Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Electrochemical Measurements
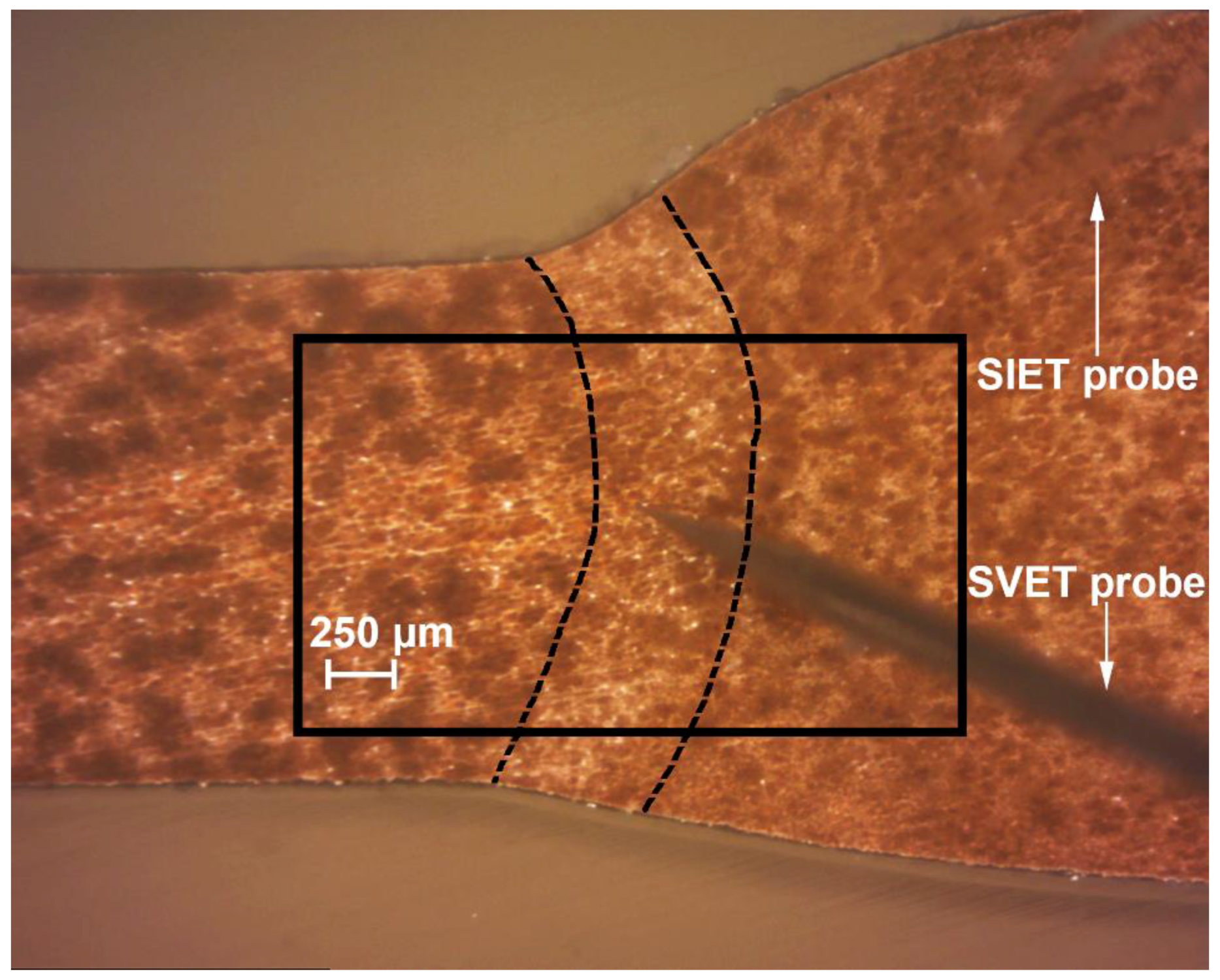
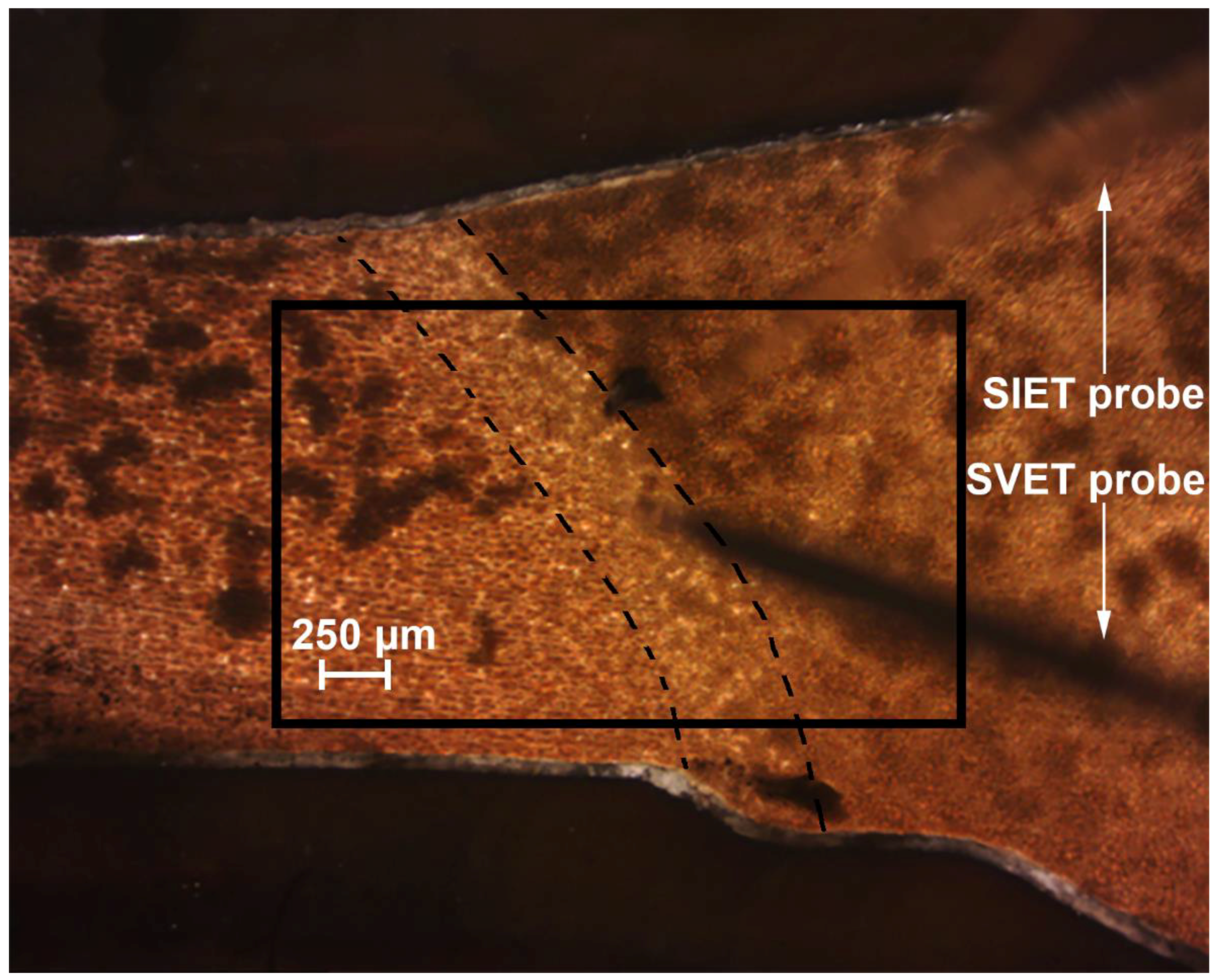
2.2.1. SVET and SIET Measurements
2.2.2. Electrochemical Impedance Spectroscopy and Potentiodynamic Polarization Measurements
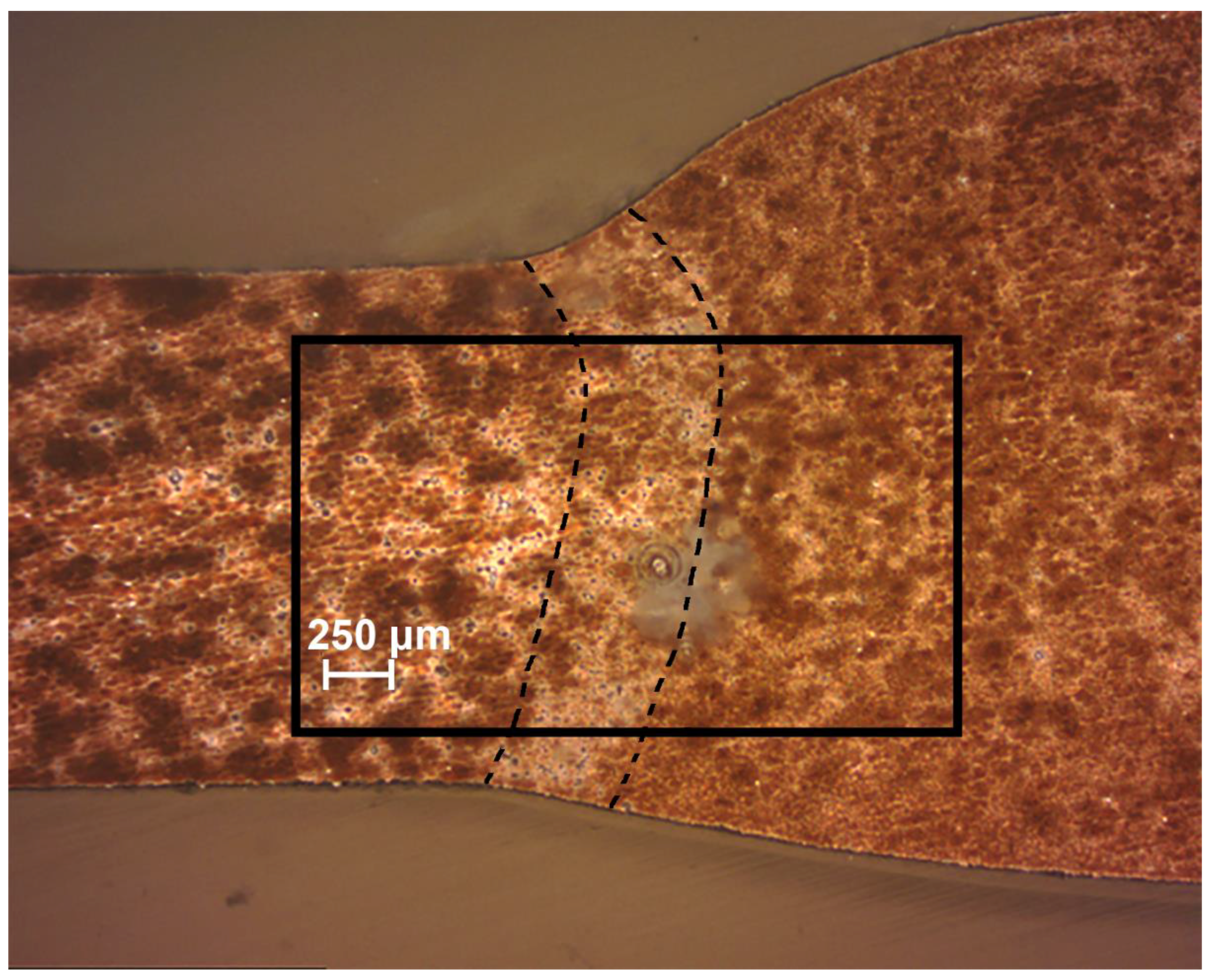
2.3. Cross-Section Preparation
3. Results and Discussion
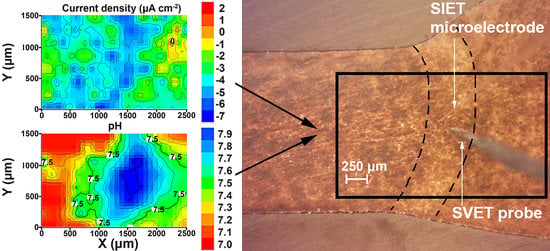
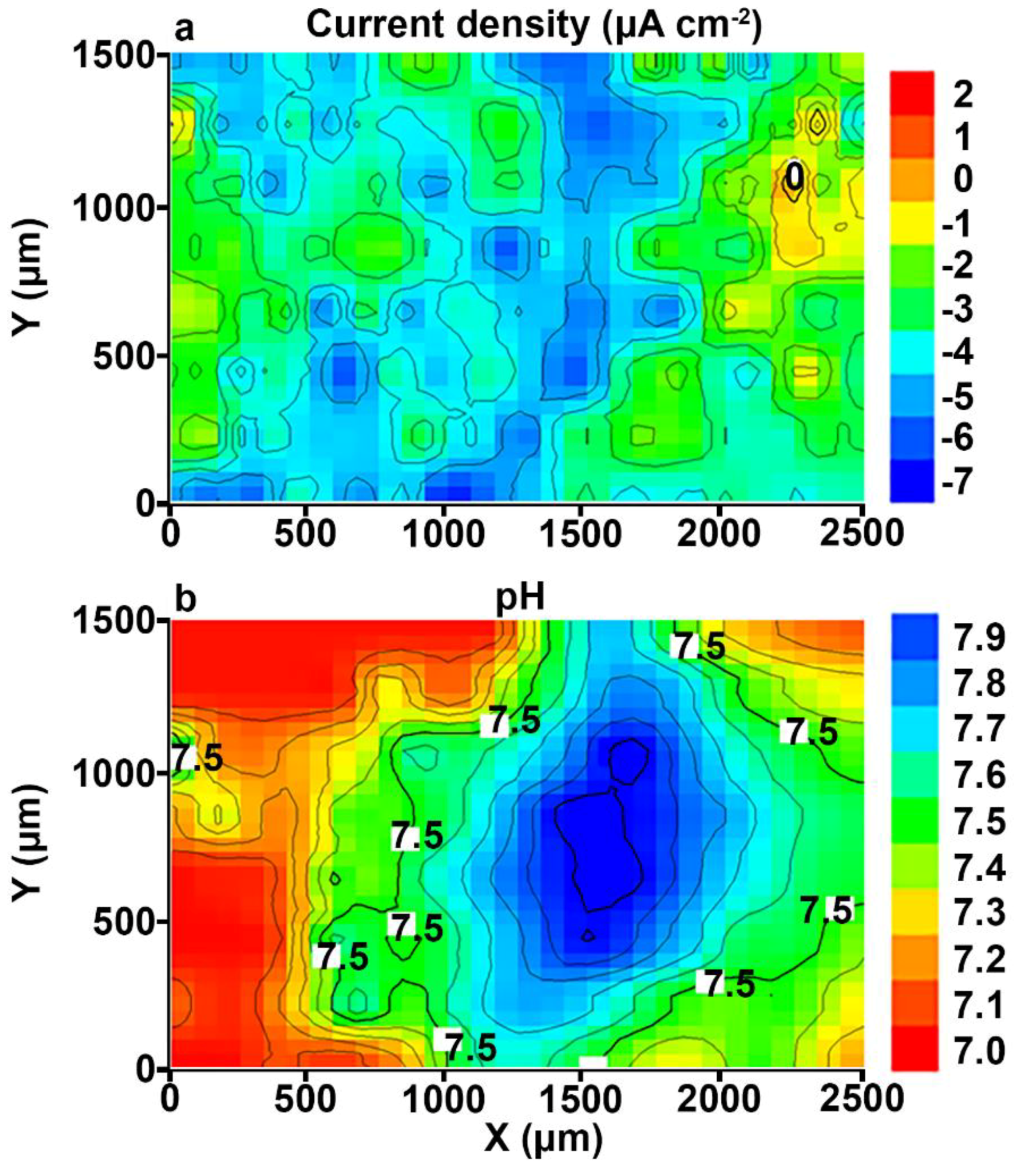
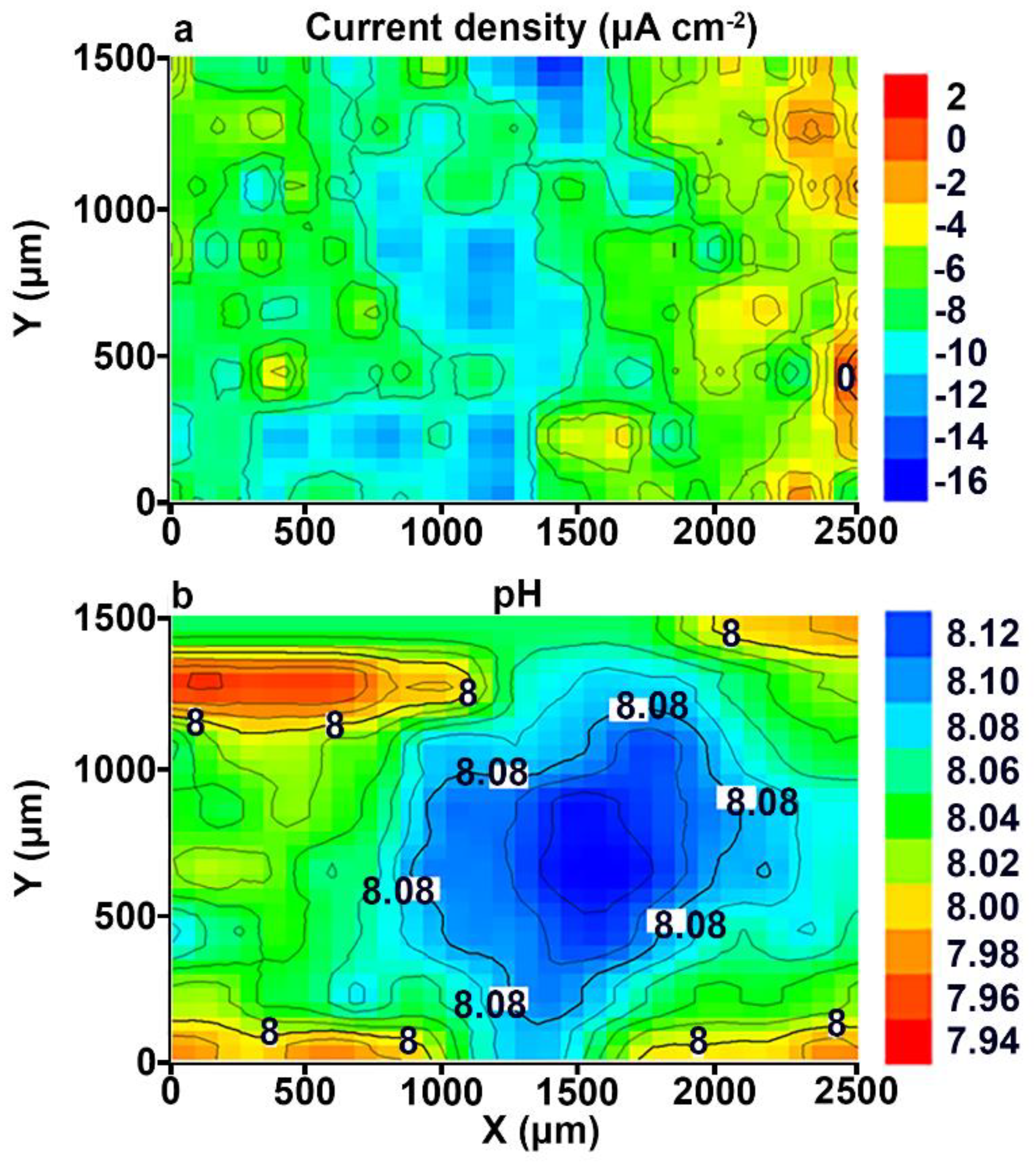
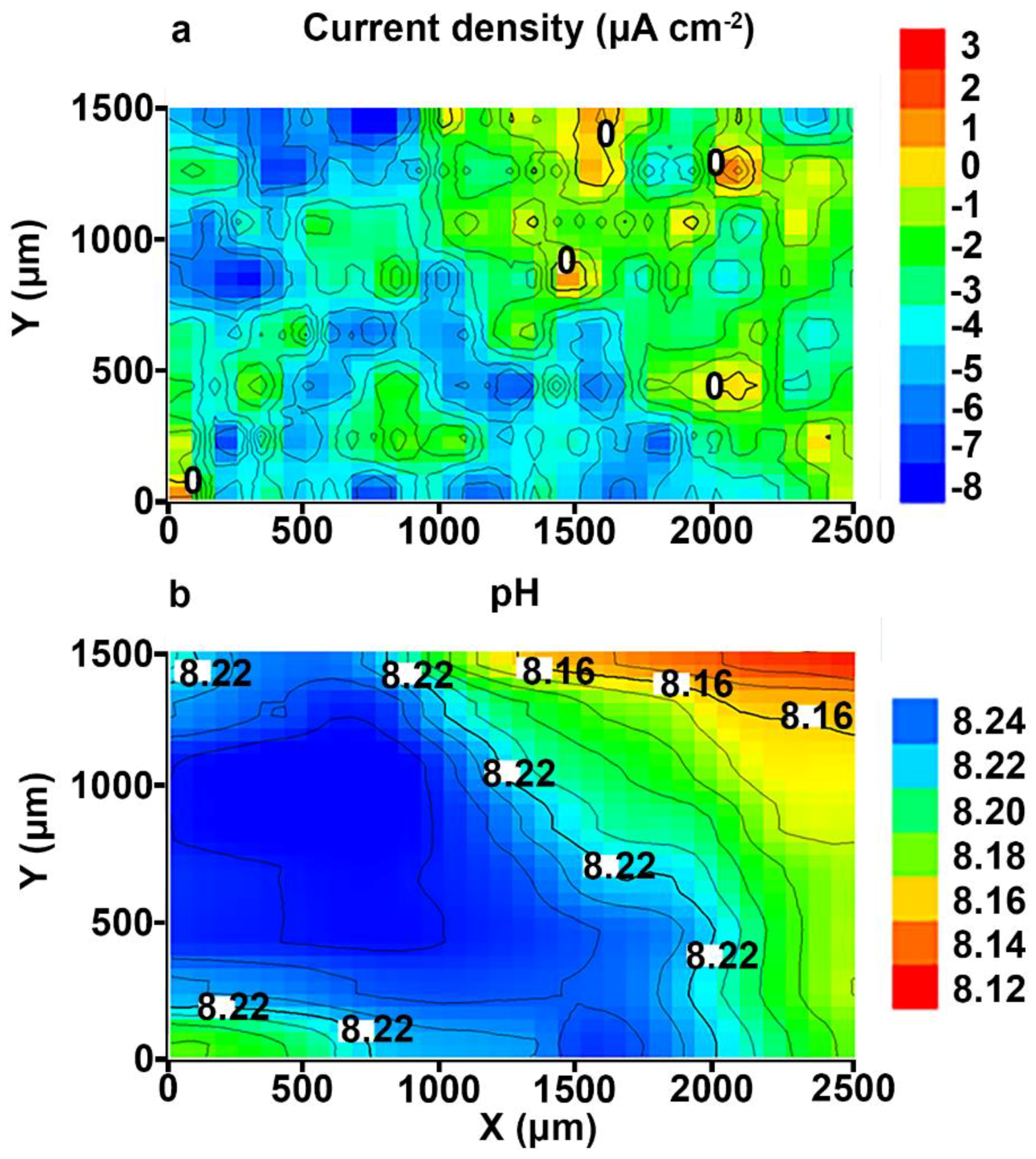
3.1. Study of the Localized Corrosion Process on the 1579 Al Alloy Sample with PEO-Coating
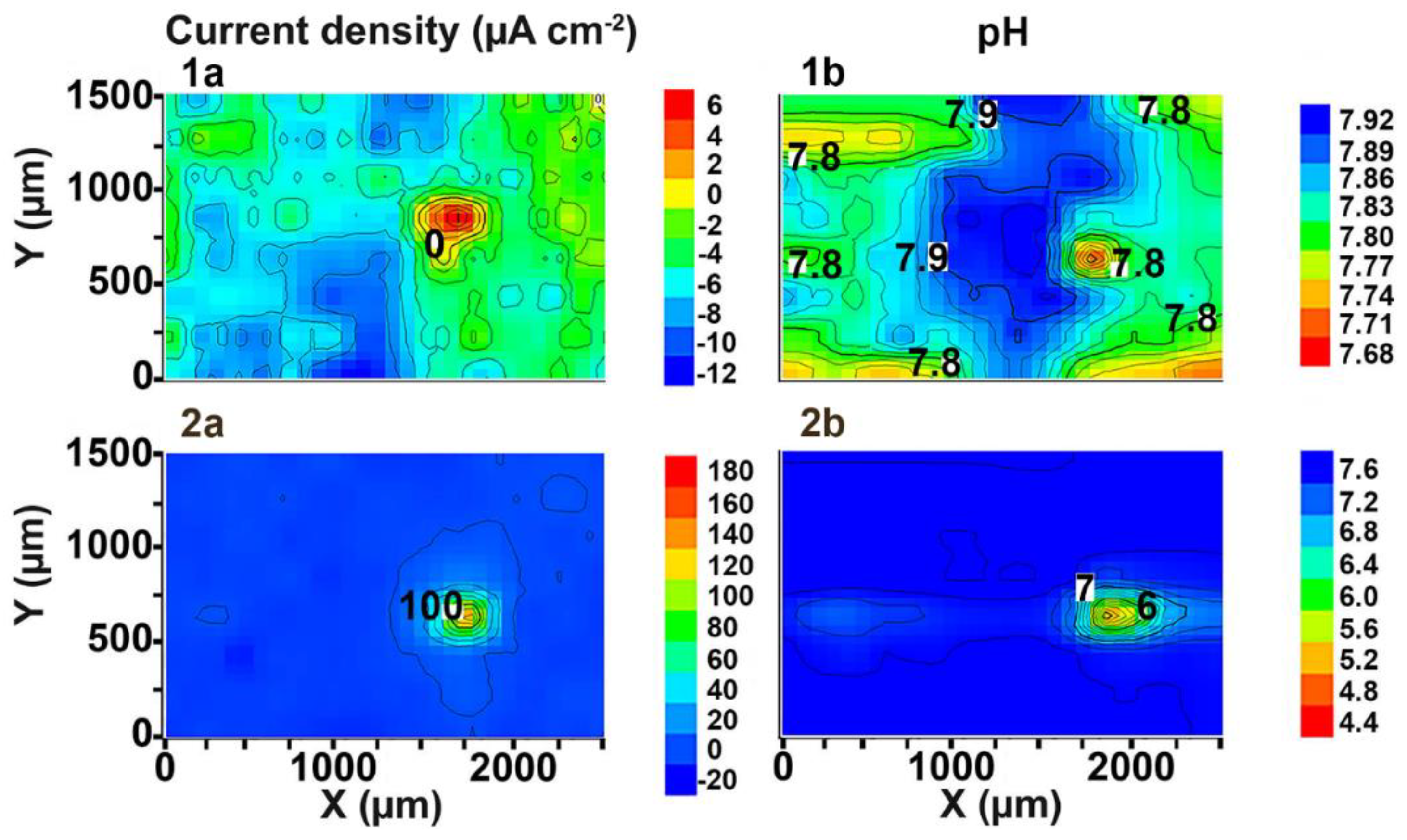
3.2. Study of the Localized Corrosion Process on the 1579 Al Alloy Sample with the Composite Polymer-Containing Coating
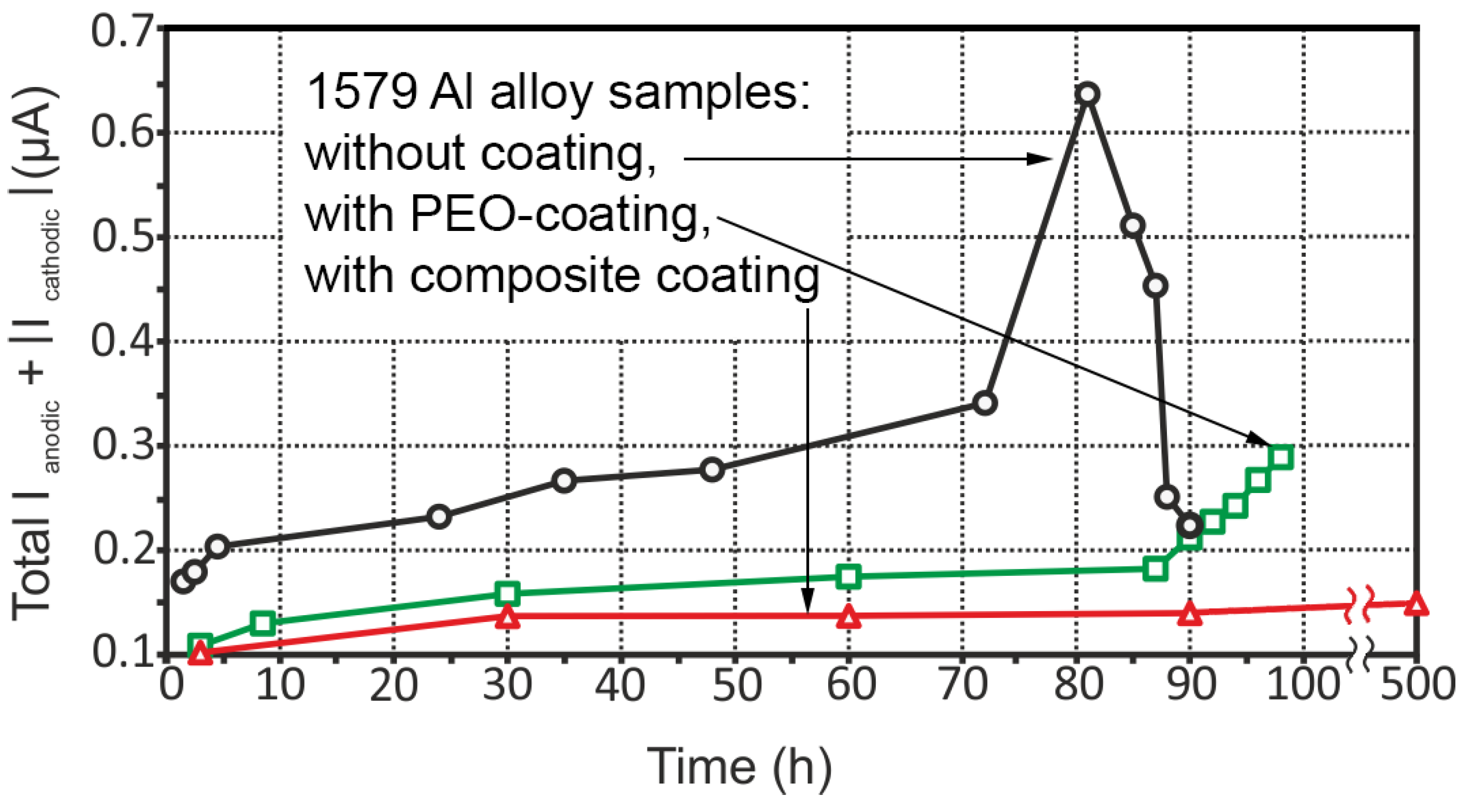
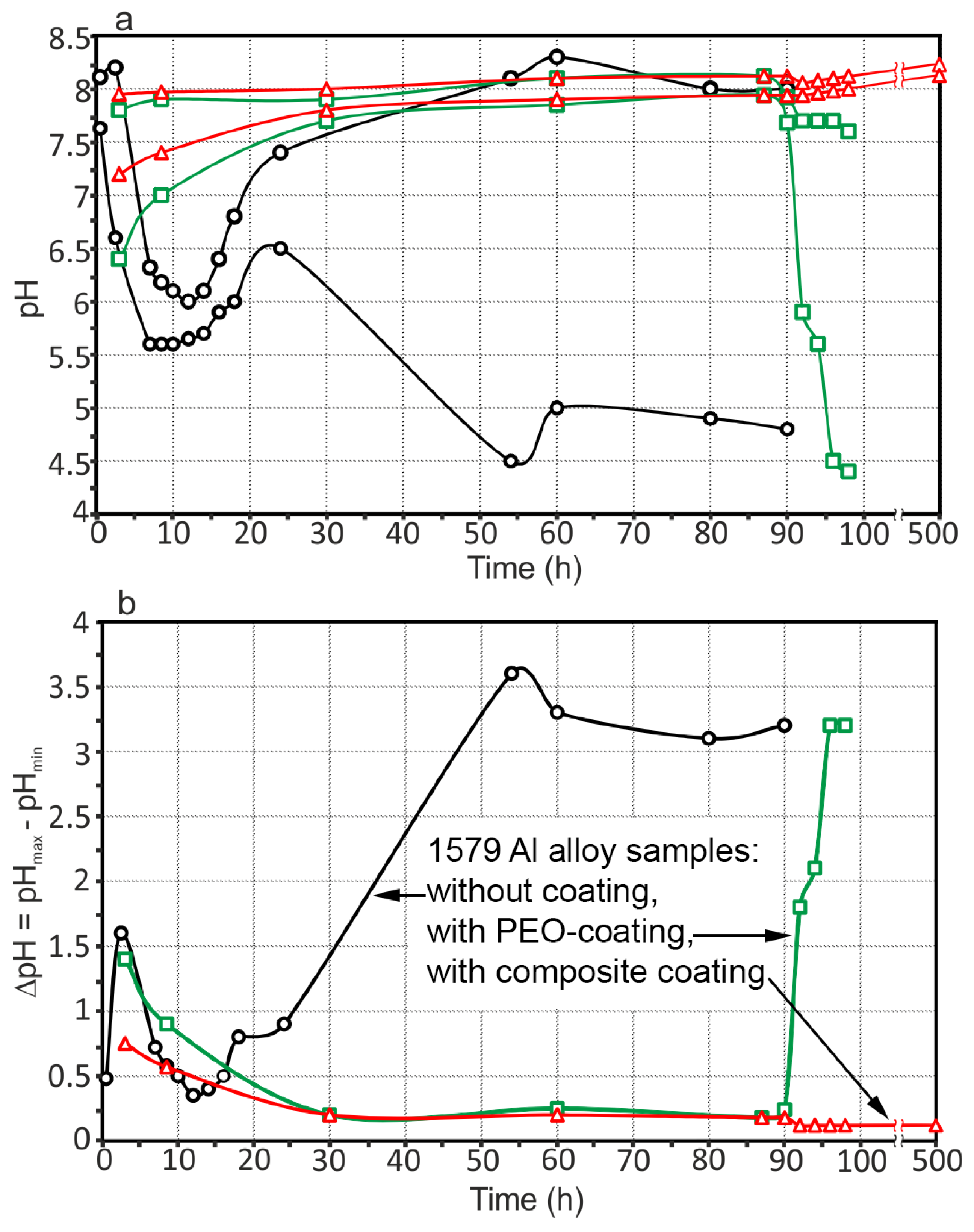
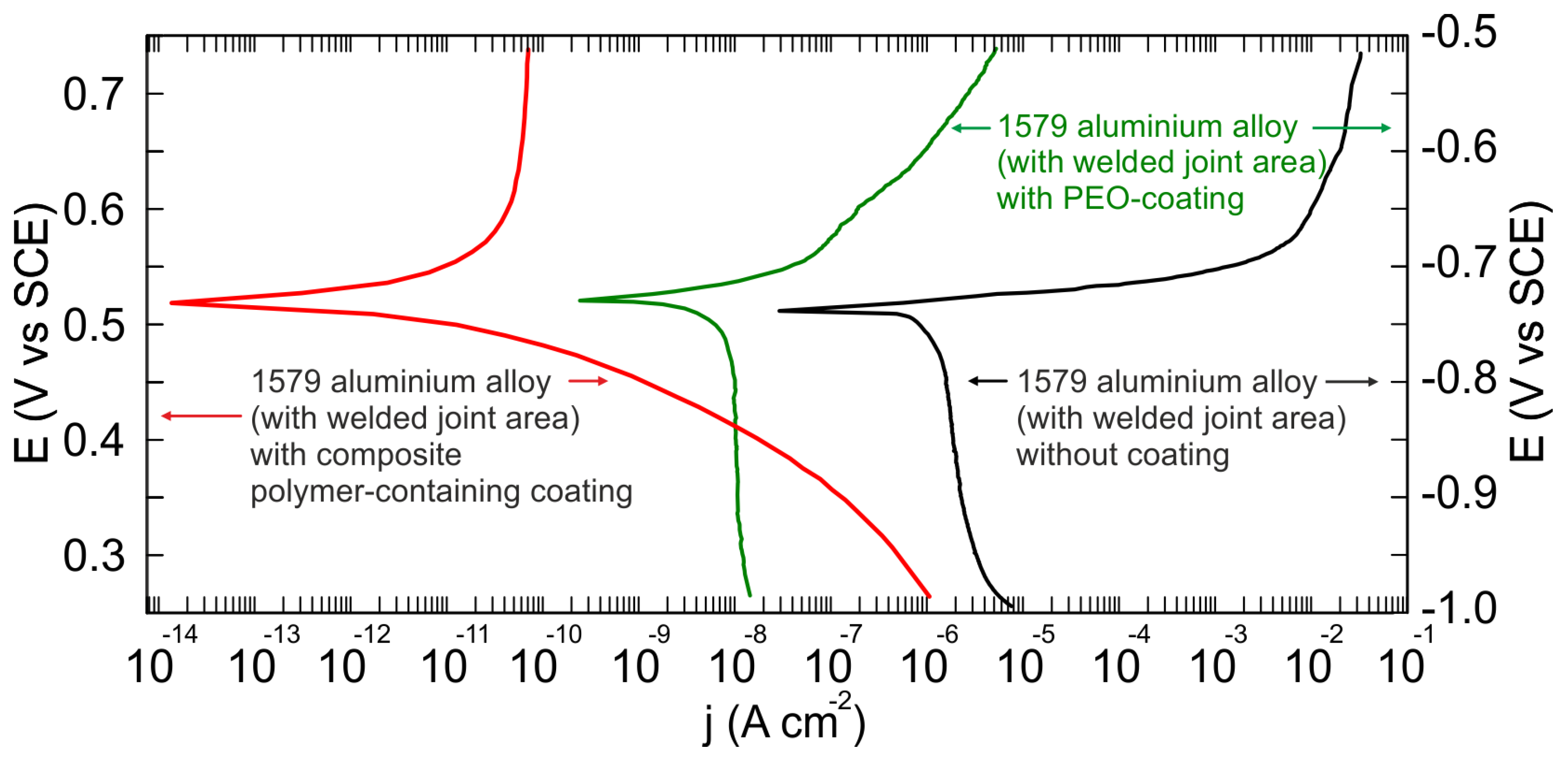
3.3. Corrosion Performance of the 1579 Al Alloy Samples
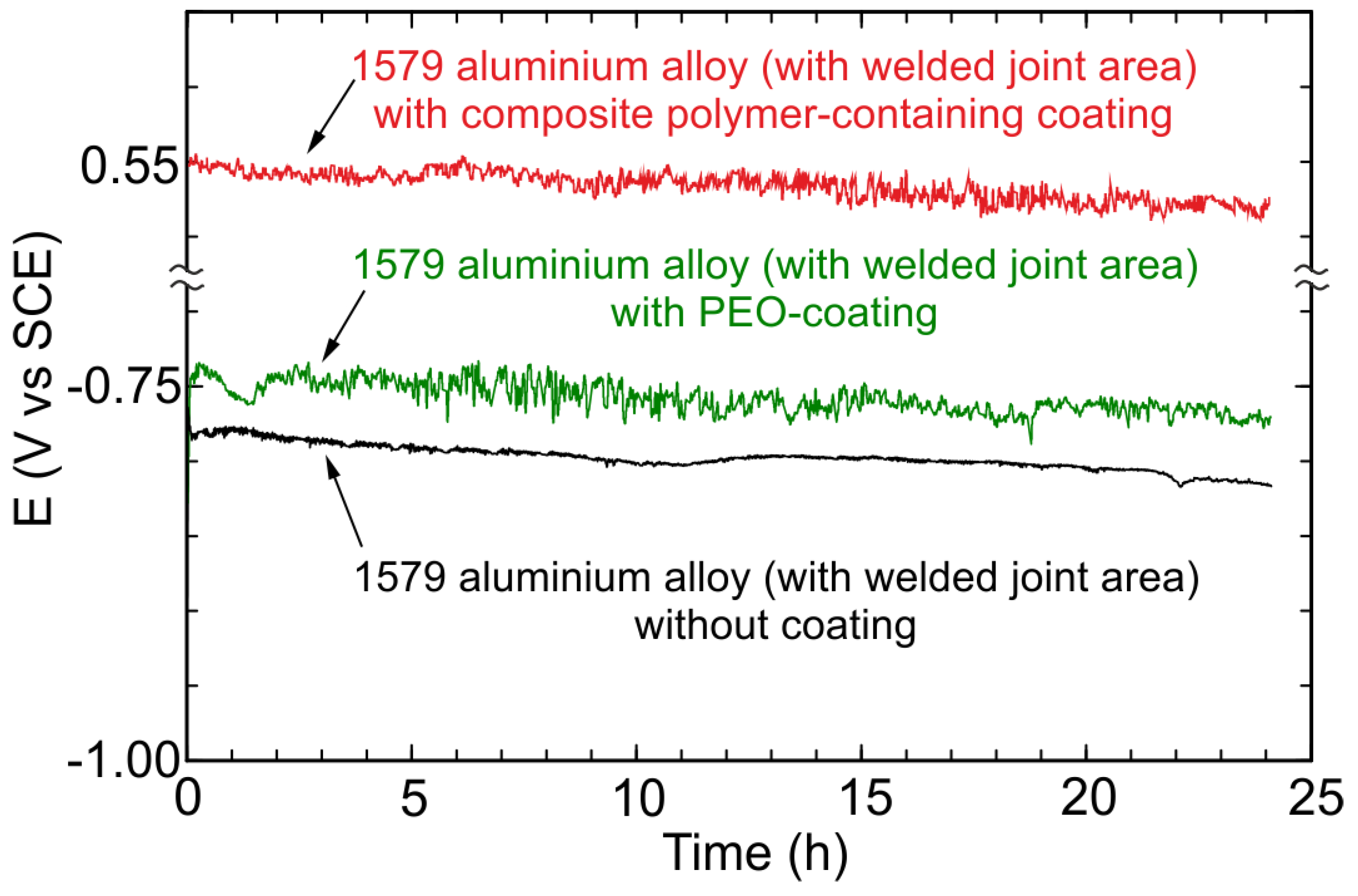
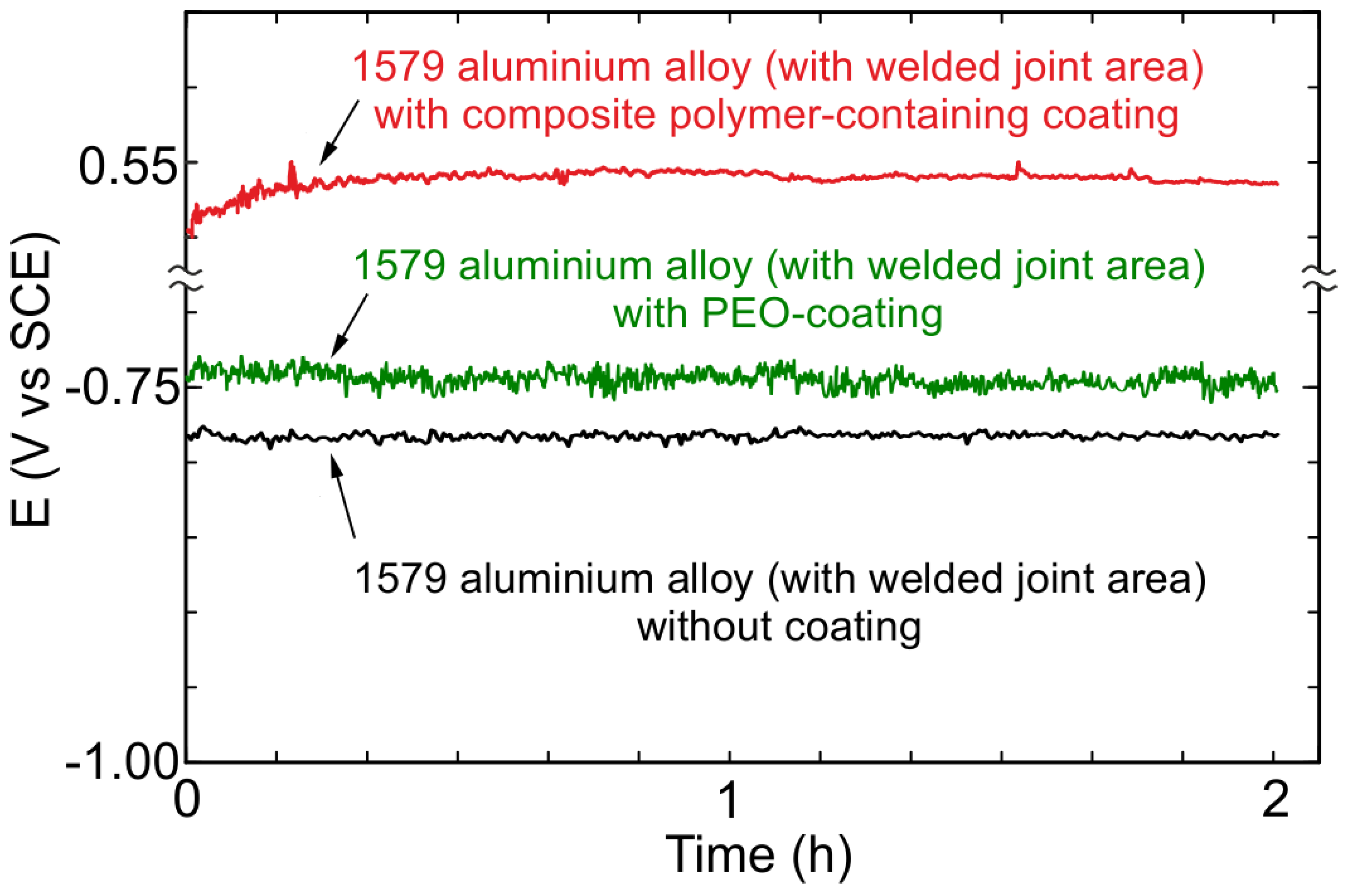
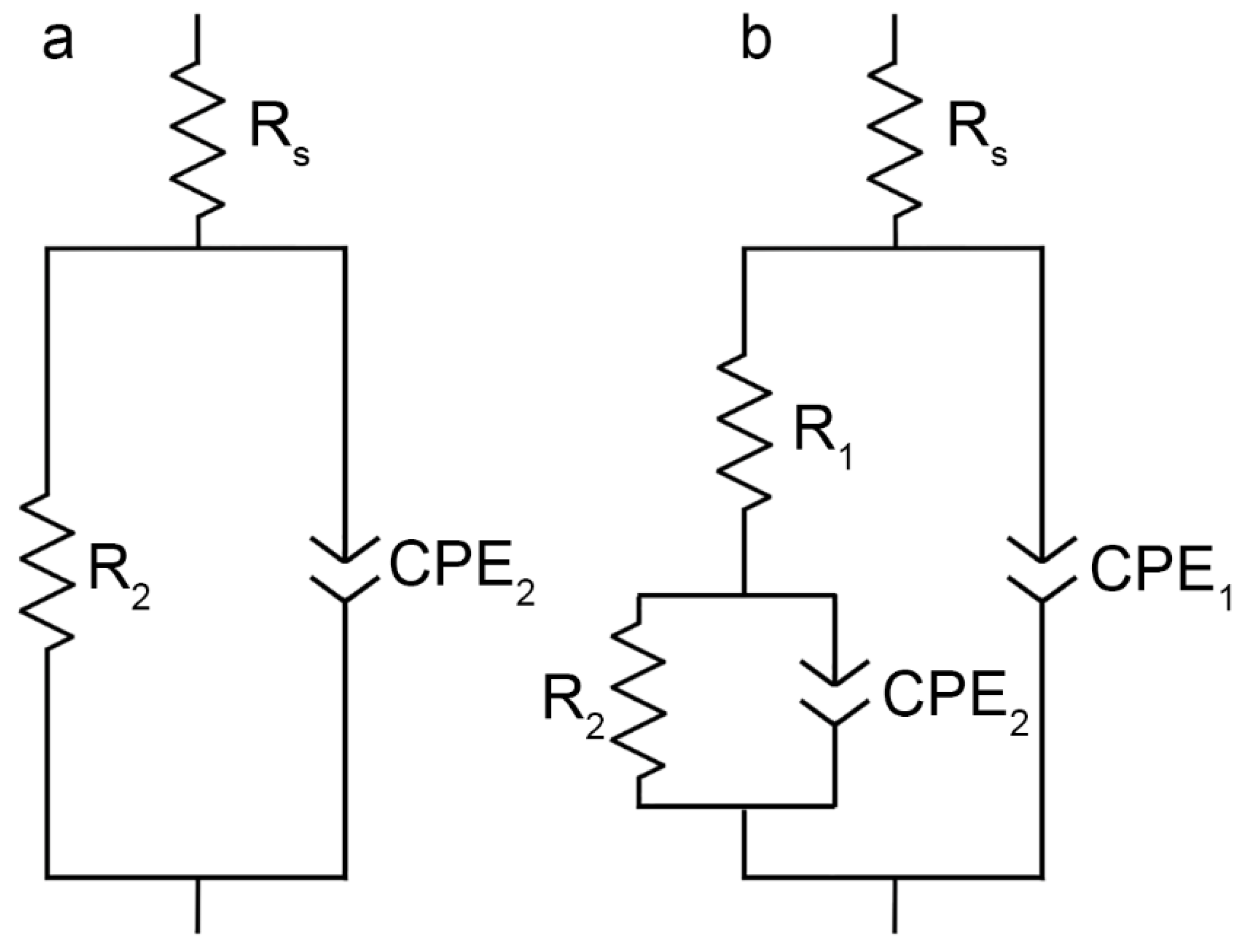
3.4. EIS, OCP, and PDP Studies of Electrochemical Properties of the 1579 Al Alloy Samples
4. Conclusions
- The coating obtained by the PEO method in tartrate-fluoride electrolyte decreases the corrosion intensity of the studied sample. The current density difference between anodic and cathodic areas for the PEO treated sample decreased by more than one order of magnitude as compared to the bare 1579 aluminium alloy, from 400 μA cm−2 down to 18 μA cm−2, according to SVET and SIET.
- According to SVET/SIET data, the area of the welded joint, which is an activator of the corrosion process in contact with the aggressive environment, became a cathodic zone due to the PEO-layer, which prevented the corrosion destruction of the studied material.
- The promising method of PEO-layer modification using superdispersed polytetrafluoroethylene (SPTFE) was applied. It has been established that single SPTFE treatment enables one to significantly improve the protective properties of the PEO-coated 1579 Al alloy (more than 5.5-fold), see the SVET/SIET data.
- Electrochemical methods (OCP, EIS, and PDP) showed a high level of sample protection using PEO-based coatings. The value of the impedance modulus at low frequency as well as that of the corrosion current density corroborated a significant corrosion inhibition in chloride-containing media for the Al alloy sample with a welded joint through the PEO-layer (240 kΩ cm2, 10 nA/cm2) and composite polymer-containing coating (93 MΩ cm2, 28 pA/cm2) formation.
- The combined results of Part 1 and Part 2 of this study indicate that SVET and SIET represent useful methods to characterize and to compare corrosion behaviour of the coated and uncoated samples with a welded joint in chloride-containing media. The data obtained using localized and conventional electrochemical methods enabled us to choose the appropriate multiplicity of PEO-coating treatment with polymer.
Author Contributions
Funding
Conflicts of Interest
References
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development. Materials 2018, 11, 2053. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-Y.; Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 1: Improved corrosion performance. Corros. Sci. 2015, 92, 118–126. [Google Scholar] [CrossRef]
- Jiang, M.-Y.; Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 2: Mechanistic investigations. Corros. Sci. 2015, 92, 127–135. [Google Scholar] [CrossRef]
- Coelho, L.B.; Cossement, D.; Olivier, M.-G. Benzotriazole and cerium chloride as corrosion inhibitors for AA2024-T3: An EIS investigation supported by SVET and ToF-SIMS analysis. Corros. Sci. 2018, 130, 177–189. [Google Scholar] [CrossRef]
- Agureev, L.; Savushkina, S.; Ashmarin, A.; Borisov, A.; Apelfeld, A.; Anikin, K.; Tkachenko, N.; Gerasimov, M.; Shcherbakov, A.; Ignatenko, V.; et al. Study of Plasma Electrolytic Oxidation Coatings on Aluminum Composites. Metals (Basel) 2018, 8, 459. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Bouali, A.; Höche, D.; Kuznetsov, B.; Karpushenkov, S.; Blawert, C.; Zheludkevich, M. Role of Phase Composition of PEO Coatings on AA2024 for In-Situ LDH Growth. Coatings 2017, 7, 190. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the magnesium alloys corrosion in the chloride-containing media. Solid State Phenom. 2014, 213, 143–148. [Google Scholar] [CrossRef]
- Němcová, A.; Skeldon, P.; Thompson, G.E.; Morse, S.; Čížek, J.; Pacal, B. Influence of plasma electrolytic oxidation on fatigue performance of AZ61 magnesium alloy. Corros. Sci. 2014, 82, 58–66. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S. Composite Protective Coatings on Nitinol Surface. Mater. Manuf. Process. 2008, 23, 879–883. [Google Scholar] [CrossRef]
- Shoaei-Rad, V.; Bayati, M.R.; Zargar, H.R.; Javadpour, J.; Golestani-Fard, F. In situ growth of ZrO2–Al2O3 nano-crystalline ceramic coatings via micro arc oxidation of aluminum substrates. Mater. Res. Bull. 2012, 47, 1494–1499. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Ivanou, D.K.; Yasakau, K.A.; Kallip, S.; Lisenkov, A.D.; Starykevich, M.; Lamaka, S.V.; Ferreira, M.G.S.; Zheludkevich, M.L.; Aparicio, M. Active corrosion protection coating for a ZE41 magnesium alloy created by combining PEO and sol–gel techniques. RSC Adv. 2016, 6, 12553–12560. [Google Scholar] [CrossRef]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.C.; Ferreira, M.G.S.; Zheludkevich, M.L. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Chizhikov, R.G. Increasing thickness and protective properties of PEO-coatings on aluminum alloy. Surf. Coat. Technol. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Chen, Y.; Song, X.; Zhou, J.; Liu, H.; Yang, Y. The Study on the Overall Plasma Electrolytic Oxidation for 6061–7075 Dissimilar Aluminum Alloy Welded Parts Based on the Dielectric Breakdown Theory. Materials (Basel) 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Matykina, E.; Arrabal, R.; Mohamed, A.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of pre-anodized aluminium. Corros. Sci. 2009, 51, 2897–2905. [Google Scholar] [CrossRef]
- Monfort, F.; Berkani, A.; Matykina, E.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte. Corros. Sci. 2007, 49, 672–693. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Belca, I.; Petkovic, M.; Kasalica, B.; Nedic, Z.; Zekovic, L. Characterization of the plasma electrolytic oxidation of aluminium in sodium tungstate. Corros. Sci. 2010, 52, 3258–3265. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S.; Vyaliy, I.E.; Mashtalyar, D.V.; Egorkin, V.S. Plasma electrolytic oxidation coatings on titanium formed with microsecond current pulses. Solid State Phenom. 2014, 213, 149–153. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Du, N.; Li, X.; Zhao, Q. Evolution of the Three-Dimensional Structure and Growth Model of Plasma Electrolytic Oxidation Coatings on 1060 Aluminum Alloy. Coatings 2018, 8, 105. [Google Scholar] [CrossRef]
- Sieber, M.; Simchen, F.; Morgenstern, R.; Scharf, I.; Lampke, T. Plasma Electrolytic Oxidation of High-Strength Aluminium Alloys—Substrate Effect on Wear and Corrosion Performance. Metals (Basel) 2018, 8, 356. [Google Scholar] [CrossRef]
- Zhang, Y.; Blawert, C.; Tang, S.; Hu, J.; Mohedano, M.; Zheludkevich, M.L.; Kainer, K.U. Influence of surface pre-treatment on the deposition and corrosion properties of hydrophobic coatings on a magnesium alloy. Corros. Sci. 2016, 112, 483–494. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Guo, L.; Ouyang, J.-H. Microstructure and corrosion resistance of modified 2024 Al alloy using surface mechanical attrition treatment combined with microarc oxidation process. Corros. Sci. 2011, 53, 473–480. [Google Scholar] [CrossRef]
- Ivanou, D.K.; Starykevich, M.; Lisenkov, A.D.; Zheludkevich, M.L.; Xue, H.B.; Lamaka, S.V.; Ferreira, M.G.S. Plasma anodized ZE41 magnesium alloy sealed with hybrid epoxy-silane coating. Corros. Sci. 2013, 73, 300–308. [Google Scholar] [CrossRef]
- Li, Z.; Jing, X.; Yuan, Y.; Zhang, M. Composite coatings on a Mg-Li alloy prepared by combined plasma electrolytic oxidation and sol-gel techniques. Corros. Sci. 2012, 63, 358–366. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Insights into plasma electrolytic oxidation treatment with particle addition. Corros. Sci. 2015, 101, 201–207. [Google Scholar] [CrossRef]
- Cui, X.; Lin, X.; Liu, C.; Yang, R.; Zheng, X.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Guo, X.; Du, K.; Guo, Q.; Wang, Y.; Wang, R.; Wang, F. Effect of phytic acid on the corrosion inhibition of composite film coated on Mg-Gd-Y alloy. Corros. Sci. 2013, 76, 129–141. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Zhang, C. Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci. 2017, 114, 146–155. [Google Scholar] [CrossRef]
- Guo, X.; Du, K.; Guo, Q.; Wang, Y.; Wang, F. Experimental study of corrosion protection of a three-layer film on AZ31B Mg alloy. Corros. Sci. 2012, 65, 367–375. [Google Scholar] [CrossRef]
- Ghafaripoor, M.; Raeissi, K.; Santamaria, M.; Hakimizad, A. The corrosion and tribocorrosion resistance of PEO composite coatings containing α-Al2O3 particles on 7075 Al alloy. Surf. Coat. Technol. 2018, 349, 470–479. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Liskiewicz, T.; Yerokhin, A.; Korenyi-Both, A.; Zabinski, J.; Lin, M.; Matthews, A.; Voevodin, A.A. Fretting wear behavior of duplex PEO/chameleon coating on Al alloy. Surf. Coat. Technol. 2018, 352, 238–246. [Google Scholar] [CrossRef]
- Sung, D.; Kim, D.; Park, J.-H.; Kim, Y.; Chung, W. Effect of composite PEO film containing titanium oxides on the corrosion resistance of Al 6061 alloy. Surf. Coat. Technol. 2017, 309, 698–702. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Sidorova, M.V.; Gnedenkov, A.S. Composite polymer-containing protective coatings on magnesium alloy MA8. Corros. Sci. 2014, 85, 52–59. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Zavidnaya, A.G.; Sinebrukhov, S.L.; Kovryanov, A.N.; Scorobogatova, T.M.; Gordienko, P.S. Production of hard and heat-resistant coatings on aluminium using a plasma micro-discharge. Surf. Coat. Technol. 2000, 123, 24–28. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective properties of inhibitor-containing composite coatings on a Mg alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Taryba, M.; Montemor, M.F.; Isaacs, H.S.; Ferreira, M.G.S. Quasi-simultaneous measurements of ionic currents by vibrating probe and pH distribution by ion-selective microelectrode. Electrochem. Commun. 2011, 13, 20–23. [Google Scholar] [CrossRef]
- Alvarez-Pampliega, A.; Lamaka, S.V.; Taryba, M.G.; Madani, M.; De Strycker, J.; Tourwé, E.; Ferreira, M.G.S.; Terryn, H. Cut-edge corrosion study on painted aluminum rich metallic coated steel by scanning vibrating electrode and micro-potentiometric techniques. Electrochim. Acta 2012, 61, 107–117. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An innovative specimen configuration for the study of Mg corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Hofstetter, J.; Uggowitzer, P.J.; Song, G.; Liu, M.; Atrens, A. Corrosion of ultra-high-purity Mg in 3.5% NaCl solution saturated with Mg(OH)2. Corros. Sci. 2013, 75, 78–99. [Google Scholar] [CrossRef]
- Golestani-Fard, F.; Bayati, M.R.; Zargar, H.R.; Abbasi, S.; Rezaei, H.R. MAO-preparation of nanocrystalline hydroxyapatite–titania composite films: Formation stages and effect of the growth time. Mater. Res. Bull. 2011, 46, 2422–2426. [Google Scholar] [CrossRef]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Cerium-based sealing of PEO coated AM50 magnesium alloy. Surf. Coat. Technol. 2015, 269, 145–154. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Microscale morphology and properties of the PEO-coating surface. Phys. Procedia 2012, 23, 98–101. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L. Electrochemical Impedance Spectroscopy of Oxide Layers on the Titanium Surface. Russ. J. Electrochem. 2005, 41, 858–865. [Google Scholar] [CrossRef]
- Sobolev, A.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. An Investigation of Oxide Coating Synthesized on an Aluminum Alloy by Plasma Electrolytic Oxidation in Molten Salt. Appl. Sci. 2017, 7, 889. [Google Scholar] [CrossRef]
- Liu, H.; Pan, W.; Si, F.; Huang, K.; Liu, Y.; Liu, J. Enhanced Compressive Property of Al Composite Foams at Elevated Temperatures via Plasma Electrolytic Oxidation. Metals (Basel) 2018, 8, 118. [Google Scholar] [CrossRef]
- Arrabal, R.; Mota, J.M.; Criado, A.; Pardo, A.; Mohedano, M.; Matykina, E. Assessment of duplex coating combining plasma electrolytic oxidation and polymer layer on AZ31 magnesium alloy. Surf. Coat. Technol. 2012, 206, 4692–4703. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II.: EIS interpretation of corrosion behaviour. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Barchiche, C.-E.; Rocca, E.; Hazan, J. Corrosion behaviour of Sn-containing oxide layer on AZ91D alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2008, 202, 4145–4152. [Google Scholar] [CrossRef]
- Akbari, E.; Franco, F.D.; Ceraolo, P.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Electrochemically-induced TiO2 incorporation for enhancing corrosion and tribocorrosion resistance of PEO coating on 7075 Al alloy. Corros. Sci. 2018, 143, 314–328. [Google Scholar] [CrossRef]
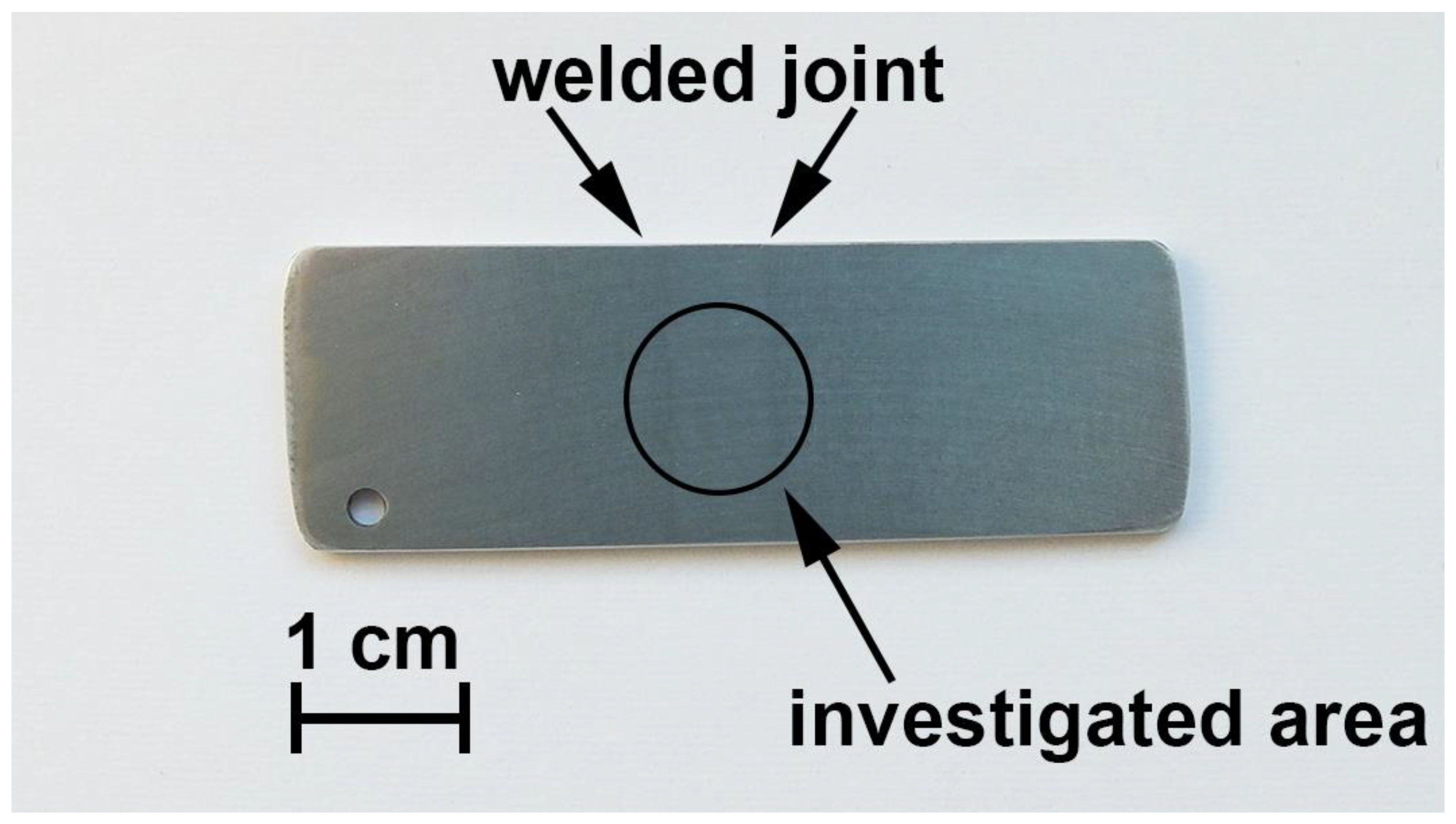

| Mg | Zn | Cu | Si | Fe | Zr | Sc | Cr | Ni | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 6.78 | 0.62 | 0.14 | 0.51 | 0.15 | 0.13 | 0.13 | 0.17 | 0.10 | 0.02 | balance |
| Sample # | Type of Sample Surface | |Z|f=0.1Hz (Ω cm2) | IC (A/cm2) | EC (V vs. SCE) | βa (mV/Decade) | −βc (mV/Decade) |
|---|---|---|---|---|---|---|
| 1 | Without coating | 1.3 × 103 | 1.4 × 10−6 | −0.739 | 16.7 | 1114.8 |
| 2 | PEO-layer | 2.4 × 105 | 1.0 × 10−8 | −0.728 | 63.8 | 1320.0 |
| 3 | Composite polymer-containing coating | 9.3 × 107 | 2.8 × 10−11 | 0.523 | 285.24 | 89.9 |
| Sample # | Type of Sample Surface | CPE1 | R1 (Ω cm2) | CPE2 | R2 (Ω cm2) | ||
|---|---|---|---|---|---|---|---|
| Q (S cm−2 sn) | n | Q (S cm−2 sn) | n | ||||
| 1 | Without coating | – | – | – | 1.2 × 10−5 | 0.95 | 4.3 × 103 |
| 2 | PEO-layer | 4.4 × 10−7 | 0.76 | 2.7 × 102 | 8.9 × 10−7 | 0.85 | 2.7 × 105 |
| 3 | Composite polymer-containing coating | 3.7 × 10−10 | 0.97 | 7.9 × 105 | 1.9 × 10−8 | 0.87 | 6.8 × 108 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials 2018, 11, 2177. https://doi.org/10.3390/ma11112177
Gnedenkov AS, Sinebryukhov SL, Mashtalyar DV, Vyaliy IE, Egorkin VS, Gnedenkov SV. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials. 2018; 11(11):2177. https://doi.org/10.3390/ma11112177
Chicago/Turabian StyleGnedenkov, Andrey S., Sergey L. Sinebryukhov, Dmitry V. Mashtalyar, Igor E. Vyaliy, Vladimir S. Egorkin, and Sergey V. Gnedenkov. 2018. "Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection" Materials 11, no. 11: 2177. https://doi.org/10.3390/ma11112177
APA StyleGnedenkov, A. S., Sinebryukhov, S. L., Mashtalyar, D. V., Vyaliy, I. E., Egorkin, V. S., & Gnedenkov, S. V. (2018). Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials, 11(11), 2177. https://doi.org/10.3390/ma11112177