Sequential Slot-Die Deposition of Perovskite Solar Cells Using Dimethylsulfoxide Lead Iodide Ink
Abstract
:1. Introduction
2. Results
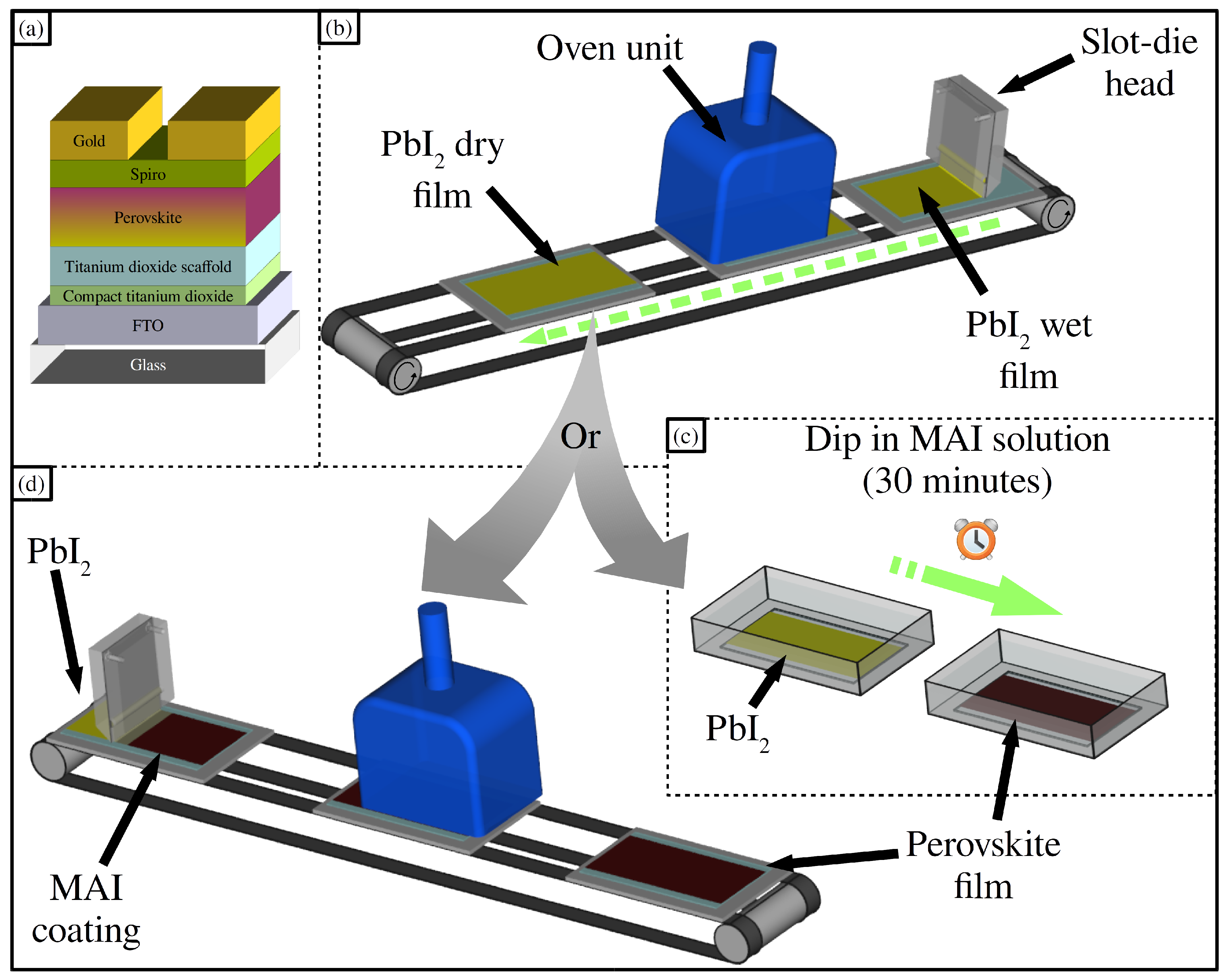
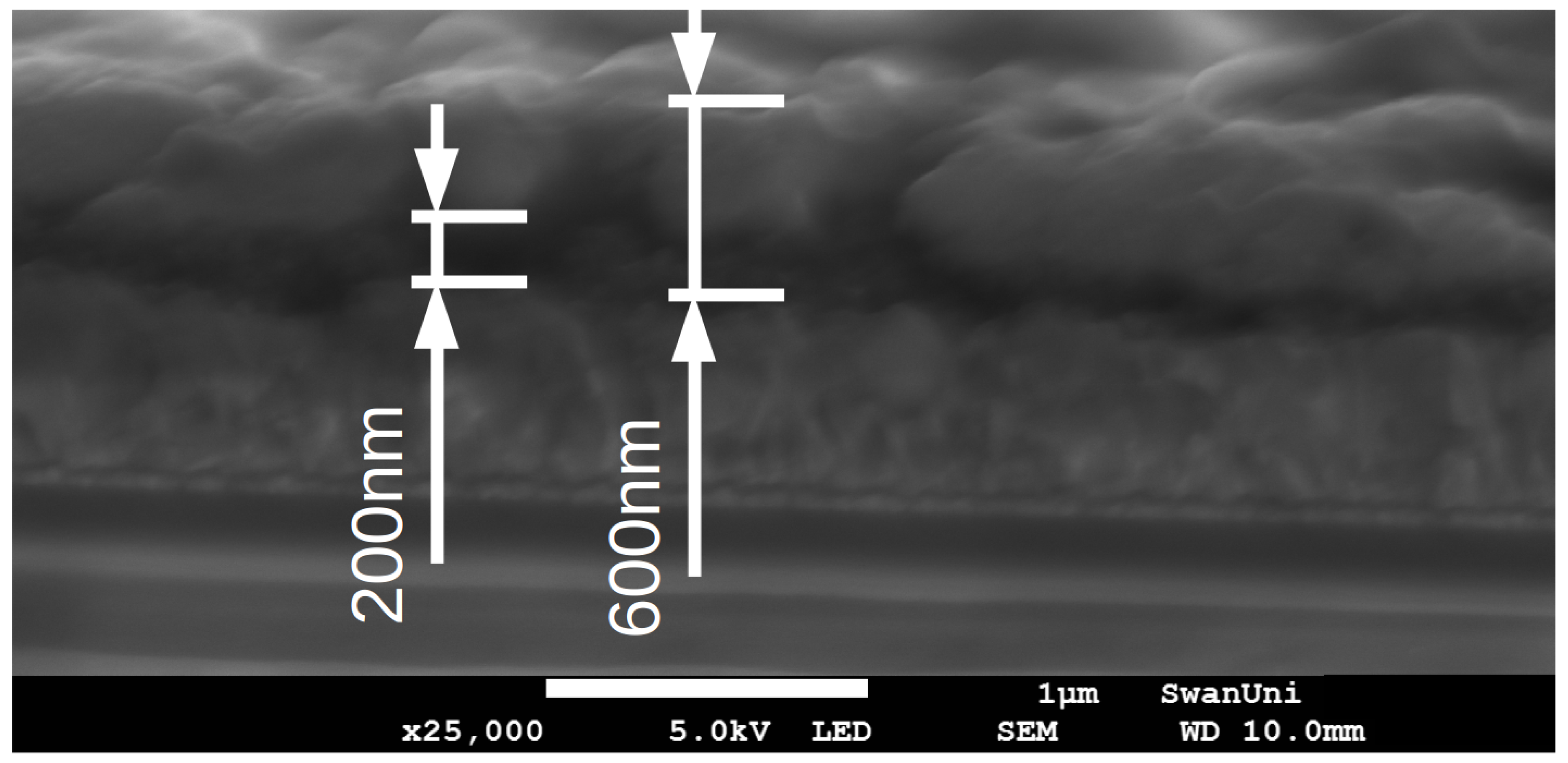
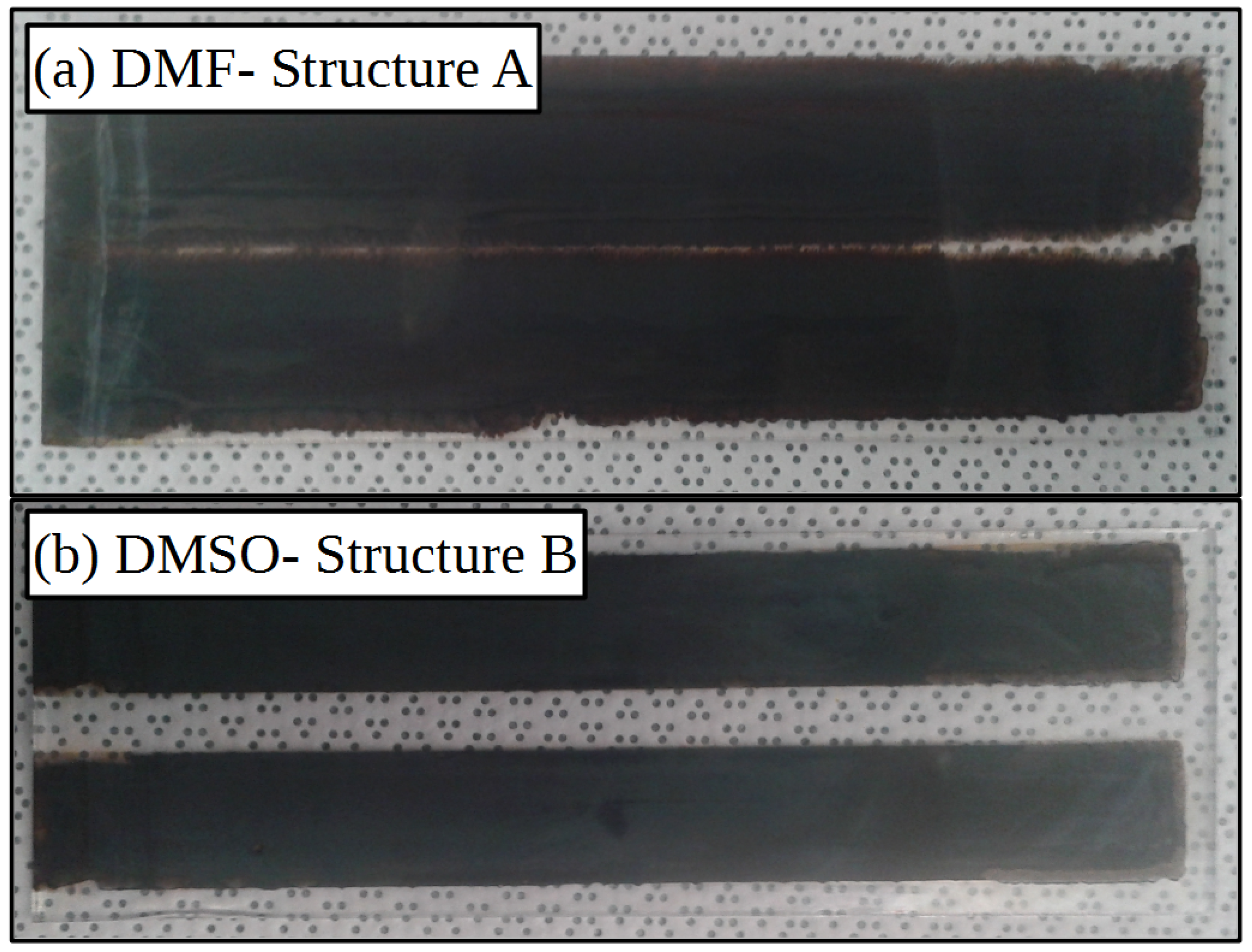
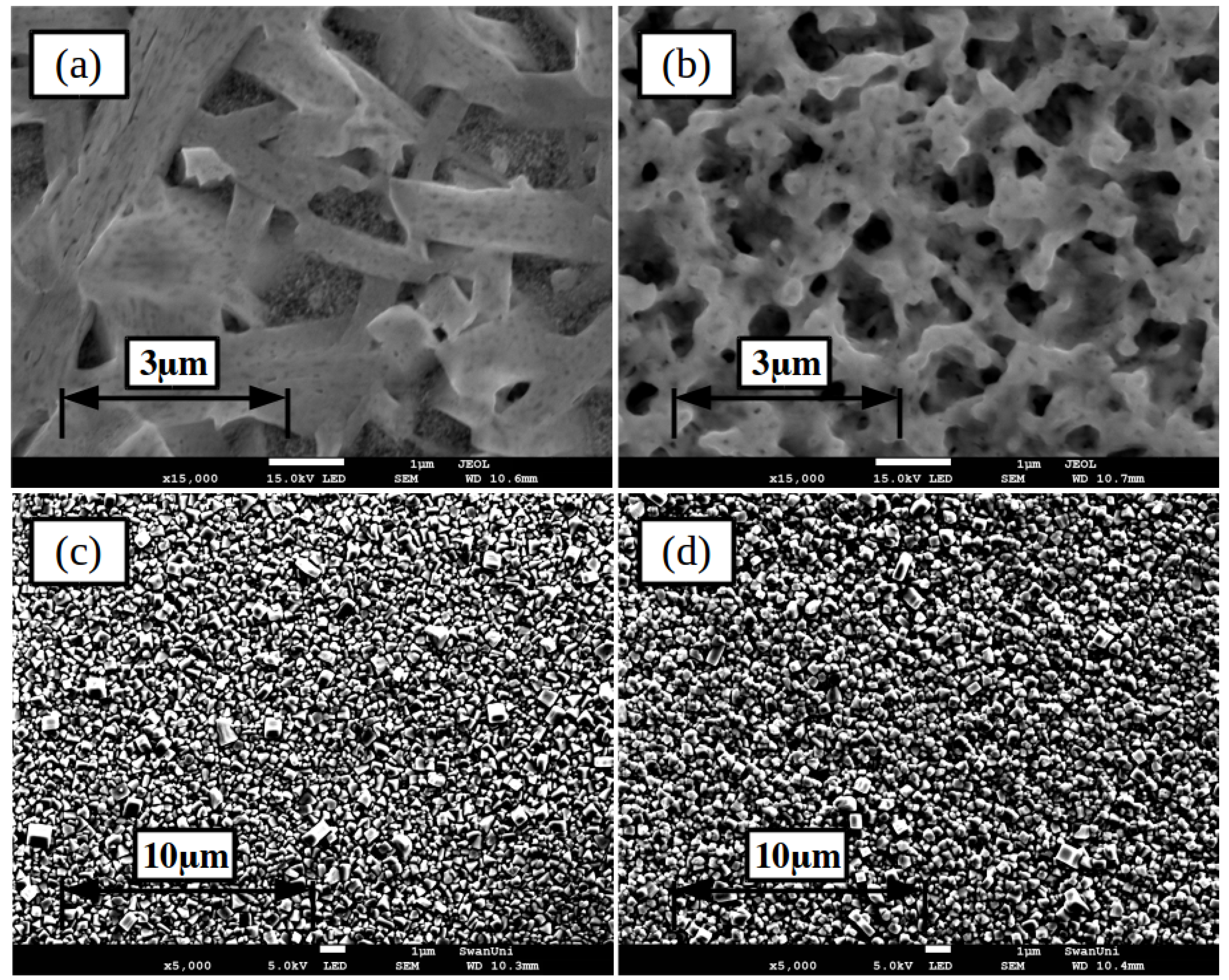
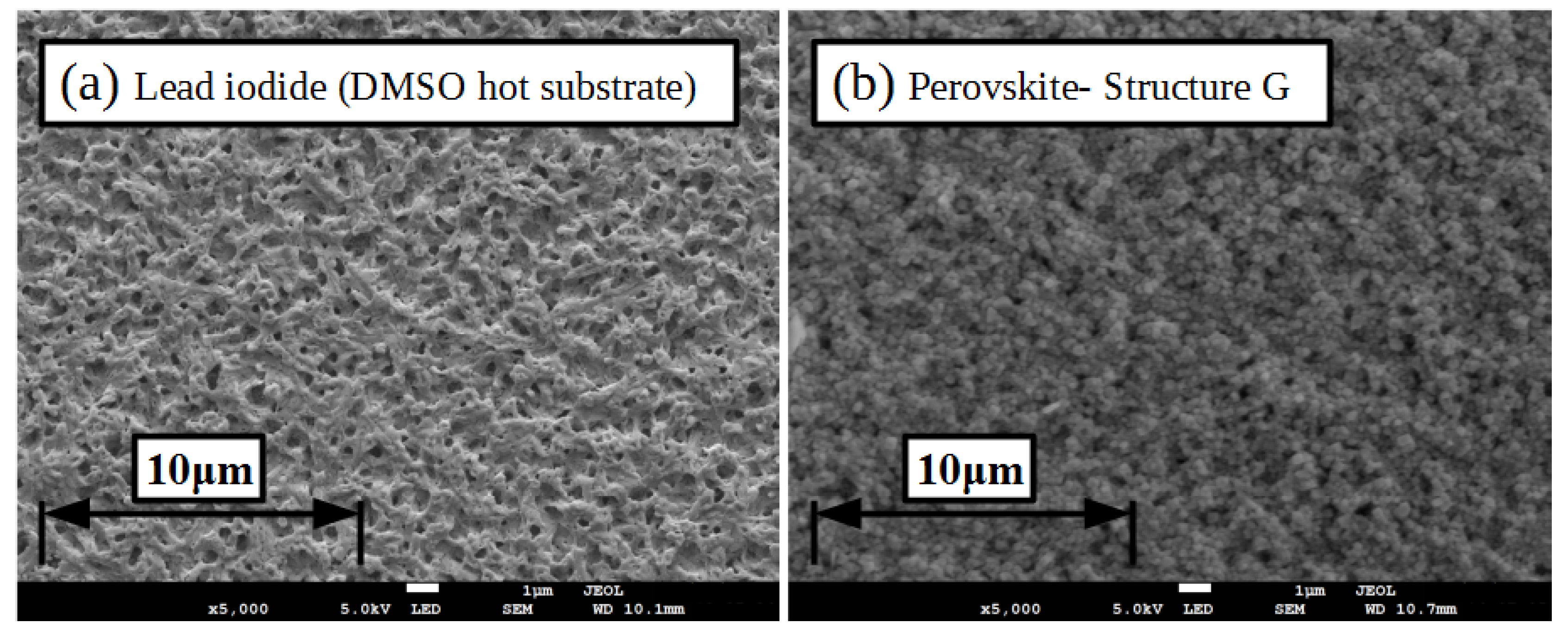
2.1. Lead Iodide Layer Slot-Die Coating
2.2. Methylammonium Iodide Slot-Die Coating
3. Materials and Methods
3.1. Device Fabrication Methods
3.2. Test Methods
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mouafi, Y.B.; Zuschlag, A.; Pichon, P.Y.; Fritz, J.; Schönecker, A.; Hahn, G. Novel RGS materials with high fill factors and no material-induced shunts with record solar cell efficiencies exceeding 16%. Sol. Energy Mater. Sol. Cells 2016, 146, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Derbouz, A.; Slaoui, A.; Jolivet, E.; de Moro, F.; Belouet, C. N-type silicon RST ribbon solar cells. Sol. Energy Mater. Sol. Cells 2012, 107, 212–218. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Cui, H.; Liu, F.; Hao, X.; Conibeer, G.; Mitzi, D.B.; Green, M. The current status and future prospects of kesterite solar cells: A brief review. Prog. Photovolt. Res. Appl. 2016, 24, 879–898. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; McElvany, C.L.; Phillips, A.B.; Celik, I.; Krantz, P.W.; Watthage, S.C.; Liyanage, G.K.; Apul, D.; Heben, M.J. A technoeconomic analysis of perovskite solar module manufacturing with low-cost materials and techniques. Energy Environ. Sci. 2017, 10, 1297–1305. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Cost-Performance Analysis of Perovskite Solar Modules. Adv. Sci. 2017, 4, 1600269. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.L.; Ho-Baillie, A.W.Y.; Vak, D.; Gao, M.; Green, M.A.; Egan, R.J. Manufacturing cost and market potential analysis of demonstrated roll-to-roll perovskite photovoltaic cell processes. Sol. Energy Mater. Sol. Cells 2018, 174, 314–324. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Eames, C.; Frost, J.M.; Barnes, P.R.F.; O’Regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calado, P.; Telford, A.M.; Bryant, D.; Li, X.; Nelson, J.; O’Regan, B.C.; Barnes, P.R.F. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 2016, 7, 13831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pockett, A.; Carnie, M.J. Ionic Influences on Recombination in Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1683–1689. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide—Based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.A.; Mouhamad, Y.; Hooper, K.E.A.; Burkitt, D.; Geoghegan, M.; Watson, T.M. From spin coating to roll-to-roll: Investigating the challenge of upscaling lead halide perovskite solar cells. IET Renew. Power Gener. 2017, 11, 546–549. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; Grätzel, M.; Han, H. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ku, Z.; Rong, Y.; Xu, M.; Liu, T.; Han, H. Full Printable Processed Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells with Carbon Counter Electrode. Sci. Rep. 2013, 2013. 3, 3132. [Google Scholar] [CrossRef]
- Li, S.G.; Jiang, K.J.; Su, M.J.; Cui, X.P.; Huang, J.H.; Zhang, Q.Q.; Zhou, X.Q.; Yang, L.M.; Song, Y.L. Inkjet printing of CH3NH3PbI3 on a mesoscopic TiO2 film for highly efficient perovskite solar cells. J. Mater. Chem. A 2015, 3, 9092–9097. [Google Scholar] [CrossRef]
- Bag, M.; Jiang, Z.; Renna, L.A.; Jeong, S.P.; Rotello, V.M.; Venkataraman, D. Rapid combinatorial screening of inkjet-printed alkyl-ammonium cations in perovskite solar cells. Mater. Lett. 2016, 164, 472–475. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Chen, H.; Yan, K.; Yang, S. Inkjet Printing and Instant Chemical Transformation of a CH3NH3PbI3/Nanocarbon Electrode and Interface for Planar Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 13239–13243. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Yang, B.; Gu, G.; Joshi, P.C.; Ivanov, I.N.; Rouleau, C.M.; Aytug, T.; Geohegan, D.B.; Xiao, K. High-Performance Flexible Perovskite Solar Cells by Using a Combination of Ultrasonic Spray-Coating and Low Thermal Budget Photonic Curing. ACS Photonics 2015, 2, 680–686. [Google Scholar] [CrossRef]
- Mohamad, D.K.; Griffin, J.; Bracher, C.; Barrows, A.T.; Lidzey, D.G. Spray-Cast Multilayer Organometal Perovskite Solar Cells Fabricated in Air. Adv. Energy Mater. 2016, 6, 1600994. [Google Scholar] [CrossRef] [Green Version]
- Bishop, J.E.; Mohamad, D.K.; Wong-Stringer, M.; Smith, A.; Lidzey, D.G. Spray-cast multilayer perovskite solar cells with an active-area of 1.5 cm2. Sci. Rep. 2017, 7, 7962. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Peng, E.; Shao, Y.; Xiao, Z.; Dong, Q.; Huang, J. Scalable fabrication of efficient organolead trihalide perovskite solar cells with doctor-bladed active layers. Energy Environ. Sci. 2015, 8, 1544–1550. [Google Scholar] [CrossRef]
- Zhong, Y.; Munir, R.; Li, J.; Tang, M.C.; Niazi, M.R.; Smilgies, D.M.; Zhao, K.; Amassian, A. Blade-Coated Hybrid Perovskite Solar Cells with Efficiency >17%: An In Situ Investigation. ACS Energy Lett. 2018, 3, 1078–1085. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Reese, M.O.; Reid, O.G.; Kim, D.H.; Siol, S.; Klein, T.R.; Yan, Y.; Berry, J.J.; van Hest, M.F.A.M.; Zhu, K. Perovskite ink with wide processing window for scalable high-efficiency solar cells. Nat. Energy 2017, 2, 17038. [Google Scholar] [CrossRef]
- Vak, D.; Hwang, K.; Faulks, A.; Jung, Y.S.; Clark, N.; Kim, D.Y.; Wilson, G.J.; Watkins, S.E. 3D Printer Based Slot-Die Coater as a Lab-to-Fab Translation Tool for Solution-Processed Solar Cells. Adv. Energy Mater. 2015, 5, 1401539. [Google Scholar]
- Hwang, K.; Jung, Y.S.; Heo, Y.J.; Scholes, F.H.; Watkins, S.E.; Subbiah, J.; Jones, D.J.; Kim, D.Y.; Vak, D. Toward Large Scale Roll-to-Roll Production of Fully Printed Perovskite Solar Cells. Adv. Mater. 2015, 27, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.M.; Larsen-Olsen, T.T.; Carlé, J.E.; Angmo, D.; Krebs, F.C. Upscaling of Perovskite Solar Cells: Fully Ambient Roll Processing of Flexible Perovskite Solar Cells with Printed Back Electrodes. Adv. Energy Mater. 2015, 5, 1500569. [Google Scholar] [CrossRef]
- Gu, Z.; Zuo, L.; Larsen-Olsen, T.T.; Ye, T.; Wu, G.; Krebs, F.C.; Chen, H. Interfacial engineering of self-assembled monolayer modified semi-roll-to-roll planar heterojunction perovskite solar cells on flexible substrates. J. Mater. Chem. A 2015, 3, 24254–24260. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.S.; Hwang, K.; Heo, Y.J.; Kim, J.E.; Lee, D.; Lee, C.H.; Joh, H.I.; Yeo, J.S.; Kim, D.Y. One-Step Printable Perovskite Films Fabricated under Ambient Conditions for Efficient and Reproducible Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 27832–27838. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Huang, W.; Kim, J.E.; Vak, D.; Forsyth, C.; McNeill, C.R.; Cheng, Y.B. Amorphous hole-transporting layer in slot-die coated perovskite solar cells. Nano Energy 2017, 31, 210–217. [Google Scholar] [CrossRef]
- Cotella, G.; Baker, J.; Worsley, D.; Rossi, F.D.; Pleydell-Pearce, C.; Carnie, M.; Watson, T. One-step deposition by slot-die coating of mixed lead halide perovskite for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2017, 159, 362–369. [Google Scholar] [CrossRef]
- Heo, Y.J.; Kim, J.E.; Weerasinghe, H.; Angmo, D.; Qin, T.; Sears, K.; Hwang, K.; Jung, Y.S.; Subbiah, J.; Jones, D.J.; et al. Printing-friendly sequential deposition via intra-additive approach for roll-to-roll process of perovskite solar cells. Nano Energy 2017, 41, 443–451. [Google Scholar] [CrossRef]
- Ciro, J.; Mejía-Escobar, M.A.; Jaramillo, F. Slot-die processing of flexible perovskite solar cells in ambient conditions. Sol. Energy 2017, 150, 570–576. [Google Scholar] [CrossRef]
- Cai, L. Large area perovskite solar cell module. J. Semicond. 2017, 38, 014006. [Google Scholar] [CrossRef]
- Lee, D.; Jung, Y.S.; Heo, Y.J.; Lee, S.; Hwang, K.; Jeon, Y.J.; Kim, J.E.; Park, J.; Jung, G.Y.; Kim, D.Y. Slot-Die Coated Perovskite Films Using Mixed Lead Precursors for Highly Reproducible and Large-Area Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 16133–16139. [Google Scholar] [CrossRef] [PubMed]
- Giacomo, F.D.; Shanmugam, S.; Fledderus, H.; Bruijnaers, B.J.; Verhees, W.J.; Dorenkamper, M.S.; Veenstra, S.C.; Qiu, W.; Gehlhaar, R.; Merckx, T.; et al. Up-scalable sheet-to-sheet production of high efficiency perovskite module and solar cells on 6-in. substrate using slot die coating. Sol. Energy Mater. Sol. Cells 2018, 181, 53–59. [Google Scholar] [CrossRef]
- Kim, J.E.; Jung, Y.S.; Heo, Y.J.; Hwang, K.; Qin, T.; Kim, D.Y.; Vak, D. Slot die coated planar perovskite solar cells via blowing and heating assisted one step deposition. Sol. Energy Mater. Sol. Cells 2018, 179, 80–86. [Google Scholar] [CrossRef]
- Remeika, M.; Ono, L.K.; Maeda, M.; Hu, Z.; Qi, Y. High-throughput surface preparation for flexible slot die coated perovskite solar cells. Org. Electron. 2018, 54, 72–79. [Google Scholar] [CrossRef]
- Zuo, C.; Vak, D.; Angmo, D.; Ding, L.; Gao, M. One-step roll-to-roll air processed high efficiency perovskite solar cells. Nano Energy 2018, 46, 185–192. [Google Scholar] [CrossRef]
- Burkitt, D.; Searle, J.; Watson, T. Perovskite solar cells in N-I-P structure with four slot-die-coated layers. R. Soc. Open Sci. 2018, 5, 172158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.Y.; Park, E.Y.; Yang, T.Y.; Noh, J.H.; Shin, T.J.; Jeon, N.J.; Seo, J. Fast two-step deposition of perovskite via mediator extraction treatment for large-area, high-performance perovskite solar cells. J. Mater. Chem. A 2018, 6, 12447–12454. [Google Scholar] [CrossRef]
- Whitaker, J.B.; Kim, D.H.; Larson, B.; Zhang, F.; Berry, J.J.; van Hest, M.F.A.M.; Zhu, K. Scalable slot-die coating of high performance perovskite solar cells. Sustain. Energy Fuels 2018. [Google Scholar] [CrossRef]
- Lin, C.F.; Wang, B.K.; Lo, S.H.; Wong, D.S.H.; Liu, T.J.; Tiu, C. Operating windows of stripe coating. Asia-Pac. J. Chem. Eng. 2014, 9, 134–145. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.; Shin, K. Statistical analysis for the manufacturing of multi-strip patterns by roll-to-roll single slot-die systems. Robot. Comput.-Integr. Manuf. 2014, 30, 363–368. [Google Scholar] [CrossRef]
- Raupp, S.M.; Schmitt, M.; Walz, A.L.; Diehm, R.; Hummel, H.; Scharfer, P.; Schabel, W. Slot die stripe coating of low viscous fluids. J. Coat. Technol. Res. 2018, 15, 899–911. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Schmitt, M.; Scharfer, P.; Schabel, W. Slot die coating of lithium-ion battery electrodes: Investigations on edge effect issues for stripe and pattern coatings. J. Coat. Technol. Res. 2014, 11, 57–63. [Google Scholar] [CrossRef]
- Schmitt, M.; Diehm, R.; Scharfer, P.; Schabel, W. An experimental and analytical study on intermittent slot die coating of viscoelastic battery slurries. J. Coat. Technol. Res. 2015, 12, 927–938. [Google Scholar] [CrossRef]
- Maza, D.; Carvalho, M.S. Trailing edge formation during slot coating of rectangular patches. J. Coat. Technol. Res. 2017, 14, 1003–1013. [Google Scholar] [CrossRef]
- Jakubka, F.; Heyder, M.; Machui, F.; Kaschta, J.; Eggerath, D.; Lövenich, W.; Krebs, F.C.; Brabec, C.J. Determining the coating speed limitations for organic photovoltaic inks. Sol. Energy Mater. Sol. Cells 2013, 109, 120–125. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological Control for High Performance, Solution-Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Chu, Z.; Yang, M.; Schulz, P.; Wu, D.; Ma, X.; Seifert, E.; Sun, L.; Li, X.; Zhu, K.; Lai, K. Impact of grain boundaries on efficiency and stability of organic–inorganic trihalide perovskites. Nat. Commun. 2017, 8, 2230. [Google Scholar] [CrossRef] [PubMed]
- Sherkar, T.S.; Momblona, C.; Gil-Escrig, L.; Ávila, J.; Sessolo, M.; Bolink, H.J.; Koster, L.J.A. Recombination in Perovskite Solar Cells: Significance of Grain Boundaries, Interface Traps, and Defect Ions. ACS Energy Lett. 2017, 2, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Mitzi, D.B.; Prikas, M.T. Synthesis and Characterization of Organic Inorganic Perovskite Thin Films Prepared Using a Versatile Two Step Dipping Technique. Chem. Mater. 1998, 10, 403–411. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Wark, M. Recent Progress in the Solution-Based Sequential Deposition of Planar Perovskite Solar Cells. Cryst. Growth Des. 2018, 18, 4790–4806. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high performance inorganic organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, H.; Yuan, H.; Yang, Z.; Fan, J.Z.; Kim, J.; Voznyy, O.; Gong, X.; Quan, L.N.; Tan, C.S.; et al. Perovskite seeding growth of formamidinium-lead-iodide-based perovskites for efficient and stable solar cells. Nat. Commun. 2018, 9, 1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Chen, M.; Ding, X.; Wang, M.; Liu, G.; Wang, X. Nucleation mechanism of CH3NH3PbI3 with two-step method for rational design of high performance perovskite solar cells. J. Alloys Compd. 2017, 697, 374–379. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Kheshgi, H.S. Low-flow limit in slot coating: Theory and experiments. AIChE J. 2000, 46, 1907–1917. [Google Scholar] [CrossRef]
- Liao, H.C.; Tsao, C.S.; Jao, M.H.; Shyue, J.J.; Hsu, C.P.; Huang, Y.C.; Tian, K.Y.; Chen, C.Y.; Su, C.J.; Su, W.F. Hierarchical i-p and i-n porous heterojunction in planar perovskite solar cells. J. Mater. Chem. A 2015, 3, 10526–10535. [Google Scholar] [CrossRef]
- Wagner, L.; Mundt, L.E.; Mathiazhagan, G.; Mundus, M.; Schubert, M.C.; Mastroianni, S.; Würfel, U.; Hinsch, A.; Glunz, S.W. Distinguishing crystallization stages and their influence on quantum efficiency during perovskite solar cell formation in real-time. Sci. Rep. 2017, 7, 14899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Hwang, T.; Lee, S.; Lee, B.; Kim, J.; Jang, G.S.; Nam, S.; Park, B. Solvent and Intermediate Phase as Boosters for the Perovskite Transformation and Solar Cell Performance. Sci. Rep. 2016, 6, 25648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Chen, Y.; Zheng, Y.C.; Chen, X.; Hou, Y.; Yang, H.G. Formation of high-quality perovskite thin film for planar heterojunction solar cells. RSC Adv. 2015, 5, 69502–69508. [Google Scholar] [CrossRef]
- Becker, M.; Wark, M. Controlling the crystallization and grain size of sequentially deposited planar perovskite films via the permittivity of the conversion solution. Org. Electron. 2017, 50, 87–93. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Liu, M.; Wu, J.; Gan, Y.; Hanaor, D.A.H.; Chen, C.Q. Evaporation Limited Radial Capillary Penetration in Porous Media. Langmuir 2016, 32, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, T.J.; Correa-Baena, J.P.; Halvani Anaraki, E.; Philippe, B.; Stranks, S.D.; Bouduban, M.E.F.; Tress, W.; Schenk, K.; Teuscher, J.; Moser, J.E.; et al. Unreacted PbI2 as a Double-Edged Sword for Enhancing the Performance of Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 10331–10343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, J.H.; Jang, I.H.; Pellet, N.; Grätzel, M.; Park, N.G. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, J.; Harris, T.A. A review of the operating limits in slot die coating processes. AIChE J. 2016, 62, 2508–2524. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Dubale, A.A.; Hwang, B.J. Organometal halide perovskite solar cells: degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Wang, S.; Sina, M.; Parikh, P.; Uekert, T.; Shahbazian, B.; Devaraj, A.; Meng, Y.S. Role of 4-tert-Butylpyridine as a Hole Transport Layer Morphological Controller in Perovskite Solar Cells. Nano Lett. 2016, 16, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J.; Grätzel, M. Enhanced charge mobility in a molecular hole transporter via addition of redox inactive ionic dopant: Implication to dye-sensitized solar cells. Appl. Phys. Lett. 2006, 89, 262114. [Google Scholar] [CrossRef]
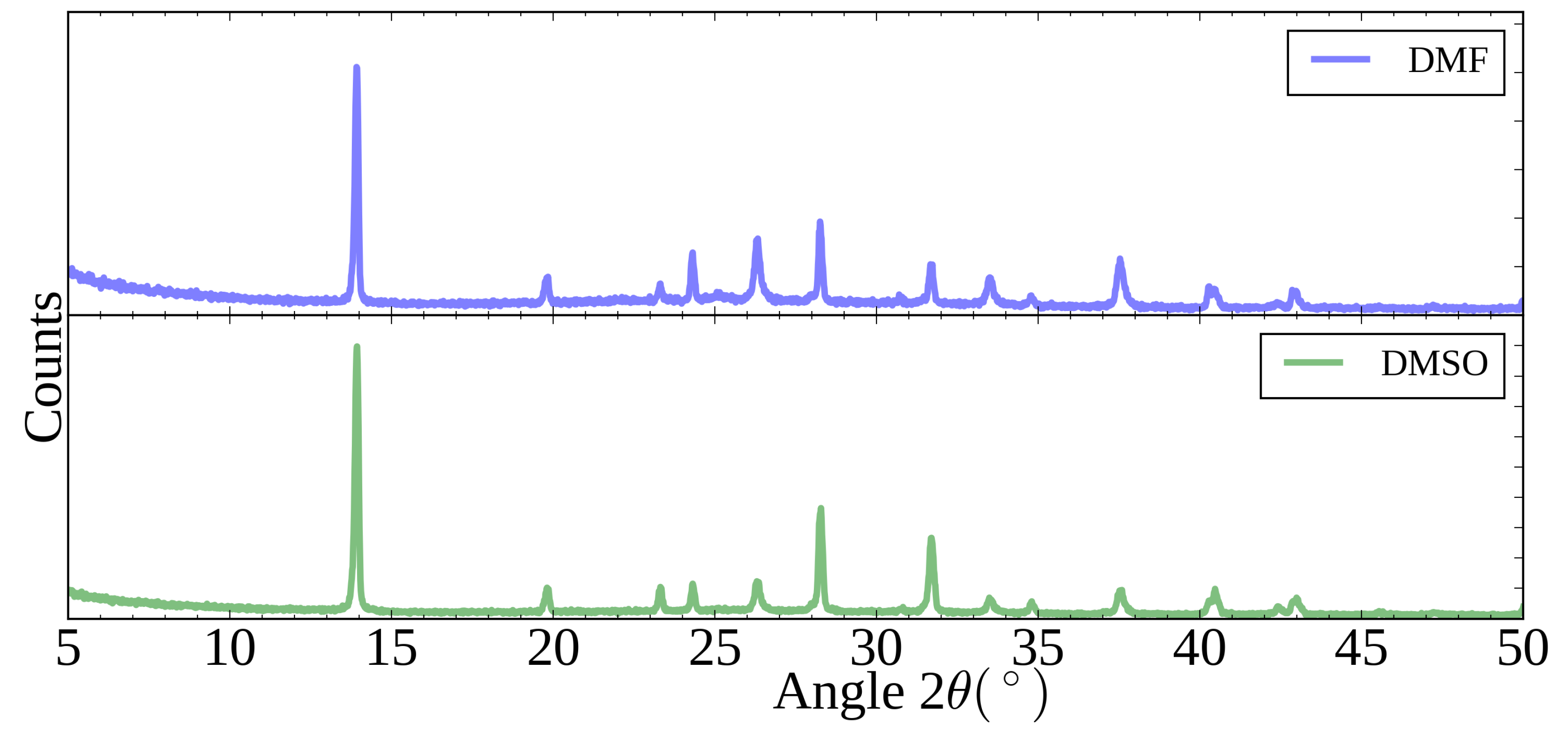
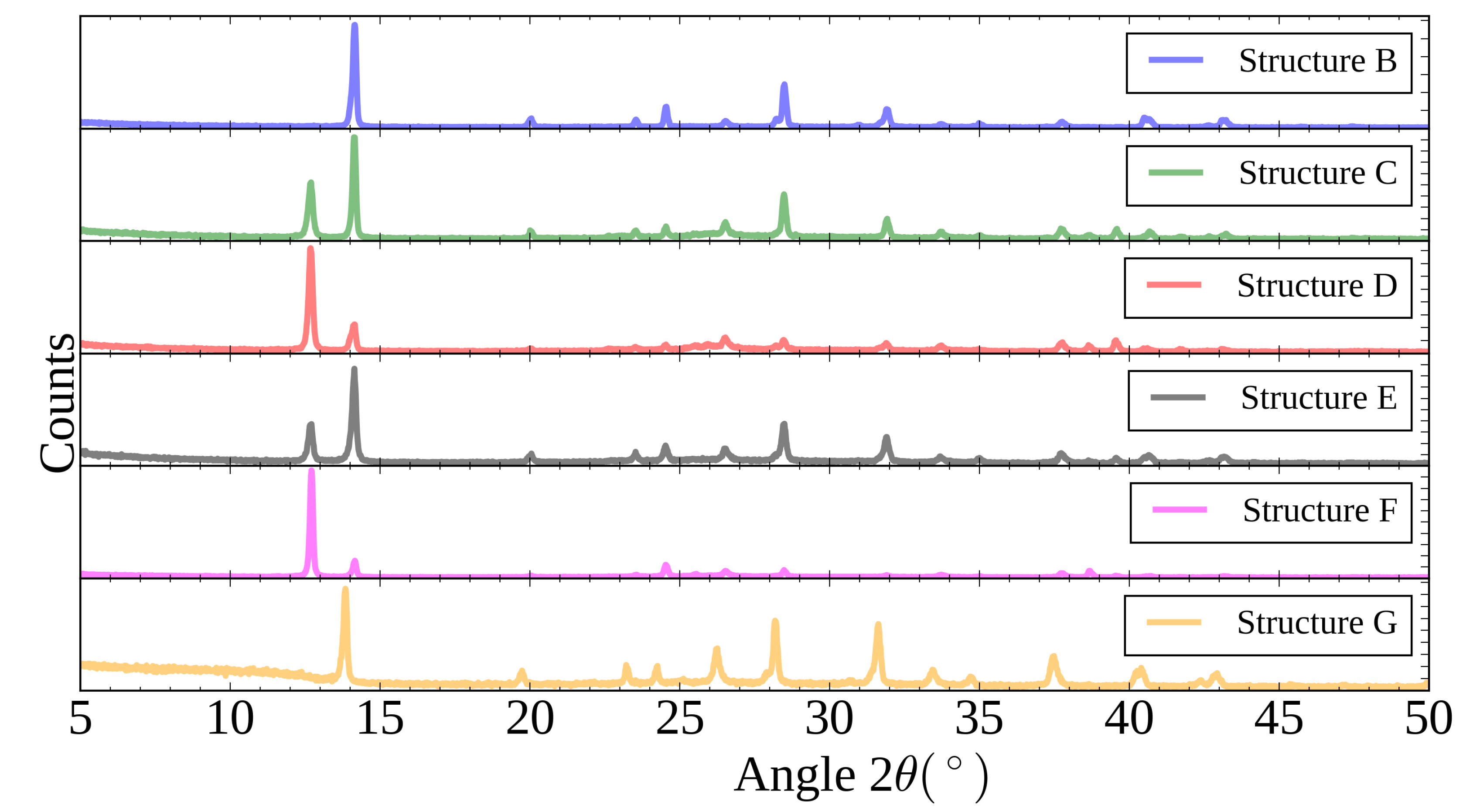
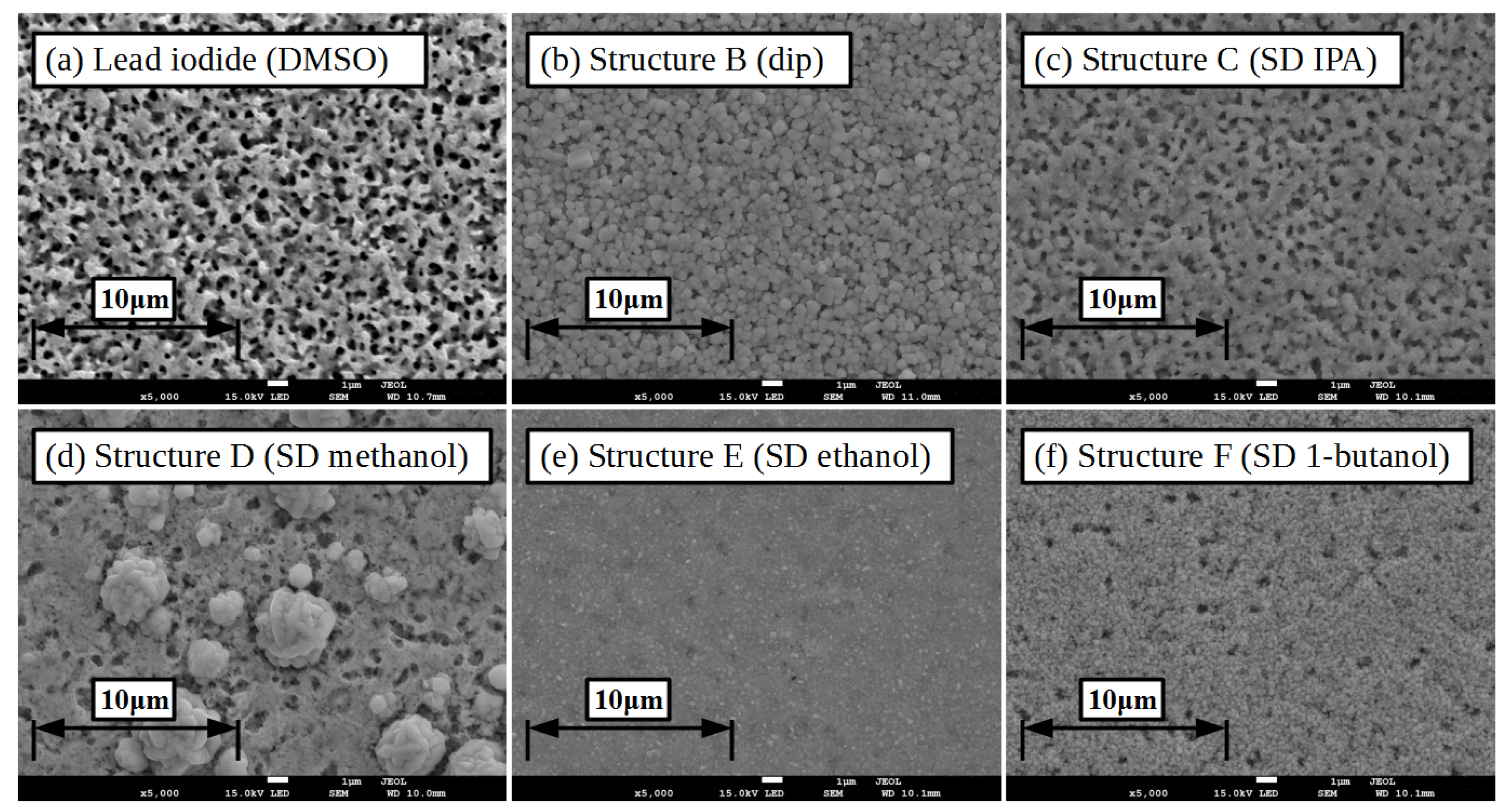
| Solvent | Contact Angle | Contact Angle |
|---|---|---|
| Mesoporous Surface | Blocking Layer Surface | |
| (Degrees/) | (Degrees/) | |
| Instant/Static | Instant/Static | |
| DMF | 9.9/<5 | 16.5/<5 |
| DMSO | 20.0/<5 | 23.0/22.5 |
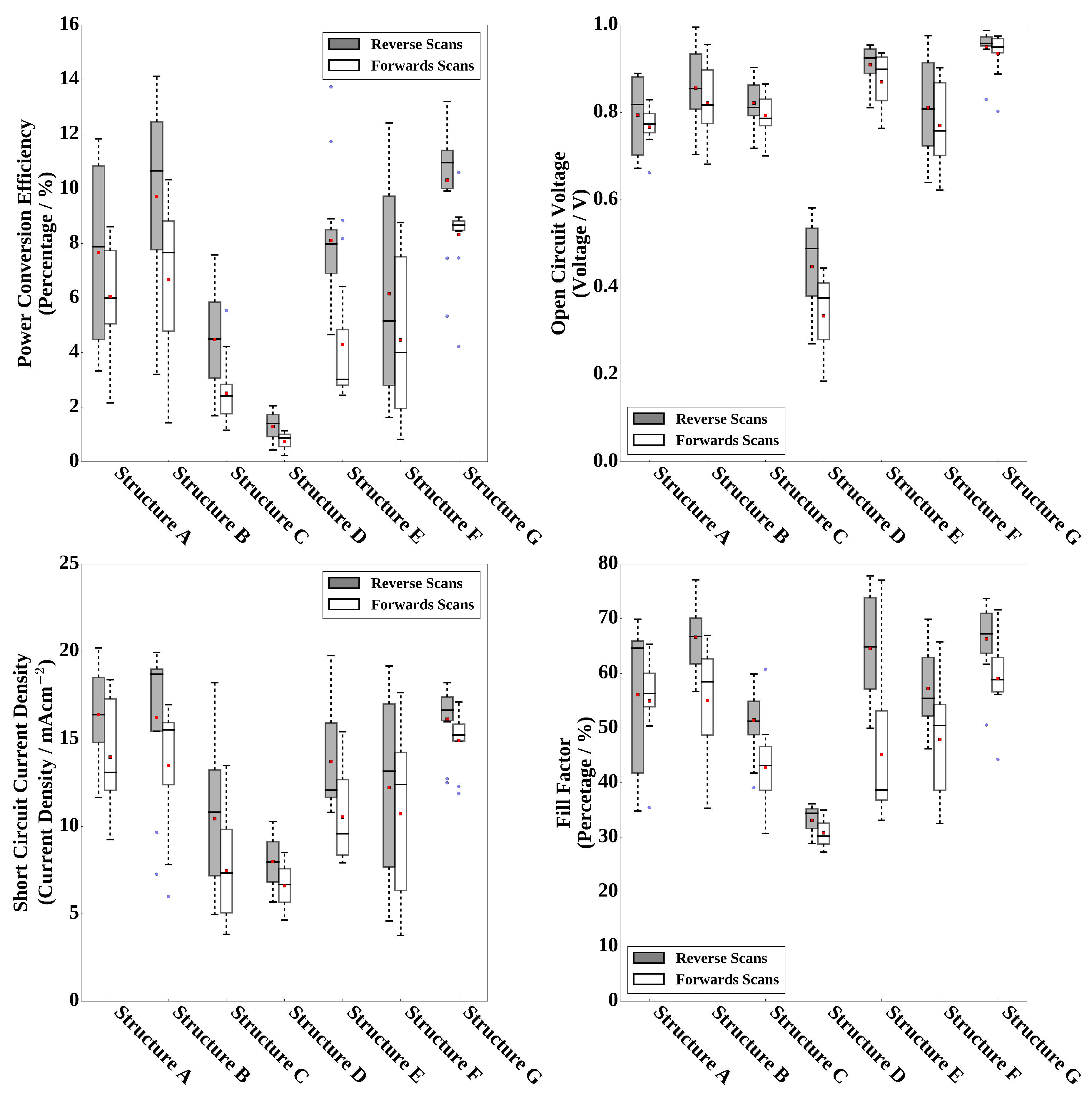
| Structure | Solvent | Coating Method | Solvent | Scan Direction | Voc | Jsc | FF | PCE | Hero PCE |
|---|---|---|---|---|---|---|---|---|---|
| Lead Iodide | MAI | MAI | (V) | mA·cm | (%) | (%) | (%) | ||
| A | DMF | Dip | IPA | Reverse | 0.82 | 16.4 | 64.6 | 7.9 | 11.8 |
| Forwards | 0.77 | 13.1 | 56.4 | 6.0 | 8.6 | ||||
| B | DMSO | Dip | IPA | Reverse | 0.86 | 18.7 | 66.7 | 10.7 | 14.1 |
| Forwards | 0.82 | 15.5 | 58.5 | 7.7 | 10.3 | ||||
| C | DMSO | Slot-die | IPA | Reverse | 0.81 | 10.8 | 51.3 | 4.5 | 7.6 |
| Forwards | 0.79 | 7.3 | 43.2 | 2.5 | 5.5 | ||||
| D | DMSO | Slot-die | Methanol | Reverse | 0.49 | 8.0 | 34.4 | 1.4 | 2.1 |
| Forwards | 0.38 | 6.7 | 30.2 | 0.9 | 1.1 | ||||
| E | DMSO | Slot-die | Ethanol | Reverse | 0.93 | 12.1 | 64.9 | 8.0 | 13.7 |
| Forwards | 0.90 | 9.6 | 38.7 | 3.0 | 8.9 | ||||
| F | DMSO | Slot-die | 1-Butanol | Reverse | 0.81 | 13.2 | 55.5 | 5.2 | 12.4 |
| Forwards | 0.76 | 12.4 | 50.5 | 4.0 | 8.8 | ||||
| G | DMSO | Slot-die | Ethanol | Reverse | 0.96 | 16.7 | 67.2 | 11.0 | 13.2 |
| 100 C substrate | Forwards | 0.95 | 15.2 | 58.9 | 8.7 | 10.6 |
| Formulation Solvent | Viscosity at 25 s | Surface Tension |
|---|---|---|
| (mPa·s) | (m·Nm) | |
| Methanol | 0.44 | 21.52 |
| Ethanol | 1.00 | 20.13 |
| IPA | 1.69 | 19.52 |
| 1-butanol | 2.66 | 23.23 |
| MAI Coating Method | Dip | Slot-die | ||
|---|---|---|---|---|
| Structure B | Structure G | |||
| Parameter | Day 1 | Day 442 | Day 1 | Day 442 |
| PCE (%) | 12.0 | 7.2 | 11.3 | 8.3 |
| Voc (V) | 0.95 | 0.95 | 0.96 | 0.93 |
| Jsc (mAcm) | 16.8 | 12.2 | 17.2 | 13.5 |
| FF (%) | 75 | 62 | 68 | 66 |
| Rs (Ohms·cm) | 5.1 | 6.3 | 5.0 | 8.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkitt, D.; Searle, J.; Worsley, D.A.; Watson, T. Sequential Slot-Die Deposition of Perovskite Solar Cells Using Dimethylsulfoxide Lead Iodide Ink. Materials 2018, 11, 2106. https://doi.org/10.3390/ma11112106
Burkitt D, Searle J, Worsley DA, Watson T. Sequential Slot-Die Deposition of Perovskite Solar Cells Using Dimethylsulfoxide Lead Iodide Ink. Materials. 2018; 11(11):2106. https://doi.org/10.3390/ma11112106
Chicago/Turabian StyleBurkitt, Daniel, Justin Searle, David A. Worsley, and Trystan Watson. 2018. "Sequential Slot-Die Deposition of Perovskite Solar Cells Using Dimethylsulfoxide Lead Iodide Ink" Materials 11, no. 11: 2106. https://doi.org/10.3390/ma11112106
APA StyleBurkitt, D., Searle, J., Worsley, D. A., & Watson, T. (2018). Sequential Slot-Die Deposition of Perovskite Solar Cells Using Dimethylsulfoxide Lead Iodide Ink. Materials, 11(11), 2106. https://doi.org/10.3390/ma11112106






