Time-Resolved Tomographic Quantification of the Microstructural Evolution of Ice Cream
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ice Cream Samples
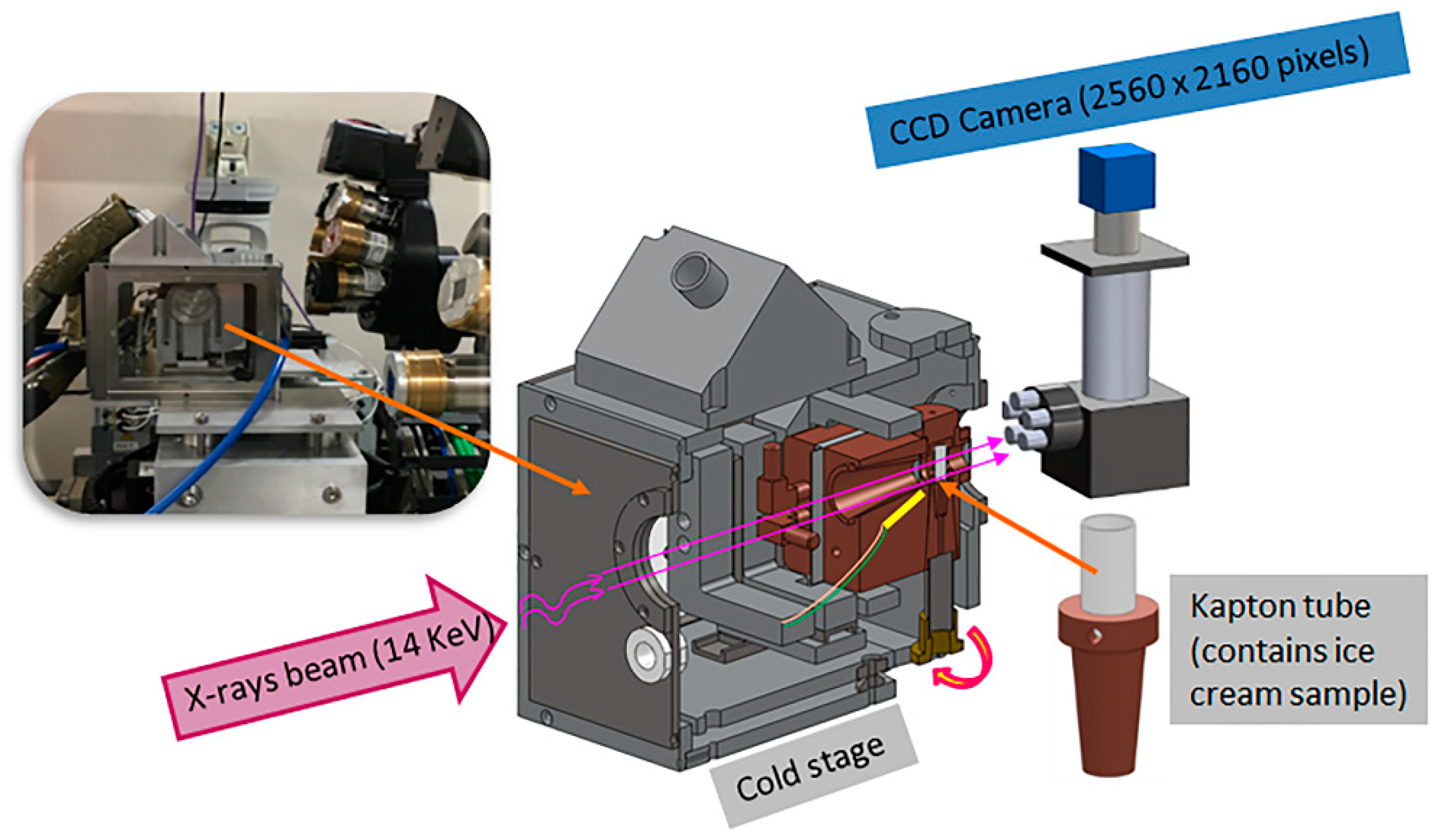
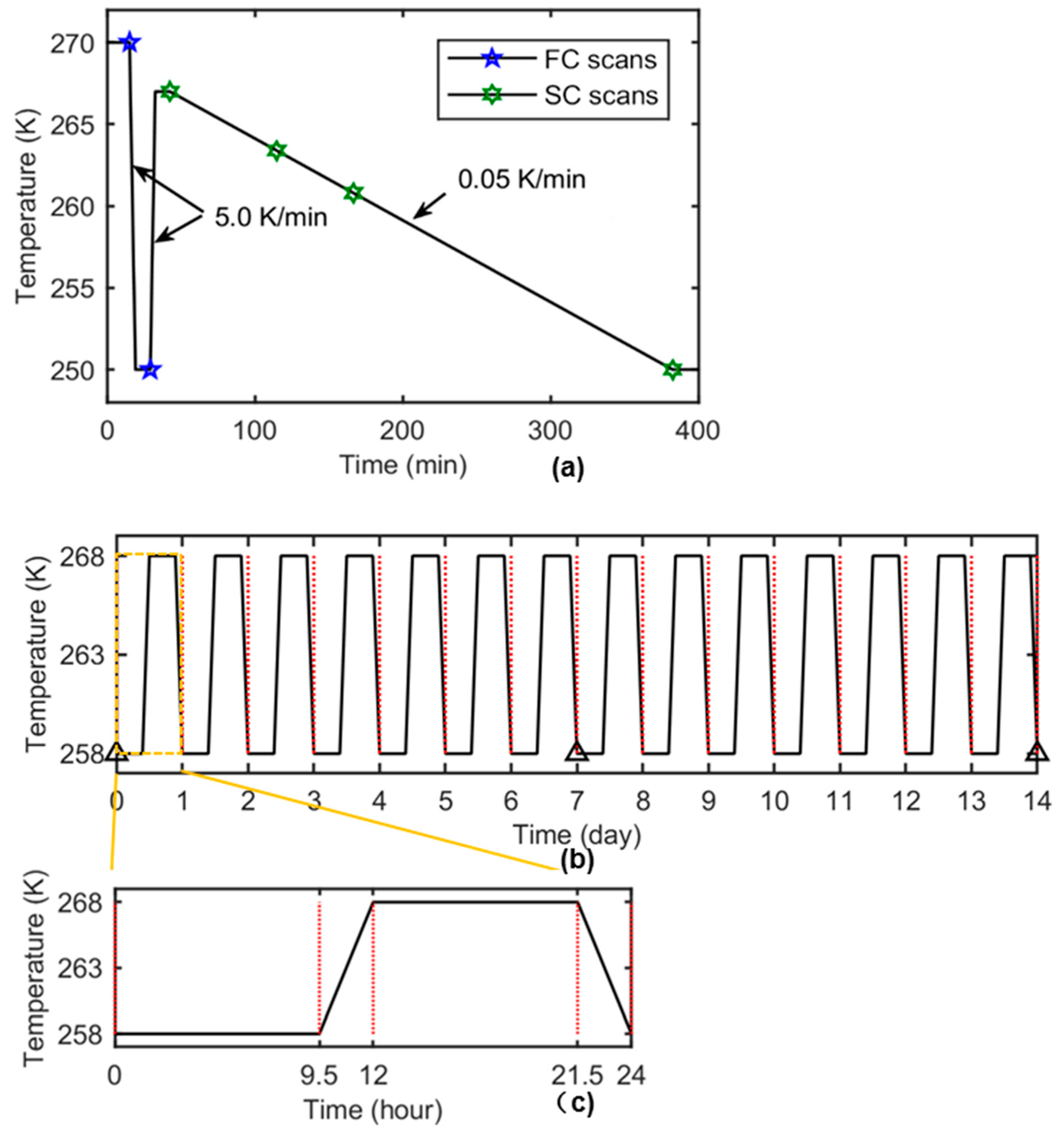
2.2. Cold Stage Experimental Setup and Thermal Cycling for Ex Situ and In Situ sCT
2.3. Microstructural Characterization Using Synchrotron X-ray Computed Tomography (sCT)
2.4. Volume Data Reconstruction and Pre-Processing
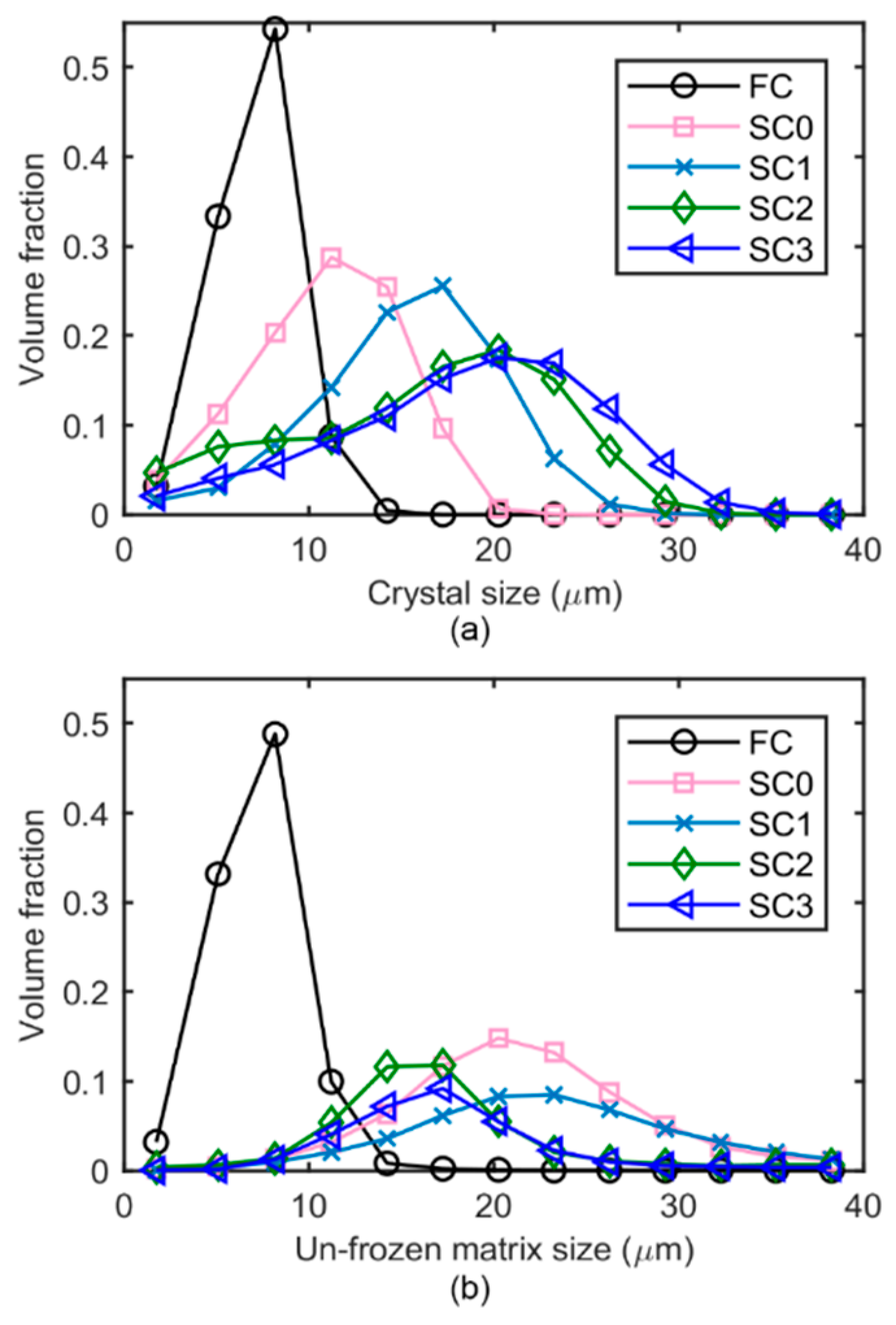
2.5. 3D Based Quantification of Ice Crystal Dimensions
- A 3D distance map was first created by a Euclidean distance transform from the binarized image.
- For the current samples, 13 sampling spheres with a diameter equally spaced between 5 µm and 40 µm were chosen to balance the quantification accuracy and computational expenses.
- The radius of each sampling sphere (in voxels) was compared to the voxel intensities in the distance map, determining the centers of the spheres of a radius that can be completely placed within the ice phase.
- A dilation algorithm was then applied to the voxels correlated to the sphere centers, using a spherical kernel of the same radius. The volume after dilation is the volume correlated to the sampling sphere.
- Step 3–4 were repeated for all the sampling sphere dimensions chosen in step 2, providing a range of reachable volumes corresponding to increasing sphere diameters.
- The volume fraction was calculated by dividing each volume by the total volume of the ice phase. Then an ice crystal size distribution can be calculated as the differential of the area under the cumulative volume percentage curve.
3. Results and Discussion
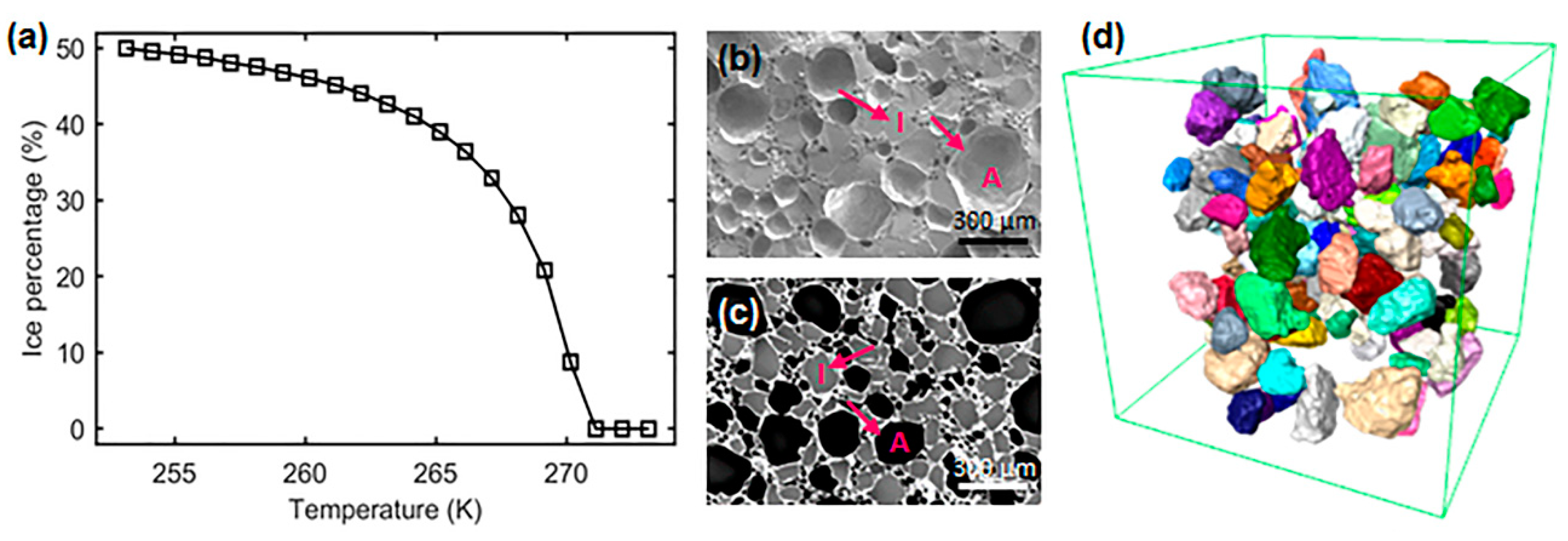
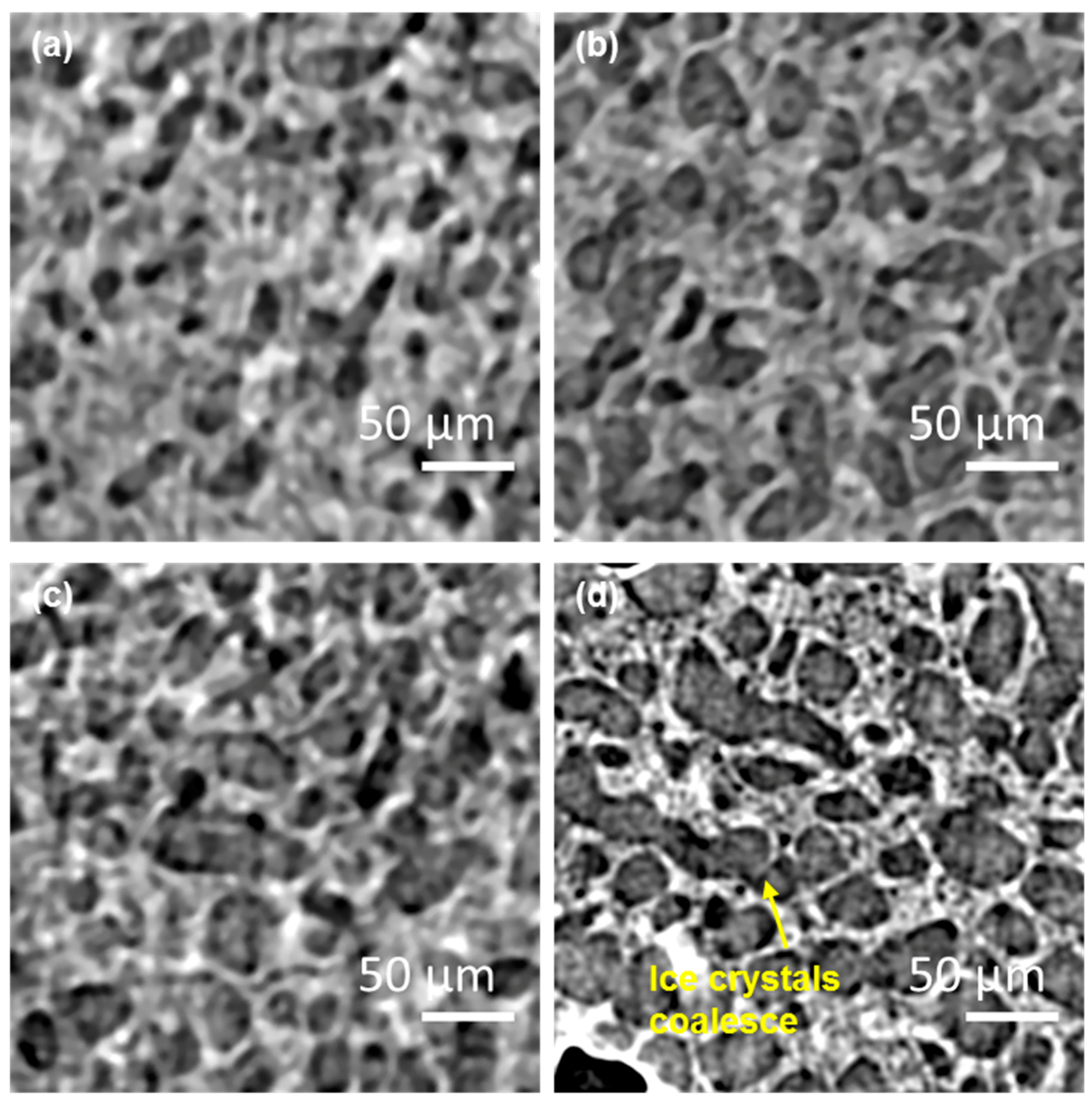
3.1. In Situ: Microstructural Evolution—2D Images
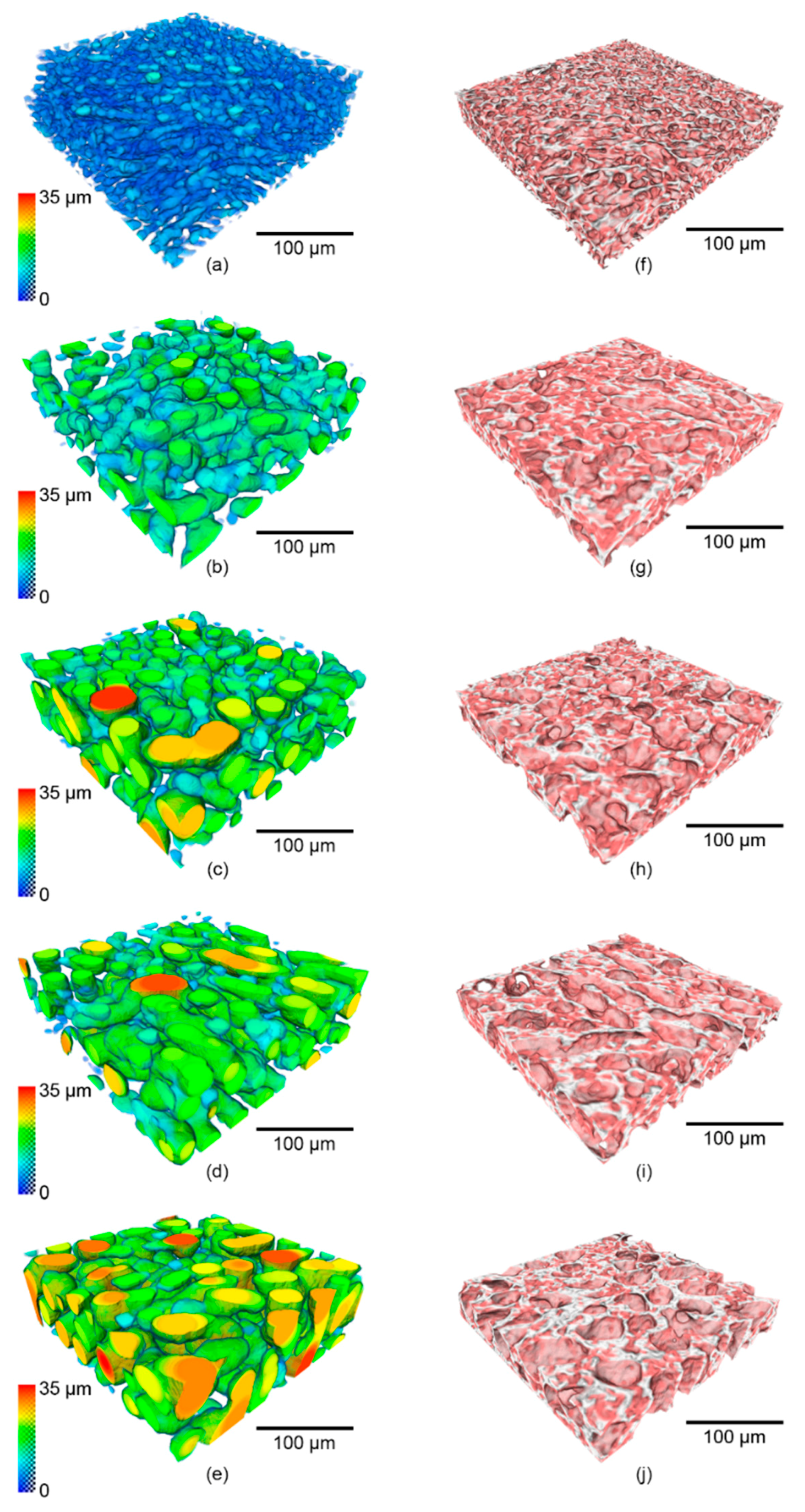
3.2. In situ: 3D Microstructural Evolution as a Function of Temperature and Time
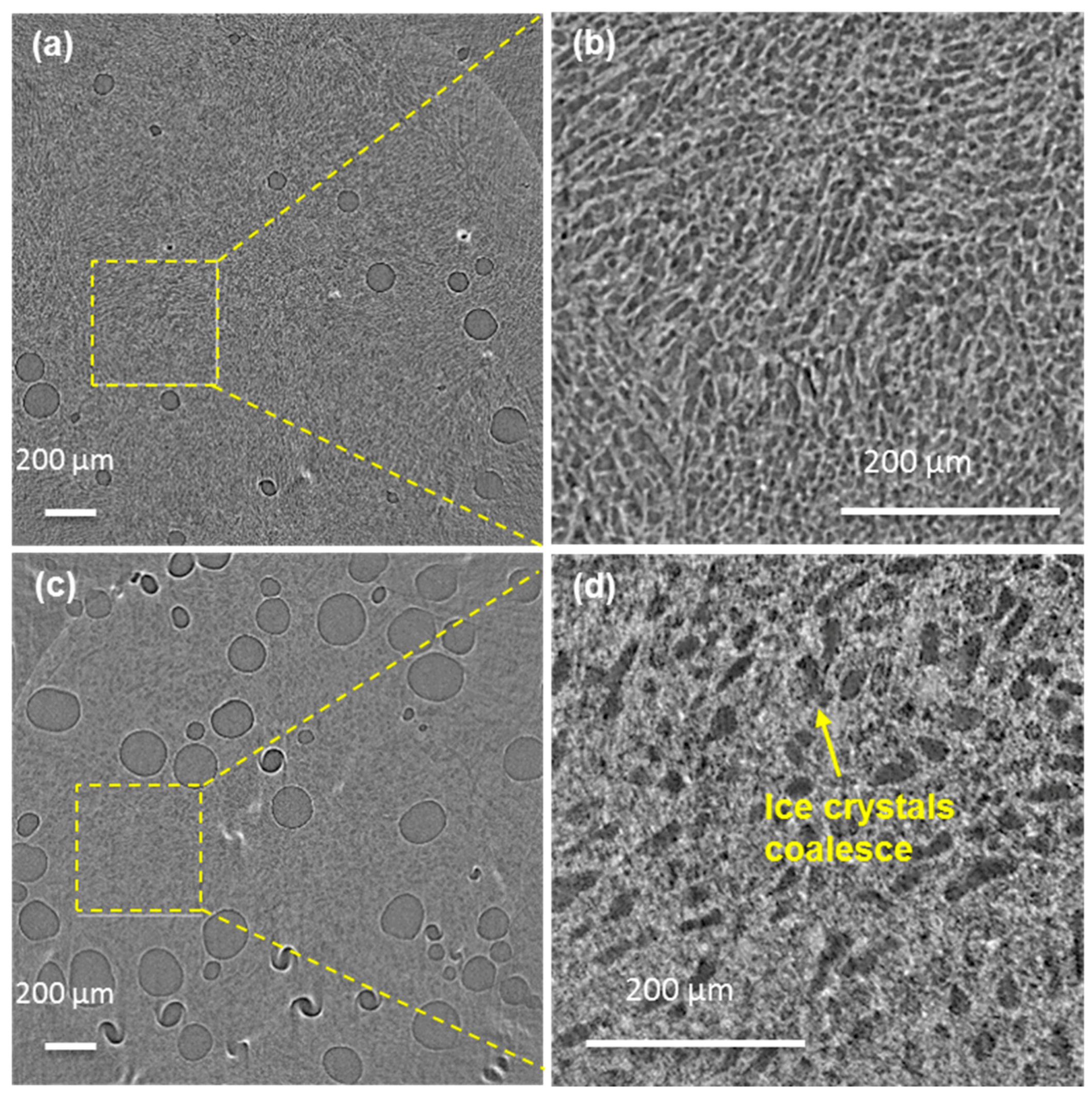
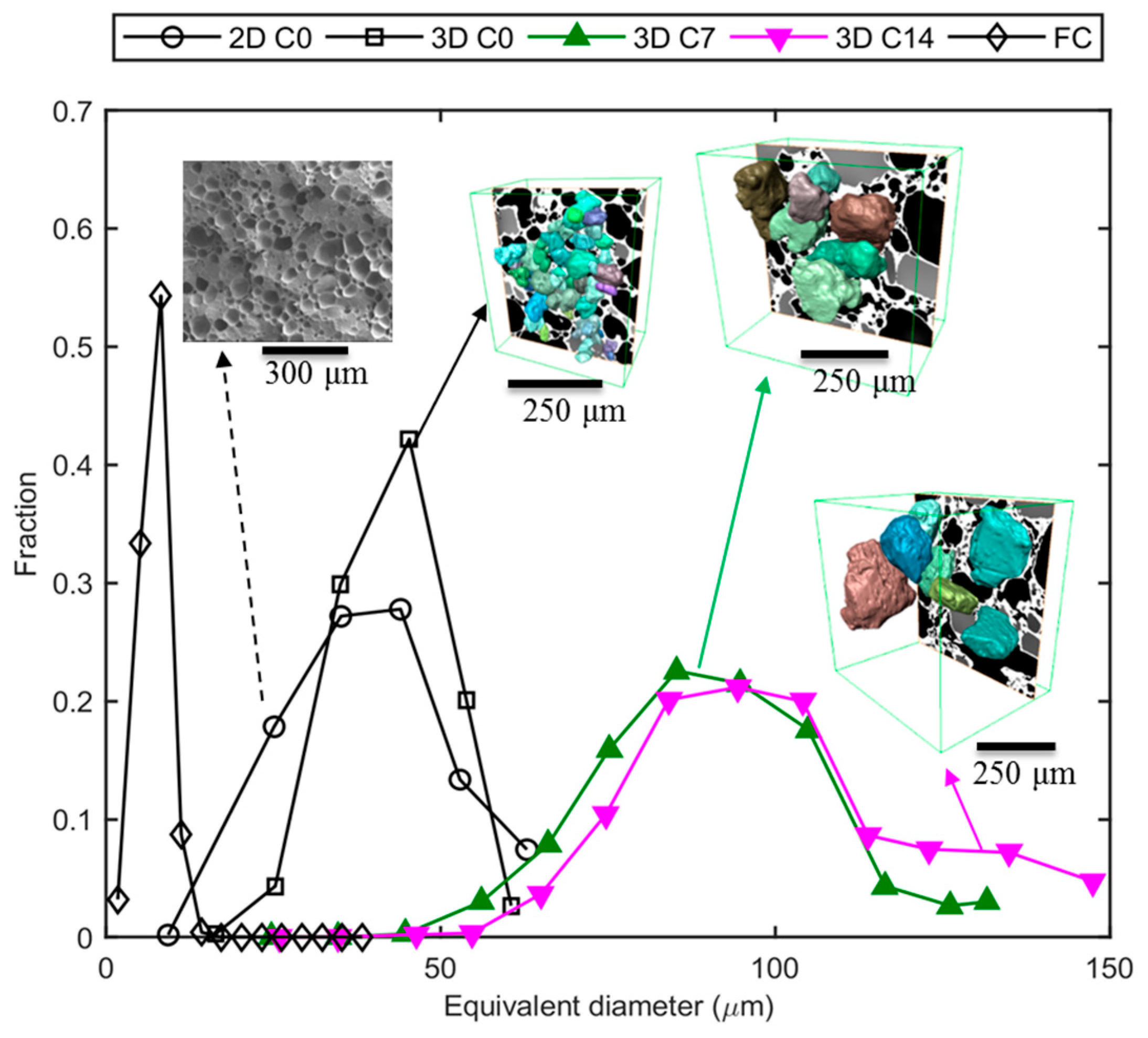
3.3. Ex Situ: Microstructural Evolution of Ice Crystals Following Long-Term Thermal Cycling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarke, C. The Science of Ice Cream; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Guo, E.; Kazantsev, D.; Mo, J.; Bent, J.; Van Dalen, G.; Schuetz, P.; Rockett, P.; StJohn, D.; Lee, P.D. Revealing the microstructural stability of a three-phase soft solid (ice cream) by 4D synchrotron X-ray tomography. J. Food Eng. 2018, 237, 204–214. [Google Scholar] [CrossRef]
- Clarke, C. The physics of Ice Cream. Phys. Educ. 2003, 38, 248–253. [Google Scholar] [CrossRef]
- Cook, K.L.K.; Hartel, R.W. Effect of freezing temperature and warming rate on dendrite break-up when freezing ice cream mix. Int. Dairy J. 2011, 21, 447–453. [Google Scholar] [CrossRef]
- Chang, Y.; Hartel, R.W. Measurement of air cell distributions in dairy foams. Int. Dairy J. 2002, 12, 463–472. [Google Scholar] [CrossRef]
- Sofjan, R.P.; Hartel, R.W. Effects of overrun on structural and physical characteristics of ice cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Guo, E.Y.; Zeng, G.; Kazantsev, D.; Rockett, P.; Bent, J.; Kirkland, M.; Van Dalen, G.; Eastwood, D.S.; StJohn, D.; Lee, P.D. Synchrotron X-ray tomographic quantification of microstructural evolution in ice cream—A multiphase soft solid. Rsc. Adv. 2017, 7, 15561–15573. [Google Scholar] [CrossRef]
- Goff, H.D.; Verespej, E.; Smith, A.K. A study of fat and air structures in ice cream. Int. Dairy J. 1999, 9, 817–829. [Google Scholar] [CrossRef]
- Cook, K.L.K.; Hartel, R.W. Mechanisms of Ice Crystallization in Ice Cream Production. Compr. Rev. Food Sci. Food Saf. 2010, 9, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Flemings, M.C.; Riek, R.; Young, K. Rheocasting. Mater. Sci. Eng. 1976, 25, 103–117. [Google Scholar] [CrossRef]
- Van Dalen, G. A study of bubbles in foods by X-ray microtomography and image analysis. Microsc. Anal. 2012, 26, S8–S12. [Google Scholar]
- Eisner, M.D.; Wildmoser, H.; Windhab, E.J. Air cell microstructuring in a high viscous ice cream matrix. Colloids Surf. A: Physicochem. Eng. Asp. 2005, 263, 390–399. [Google Scholar] [CrossRef]
- Méndez-Velasco, C.; Goff, H.D. Fat structure in ice cream: A study on the types of fat interactions. Food Hydrocoll. 2012, 29, 152–159. [Google Scholar] [CrossRef]
- Chang, Y.; Hartel, R. Stability of air cells in ice cream during hardening and storage. J. Food Eng. 2002, 55, 59–70. [Google Scholar] [CrossRef]
- Chang, Y.; Hartel, R.W. Development of air cells in a batch ice cream freezer. J. Food Eng. 2002, 55, 71–78. [Google Scholar] [CrossRef]
- Caillet, A.; Cogné, C.; Andrieu, J.; Laurent, P.; Rivoire, A. Characterization of ice cream structure by direct optical microscopy. Influence of freezing parameters. LWT-Food Sci. Technol. 2003, 36, 743–749. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of milk protein-polysaccharide interactions on the stability of ice cream mix model systems. Food Hydrocoll. 2015, 45, 327–336. [Google Scholar] [CrossRef]
- Pinzer, B.R.; Medebach, A.; Limbach, H.J.; Dubois, C.; Stampanoni, M.; Schneebeli, M. 3D-characterization of three-phase systems using X-ray tomography: Tracking the microstructural evolution in ice cream. Soft Matter 2012, 8, 4584–4594. [Google Scholar] [CrossRef]
- Vicent, V.; Verboven, P.; Ndoye, F.T.; Alvarez, G.; Nicolai, B. A new method developed to characterize the 3D microstructure of frozen apple using X-ray micro-CT. J. Food Eng. 2017, 212, 154–164. [Google Scholar] [CrossRef]
- Lee, P.; Hunt, J. Hydrogen porosity in directional solidified aluminium-copper alloys: In situ observation. Acta Mater. 1997, 45, 4155–4169. [Google Scholar] [CrossRef]
- Leung, C.L.A.; Marussi, S.; Atwood, R.C.; Towrie, M.; Withers, P.J.; Lee, P.D. In situ X-ray imaging of defect and molten pool dynamics in laser additive manufacturing. Nat. Commun. 2018, 9, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, S. Recent advances in X-ray microtomography applied to materials. Int. Mater. Rev. 2008, 53, 129–181. [Google Scholar] [CrossRef]
- Maire, E.; Withers, P.J. Quantitative X-ray tomography. Int. Mater. Rev. 2014, 59, 1–43. [Google Scholar] [CrossRef]
- Bhreasail, Á.N.; Lee, P.; O’Sullivan, C.; Fenton, C.; Hamilton, R.; Rockett, P.; Connolley, T. In-Situ Observation of Cracks in Frozen Soil using Synchrotron Tomography. Permafr. Periglac. Process. 2012, 23, 170–176. [Google Scholar] [CrossRef]
- Figueroa Pilz, F.; Dowey, P.J.; Fauchille, A.L.; Courtois, L.; Bay, B.; Ma, L.; Taylor, T.G.; Mecklenburgh, J.; Lee, P. Synchrotron tomographic quantification of strain and fracture during simulated thermal maturation of an organic-rich shale, UK Kimmeridge Clay. J. Geophys. Res. Solid Earth 2017, 122, 2553–2564. [Google Scholar] [CrossRef] [Green Version]
- Polacci, M.; Arzilli, F.; La Spina, G.; Le Gall, N.; Cai, B.; Hartley, M.E.; Genova, D.D.; Vo, N.T.; Nonni, S.; Atwood, R.C.; et al. Crystallisation in basaltic magmas revealed via in situ 4D synchrotron X-ray microtomography. Sci. Rep. 2018, 8, 8377–8383. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.K.; Wilde, F.; Kozhar, S.; Beckmann, F.; Vieira, J.; Heinrich, S.; Palzer, S. Synchrotron X-ray microtomography reveals interior microstructure of multicomponent food materials such as chocolate. J. Food. Eng. 2016, 174, 37–46. [Google Scholar] [CrossRef]
- Kareh, K.M.; Lee, P.D.; Atwood, R.C.; Connolley, T.; Gourlay, C.M. Revealing the micromechanisms behind semi-solid metal deformation with time-resolved X-ray tomography. Nat. Commun. 2014, 5, 4464. [Google Scholar] [CrossRef] [PubMed]
- Karagadde, S.; Lee, P.D.; Cai, B.; Fife, J.L.; Azeem, M.A.; Kareh, K.M.; Puncreobutr, C.; Puncreobutr, D.; Puncreobutr, T.; Puncreobutr, R.C. Transgranular liquation cracking of grains in the semi-solid state. Nat. Commun. 2015, 6, 8300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.Y.; Hill, R.G.; Yue, S.; Nightingale, D.; Lee, P.D.; Jones, J.R. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater. 2011, 7, 1807–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwood, R.C.; Jones, J.R.; Lee, P.D.; Hench, L.L. Analysis of pore interconnectivity in bioactive glass foams using X-ray microtomography. Scr. Mater. 2004, 51, 1029–1033. [Google Scholar] [CrossRef]
- Yue, S.; Lee, P.D.; Poologasundarampillai, G.; Jones, J.R. Evaluation of 3-D bioactive glass scaffolds dissolution in a perfusion flow system with X-ray microtomography. Acta Biomater. 2011, 7, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.L.; Mao, H.K.; Meng, Y.; Eng, P.J.; Hu, M.Y.; Chow, P.; Cai, Y.Q.; Shu, J.; Hemley, R.J. X-ray-induced dissociation of H2O and formation of an O-2-H-2 alloy at high pressure. Science 2006, 314, 636–638. [Google Scholar] [CrossRef] [PubMed]
| Cooling Rate | Temperature (K) | Ice Volume Fraction (%) | specific Interface Area (Ss) (mm−1) |
|---|---|---|---|
| FC | 250.0 | 38 | 599 |
| SC0 | 266.8 | 28 | 301 |
| SC1 | 263.4 | 40 | 247 |
| SC2 | 260.3 | 36 | 233 |
| SC3 | 250.0 | 46 | 193 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, J.; Guo, E.; McCartney, D.G.; Eastwood, D.S.; Bent, J.; Van Dalen, G.; Schuetz, P.; Rockett, P.; Lee, P.D. Time-Resolved Tomographic Quantification of the Microstructural Evolution of Ice Cream. Materials 2018, 11, 2031. https://doi.org/10.3390/ma11102031
Mo J, Guo E, McCartney DG, Eastwood DS, Bent J, Van Dalen G, Schuetz P, Rockett P, Lee PD. Time-Resolved Tomographic Quantification of the Microstructural Evolution of Ice Cream. Materials. 2018; 11(10):2031. https://doi.org/10.3390/ma11102031
Chicago/Turabian StyleMo, Jingyi, Enyu Guo, D. Graham McCartney, David S. Eastwood, Julian Bent, Gerard Van Dalen, Peter Schuetz, Peter Rockett, and Peter D. Lee. 2018. "Time-Resolved Tomographic Quantification of the Microstructural Evolution of Ice Cream" Materials 11, no. 10: 2031. https://doi.org/10.3390/ma11102031
APA StyleMo, J., Guo, E., McCartney, D. G., Eastwood, D. S., Bent, J., Van Dalen, G., Schuetz, P., Rockett, P., & Lee, P. D. (2018). Time-Resolved Tomographic Quantification of the Microstructural Evolution of Ice Cream. Materials, 11(10), 2031. https://doi.org/10.3390/ma11102031





