Lubrication Performance of Graphene as Lubricant Additive in 4-n-pentyl-4′-cyanobiphyl Liquid Crystal (5CB) for Steel/Steel Contacts
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Graphene/5CB Suspension
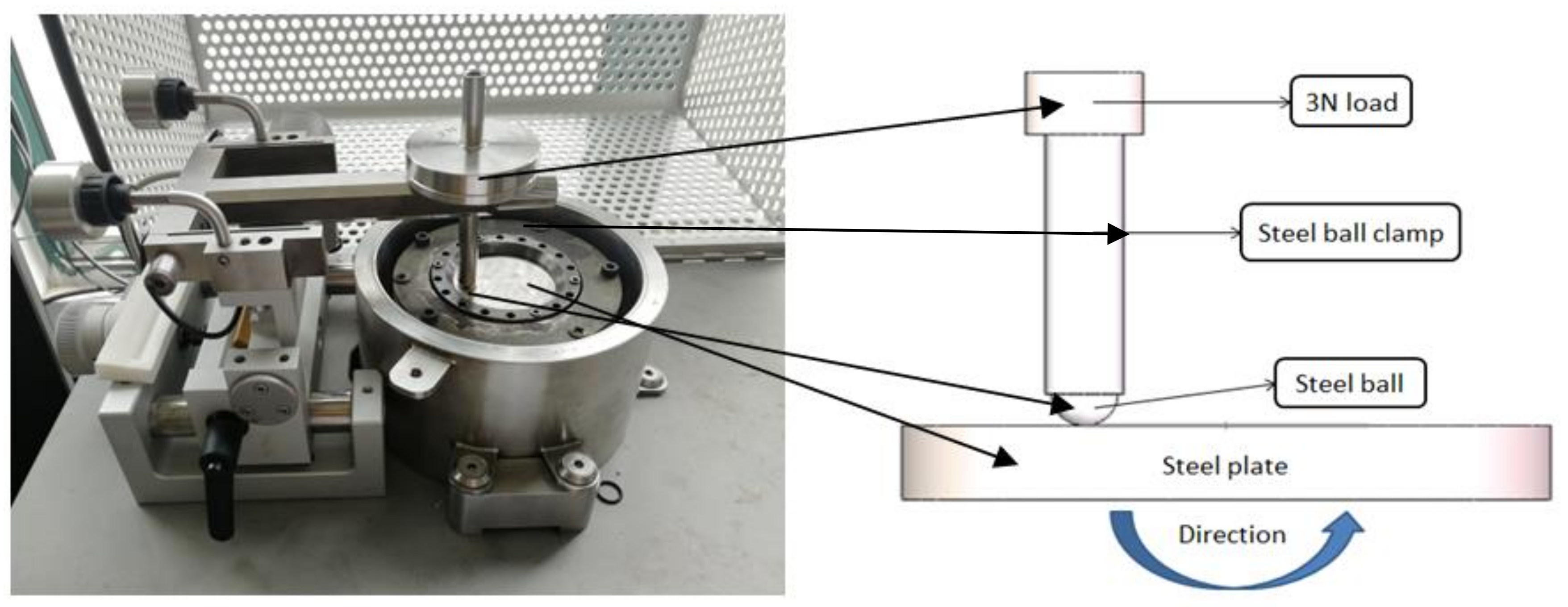
2.3. Friction Tests
2.4. Surface Analyses
3. Results and Discussion
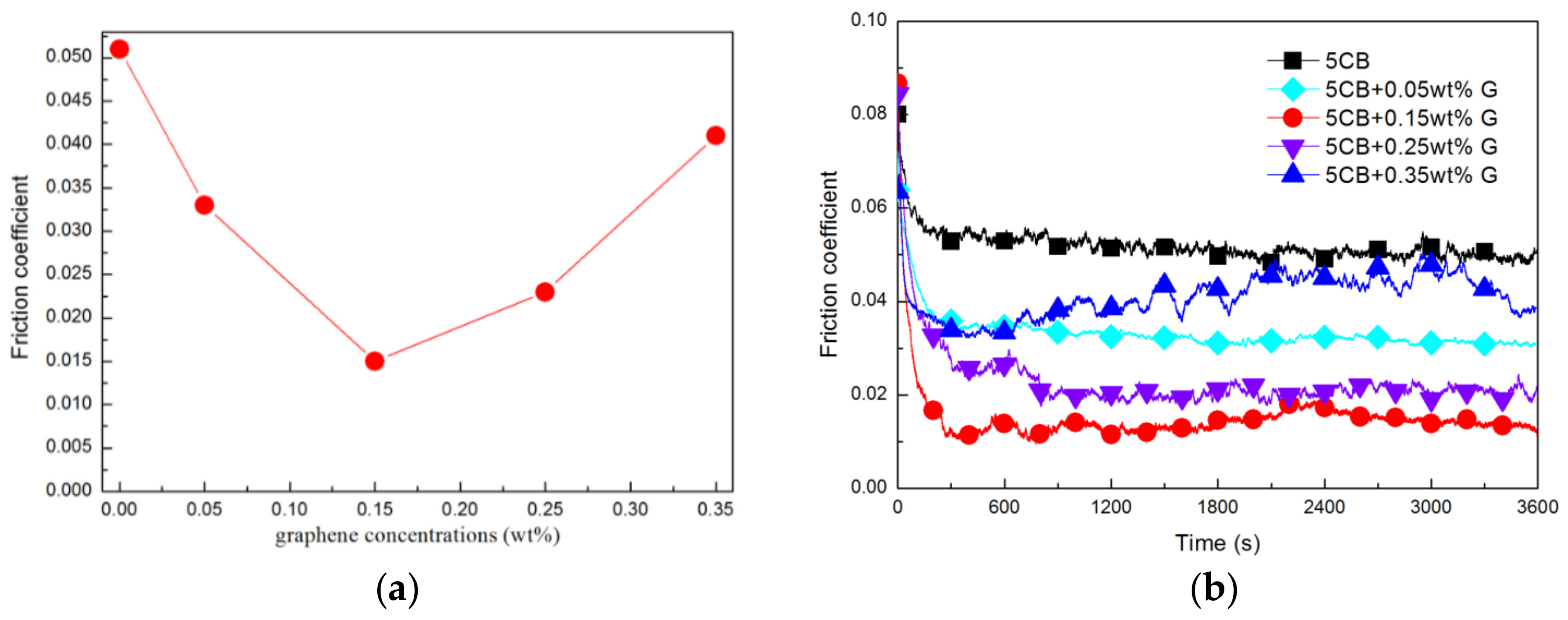
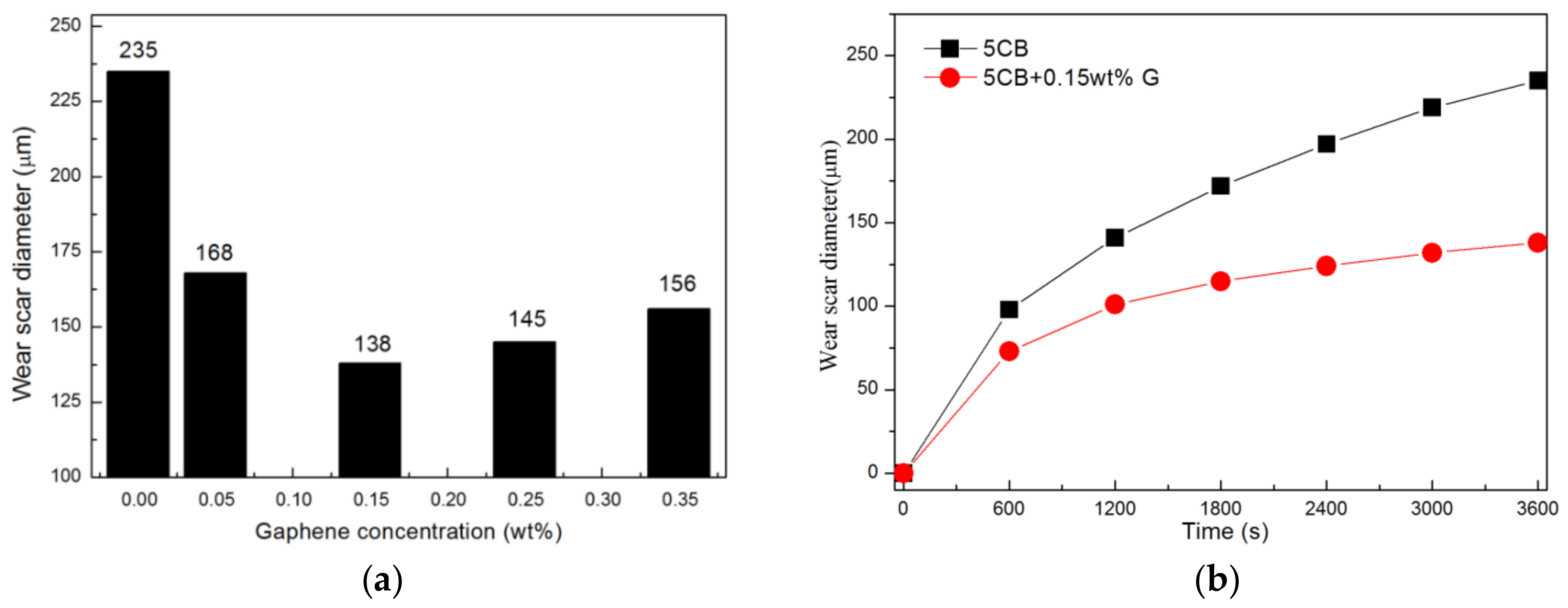
3.1. Influence of Graphene Concentration on Lubrication Performance
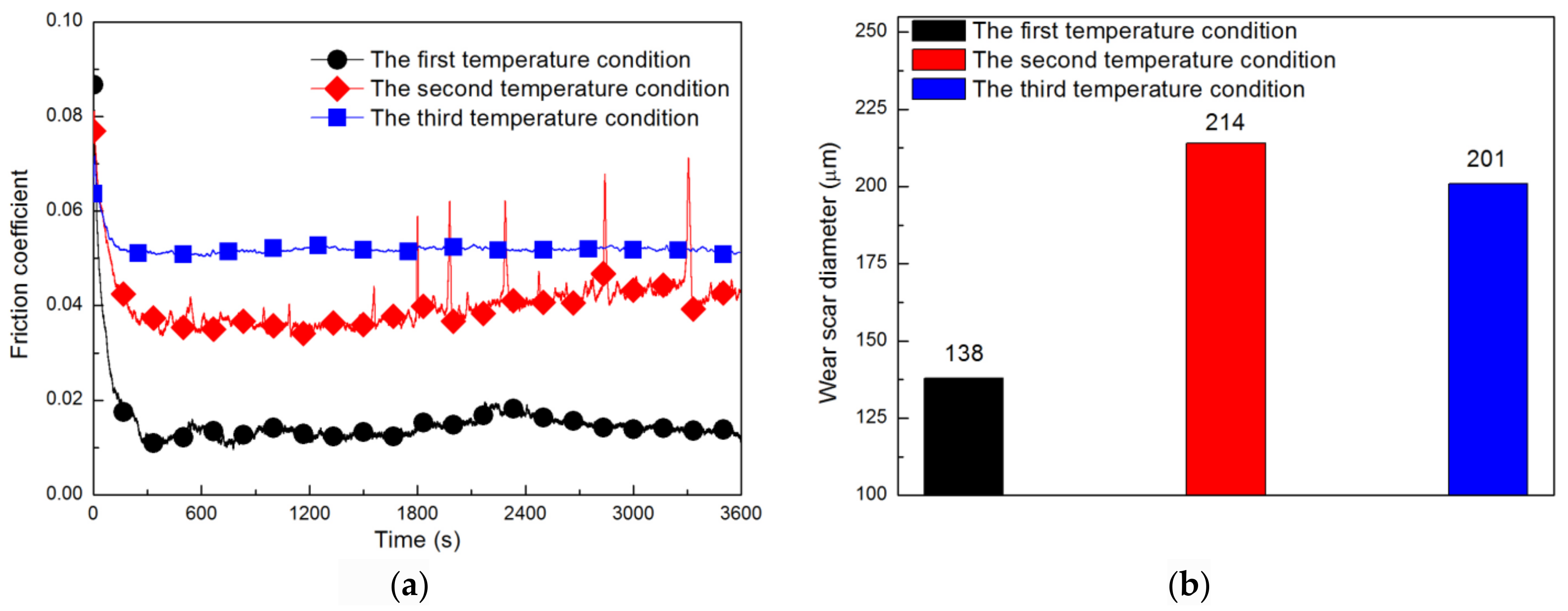
3.2. Influence of Temperature on Lubrication Performance
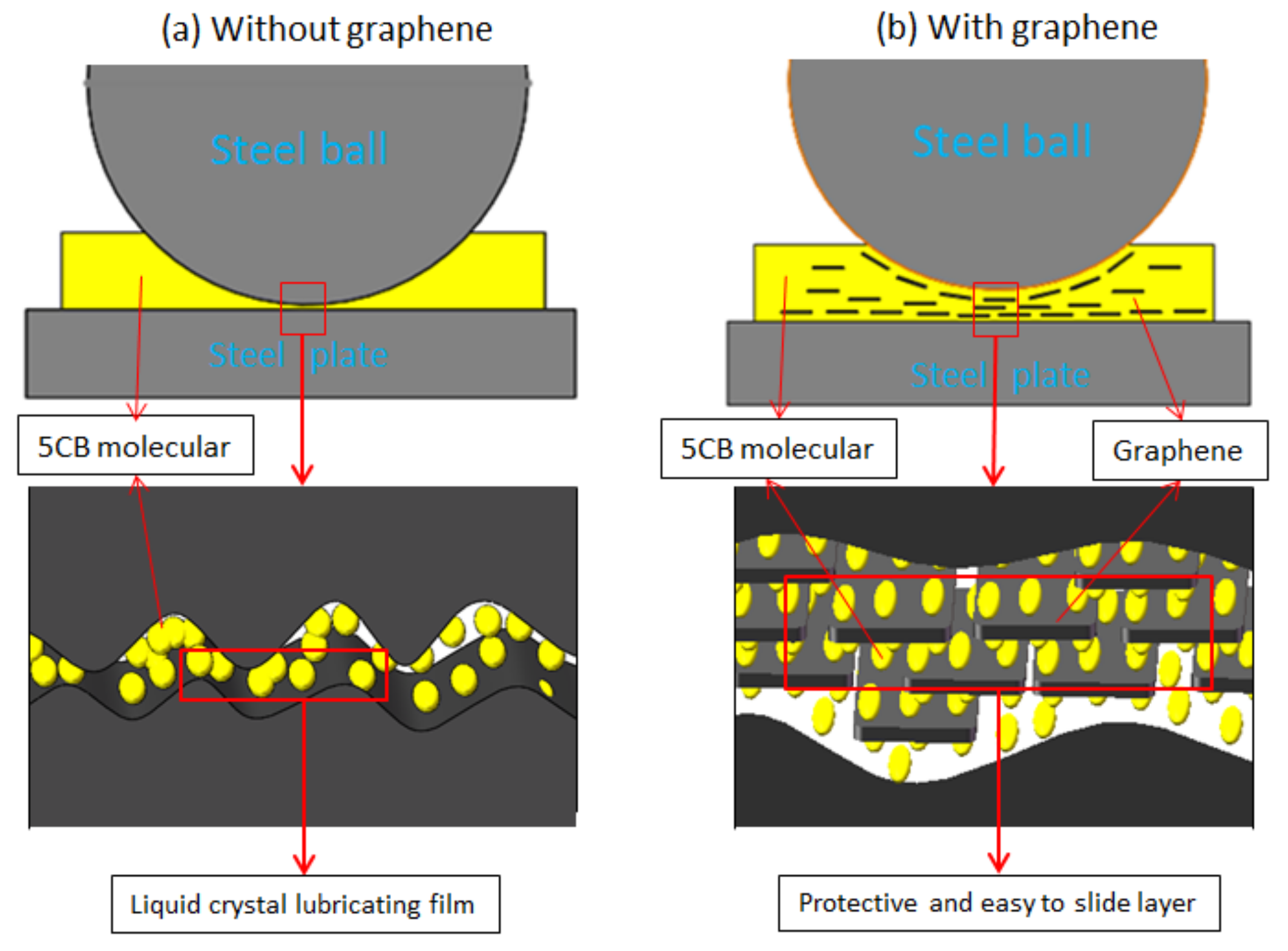
3.3. Analysis and Discussion on Lubrication Mechanism
3.3.1. Wear Surface Morphology of Steel Balls under Optical Microscope
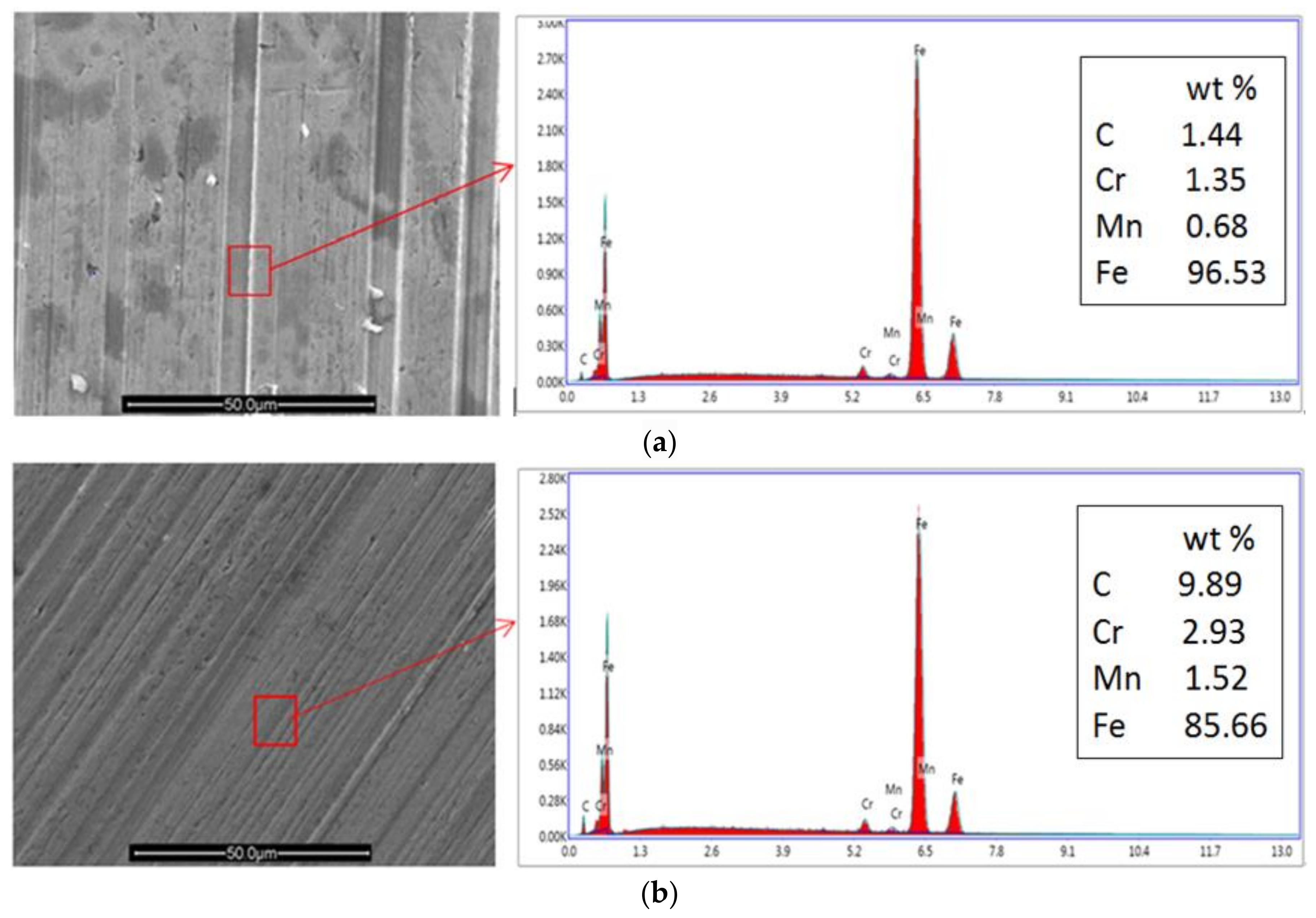
3.3.2. SEM and EDS Analysis
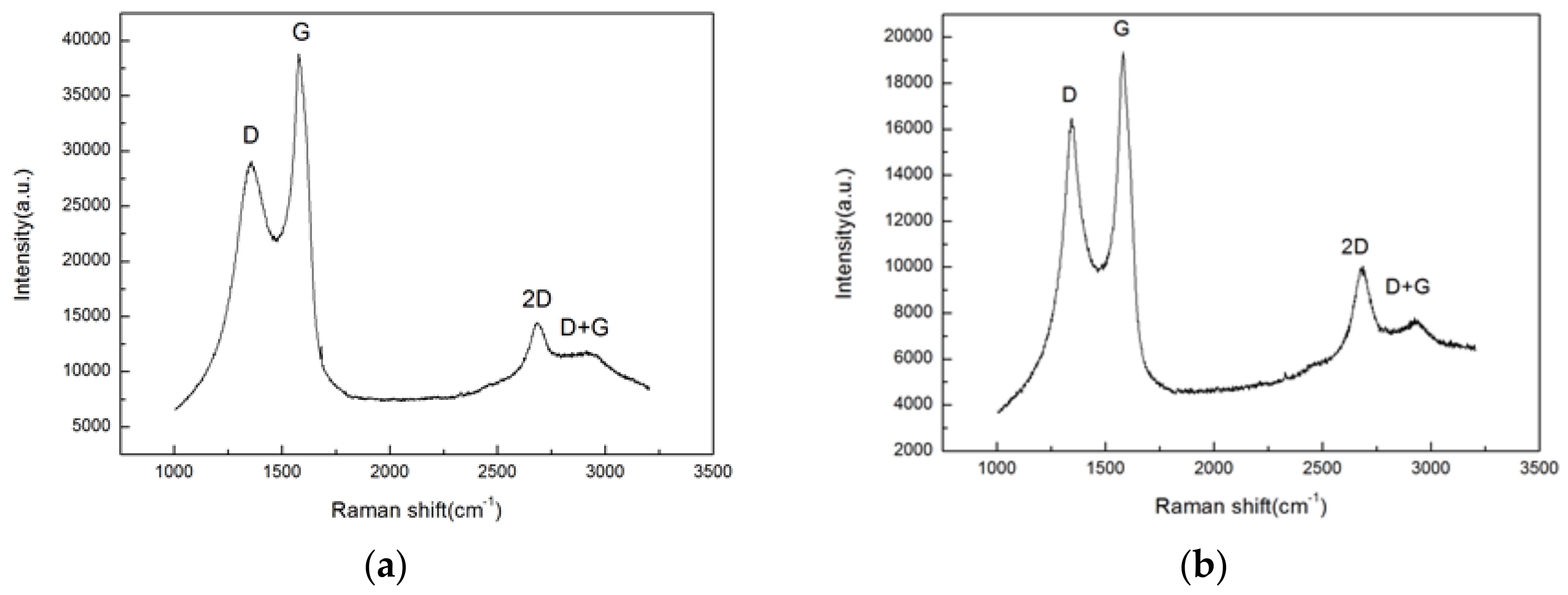
3.3.3. Raman Spectroscopy Analysis
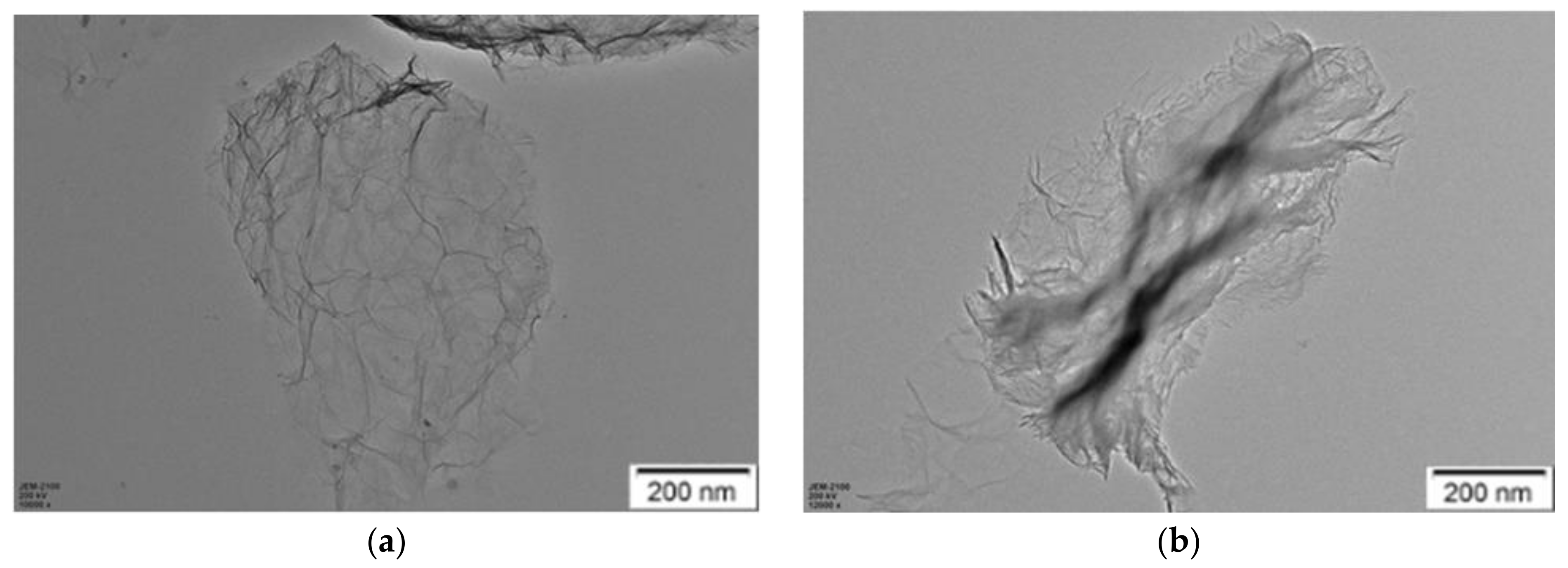
3.3.4. TEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tung, S.C.; McMillan, M.L. Automotive tribology overview of current advances and challenges for the future. Tribol. Int. 2004, 37, 517–536. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, C.; Hwang, Y.; Park, M.; Lee, J.; Choi, C.; Jung, M. Tribological behavior of copper nanoparticles as additives in oil. Curr. Appl. Phys. 2009, 9, e124e7. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E. Materials: Introduction and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 242–248. ISBN 978-0-470-52379-7. [Google Scholar]
- Fischer, T.E.; Bhattacharya, S.; Salher, R.; Lauer, J.L.; Ahn, Y.J. Lubrication by a Smectic Liquid Crystal. Tribol. Trans. 1988, 31, 442–448. [Google Scholar] [CrossRef]
- Mansare, T.; Decressain, R.; Gors, C.; Dolganov, V.K. Phase Transformations and Dynamics of 4-cyano-4′-pentylbiphenyl (5CB) By Nuclear Magnetic Resonance, Analysis Differential Scanning Calorimetry, And Wideangle X-Ray Diffraction Analysis. Mol. Cryst. Liq. Cryst. 2002, 382, 97–111. [Google Scholar] [CrossRef]
- Tsai, T.R.; Chen, C.Y.; Pan, C.L.; Pan, R.P.; Zhang, X.C. Terahertz time-domain spectroscopy studies of the optical constants of the nematic liquid crystal 5CB. Appl. Opt. 2003, 42, 2372–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, H.; Wang, J.; Ye, X.; Lei, W.; Xue, M.; Tang, H.; Li, C. Synthesis of Ultrathin WS2 Nanosheets and Their Tribological Properties as Lubricant Additives. Nanoscale Res. Lett. 2016, 11, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhang, C. The synthesis of MoS2 particles with different morphologies for tribological applications. Tribol. Int. 2017, 116, 285–294. [Google Scholar] [CrossRef]
- Podgornik, B.; Kafexhiu, F.; Kosec, T.; Jerina, J.; Kalin, M. Friction and anti-galling properties of hexagonal boron nitride (h-BN) in aluminium forming. Wear 2017, 388–389, 2–8. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Physics 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Physics 2010, 6, 11–19. [Google Scholar] [CrossRef]
- Kasar, A.K.; Menezes, P.L. Synthesis and recent advances in tribological applications of graphene. Int. J. Adv. Manuf. Technol. 2018, 97, 3999–4019. [Google Scholar] [CrossRef]
- Ismail, N.A.; Bagheri, S. Highly oil-dispersed functionalized reduced graphene oxide nanosheets as lube oil friction modifier. Mater. Sci. Eng. B 2017, 222, 34–42. [Google Scholar] [CrossRef]
- Zheng, D.; Cai, Z.-B.; Shen, M.-X.; Li, Z.-Y.; Zhu, M.-H. Investigation of the tribology behaviour of the graphene nanosheets as oil additives on textured alloy cast iron surface. Appl. Surf. Sci. 2016, 387, 66–75. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R.; Singh, R.K.; Singh, M. Novel uses of alumina/graphene hybrid nanoparticle additives for improved tribological properties of lubricant in turning operation. Tribol. Int. 2018, 119, 99–111. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wang, L.; Li, W. Multilayer Graphene as a Lubricating Additive in Bentone Grease. Tribol. Lett. 2014, 55, 455–464. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S. The Tribological Properties of Multi-Layered Graphene as Additives of PAO2 Oil in Steel–Steel Contacts. Lubricants 2016, 4, 30. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-Based Engine Oil Nanofluids for Tribological Applications. ACS Appl. Mater. Interfaces 2011, 9, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jiang, B.; Dai, J.; Peng, C.; Li, C.; Li, Q.; Pan, F. Tribological Behaviors of Graphene and Graphene Oxide as Water-Based Lubricant Additives for Magnesium Alloy/Steel Contacts. Materials 2018, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Restuccia, P.; Righi, M.C. Tribochemistry of graphene on iron and its possible role in lubrication of steel. Carbon 2016, 106, 118–124. [Google Scholar] [CrossRef]
- Marchetto, D.; Restuccia, P.; Ballestrazzi, A.; Righi, M.C.; Rota, A.; Valeri, S. Surface passivation by graphene in the lubrication of iron: A comparison with bronze. Carbon 2017, 116, 375–380. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Titovich, K.A.; Ivanovich, K.V.; Vyacheslavovich, S.A.; Dash, S. Lubrication properties of chemically aged reduced graphene-oxide additives. Surf. Interfaces 2017, 7, 6–13. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P.; et al. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303–205306. [Google Scholar] [CrossRef]
- Bordignon, R.; Salvaro, D.; Binder, C.; Klein, A.N.; Drago, V.; Mello, J.D.B. Tribological Behaviour of Plasma-Functionalized Graphene as Low-Viscosity Oil Additive. Tribol. Lett. 2018, 66, 114. [Google Scholar] [CrossRef]
- Dou, X.; Koltonow, A.R.; He, X.; Jang, H.D.; Wang, Q.; Chung, Y.W.; Huang, J. Self-dispersed crumpled graphene balls in oil for friction and wear reduction. Proc. Natl. Acad. Sci. USA 2016, 113, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, Y.H.; Jeong, H.S.; Jung, H.T. Direct visualization of large-area graphene domains and boundaries by optical birefringency. Nat. Nanotechnol. 2012, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Ha, D.H.; Kim, J.H. Mapping of the atomic lattice orientation of a graphite flake using macroscopic liquid crystal texture. Nanotechnology 2012, 23, 395704. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Iannacchione, G.S. Nematic anchoring on carbon nanotubes. Appl. Phys. Lett. 2009, 95, 173113. [Google Scholar] [CrossRef]
- Li, S.; Fu, M.; Sun, H.; Zhao, Y.; Liu, Y.; He, D.; Wang, Y. Enhanced Photorefractive and Third-Order Nonlinear Optical Properties of 5CB-Based Polymer-Dispersed Liquid Crystals by Graphene Doping. J. Phys. Chem. C 2014, 118, 18015–18020. [Google Scholar] [CrossRef]
- Gwizdała, W.; Górny, K.; Gburski, Z. The dynamics of 4-cyano-4-n-pentylbiphenyl (5CB) mesogen molecules located between graphene layers—MD study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Kalyani; Umrao, S.; Rastogi, R.B.; Kumar, R.; Srivastava, A. Synthesis, Characterization, and Tribological Evaluation of TiO2-Reinforced Boron and Nitrogen co-Doped Reduced Graphene Oxide Based Hybrid Nanomaterials as Efficient Antiwear Lubricant Additives. ACS Appl. Mater. Interfaces 2016, 8, 11698–11710. [Google Scholar] [CrossRef] [PubMed]
- Shahnazar, S.; Bagheri, S.; Hamid, S.B.A. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrog. Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Brostow, W.; Lobland, H.E.; Hnatchuk, N.; Perez, J.M. Improvement of Scratch and Wear Resistance of Polymers by Fillers Including Nanofillers. Nanomaterials 2017, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Tang, C.; He, Y.; Wang, Y.; Chen, J.; Mao, J.; Zhou, Q.; Wang, B.; Wei, F.; et al. Highly Exfoliated Reduced Graphite Oxide Powders as Efficient Lubricant Oil Additives. Adv. Mater. Interfaces. 2016, 3, 1600700. [Google Scholar] [CrossRef]
- Kumar, P.; Wani, M.F. Tribological Characterisation of Graphene Oxide as Lubricant Additive on Hypereutectic Al-25Si/Steel Tribopair. Tribol. Trans. 2018, 61, 335–346. [Google Scholar] [CrossRef]
- Thirumalai Kannan, K.; RameshBabu, S. Tribological behavior of modified jojoba oil with graphene nanoparticle asadditive in SAE20W40 oil using pin on disc tribometer. Energy Source Part A 2017, 39, 1842–1848. [Google Scholar] [CrossRef]
- Shah, R.; Datashvili, T.; Cai, T.; Wahrmund, J.; Menard, B.; Menard, K.P.; Brostow, W.; Perez, J.M. Effects of functionalised reduced graphene oxide on frictional and wear properties of epoxy resin. Mater. Res. Innov. 2015, 19, 97–106. [Google Scholar] [CrossRef]
- Song, H.; Wang, Z.; Yang, J. Tribological properties of graphene oxide and carbon spheres as lubricating additives. Appl. Phys. A 2016, 122, 933–941. [Google Scholar] [CrossRef]
- Xu, J.; Kato, K. Formation of tribochemical layer of ceramics sliding in water and its role for low friction. Wear 2000, 245, 61–75. [Google Scholar] [CrossRef]
- Song, H.J.; Li, N. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive. Appl. Phys. A Mater. Sci. Process. 2011, 105, 827–832. [Google Scholar] [CrossRef]
- Cognard, J. Lubrication with liquid-crystals. ACS Sym. Ser. 1990, 441, 1–47. [Google Scholar] [CrossRef]
- Wen, S.; Huang, P. Princilies of Tribology, 4th ed.; Tsinghua University Press: Beijing, China, 2012; pp. 131–145. ISBN 978-7-302-301-88-2. [Google Scholar]
- Li, J.; Zhang, C.; Deng, M.; Luo, J. Superlubricity of silicone oil achieved between two surfaces by running-in with acid solution. RSC Adv. 2015, 5, 30861–30868. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, L.; Luo, J. Pitted Surfaces Produced by Lactic Acid Lubrication and Their Effect on Ultra-Low Friction. Tribol. Lett. 2015, 57, 20. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Park, J.S.; Reina, A.; Saito, R.; Kong, J.; Dresselhaus, G.; Dresselhaus, M.S. G’ band Raman spectra of single, double and triple layer graphene. Carbon 2009, 47, 1303–1310. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Ramam spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Guo, S.J.; Fang, Y.; Dong, S. Reducing sugar: New functionational molecules for the greens synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, C.; Xiao, G.; Zhang, J.; Chen, Z.; Yi, M. Lubrication Performance of Graphene as Lubricant Additive in 4-n-pentyl-4′-cyanobiphyl Liquid Crystal (5CB) for Steel/Steel Contacts. Materials 2018, 11, 2110. https://doi.org/10.3390/ma11112110
Li Z, Xu C, Xiao G, Zhang J, Chen Z, Yi M. Lubrication Performance of Graphene as Lubricant Additive in 4-n-pentyl-4′-cyanobiphyl Liquid Crystal (5CB) for Steel/Steel Contacts. Materials. 2018; 11(11):2110. https://doi.org/10.3390/ma11112110
Chicago/Turabian StyleLi, Zhiliang, Chonghai Xu, Guangchun Xiao, Jingjie Zhang, Zhaoqiang Chen, and Mingdong Yi. 2018. "Lubrication Performance of Graphene as Lubricant Additive in 4-n-pentyl-4′-cyanobiphyl Liquid Crystal (5CB) for Steel/Steel Contacts" Materials 11, no. 11: 2110. https://doi.org/10.3390/ma11112110
APA StyleLi, Z., Xu, C., Xiao, G., Zhang, J., Chen, Z., & Yi, M. (2018). Lubrication Performance of Graphene as Lubricant Additive in 4-n-pentyl-4′-cyanobiphyl Liquid Crystal (5CB) for Steel/Steel Contacts. Materials, 11(11), 2110. https://doi.org/10.3390/ma11112110



