Au Doping Effect on the Secondary Electron Emission Performance of MgO Films
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussions
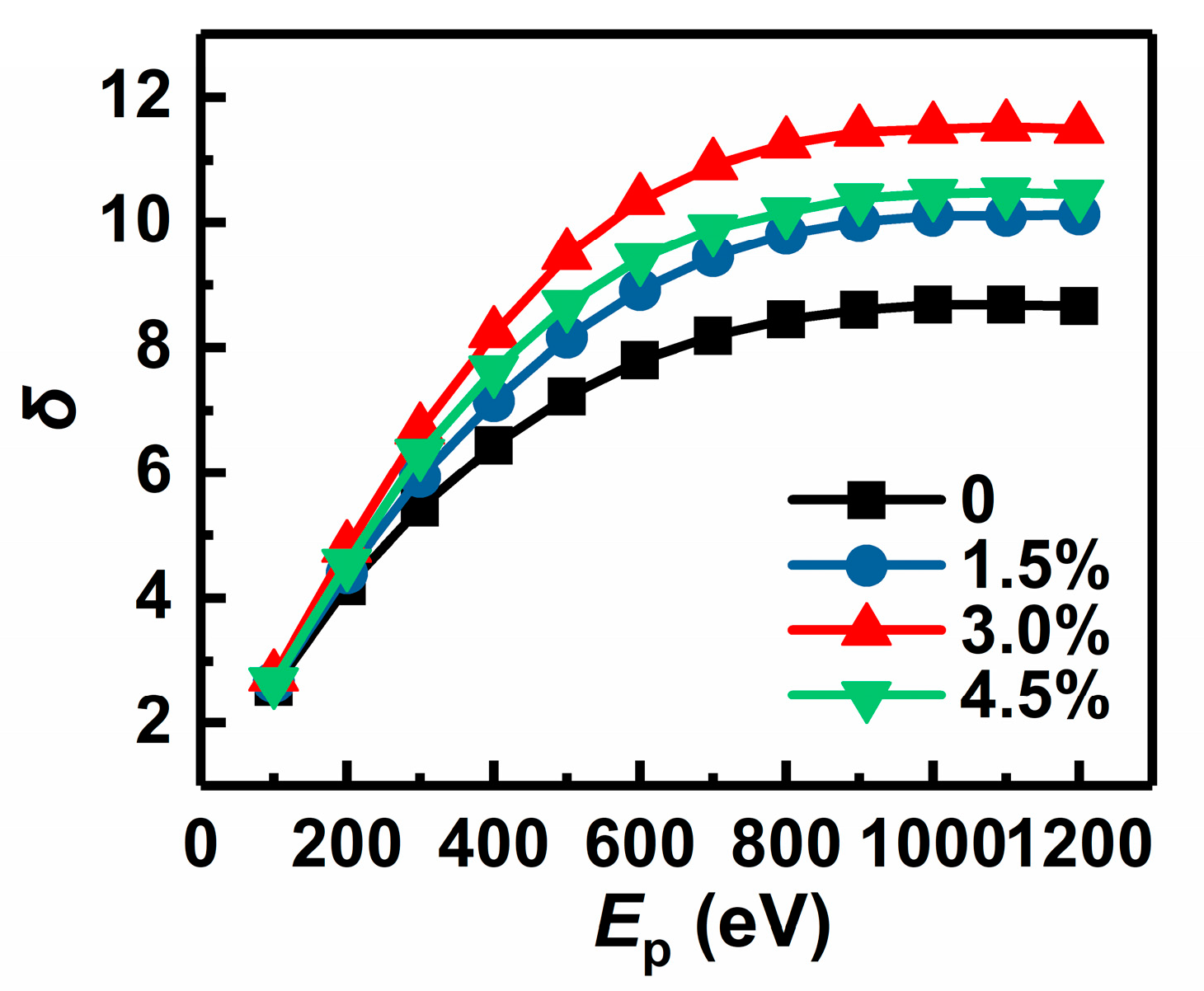
3.1. SEE Performances of Au-Doped MgO Films
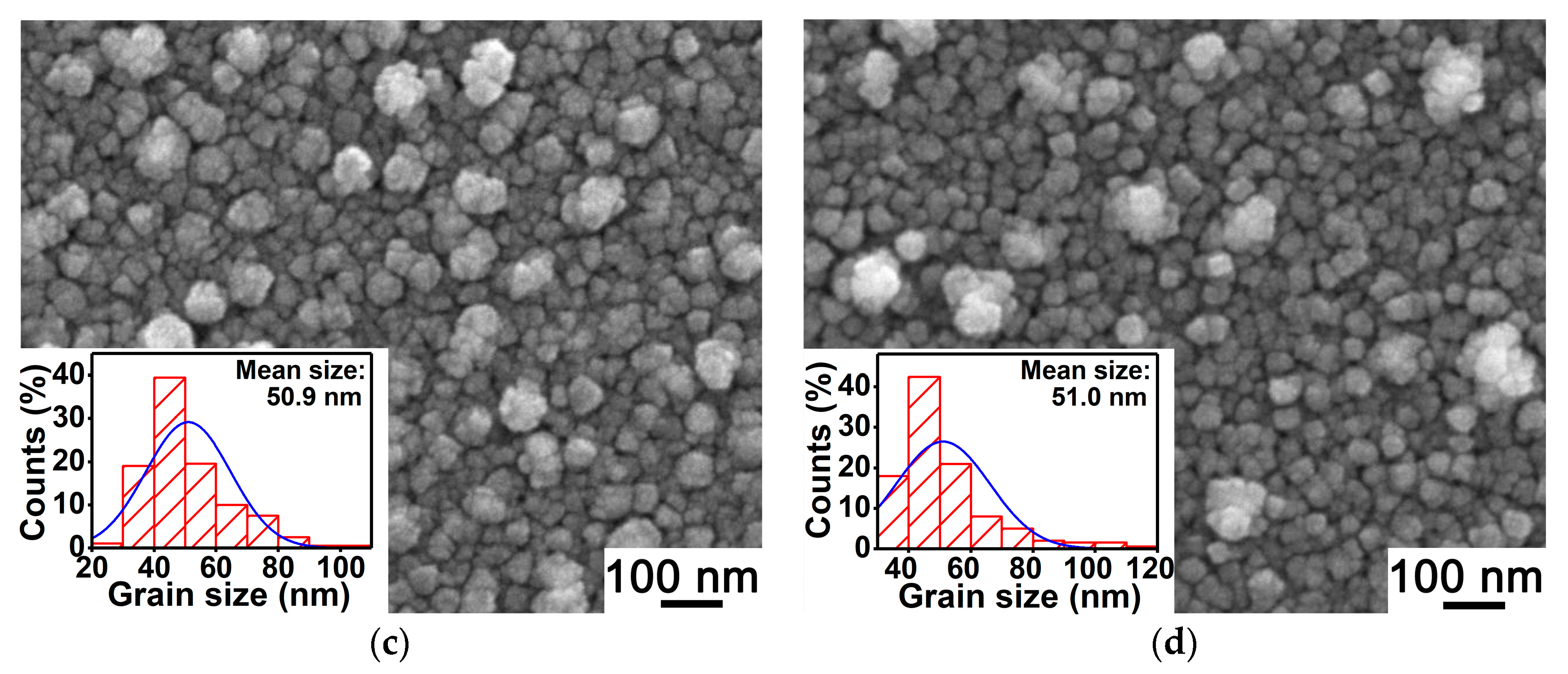
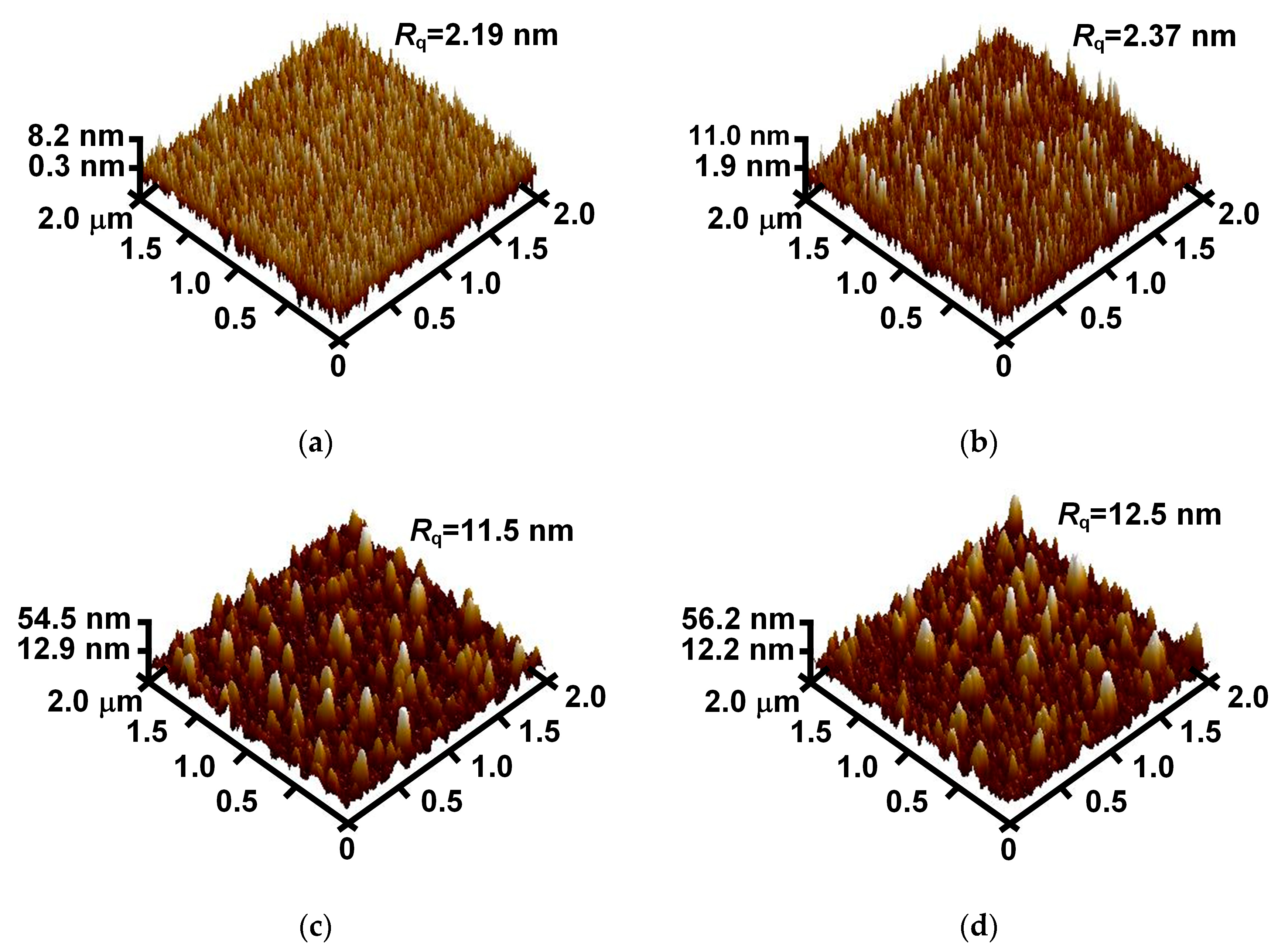
3.2. Surface Morphologies of Au-Doped MgO Films
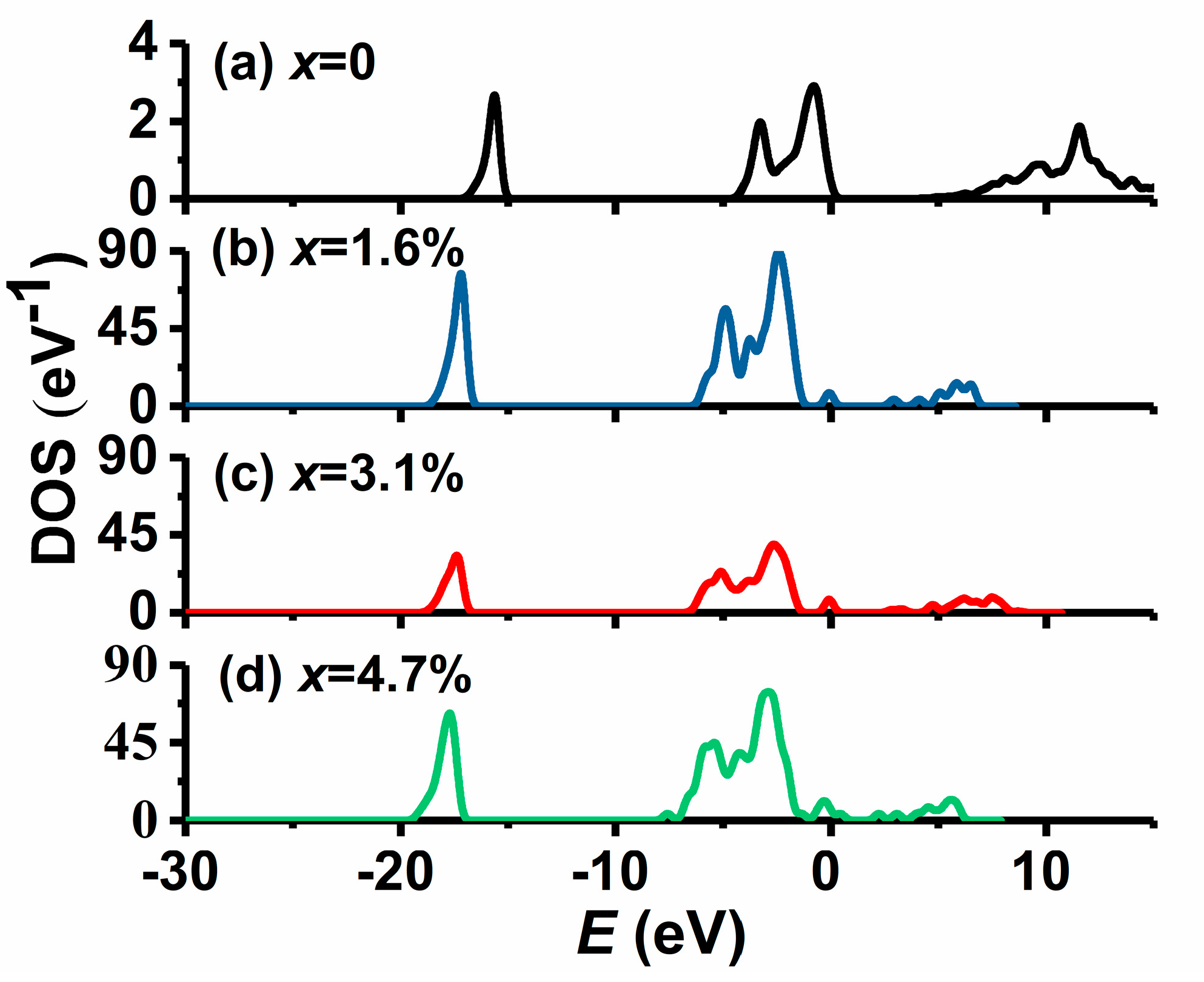
3.3. Electronic Structures of Au-Doped MgO Films
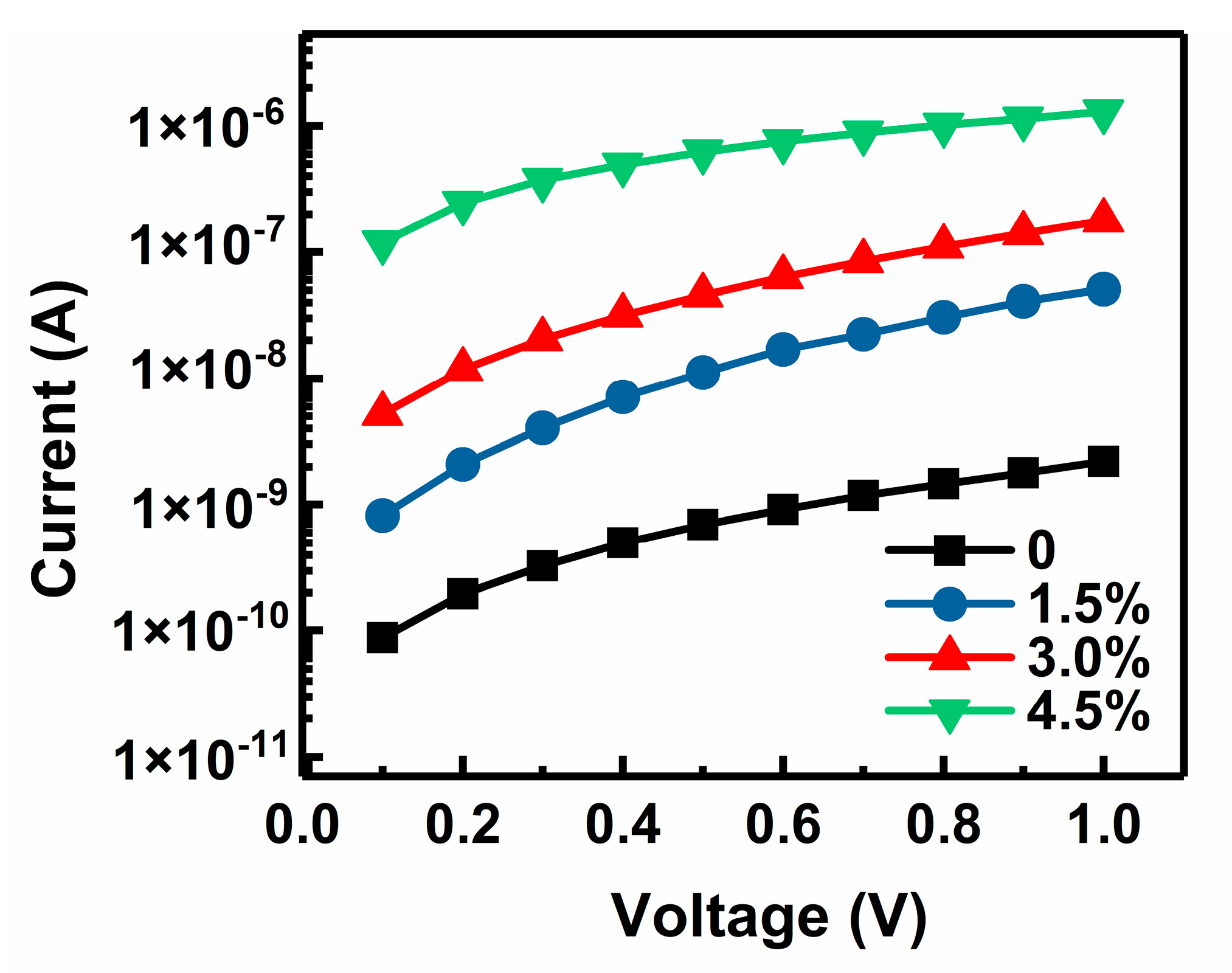
3.4. Electrical Conductivities of Au-Doped MgO Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhu, X.; Gao, L.; Chen, H. High gain, low noise, and long lifetime MCP electron multiplier for cesium clock. In China Satellite Navigation Conference (CSNC) 2017 Proceedings: Volume III, Proceedings of the China Satellite Navigation Conference, Shanghai, China, 23–25 May 2017; Springer: Singapore, 2017; pp. 575–581. [Google Scholar]
- Funsten, H.; Harper, R.; Dors, E.; Janzen, P.; Larsen, B.; Macdonald, E.; Poston, D.; Ritzau, S.; Skoug, R.; Zurbuchen, T. Comparative response of microchannel plate and channel electron multiplier detectors to penetrating radiation in space. IEEE Nucl. Sci. 2015, 62, 2283–2293. [Google Scholar] [CrossRef]
- Tao, S.; Chan, H.; Graaf, H. Secondary electron emission materials for transmission dynodes in novel photomultipliers: A review. Materials 2016, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Kirk, T.; Scholder, O.; Pietro, L.; Ramsperger, U.; Pescia, D. Evidence of nonplanar field emission via secondary electron detection in near field emission scanning electron microscopy. Appl. Phys. Lett. 2009, 94, 153502. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, E.; Wang, Y.; Xiong, L.; Liu, S. Activation treatment effects on characteristics of BeO layer and secondary electron emission properties of an activated Cu-Be alloy. Appl. Surf. Sci. 2015, 355, 19–25. [Google Scholar] [CrossRef]
- Stacey, A.; Prawer, S.; Rubanov, S.; Akhvlediani, R.; Michaelson, S.; Hoffman, A. Temperature enhancement of secondary electron emission from hydrogenated diamond films. J. Appl. Phys. 2009, 106, 063715. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Zhou, F.; Liu, W.; Wang, F.; Lai, C. Influence of activation parameters on the thickness of MgO thin film on Ag-3Mg alloy and its secondary electron emission property. Mater. Res. Bull. 2017, 96, 35–39. [Google Scholar] [CrossRef]
- Tao, S.; Theulings, A.; Smedley, J.; Graff, H. DFT study of electron affinity of alkali metal termination on clean and oxygenated β-Si3N4. Diam. Relat. Mater. 2015, 58, 214–220. [Google Scholar] [CrossRef]
- Hine, K.; Yoshimura, S.; Ikuse, K.; Kiuchi, M.; Hashimoto, J.; Terauchi, M.; Nishitani, M.; Hamaguchi, S. Measurement of magnesium oxide sputtering yields by He and Ar ions with a low-energy mass-selected ion beam system. Jpn. J. Appl. Phys. 2007, 46, L1132–L1134. [Google Scholar] [CrossRef]
- Ritz, V.; Shih, A.; Sobbcinski, B. Performance of MgO/Au cermet films as secondary electron emission cold cathodes. Surf. Interface Anal. 1992, 18, 514–524. [Google Scholar] [CrossRef]
- Yi, W.; Yu, S.; Lee, W.; Han, I.; Jeong, T.; Woo, Y.; Lee, J.; Jin, S.; Choi, W.; Heo, J.; et al. Secondary electron emission yields from MgO deposited on carbon nanotubes. J. Appl. Phys. 2001, 89, 4091–4095. [Google Scholar] [CrossRef]
- Caron, M.; Geittner, P.; Much, G. Electron induced degradation of e-beam evaporated MgO layers. Surf. Sci. 2003, 528, 97–102. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Hao, L.; Wu, S.; Zhang, J. Influence of the substrate temperature on the microstructure and electron-induced secondary electron emission properties of MgO/Au composite film. Mater. Res. Bull. 2018, 100, 308–312. [Google Scholar] [CrossRef]
- Henrich, V.; Fan, J. Effect of cesiation on secondary-electron emission from MgO/Au cermets. J. Appl. Phys. 1974, 45, 5484–5486. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, T.; Yu, S.; Jin, S.; Heo, J.; Yi, W.; Jeon, D.; Kim, J. Thickness effect on secondary electron emission of MgO layers. Appl. Surf. Sci. 2001, 174, 62–69. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, L.; Liu, S. MgO secondary electron emission film prepared by radio-frequency reactive sputtering. Acta Metall. Sin. 2016, 52, 10–16. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, F.; Wang, J.; Liu, W.; Zhang, Q.; Yin, Q. Characterization of MgO/Al2O3 composite film prepared by DC magnetron sputtering and its secondary electron emission properties. J. Electron. Mater. 2018, 47, 4116–4123. [Google Scholar] [CrossRef]
- Fan, J.; Henrich, V. Preparation and properties of sputtered MgO/Au, MgO/Ag, and MgO/Ni cermet films. J. Appl. Phys. 1974, 45, 3742–3748. [Google Scholar] [CrossRef]
- Henrich, V.; Fan, J. High-efficiency secondary-electron emission from sputtered MgO-Au cermets. Appl. Phys. Lett. 1973, 23, 7–8. [Google Scholar] [CrossRef]
- Ritz, V. Deposition of MgO/Au cermet films by triode sputtering and their characterization. Surf. Interface Anal. 1990, 15, 173–179. [Google Scholar] [CrossRef]
- Tao, S.; Theulings, A.; Prodanovic, V.; Smedley, J.; Graaf, H. Optical properties of silicon-rich silicon nitride (SixNyHz) from first principles. Computation 2015, 3, 657–669. [Google Scholar] [CrossRef]
- Cheng, Y.; Kupfer, H.; Paraian, A. Deposition of MgO films by pulsed mid-frequency magnetron sputtering. Appl. Surf. Sci. 2002, 200, 117–124. [Google Scholar] [CrossRef]
- Suharyanto; Yamano, Y.; Kobayashi, S.; Michizono, S.; Saito, Y.; Tumiran. Effect of mechanical finishes on secondary electron emission of alumina ceramics. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 620–626. [Google Scholar] [CrossRef]
- Vida, G.; Beck, I.; Josepovits, V.; Gyor, M.; Deak, P. Electron beam induced secondary emission changes investigated by work function spectroscopy. Appl. Surf. Sci. 2004, 227, 87–93. [Google Scholar] [CrossRef]
- Li, H.; Hu, W.; Wu, S.; Wei, Q. Synthesis and characterization of Microstructures of MgO/Au composite films grown by reactive RF magnetron sputtering. Chin. J. Vac. Sci. Technol. 2014, 34, 842–846. [Google Scholar] [CrossRef]


| Au Doping Concentration (%) | Work Function (eV) | |
|---|---|---|
| (200) Crystal Plane | (220) Crystal Plane | |
| 0 | 5.32 | 5.38 |
| 1.6 | 1.86 | 2.97 |
| 3.1 | 1.92 | 3.25 |
| 4.7 | 2.22 | 3.52 |
| Au Doping Concentration (%) | Band Gap (eV) |
|---|---|
| 0 | 4.76 |
| 1.6 | 2.47 |
| 3.1 | 2.26 |
| 4.7 | 1.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hu, W.; Wang, K.; Gao, B.; Li, Y.; Wu, S.; Zhang, J.; Fan, H. Au Doping Effect on the Secondary Electron Emission Performance of MgO Films. Materials 2018, 11, 2104. https://doi.org/10.3390/ma11112104
Li J, Hu W, Wang K, Gao B, Li Y, Wu S, Zhang J, Fan H. Au Doping Effect on the Secondary Electron Emission Performance of MgO Films. Materials. 2018; 11(11):2104. https://doi.org/10.3390/ma11112104
Chicago/Turabian StyleLi, Jie, Wenbo Hu, Kang Wang, Buyu Gao, Yongdong Li, Shengli Wu, Jintao Zhang, and Huiqing Fan. 2018. "Au Doping Effect on the Secondary Electron Emission Performance of MgO Films" Materials 11, no. 11: 2104. https://doi.org/10.3390/ma11112104






