A 3D MoS2/Graphene Microsphere Coated Separator for Excellent Performance Li-S Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Flower-Like MoS2
2.2. Preparation of MoS2/Graphene Composite
2.3. Preparation of MoS2/Graphene-Modified Separator
2.4. Synthesis of S/Graphene Cathode Material
2.5. Material Characterization
2.6. Electrochemical Measurements
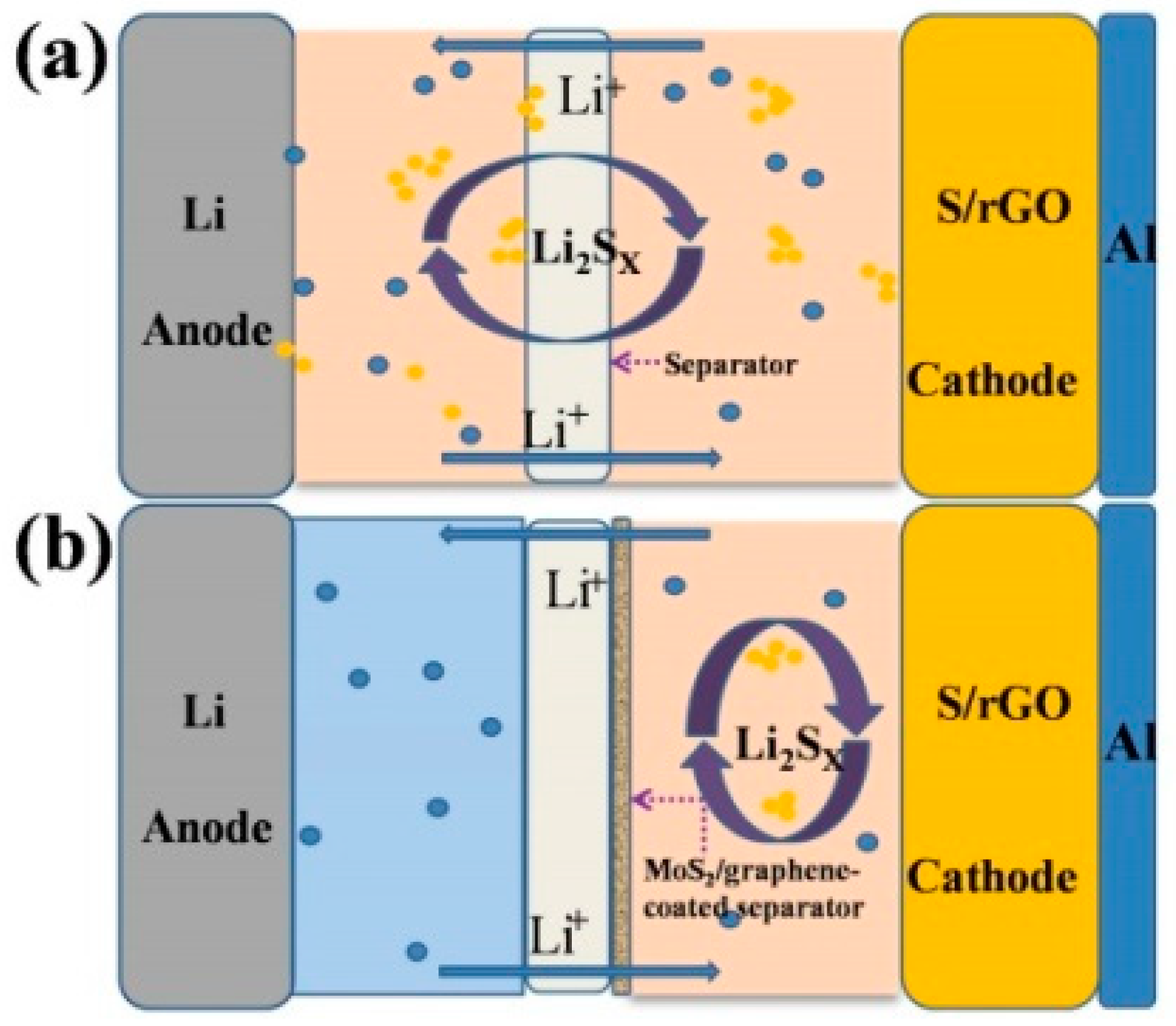
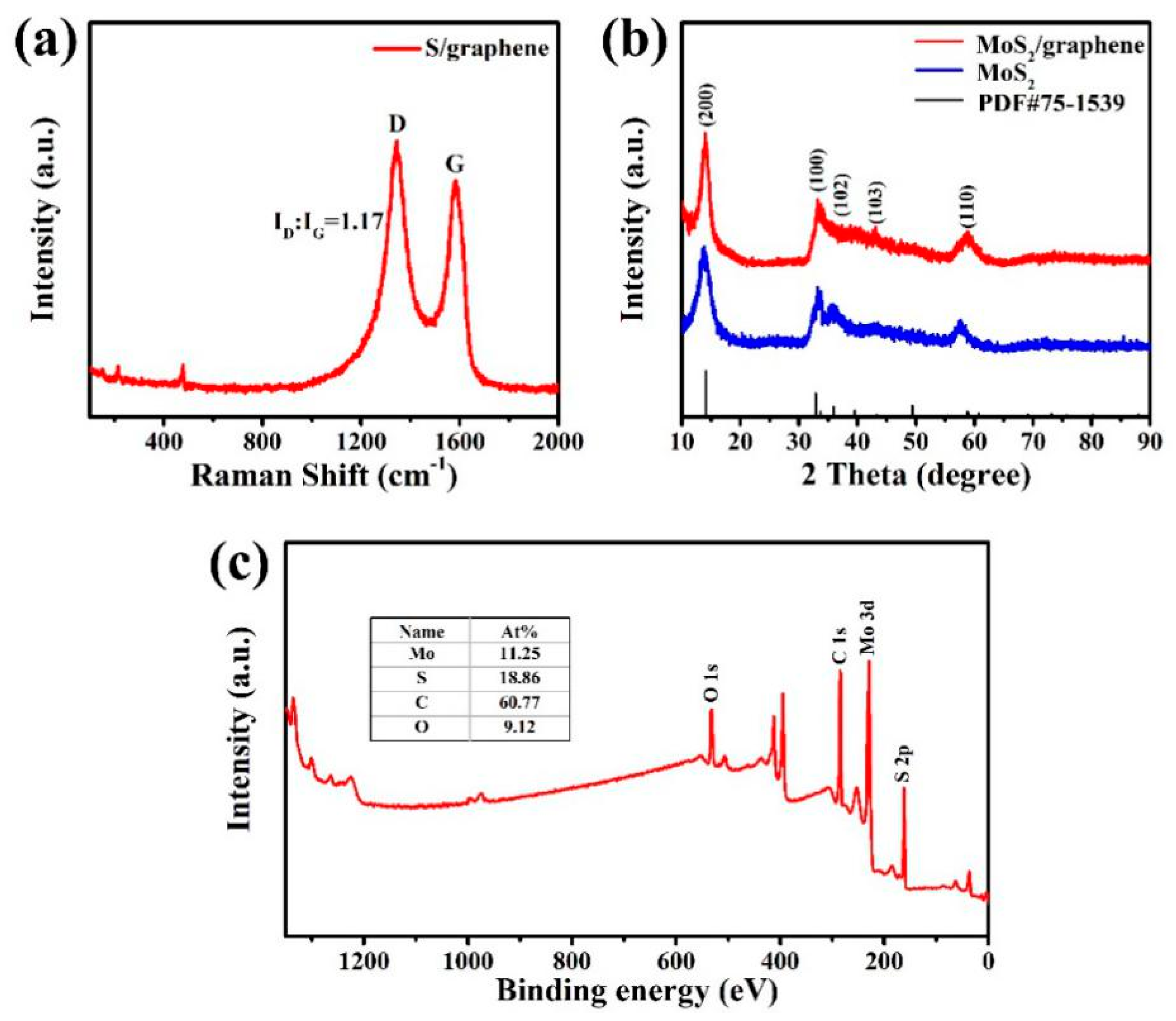
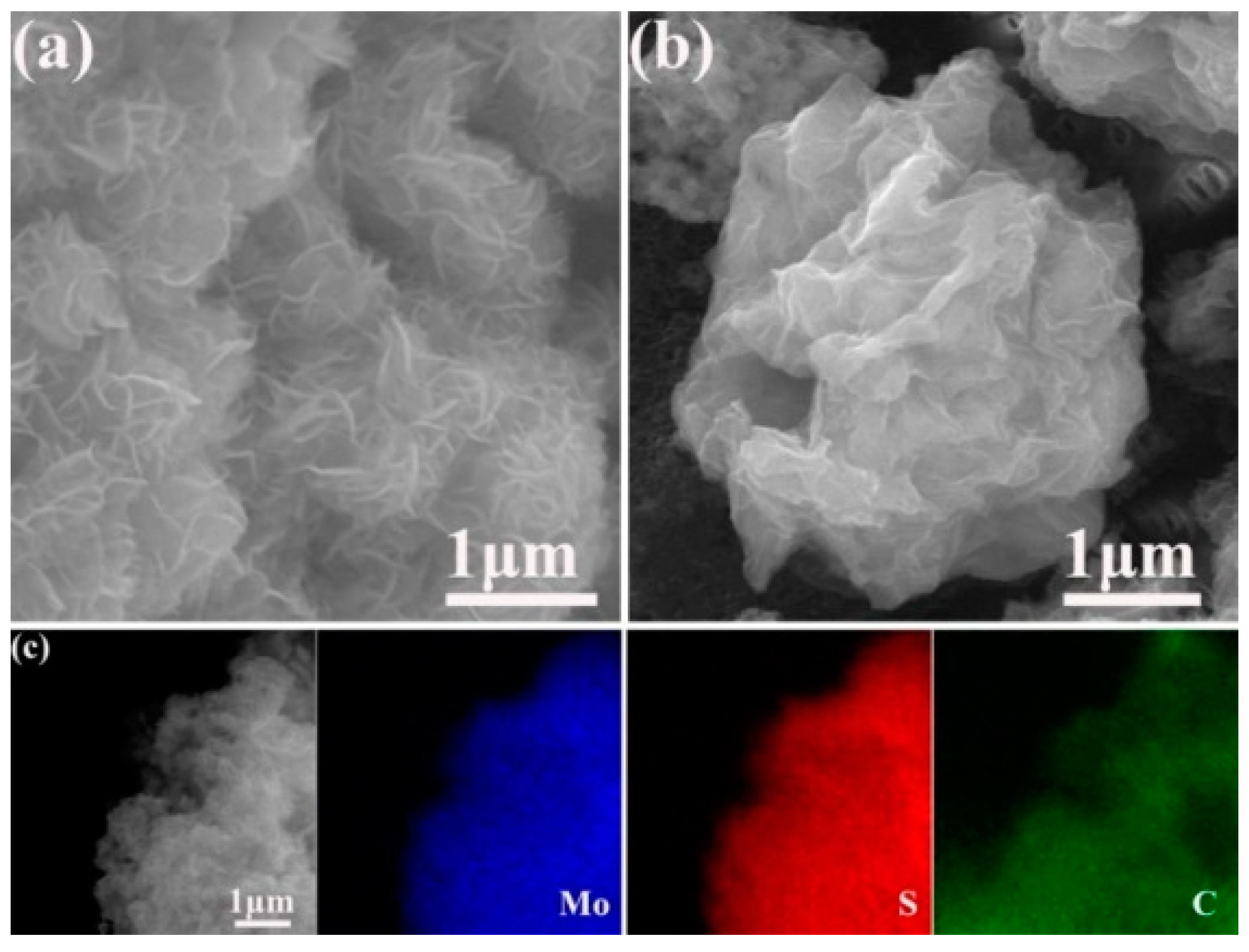
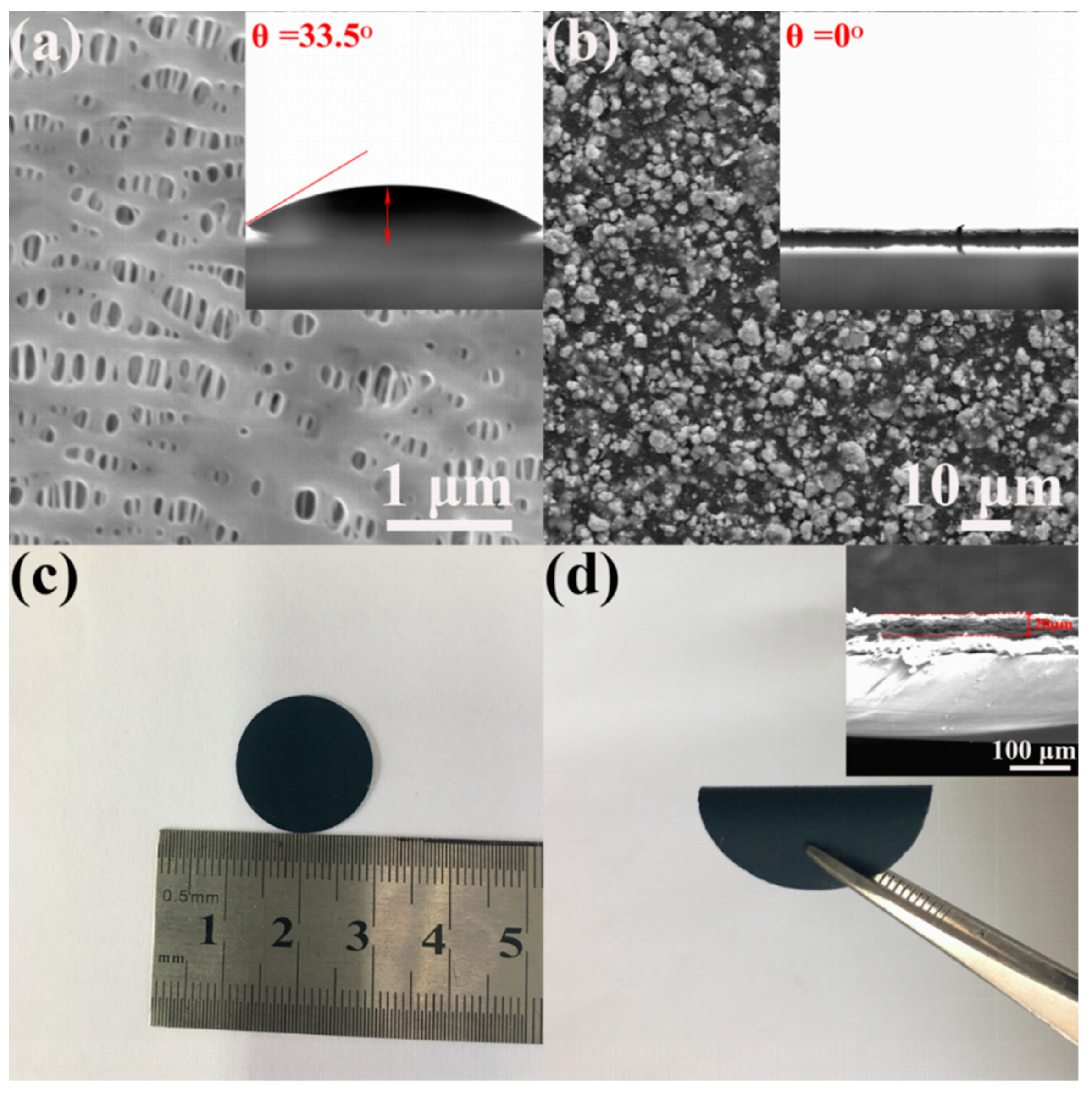
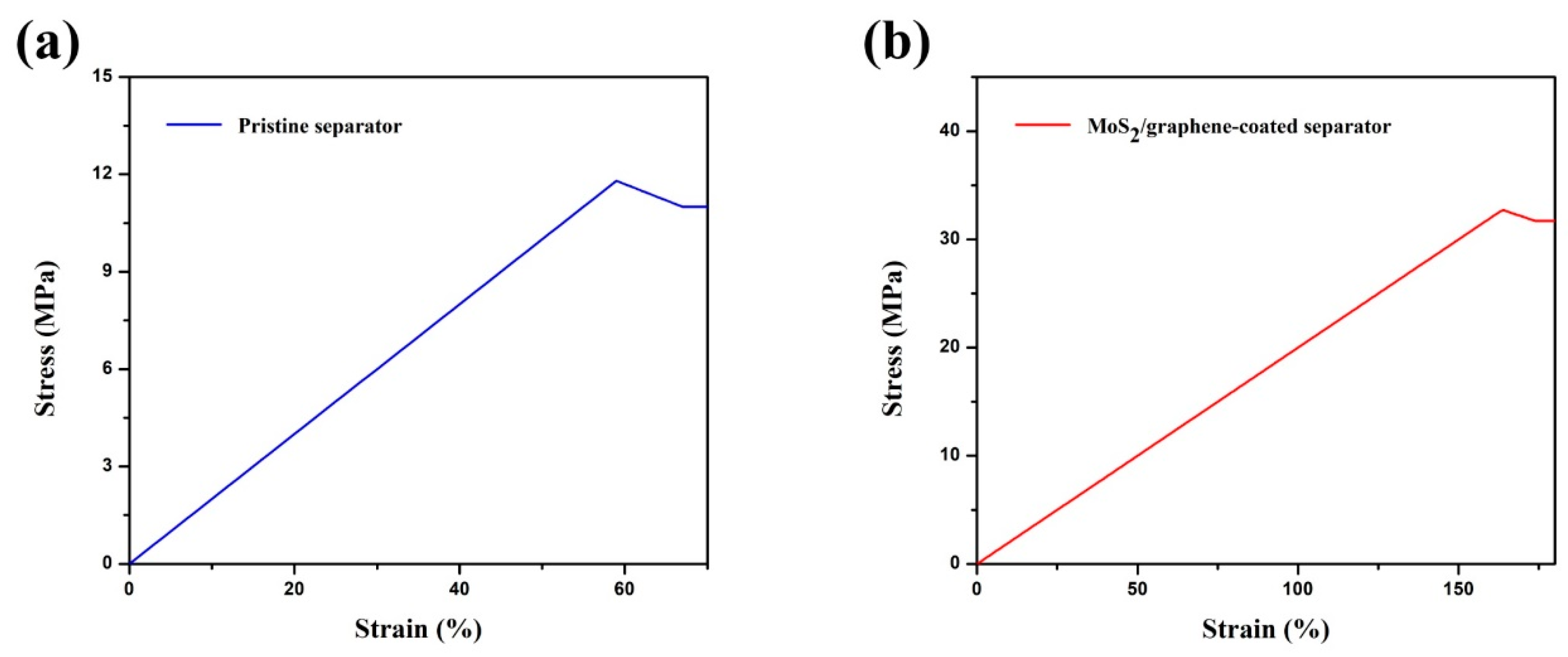
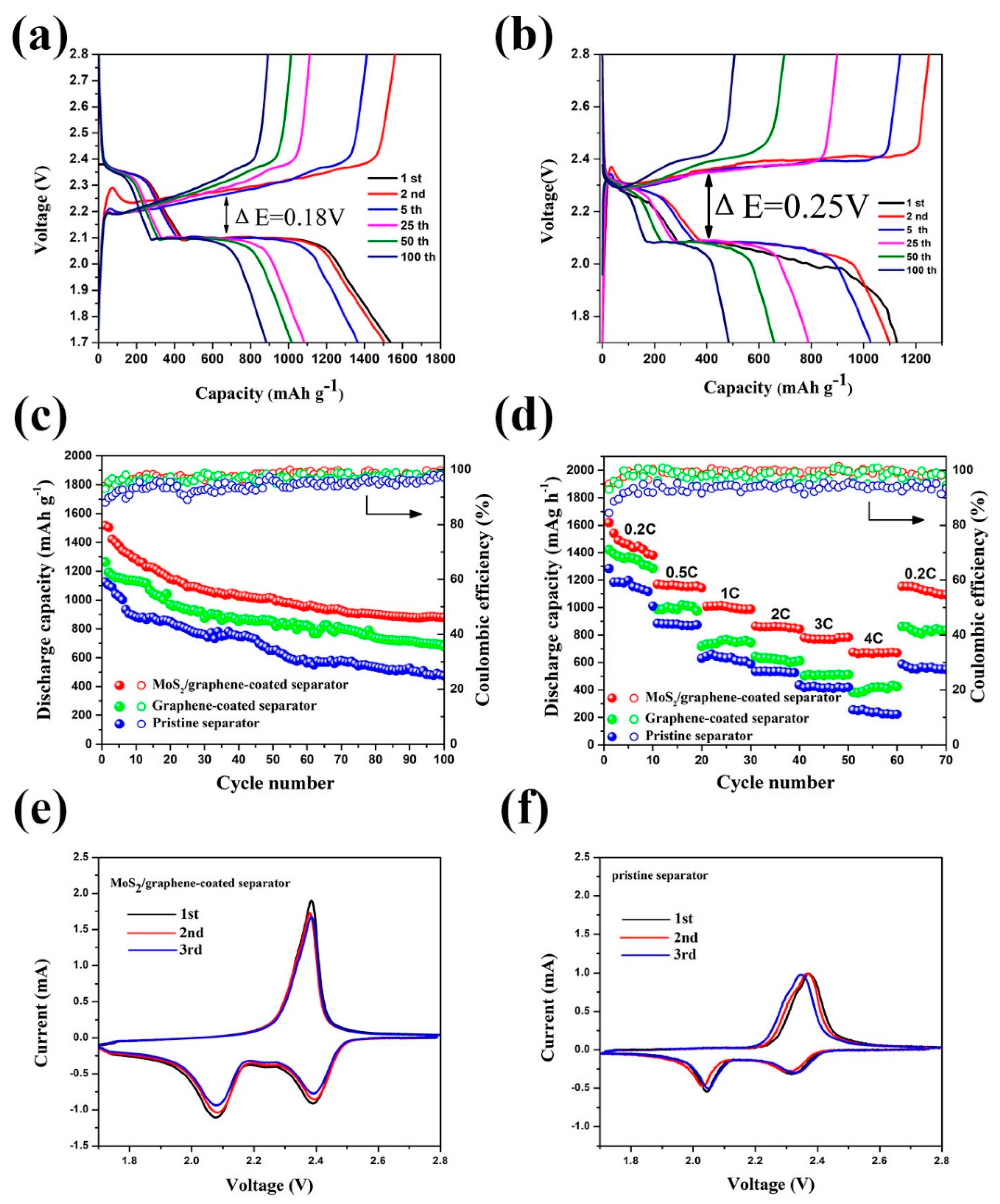
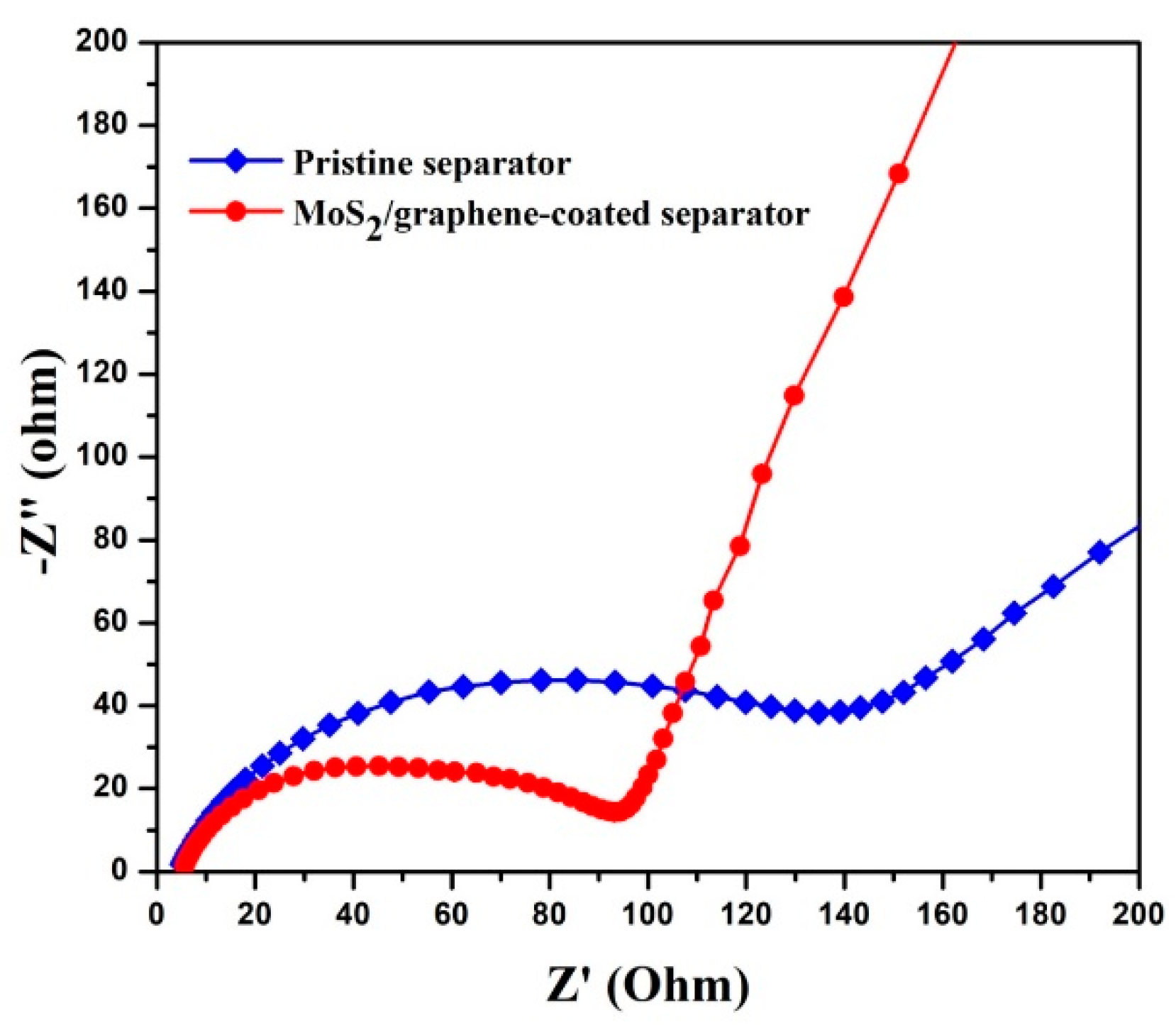
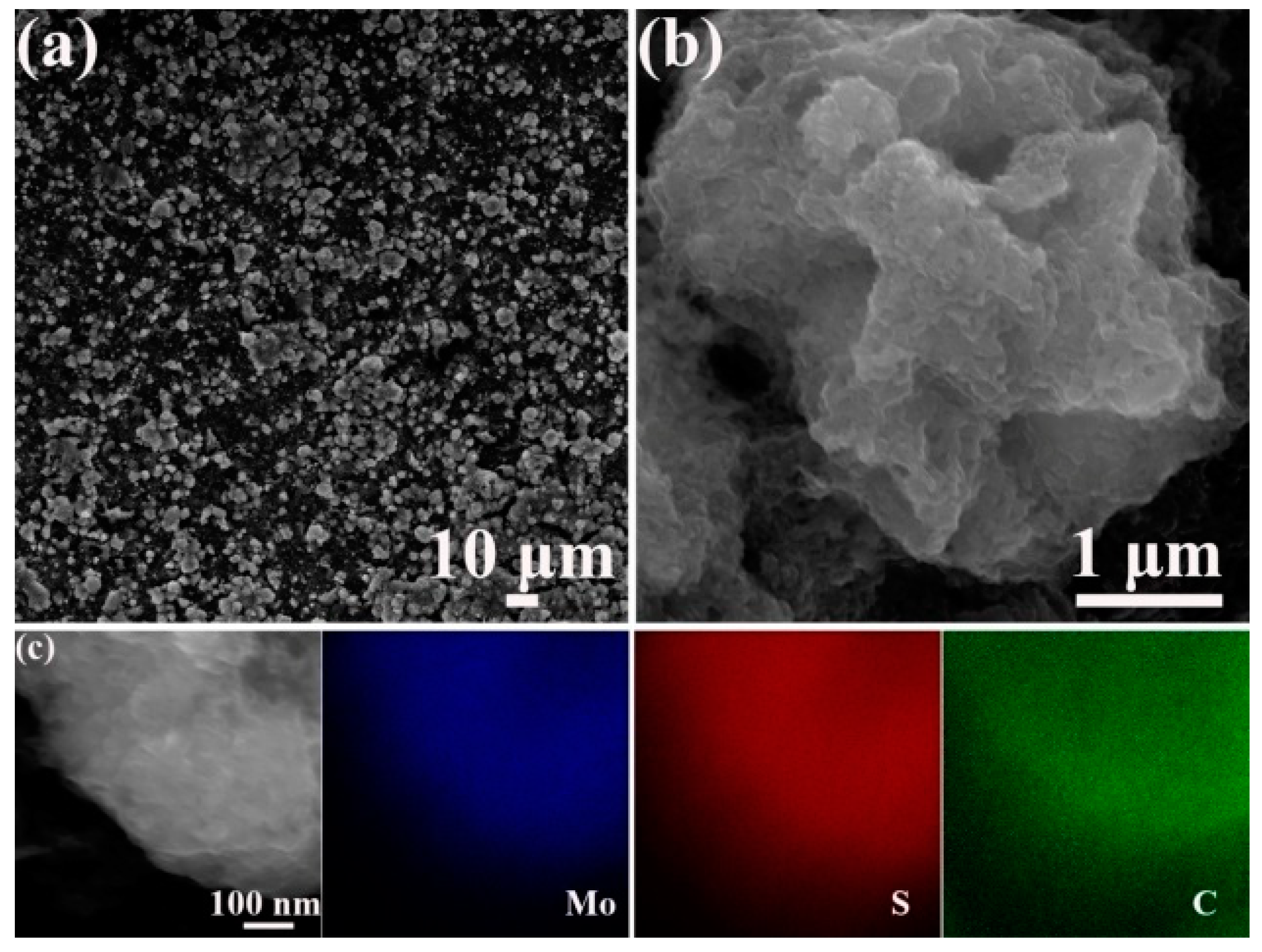
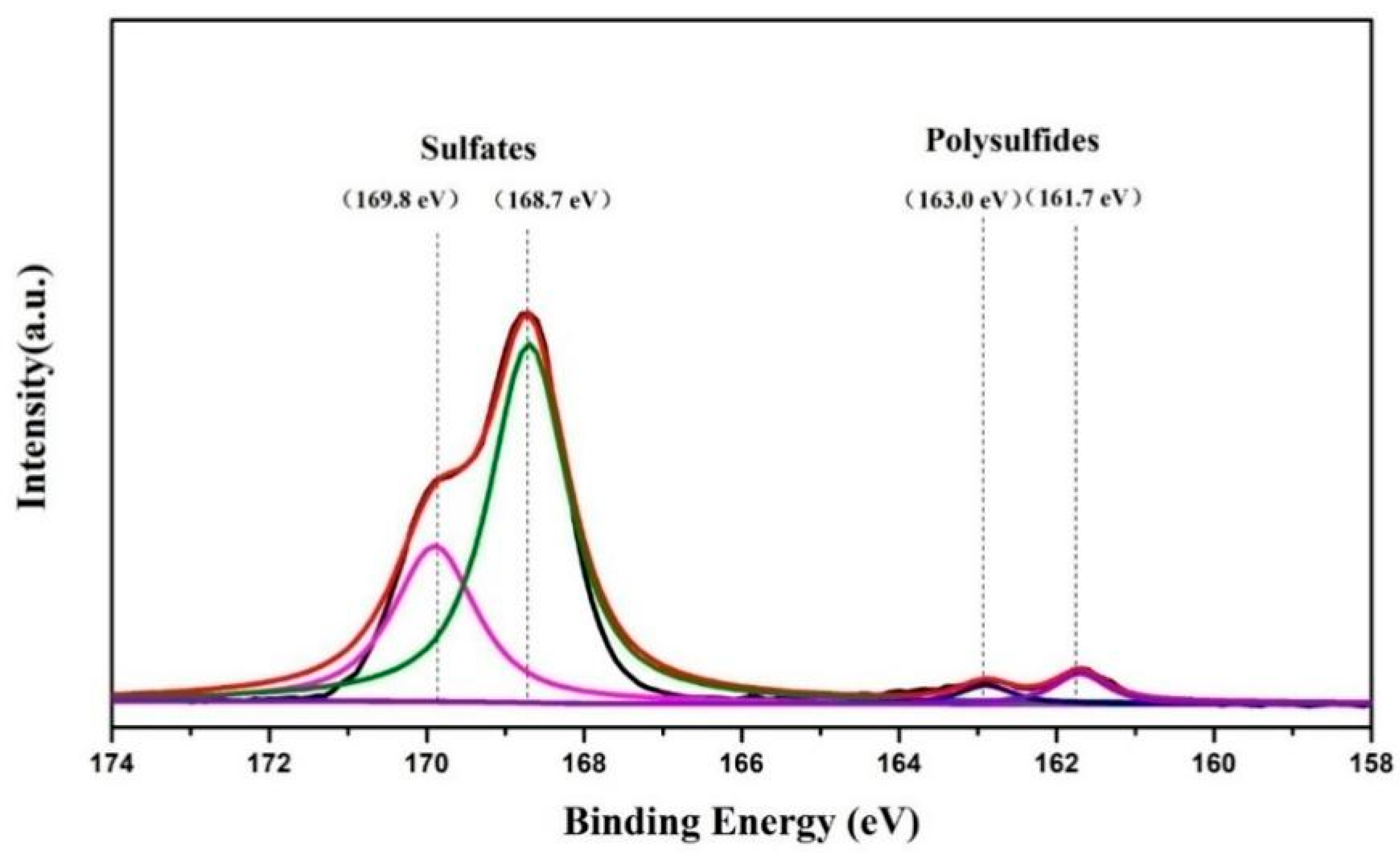
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulfur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, H.; Kim, C.S.; Zaghib, K.; Mauger, A.; Julien, C. Advances in lithium-sulfur batteries. Mater. Sci. Eng. R 2017, 121, 1–29. [Google Scholar] [CrossRef]
- Li, S.H.; Xia, X.H.; Wang, Y.D.; Wang, X.L.; Tu, J.P. Reconstruction of multidimensional carbon hosts with combined 0D, 1D and 2D networks for enhanced lithium-sulfur batteries. J. Power Sources 2017, 342, 224–230. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Y.; Yu, Z.; Gordin, M.L.; Song, J.; Wang, D. High capacity of lithium-sulfur batteries at low electrolyte/sulfur ratio enabled by an organosulfide containing electrolyte. Nano Energy 2017, 31, 418–423. [Google Scholar] [CrossRef]
- Urbonaite, S.; Poux, T.; Novák, P. Progress towards Commercially Viable Li-S Battery Cells. Adv. Energy Mater. 2015, 5, 1500118–1500137. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Hou, P.; Cheng, M.; Wang, S.; Cheng, H.; Liu, C.; Li, F. 3D Interconnected Electrode Materials with Ultrahigh Areal Sulfur Loading for Li-S Batteries. Adv. Mater. 2016, 28, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, J.; Gao, X.; Wen, Z.; Yuan, C.; Chen, J. 3D Dual-Confined Sulfur Encapsulated in Porous Carbon Nanosheets and Wrapped with Graphene Aerogels as Cathode for Advanced Lithium Sulfur Batteries. Nanoscale 2016, 8, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Ghosh, A.; Sen, U.K.; Roy, P.K.; Mitra, S.; Lochab, B. Cardanol Benzoxazine-Sulfur Copolymers for Li-S batteries: Symbiosis of Sustainability and Performance. ChemistrySelect 2016, 1, 594–600. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Wang, D.; Shan, X.; Pei, S.; Li, F.; Cheng, H. A Flexible Sulfur-Graphene-Polypropylene Separator Integrated Electrode for Advanced Li-S Batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, Y.; Ren, J.; Zhang, W.; Zhang, C.; Zhang, Y. Three-dimensionally ordered hierarchically porous polypyrrole loading sulfur as high-performance cathode for lithium/sulfur batteries. Polymer 2018, 137, 261–268. [Google Scholar] [CrossRef]
- Hagen, M.; Fanz, P.; Tübke, J. Cell energy density and electrolyte/sulfur ratio in Li-S cells. J. Power Sources 2014, 264, 30–34. [Google Scholar] [CrossRef]
- Safa, M.; Hao, Y.; Chamaani, A.; Adelowo, E.; Chawla, N.; Wang, C.; El-Zahab, B. Capacity fading mechanism in lithium-sulfur battery using poly(ionic liquid) gel electrolyte. Electrochim. Acta 2017, 258, 1284–1292. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lai, Y.Q.; Zhang, Z.A.; Li, J. A functional carbon layer-coated separator for high performance lithium sulfur batteries. Solid State Ionics 2015, 278, 166–171. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-performance Li-S batteries with an ultra-lightweight MWCNT-coated separator. J. Phys. Chem. C 2014, 5, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Chung, S.H.; Manthiram, A.; Kalra, V. A free-standing carbon nanofiber interlayer for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 4530–4538. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhuang, T.Z.; Zhang, Q.; Peng, H.J.; Chen, C.M.; Wei, F. Permselective graphene oxide membrane for highly stable and anti-self-discharge lithium sulfur batteries. ACS Nano 2015, 9, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high stable lithium-sulfur batteries: Progress and prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Chung, S.; Manthiram, A. Bifunctional Separator with a Light-Weight Carbon-Coating for Dynamically and Statically Stable Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Li, P.; He, J.; Zhao, Y.; Wang, Z.; Liu, J.; Qi, F.; Zheng, B.; Zhou, J.; et al. Enhanced Performance of Lithium Sulfur Battery with a Reduced Graphene Oxide Coating Separator. J. Electrochem. Soc. 2015, 162, A1624–A1629. [Google Scholar] [CrossRef]
- Conder, J.; Urbonaite, S.; Streich, D.; Novák, P.; Gubler, L. Taming the polysulphide shuttle in Li-S batteries by plasma-induced asymmetric functionalisation of the separator. RSC Adv. 2015, 5, 79654–79660. [Google Scholar] [CrossRef]
- Kim, J.S.; Hwang, T.H.; Kim, B.G.; Min, J.; Choi, J.W. A Lithium-Sulfur Battery with a High Areal Energy Density. Adv. Funct. Mater. 2014, 24, 5359–5367. [Google Scholar] [CrossRef]
- Li, W.; Hicksgarner, J.; Wang, J.; Liu, J.; Gross, A.F.; Sherman, E.; Graetz, J.; Vajo, J.J.; Liu, P. V2O5 Polysulfide Anion Barrier for Long-Lived Li-S Batteries. Chem. Mater. 2014, 26, 3403–3410. [Google Scholar] [CrossRef]
- Yan, L.; Luo, N.; Kong, W.; Luo, S.; Wu, H.; Jiang, K.; Li, Q.; Fan, S.; Duan, W.; Wang, J. Enhanced performance of lithium-sulfur batteries with an ultrathin and lightweight MoS2/carbon nanotube interlayer. J. Power Sources 2018, 389, 169–177. [Google Scholar] [CrossRef]
- Xu, H.; Manthiram, A. Hollow cobalt sulfide polyhedra-enabled long-life, high areal-capacity lithium-sulfur batteries. Nano Energy 2017, 33, 124–129. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, H.; Chen, L.; Fang, C.; Rui, Z.; Yang, L.; Wan, H.; Liu, J.; Zhou, Y.; Wang, P.; et al. Ferrous sulfide-assisted hollow carbon spheres as sulfur host for advanced lithium-sulfur batteries. Chem. Eng. J. 2017, 36, 1040–1047. [Google Scholar] [CrossRef]
- Zeng, Q.; Leng, X.; Wu, K.H.; Gentle, I.R.; Wang, D.W. Electroactive cellulose-supported graphene oxide interlayers for Li-S batteries. Carbon 2015, 96, 611–619. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Mass production of graphene oxide from expanded graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Su, Q.; Cheng, F.; Kong, X.; Lin, J.; Zhu, T.; Liang, S.; Pan, A. N-S co-doped C@SnS nanoflakes/graphene composite as advanced anode for sodium-ion batteries. Chem. Eng. J. 2018, 353, 606–614. [Google Scholar] [CrossRef]
- Chiochan, P.; Phattharasupakun, N.; Wutthiprom, J.; Suksomboon, M.; Kaewruang, S.; Suktha, P.; Sawangphruk, M. Core-double shell sulfur@carbon black nanosphere@oxidized carbon nanosheet composites as the cathode materials for Li-S batteries. Electrochim. Acta 2017, 237, 78–86. [Google Scholar] [CrossRef]
- Song, S.; Shi, L.; Lu, S.; Pang, Y.; Wang, Y.; Zhu, M.; Ding, D.; Ding, S. A new polysulfide blocker-poly(acrylic acid) modified separator for improved performance of lithium-sulfur battery. J. Membr. Sci. 2018, 563, 277–283. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Yang, Y.; Yue, X.; Hao, X.; Sun, W.; Rooney, D.; Sun, K. Flexible carbon nanofiber/polyvinylidene fluoride composite membranes as interlayers in high-performance Lithium Sulfur batteries. J. Power Sources 2016, 329, 305–313. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Wang, L.; Nie, H.; Zhong, M.; Lai, Q.; Xu, X.; Zhang, L.; Huang, S. Lithium-Sulfur Batteries: A Lightweight TiO2/Graphene Interlayer, Applied as a Highly Effective Polysulfide Absorbent for Fast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2015, 27, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Song, X.; Cairns, E.J. Nano-carbon/sulfur composite cathode materials with carbon nanofiber as electrical conductor for advanced secondary lithium/sulfur cells. J. Power Sources 2012, 205, 474–478. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, H.; Hong, H.; Li, Z.; Lin, Y. Novel flower-like hierarchical carbon sphere with multi-scale pores coated on PP separator for high-performance lithium-sulfur batteries. Electrochim. Acta 2017, 257, 210–216. [Google Scholar] [CrossRef]
- Hoang, V.C.; Do, V.D.; Nah, I.W.; Lee, C.; Cho, W.I.; Oh, I.H. Facile Coating of Graphene Interlayer onto Li2S as a High Electrochemical Performance Cathode for Lithium Sulfur Battery. Electrochim. Acta 2016, 210, 1–6. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, J.; Tan, T.; Zhao, Y.; Liu, N.; Li, H. A 3D MoS2/Graphene Microsphere Coated Separator for Excellent Performance Li-S Batteries. Materials 2018, 11, 2064. https://doi.org/10.3390/ma11102064
Yang S, Zhang J, Tan T, Zhao Y, Liu N, Li H. A 3D MoS2/Graphene Microsphere Coated Separator for Excellent Performance Li-S Batteries. Materials. 2018; 11(10):2064. https://doi.org/10.3390/ma11102064
Chicago/Turabian StyleYang, Shuang, Junfan Zhang, Taizhe Tan, Yan Zhao, Ning Liu, and Haipeng Li. 2018. "A 3D MoS2/Graphene Microsphere Coated Separator for Excellent Performance Li-S Batteries" Materials 11, no. 10: 2064. https://doi.org/10.3390/ma11102064
APA StyleYang, S., Zhang, J., Tan, T., Zhao, Y., Liu, N., & Li, H. (2018). A 3D MoS2/Graphene Microsphere Coated Separator for Excellent Performance Li-S Batteries. Materials, 11(10), 2064. https://doi.org/10.3390/ma11102064



