Investigation of the Localized Corrosion and Passive Behavior of Type 304 Stainless Steels with 0.2–1.8 wt % B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pitting Corrosion Resistance
2.3. Passive Film Properties
3. Results and Discussion
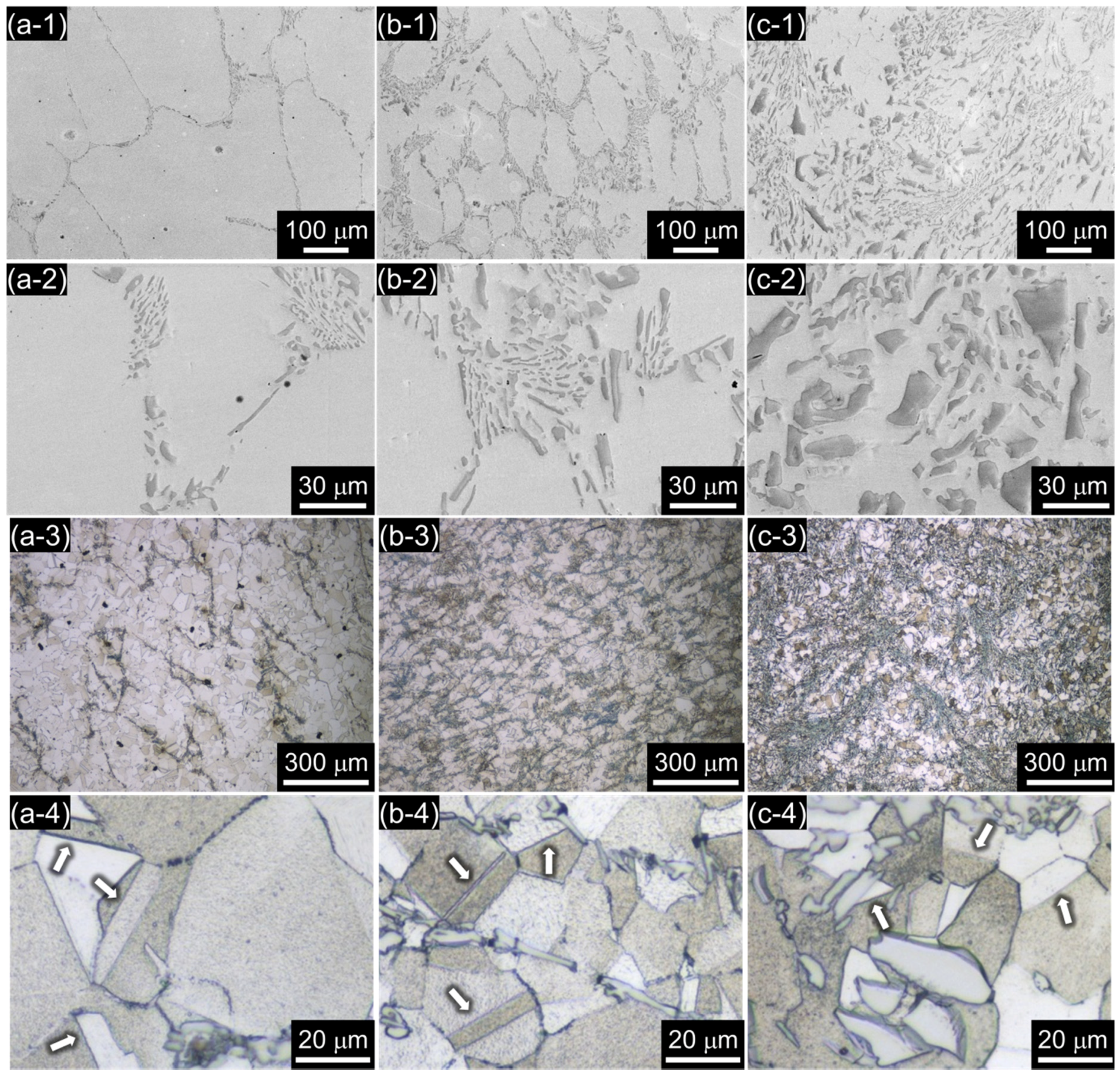
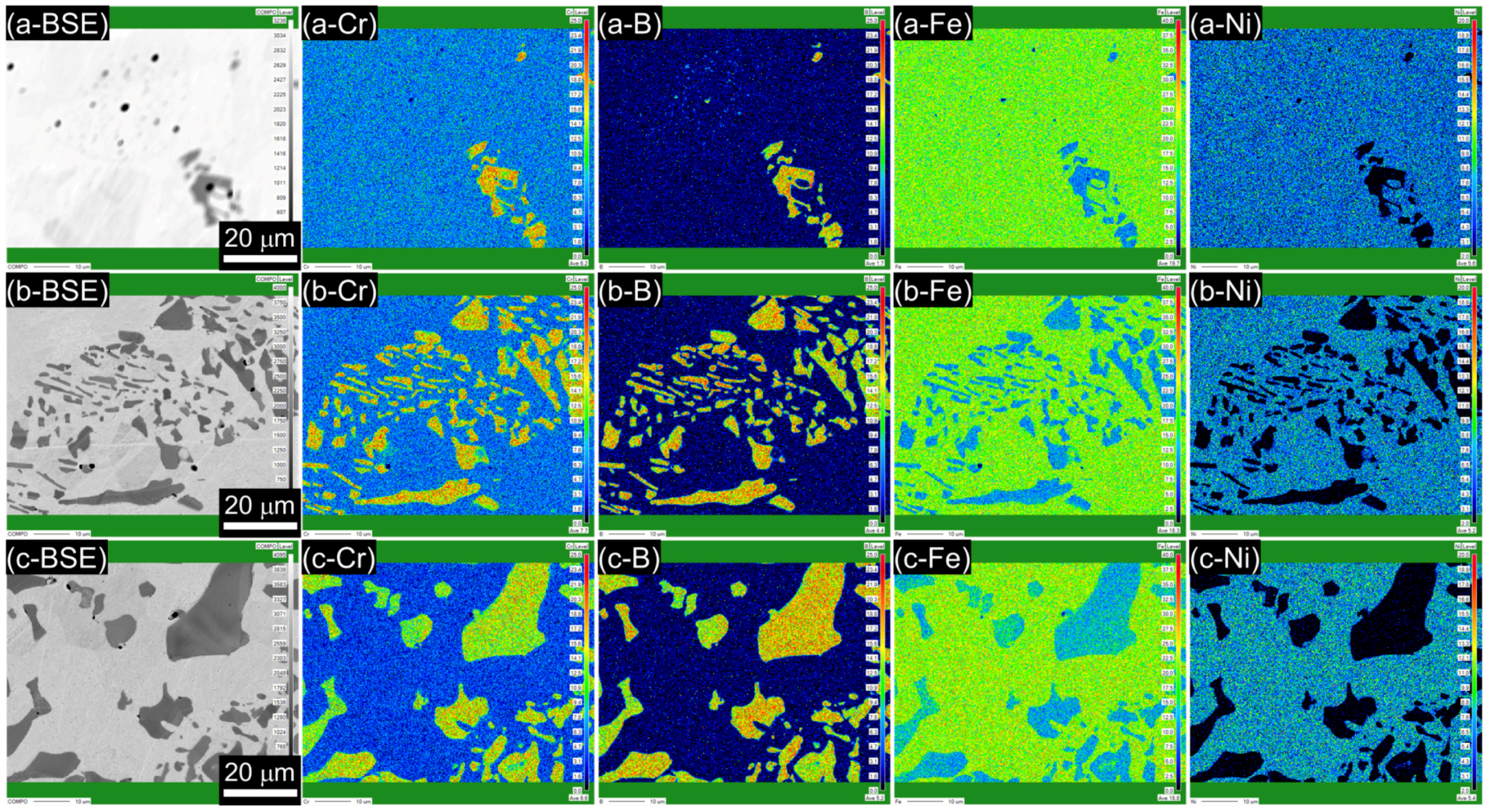
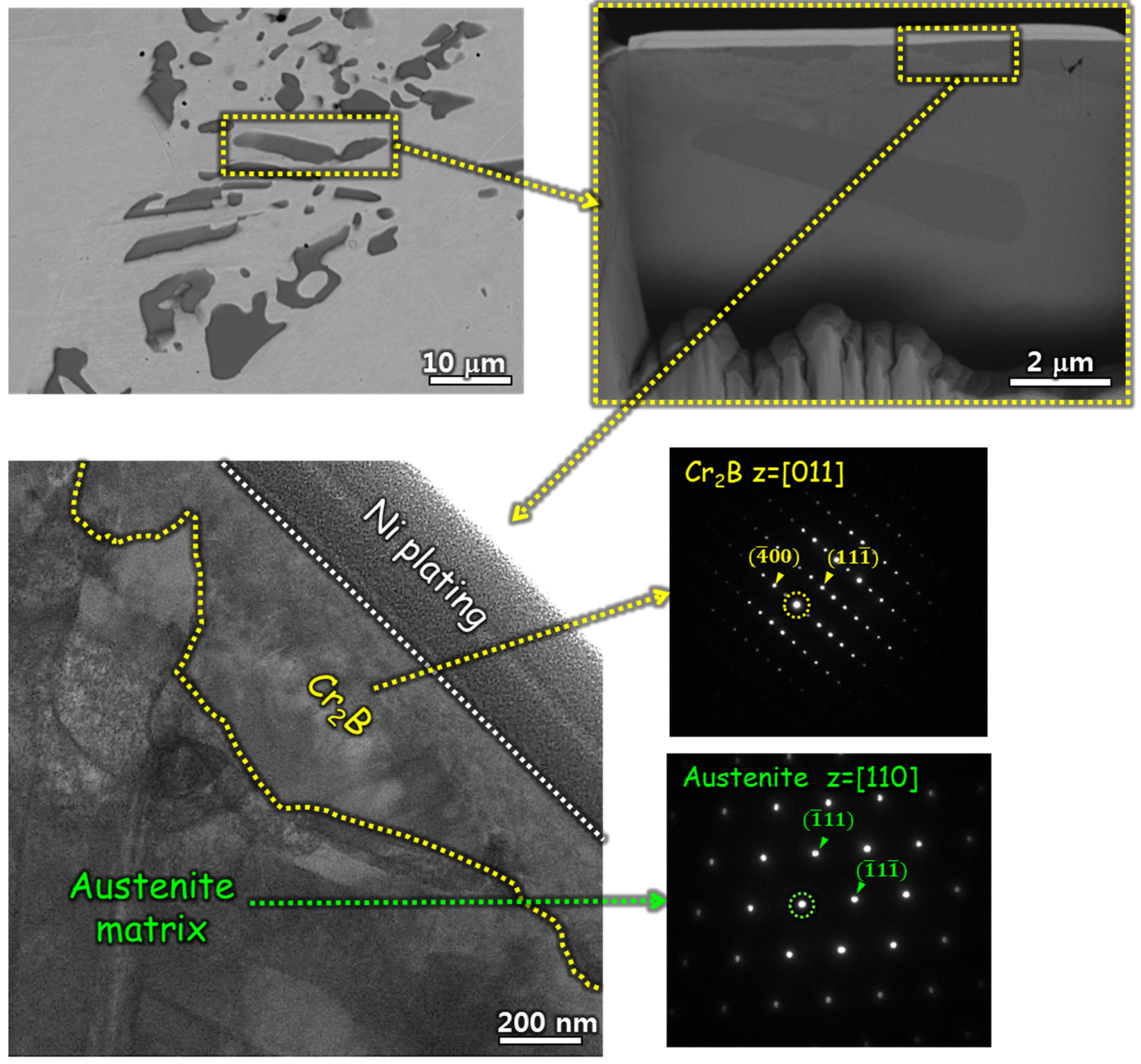
3.1. Microstructure
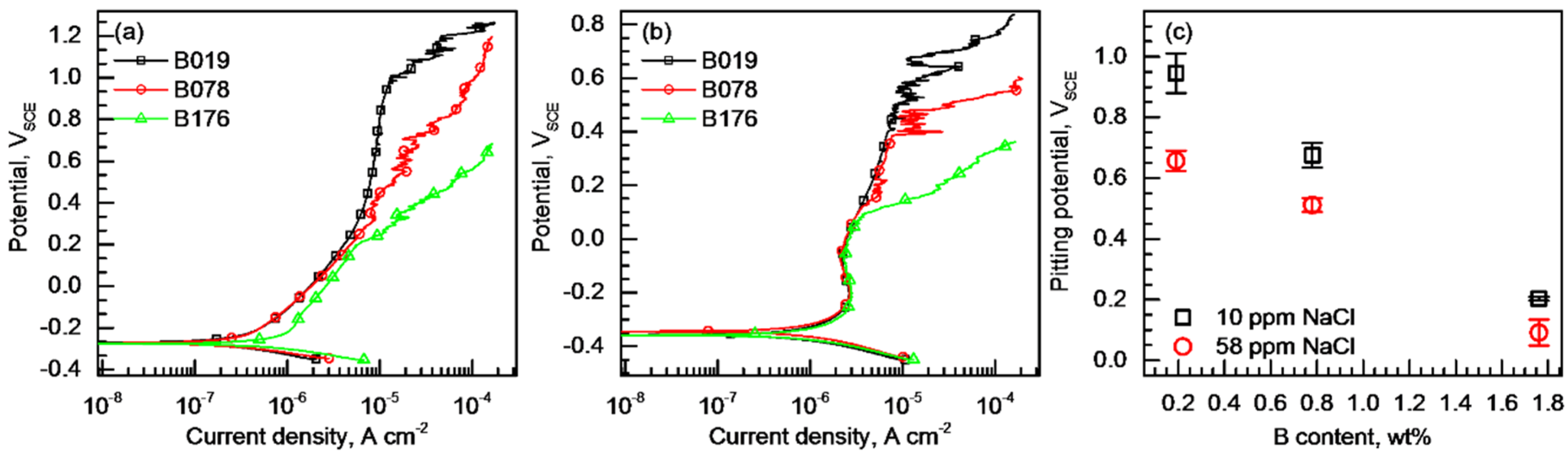
3.2. Pitting Corrosion Resistance
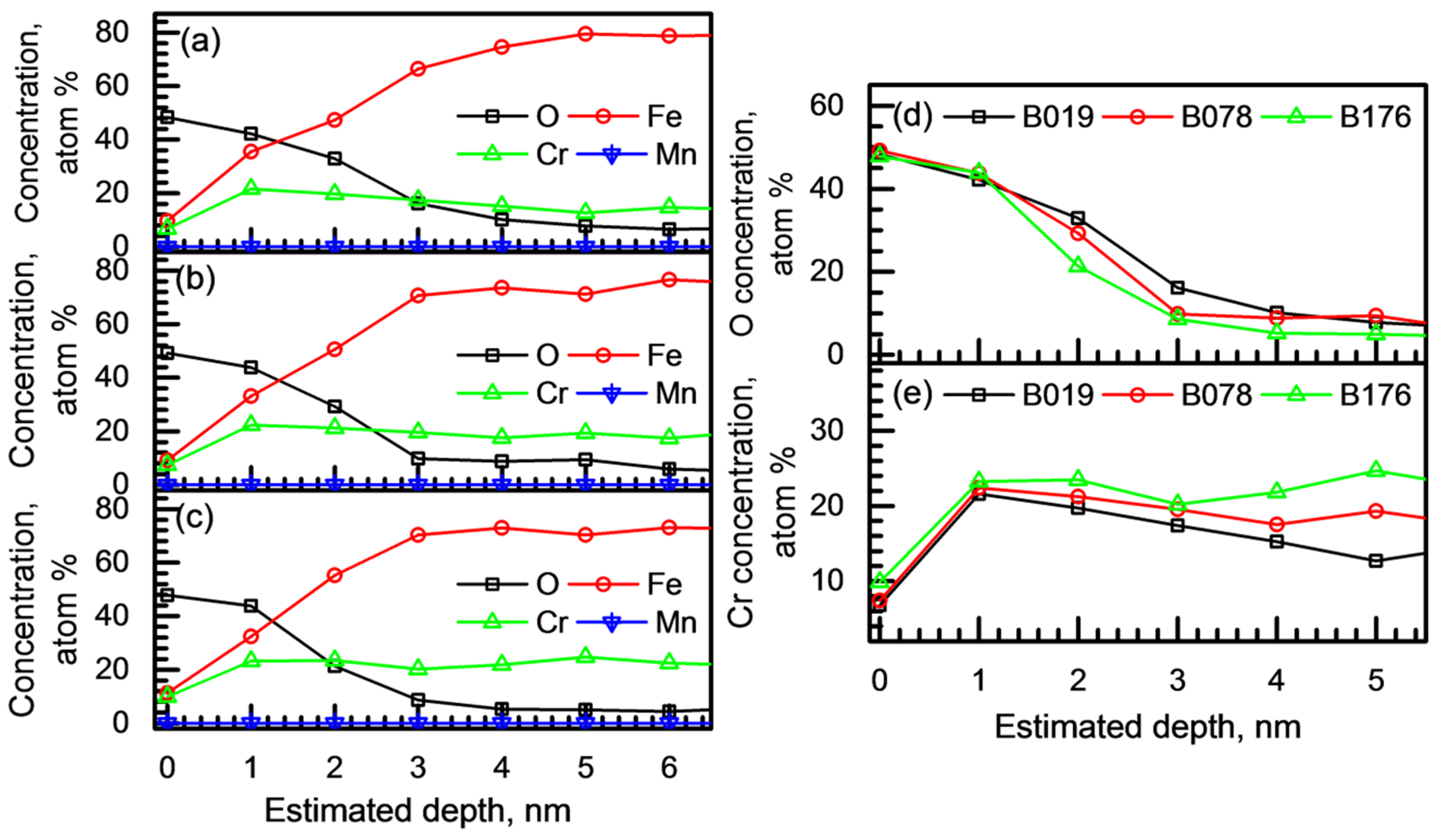
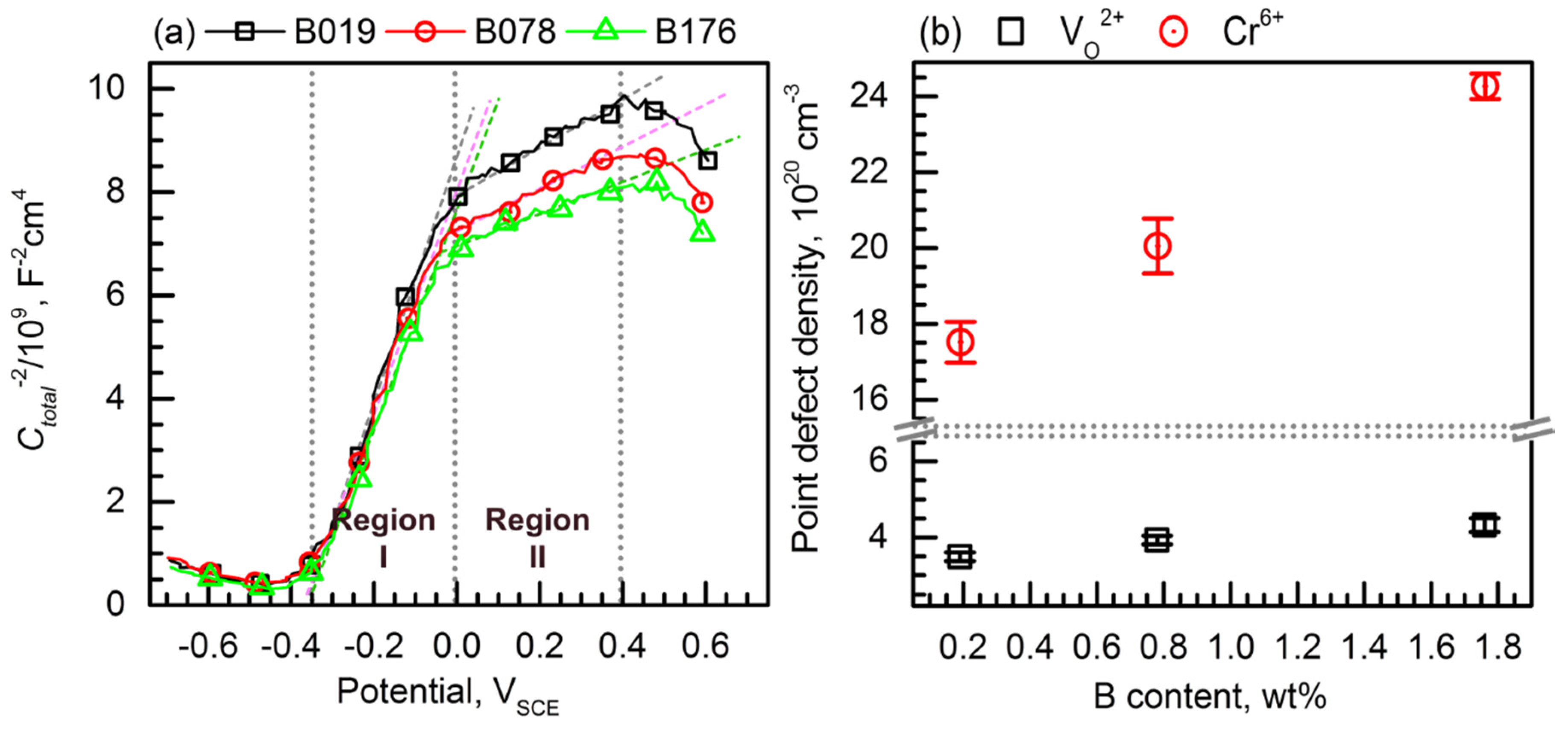
3.3. Passive Film Analysis
4. Conclusions
- The borated stainless steels were composed of austenitic matrix and Cr2B phase. As the B content increased from 0.19 to 1.76 wt %, the volume fraction of Cr2B increased from 1.68 to 22.66 vol %, and the concentration of Cr in solid solution state in the austenitic matrix was lowered from 16.67 to 9.21 wt %. In addition, the grain size of the austenite matrix decreased as the Cr2B fraction increased.
- In various NaCl solutions, lower pitting corrosion resistance was observed in the alloy with higher B content. The pits were initiated at the matrix adjacent to the Cr2B and propagated into the matrix.
- Regarding the passive behavior, the passive current density increased as the B content in the alloy increased. The passive films of the borated stainless steels formed in borate–phosphate–citric buffer solution (pH 8.5) were (Fe,Cr)-oxides. With an increase in the B addition, the passive film thickness decreased from 2.5 to 1.8 nm, and the Cr content in the passive film slightly increased. Furthermore, Mott–Schottky analysis confirmed that more defective passive film was formed on the alloy with higher B content.
- The reduced resistance to pitting corrosion of the B-bearing type 304 stainless steel along with the increase in the B content was due to the formation of a more defective and thinner passive film and a larger number of pit initiation sites in the matrix.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Machiels, A.; Lambert, R. Handbook of Neutron Absorber Materials for Spent Nuclear Fuel Transportation and Storage Applications, 2009 ed.; EPRI: Palo Alto, CA, USA, 2009; p. 2. [Google Scholar]
- Brown, R.S. Corrosion Resistance of Borated Stainless Steels; Carpenter Powder Products: Wyomissing, PA, USA, 1991; p. 657. [Google Scholar]
- Robino, C.V.; Cieslak, M.J. High-temperature metallurgy of advanced borated stainless steels. Metall. Mater. Trans. A 1995, 26, 1673–1685. [Google Scholar] [CrossRef]
- He, J.Y.; Soliman, S.E.; Bratta, A.J.; Balliett, T.A. Fracture Mechanism of Borated Stainless Steel. Nucl. Technol. 2000, 130, 218–225. [Google Scholar] [CrossRef]
- Fix, D.V.; Estill, J.C.; Wong, L.L.; Rebak, R.B. General and Localized Corrosion of Austenitic and Borated Stainless Steels in Simulated Concentrated Ground Waters. In Proceedings of the American Society of Mechanical Engineers Pressure Vessels and Piping Division (ASME-PVP) Conference, San Diego, CA, USA, 25–29 July 2004. [Google Scholar]
- Moreno, D.A.; Molina, B.; Ranninger, C.; Montero, F.; Izquierdo, J. Microstructural Characterization and Pitting Corrosion Behavior of UNS S30466 Borated Stainless Steel. Corrosion 2004, 60, 573–583. [Google Scholar] [CrossRef]
- Lister, T.E.; Mizia, R.E.; Erickson, A.W.; Matteson, B.S. General and Localized Corrosion of Borated Stainless Steels; INL/CON-07-135355; Idaho National Laboratory: Idaho Falls, ID, USA, 2008. [Google Scholar]
- He, X.; Ahn, T.; Sippel, T. Corrosion of Borated Stainless Steel in Water and Humid Air; NRC-02-07-006; Nuclear Regulatory Commission: Rockville, MD, USA, 2011. [Google Scholar]
- Shanmugarajan, B.; Chary, J.N.; Padmanabham, G.; Arivazhagan, B.; Shaju, K.A.; Bhaduri, A.K. Studies on autogenous laser welding of type 304B4 borated stainless steel. Opt. Laser Eng. 2013, 51, 1272–1277. [Google Scholar]
- RajaKumar, G.; Ram, G.D.J.; Rao, S.R.K. Microstructure and Mechanical properties of Borated Stainless Steel (304B) GTA and SMA welds. La Metall. Ital. 2015, 5, 47–52. [Google Scholar]
- Sivai Bharasi, N.; Pujar, M.G.; Nirmal, S.; Mallika, C.; Kamachi Mudali, U.; Angelo, P.C. Comparison of SCC Behavior of 304L Stainless Steels With and Without Boron Addition in Acidic Chloride Environment. J. Mater. Eng. Perform. 2016, 5, 2786–2798. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM) A 887-89. Standard Specification for Borated Stainless Steel Plate, Sheet, and Strip for Nuclear Application; ASTM: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Loria, E.A.; Isaacs, H.S. Type 304 stainless steel with 0.50% boron for storage of spent nuclear fuel. JOM 1980, 32, 10–17. [Google Scholar] [CrossRef]
- Hoffman, J.P.; De Jesus, A.S.M. The distribution of boron in stainless steels as revealed by a nuclear technique. J. S. Afr. Inst. Min. Metall. 1989, 89, 81–87. [Google Scholar]
- Levitin, V.V.; Syreishchikova, V.I. On the effect of boron on the nature of the carbide precipitation in austenitic steel on tempering. Fiz. Met. Metallovdenie 1959, 7, 308–310. [Google Scholar]
- Farrell, J.W.; Rosenthal, P.C. How boron affects corrosion of type 304 stainless steel. Met. Prog. 1960, 77, 101–103. [Google Scholar]
- Robinson, F.P.A.; Scurr, W.G. The effect of boron on the corrosion resistance of austenitic stainless steels. Corrosion 1977, 33, 408–417. [Google Scholar] [CrossRef]
- Dergach, T.A.; Sukhomlin, G.D. Effect of boron on the structure and intercrystalline-corrosion resistance of austenitic stainless steel. Prot. Met. 1990, 25, 390–394. [Google Scholar]
- Goldschmidt, H.J. Effect of Boron Additions to Austenitic Stainless Steels. J. Iron Steel Inst. 1971, 209, 900–911. [Google Scholar]
- Upadhyay, N.; Pujar, M.G.; Das, C.R.; Mallika, C.; Kamachi Mudali, U. Pitting Corrosion Studies on Solution-Annealed Borated Type 304L Stainless Steel Using Electrochemical Noise Technique. Corrosion 2014, 70, 781–795. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo-Calc Databank System. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Novak, E.; Schmit, J. White-light optical profiler with integrated primary standard. In Proceedings of the XVII IMEKO World Congress Metrology in the 3rd Millennium, Dubrovnik, Croatia, 22–27 June 2003; pp. 183–186. [Google Scholar]
- Ha, H.-Y.; Kwon, H.-S. Effects of pH Levels on the Surface Charge and Pitting Corrosion Resistance of Fe. J. Electrochem. Soc. 2012, 159, C416–C421. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Bao, Y.Z. A study of the brittleness and its improvement of high boron Cr–Ni stainless steels. Inst. Tech. Bull. 1993, 3, 194–198. [Google Scholar]
- Piao, T.; Park, S.-M. Spectroelectrochemical Studies of Passivation and Transpassive Breakdown Reactions of Stainless Steel. J. Electrochem. Soc. 1997, 144, 3371–3377. [Google Scholar] [CrossRef]
- Cho, E.A.; Kwon, H.S.; Macdonald, D.D. Photoelectrochemical analysis on the passive film formed on Fe–20Cr in pH 8.5 buffer solution. Electrochim. Acta 2002, 47, 1661–1668. [Google Scholar] [CrossRef]
- Kuczynska-Wydorska, M.; Flis-Kabulska, I.; Flis, J. Corrosion of low-temperature nitrided molybdenum-bearing stainless steels. Corros. Sci. 2011, 53, 1762–1769. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Kim, S.-J. Role of nitrogen in the active–passive transition behavior of binary Fe–Cr alloy system. Electrochim. Acta 2012, 80, 432–439. [Google Scholar] [CrossRef]
- Obayi, C.S.; Tolouei, R.; Mostavan, A.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Mantovani, D. Effect of grain sizes on mechanical properties and biodegradation behavior of pure iron for cardiovascular stent application. Biomatter 2016, 6, e959874. [Google Scholar] [CrossRef] [PubMed]
- Sabooni, S.; Rashtchi, H.; Eslami, A.; Karimzadeh, F.; Enayati, M.H.; Raeissi, K.; Ngan, A.H.W.; Imani, R.F. Dependence of corrosion properties of AISI 304L stainless steel on the austenite grain size. Int. J. Mater. Res. 2017, 108, 552–559. [Google Scholar] [CrossRef]
- Lee, C.-G.; Ha, H.-Y.; Lee, T.-H.; Cho, K.-M. Effects of Nb on Pitting Corrosion Resistance of Ni-Free FeCrMnCN-Based Stainless Steels. J. Electrochem. Soc. 2017, 164, C591–C597. [Google Scholar] [CrossRef]
- Di Schino, A.; Kenny, J.M. Effects of the grain size on the corrosion behavior of refined AISI 304 austenitic stainless steels. J. Mater. Sci. Lett. 2002, 21, 1631–1634. [Google Scholar] [CrossRef]
- Hubschmid, C.; Landolt, D.; Mathieu, H.J. XPS and AES analysis of passive films on Fe-25Cr-X (X = Mo, V, Si and Nb) model alloys. Fresenius J. Anal. Chem. 1995, 353, 234–239. [Google Scholar] [CrossRef]
- Yang, M.Z.; Luo, J.L.; Yang, Q.; Qiao, L.J.; Qin, Z.Q.; Norton, P.R. Effects of Hydrogen on Semiconductivity of Passive Films and Corrosion Behavior of 310 Stainless Steel. J. Electrochem. Soc. 1999, 146, 2107–2112. [Google Scholar] [CrossRef]
- Paola, A.D. Semiconducting properties of passive films on stainless steels. Electrochim. Acta 1989, 34, 203–210. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Jang, M.-H.; Lee, T.-H. Influences of Mn in solid solution on the pitting corrosion behaviour of Fe-23 wt %Cr-based alloys. Electrochim. Acta 2016, 19, 864–875. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Oh, C.-S.; Kim, S.-J. Effects of combined addition of carbon and nitrogen on pitting corrosion behavior of Fe–18Cr–10Mn alloys. Scr. Mater. 2009, 61, 121–124. [Google Scholar] [CrossRef]
- Kislyi, P.S.; L’vov, S.N.; Nemchenko, V.F.; Samsonov, G.V. Physical properties of the boride phases of chromium. Sov. Powder Metall. Met. Ceram. 1962, 1, 441–443. [Google Scholar] [CrossRef]




| Alloy | Fe | Cr | Ni | Mn | Al | C | Si | B |
|---|---|---|---|---|---|---|---|---|
| B019 | Balance | 17.7 | 11.9 | 1.35 | 0.024 | 0.056 | 0.25 | 0.19 |
| B078 | 18.4 | 12.3 | 1.55 | 0.035 | 0.066 | 0.26 | 0.78 | |
| B176 | 18.3 | 12.4 | 1.57 | 0.039 | 0.073 | 0.28 | 1.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, H.-Y.; Jang, J.H.; Lee, T.-H.; Won, C.; Lee, C.-H.; Moon, J.; Lee, C.-G. Investigation of the Localized Corrosion and Passive Behavior of Type 304 Stainless Steels with 0.2–1.8 wt % B. Materials 2018, 11, 2097. https://doi.org/10.3390/ma11112097
Ha H-Y, Jang JH, Lee T-H, Won C, Lee C-H, Moon J, Lee C-G. Investigation of the Localized Corrosion and Passive Behavior of Type 304 Stainless Steels with 0.2–1.8 wt % B. Materials. 2018; 11(11):2097. https://doi.org/10.3390/ma11112097
Chicago/Turabian StyleHa, Heon-Young, Jae Hoon Jang, Tae-Ho Lee, Chihyoung Won, Chang-Hoon Lee, Joonoh Moon, and Chang-Geun Lee. 2018. "Investigation of the Localized Corrosion and Passive Behavior of Type 304 Stainless Steels with 0.2–1.8 wt % B" Materials 11, no. 11: 2097. https://doi.org/10.3390/ma11112097
APA StyleHa, H.-Y., Jang, J. H., Lee, T.-H., Won, C., Lee, C.-H., Moon, J., & Lee, C.-G. (2018). Investigation of the Localized Corrosion and Passive Behavior of Type 304 Stainless Steels with 0.2–1.8 wt % B. Materials, 11(11), 2097. https://doi.org/10.3390/ma11112097






