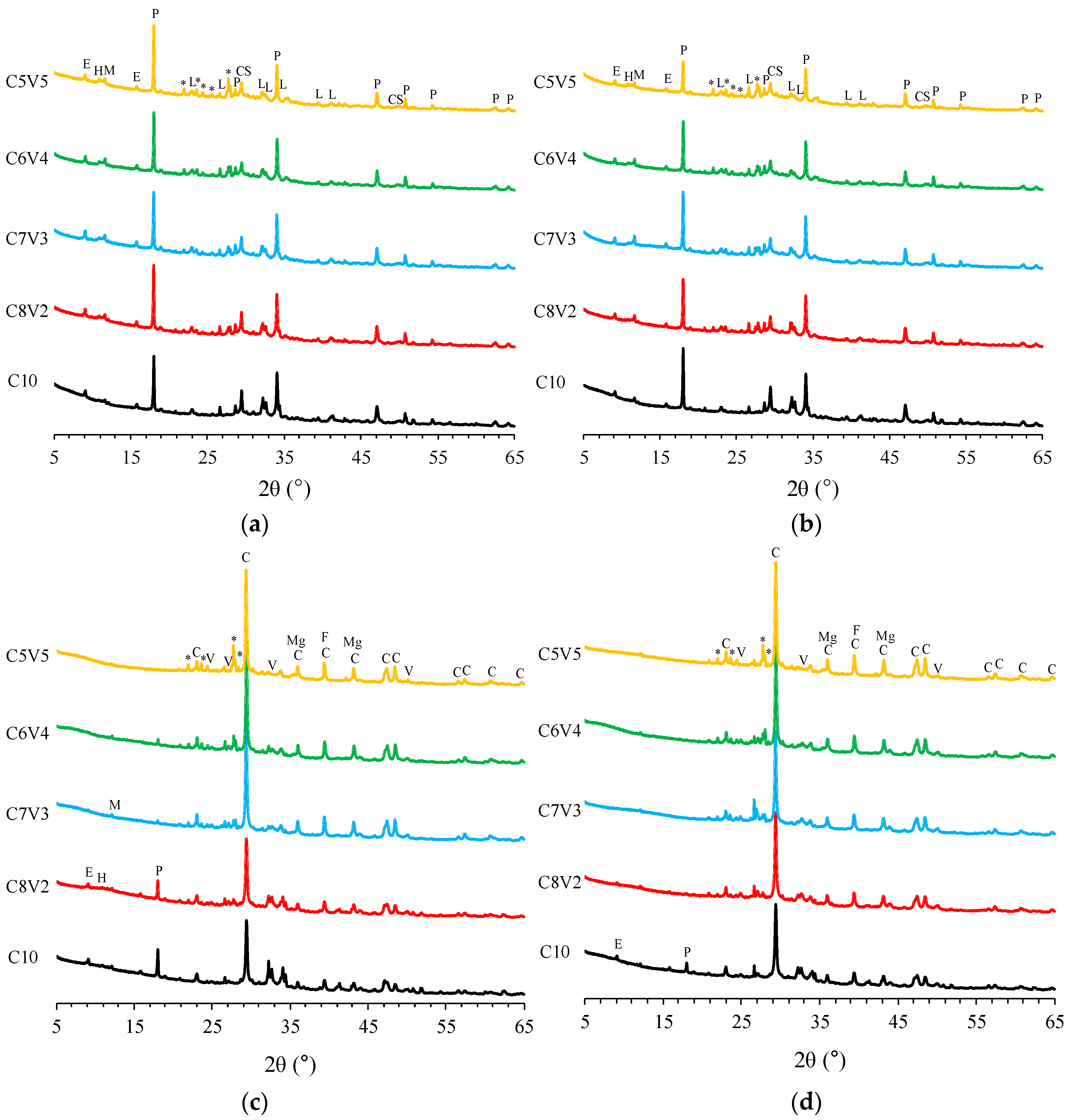
3.2. XRD
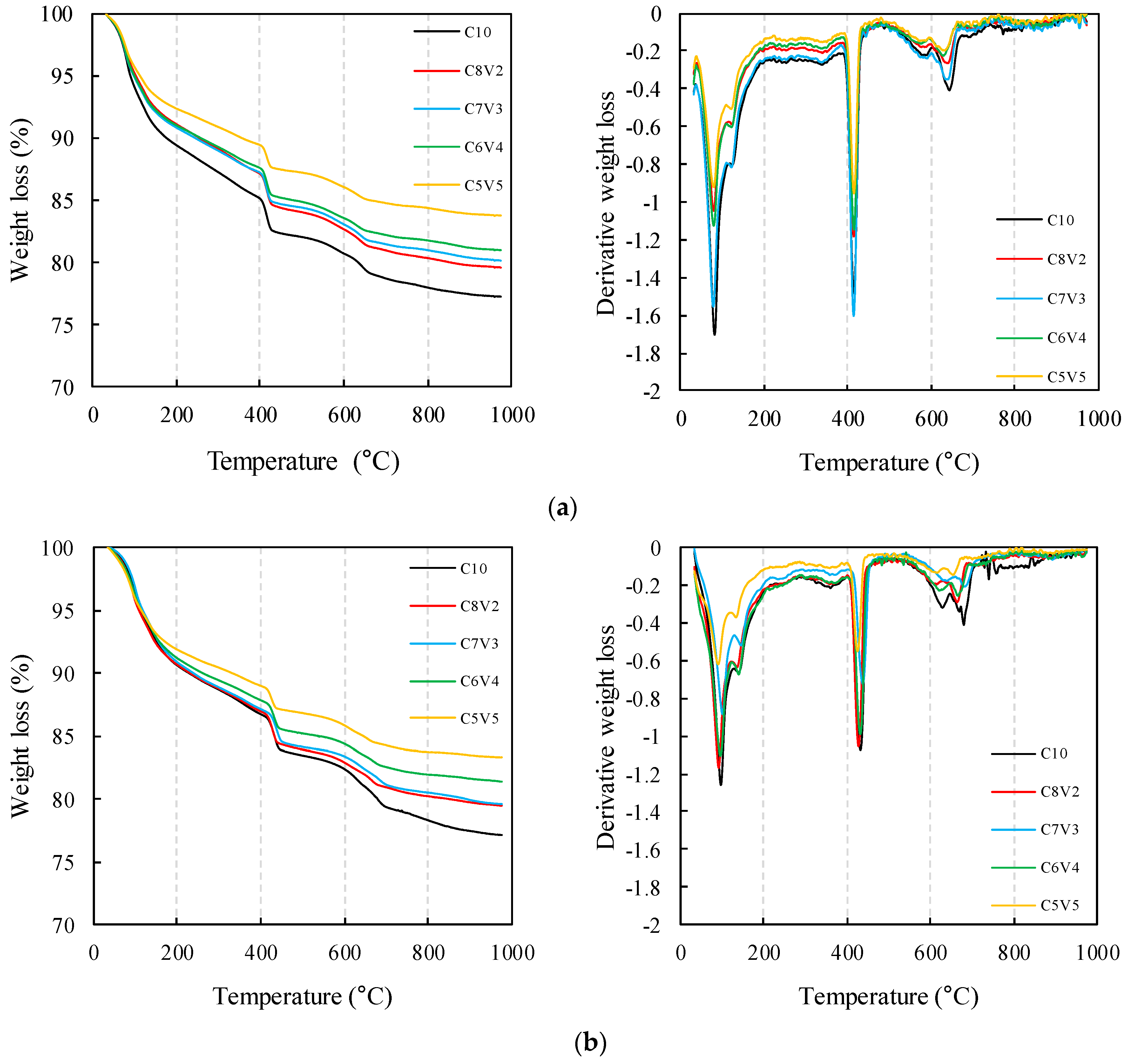
3.3. TG/DTG
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blunden, J.; Arndt, D.S.; Hartfield, G.; Sánchez-Lugo, A.; Scambos, T.A.; Schreck, C.J., III; Stammerjohn, S.; Stanitski, D.M.; Willett, K.M.; Bissolli, P.; et al. State of the Climate in 2017. Bull. Am. Meteorol. Soc. 2018, 99, Si–S270. [Google Scholar]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Link, T.; Bellmann, F.; Ludwig, H.M.; Haha, M.B. Reactivity and phase composition of Ca2SiO4 binders made by annealing of alpha-dicalcium silicate hydrate. Cem. Concr. Res. 2015, 67, 131–137. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2017. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2017. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Lee, N.K.; Lee, H.K. Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem. Concr. Res. 2016, 89, 72–79. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Chae, S.; Lee, H.K. An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials 2016, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Juenger, M.C.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Shi, C.; Palomo, A. Carbon dioxide sequestration in cementitious construction materials. Carbon 2018, 1, 474. [Google Scholar]
- Hansen, J.; Sato, M.; Kharecha, P.; Von Schuckmann, K.; Beerling, D.J.; Cao, J.; Marcott, S.; Masson-Delmotte, V.; Prather, M.J.; Rohling, E.J.; et al. Young people’s burden: Requirement of negative CO2 emissions. arXiv. 2016. Available online: https://arxiv.org/abs/1609.05878 (accessed on 19 September 2016).
- Van den Bosch, M.; Telenius, A. Supplementary Information. In Proceedings of the UNEP/UNECEGEO-6 Assessment for the Pan-European Region, Nairobi, Kenya, 28 December 2016. [Google Scholar]
- Wang, T.; Huang, H.; Hu, X.; Fang, M.; Luo, Z.; Guo, R. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Ghoshal, S. CO2 sequestration in concrete through accelerated carbonation curing in a flow-through reactor. Ind. Eng. Chem. Res. 2009, 49, 1143–1149. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation curing versus steam curing for precast concrete production. J. Mater. Civ. Eng. 2011, 24, 1221–1229. [Google Scholar] [CrossRef]
- Cheng-Yi, H.; Feldman, R.F. Hydration reactions in Portland cement-silica fume blends. Cem. Concr. Res. 1985, 15, 585–592. [Google Scholar] [CrossRef]
- Cheng-Yi, H.; Feldman, R.F. Influence of silica fume on the microstructural development in cement mortars. Cem. Concr. Res. 1985, 15, 285–294. [Google Scholar] [CrossRef]
- Hanehara, S.; Tomosawa, F.; Kobayakawa, M.; Hwang, K. Effects of water/powder ratio, mixing ratio of fly ash, and curing temperature on pozzolanic reaction of fly ash in cement paste. Cem. Concr. Res. 2001, 31, 31–39. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Hydration reactions of cement combinations containing vitrified incinerator fly ash. Cem. Concr. Res. 2004, 34, 849–856. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.-I.; Sharp, J. The chemical composition and microstructure of hydration products in blended cements. Cem. Concr. Compos. 2004, 26, 967–976. [Google Scholar] [CrossRef]
- Taylor, R.; Richardson, I.; Brydson, R. Composition and microstructure of 20-year-old ordinary Portland cement–ground granulated blast-furnace slag blends containing 0 to 100% slag. Cem. Concr. Res. 2010, 40, 971–983. [Google Scholar] [CrossRef]
- Luke, K.; Lachowski, E. Internal Composition of 20-Year-Old Fly Ash and Slag-Blended Ordinary Portland Cement Pastes. J. Am. Ceram. Soc. 2008, 91, 4084–4092. [Google Scholar] [CrossRef]
- Richardson, I.; Groves, G. The incorporation of minor and trace elements into calcium silicate hydrate (C-S-H) gel in hardened cement pastes. Cem. Concr. Res. 1993, 23, 131–138. [Google Scholar] [CrossRef]
- Wilson, I.G.; Staffell, I. Rapid fuel switching from coal to natural gas through effective carbon pricing. Nat. Energy 2018, 3, 365–372. [Google Scholar] [CrossRef]
- Laurent, D. Kingdom of Saudi Arabia Atlas: Atlas of Industrial Minerals; Alibris: Emeryville, CA, USA, 2016. [Google Scholar]
- Hossain, K.M.A.; Lachemi, M. Strength, durability and micro-structural aspects of high performance volcanic ash concrete. Cem. Concr. Res. 2007, 37, 759–766. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Hossain, K.M.A. Blended cement using volcanic ash and pumice. Cem. Concr. Res. 2003, 33, 1601–1605. [Google Scholar] [CrossRef]
- Hossain, K.M.A. Chloride induced corrosion of reinforcement in volcanic ash and pumice based blended concrete. Cem. Concr. Compos. 2005, 27, 381–390. [Google Scholar] [CrossRef]
- Younsi, A.; Turcry, P.; Rozière, E.; Aït-Mokhtar, A.; Loukili, A. Performance-based design and carbonation of concrete with high fly ash content. Cem. Concr. Compos. 2011, 33, 993–1000. [Google Scholar] [CrossRef]
- Rukzon, S.; Chindaprasirt, P. An experimental investigation of the carbonation of blended Portland cement palm oil fuel ash mortar in an indoor environment. Indoor Built Environ. 2009, 18, 313–318. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem. Concr. Res. 2015, 67, 226–236. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement: Part I. A fine non-hydraulic filler. Cem. Concr. Res. 1990, 20, 778–782. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Efficiency of mineral admixtures in mortars: Quantification of the physical and chemical effects of fine admixtures in relation with compressive strength. Cem. Concr. Res. 2006, 36, 264–277. [Google Scholar] [CrossRef]
- Alfreðsson, H.A. Water-Rock Interaction during Mineral Carbonation and Volcanic Ash Weathering. Ph.D. Thesis, University of Iceland, Reykjavik, Iceland, 17 September 2015. [Google Scholar]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Silva, D.A.D.; Roman, H.R.; Gleize, P.J.P. Evidences of chemical interaction between EVA and hydrating Portland cement. Cem. Concr. Res. 2002, 32, 1383–1390. [Google Scholar] [CrossRef]
- Borges, P.H.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.H.; Oh, J.E. Microstructural verification of the strength performance of ternary blended cement systems with high volumes of fly ash and GGBFS. Constr. Build. Mater. 2015, 95, 96–107. [Google Scholar] [CrossRef]

| Chemical Compound (%) | Portland Cement |
|---|---|
| CaO | 63.84 |
| SiO2 | 21.45 |
| Al2O3 | 6.03 |
| Fe2O3 | 3.27 |
| SO3 | 2.13 |
| L.O.I. 1 | 3.27 |
| Chemical Compound (%) | Volcanic Ash |
|---|---|
| Na2O | 5.08 |
| MgO | 3.20 |
| Al2O3 | 17.30 |
| SiO2 | 48.10 |
| P2O5 | 0.55 |
| SO3 | 0.20 |
| Cl | 0.15 |
| K2O | 2.18 |
| CaO | 7.57 |
| TiO2 | 2.41 |
| MnO | 0.26 |
| Fe2O3 | 13.10 |
| Specimen ID 1 | Cement | Volcanic Ash | Water/powder 2 Ratio |
|---|---|---|---|
| C10 | 1 | 0.0 | 0.4 |
| C8V2 | 0.8 | 0.2 | 0.4 |
| C7V3 | 0.7 | 0.3 | 0.4 |
| C6V4 | 0.6 | 0.4 | 0.4 |
| C5V5 | 0.5 | 0.5 | 0.4 |
| Curing Condition | Specimen ID | C-S-H and Ettringite (50–200 °C) | Portlandite (400–450 °C) | Calcium Carbonate (500–720 °C) |
|---|---|---|---|---|
| Uncarbonated | C10 | 9.16 | 2.83 | 4.31 |
| C8V2 | 8.94 | 2.59 | 3.18 | |
| C7V3 | 8.91 | 2.56 | 3.19 | |
| C6V4 | 8.34 | 2.28 | 2.82 | |
| C5V5 | 7.47 | 1.86 | 2.72 | |
| Carbonated | C10 | 7.20 | 0.88 | 13.1 |
| C8V2 | 5.06 | 0.69 | 12.3 | |
| C7V3 | 3.93 | 0.79 | 10.3 | |
| C6V4 | 3.05 | 0.76 | 13.7 | |
| C5V5 | 2.59 | 0.62 | 13.7 |
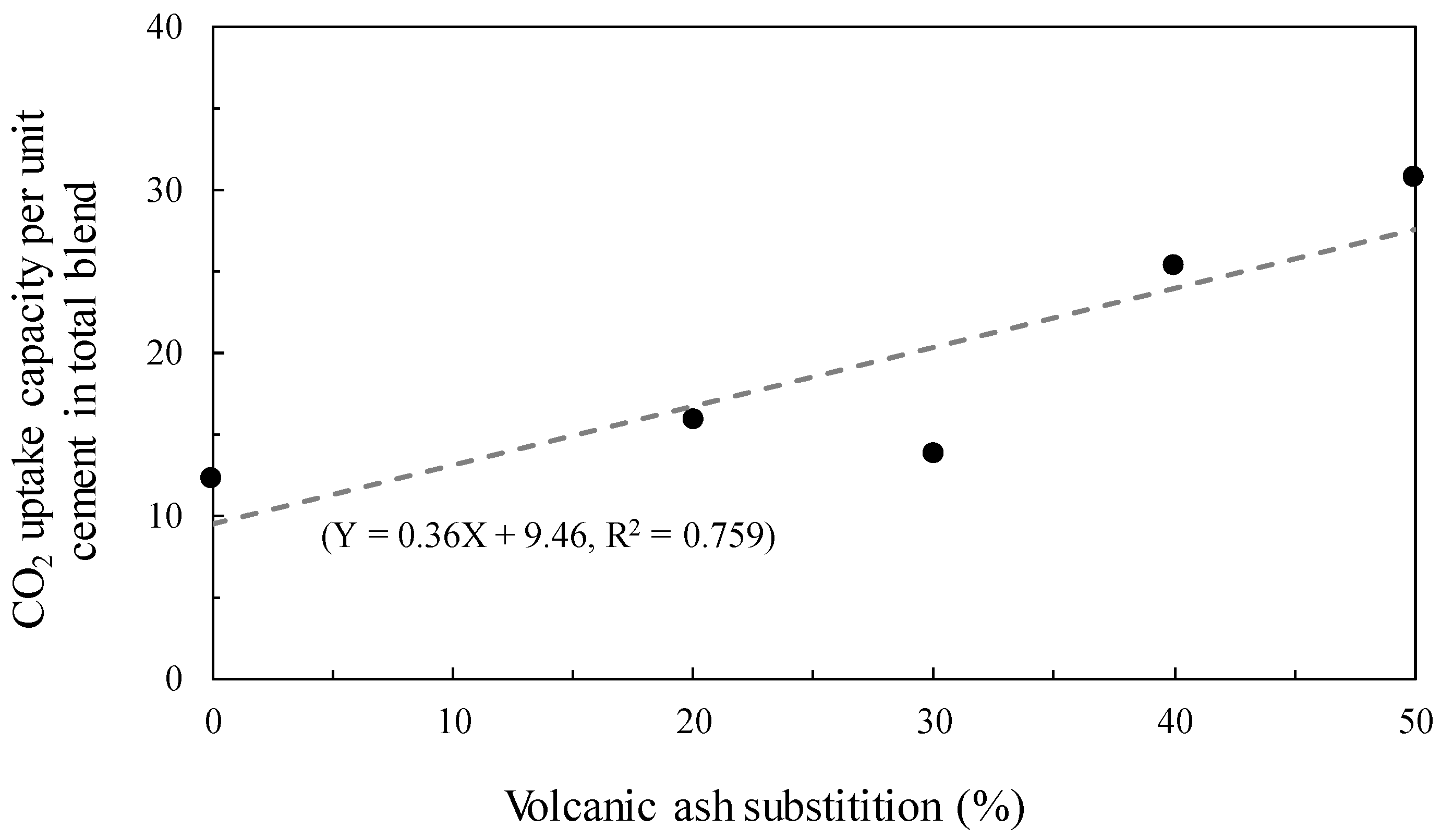
| Specimen ID | Mcarbonation-cured (a) | Muncarbonated (b) | (a–b) | CO2 Uptake Capacity Per Unit Cement in Total Blend |
|---|---|---|---|---|
| C10 | 13.1 | 4.3 | 8.8 | 12.3 |
| C8V2 | 12.3 | 3.2 | 9.1 | 15.9 |
| C7V3 | 10.1 | 3.2 | 6.9 | 13.8 |
| C6V4 | 13.7 | 2.8 | 10.9 | 25.4 |
| C5V5 | 13.7 | 2.7 | 11.0 | 30.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.H.; Amr, I.T.; Park, S.M.; Bamagain, R.A.; Fadhel, B.A.; Kim, G.M.; Hunaidy, A.S.; Lee, H.K. CO2 Uptake of Carbonation-Cured Cement Blended with Ground Volcanic Ash. Materials 2018, 11, 2187. https://doi.org/10.3390/ma11112187
Seo JH, Amr IT, Park SM, Bamagain RA, Fadhel BA, Kim GM, Hunaidy AS, Lee HK. CO2 Uptake of Carbonation-Cured Cement Blended with Ground Volcanic Ash. Materials. 2018; 11(11):2187. https://doi.org/10.3390/ma11112187
Chicago/Turabian StyleSeo, Joon Ho, Issam T. Amr, Sol Moi Park, Rami A. Bamagain, Bandar A. Fadhel, Gwang Mok Kim, Ali S. Hunaidy, and Haeng Ki Lee. 2018. "CO2 Uptake of Carbonation-Cured Cement Blended with Ground Volcanic Ash" Materials 11, no. 11: 2187. https://doi.org/10.3390/ma11112187
APA StyleSeo, J. H., Amr, I. T., Park, S. M., Bamagain, R. A., Fadhel, B. A., Kim, G. M., Hunaidy, A. S., & Lee, H. K. (2018). CO2 Uptake of Carbonation-Cured Cement Blended with Ground Volcanic Ash. Materials, 11(11), 2187. https://doi.org/10.3390/ma11112187






