Effect of Duty Cycle on Properties of Al2O3 Ceramic Coatings Fabricated on TiAl Alloy via Cathodic Plasma Electrolytic Deposition
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
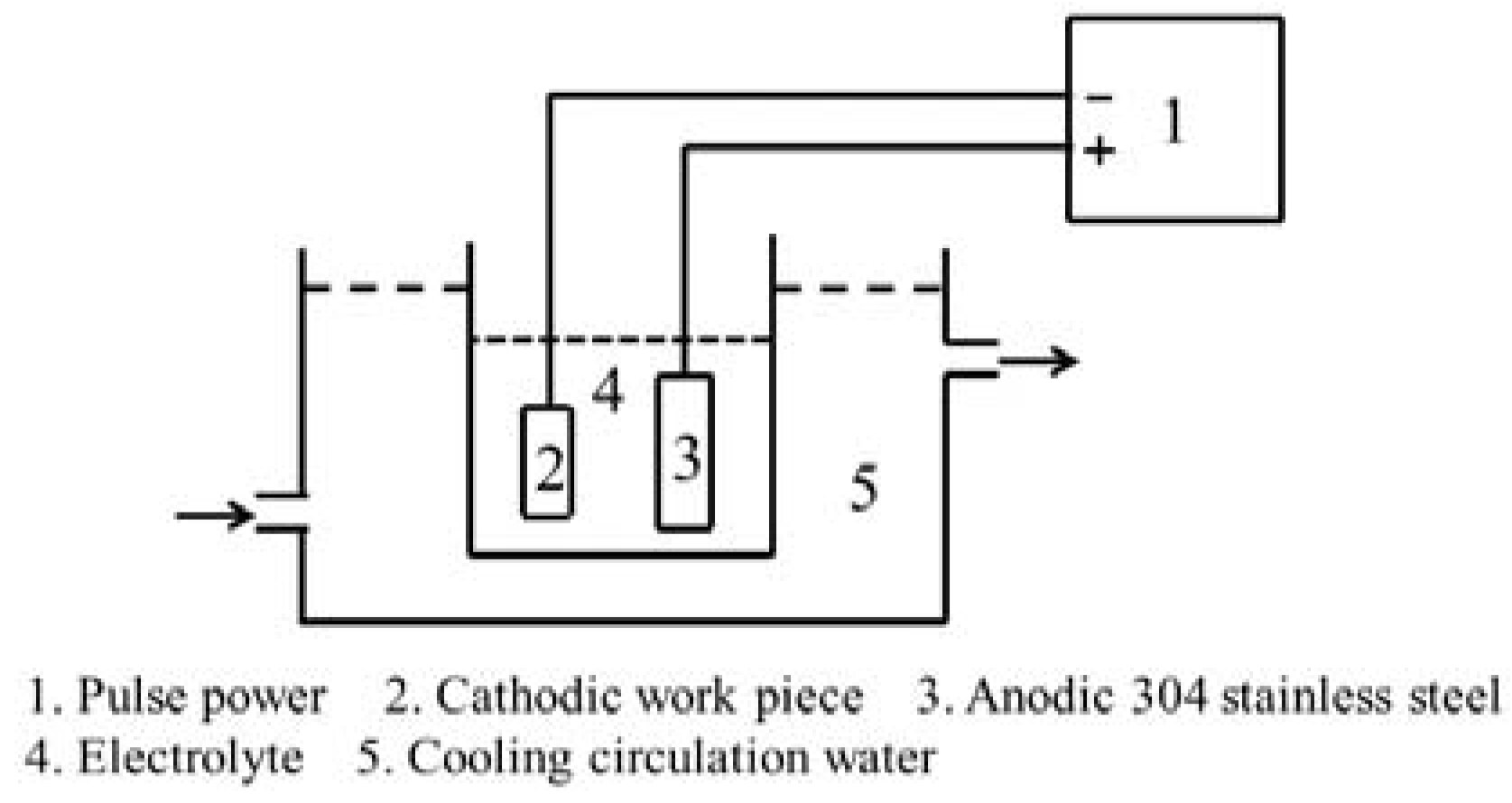
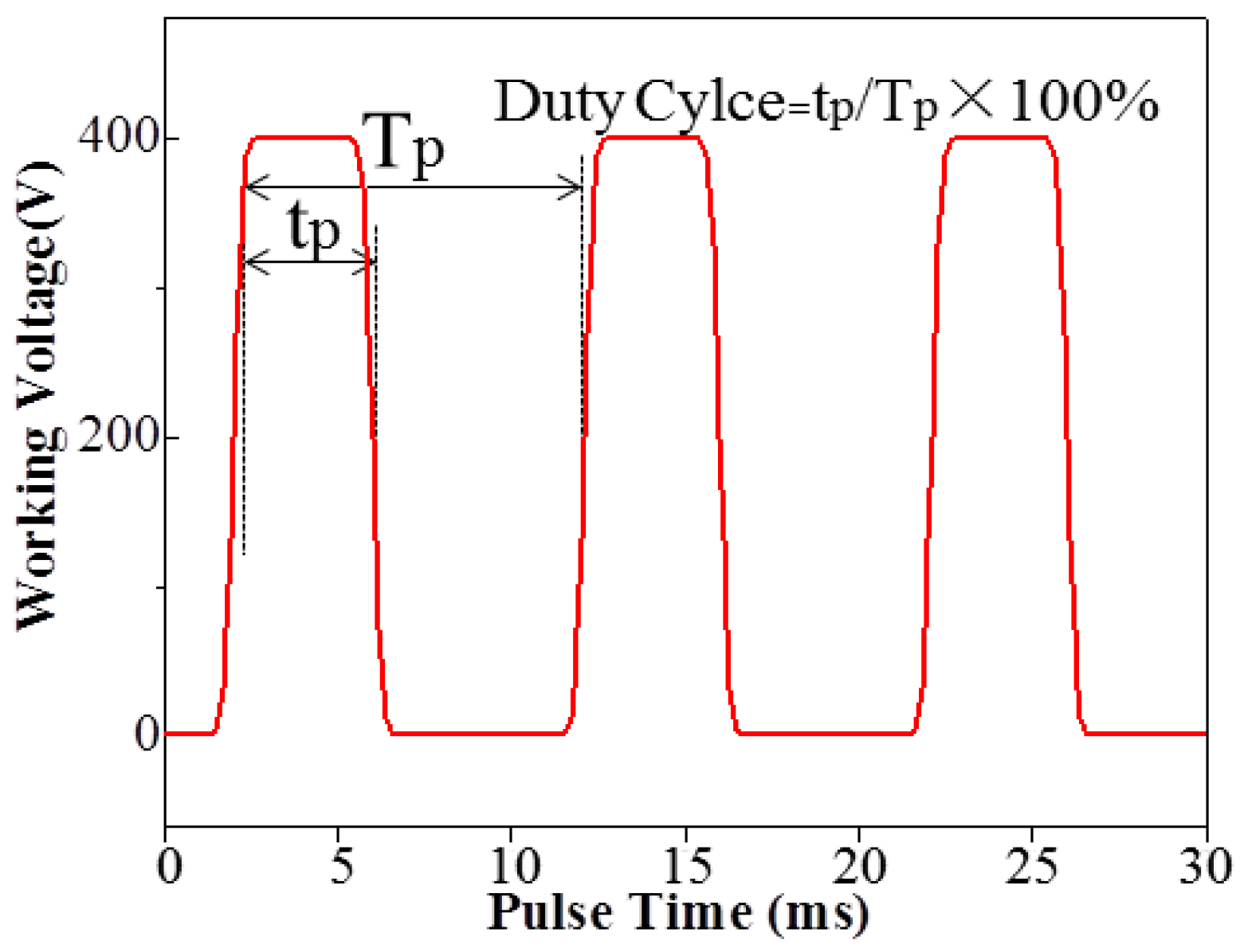
2.2. Fabrication of Al2O3 CPED Coating
2.3. Characterization of Al2O3 CPED Coating
3. Results and Discussion
3.1. Effect of Duty Cycle on the CPED Process
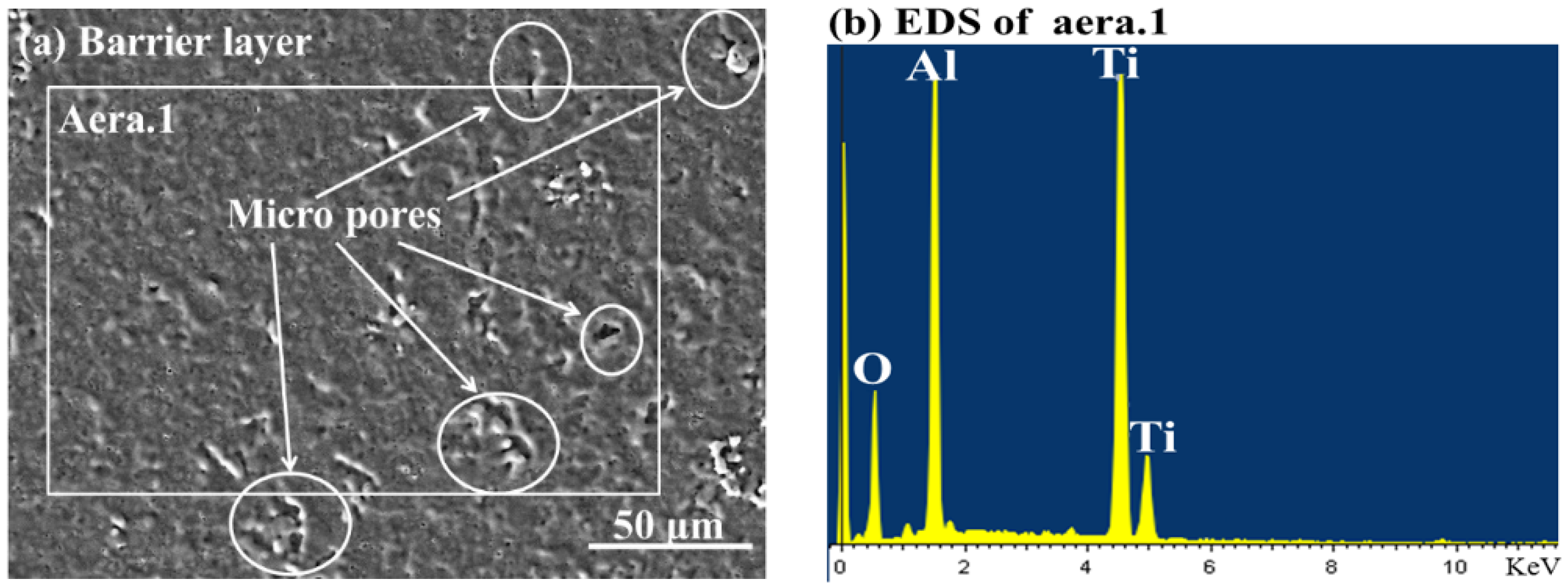
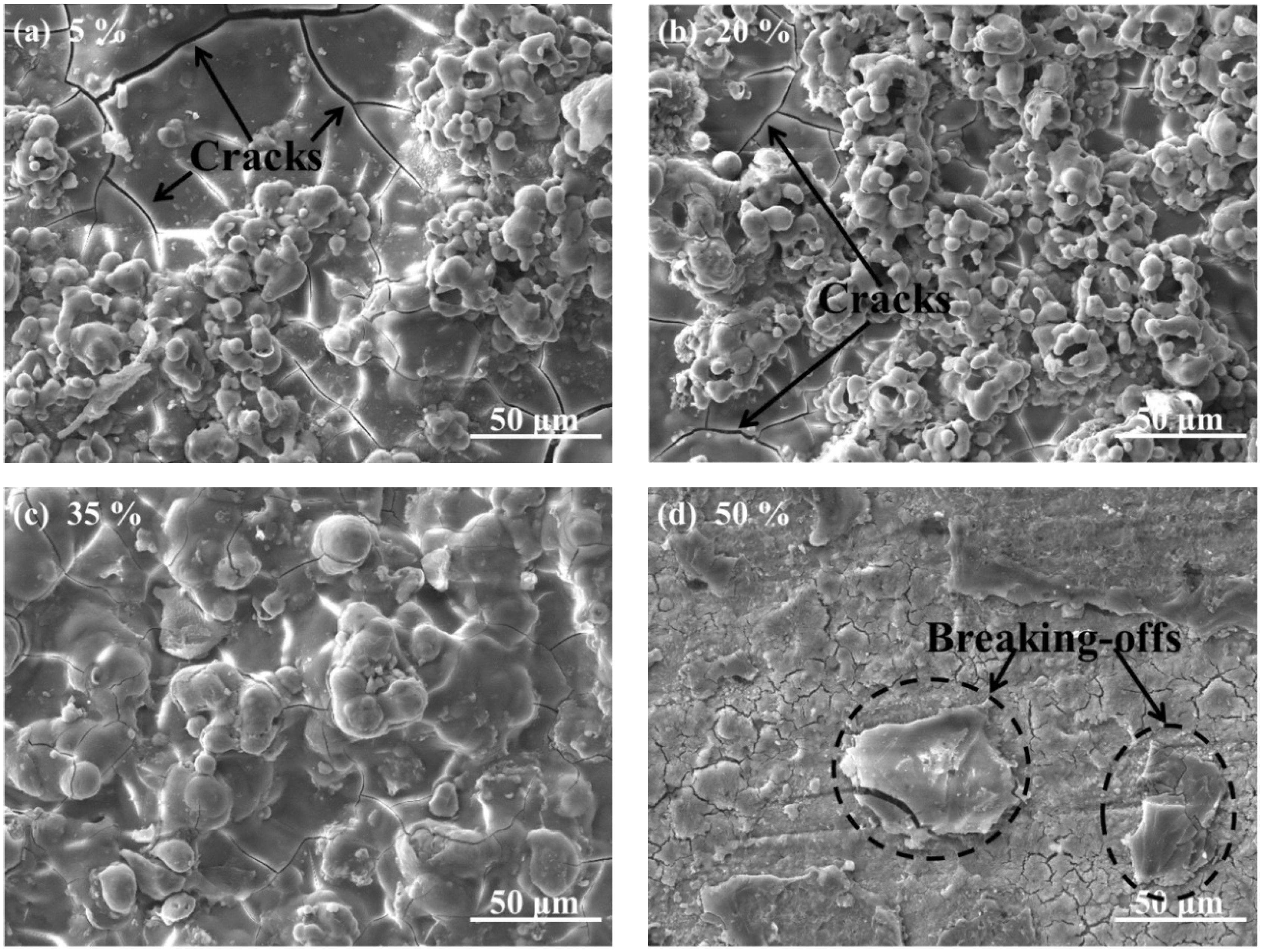
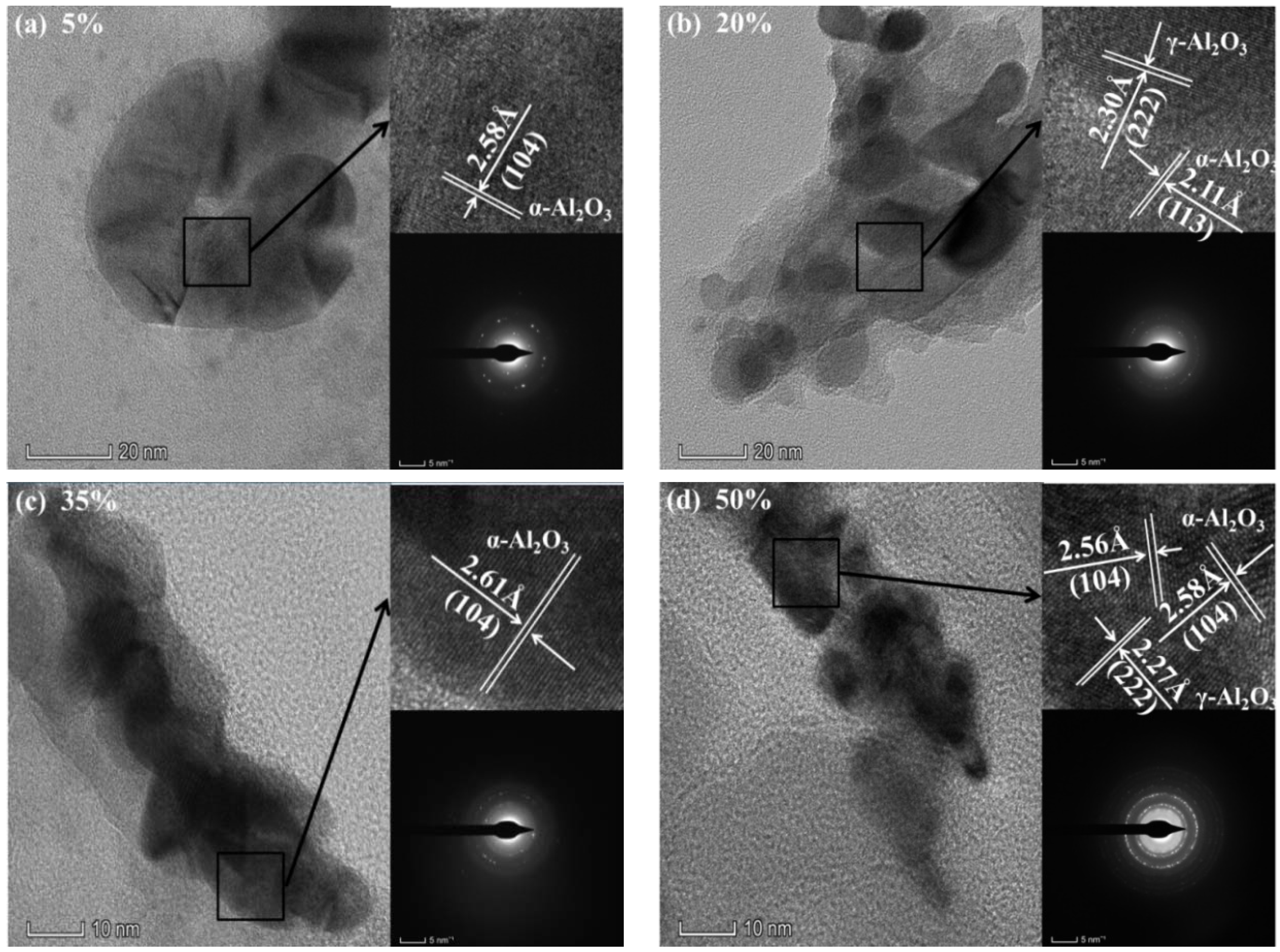
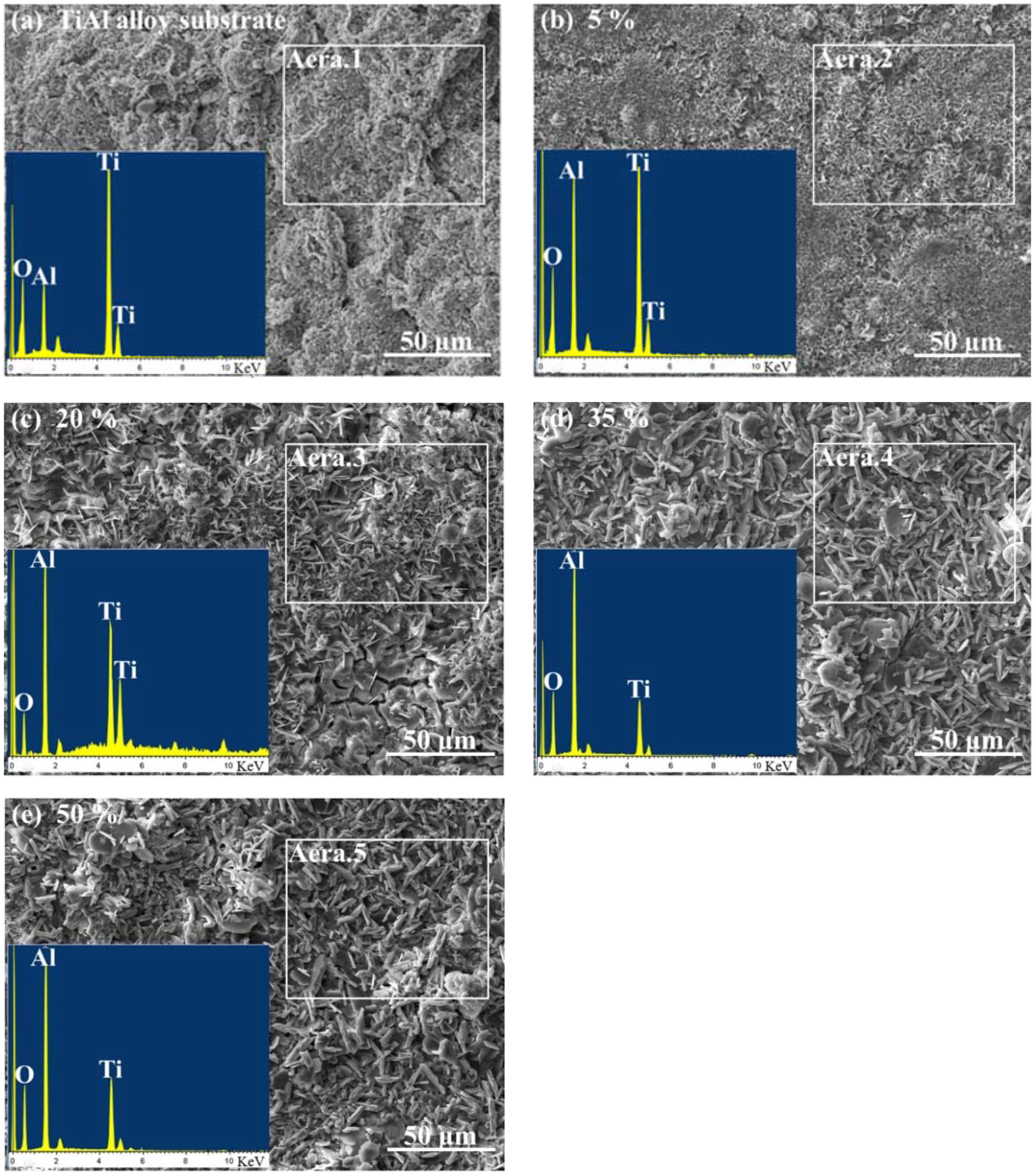
3.2. Characterizations of the CPED Coatings
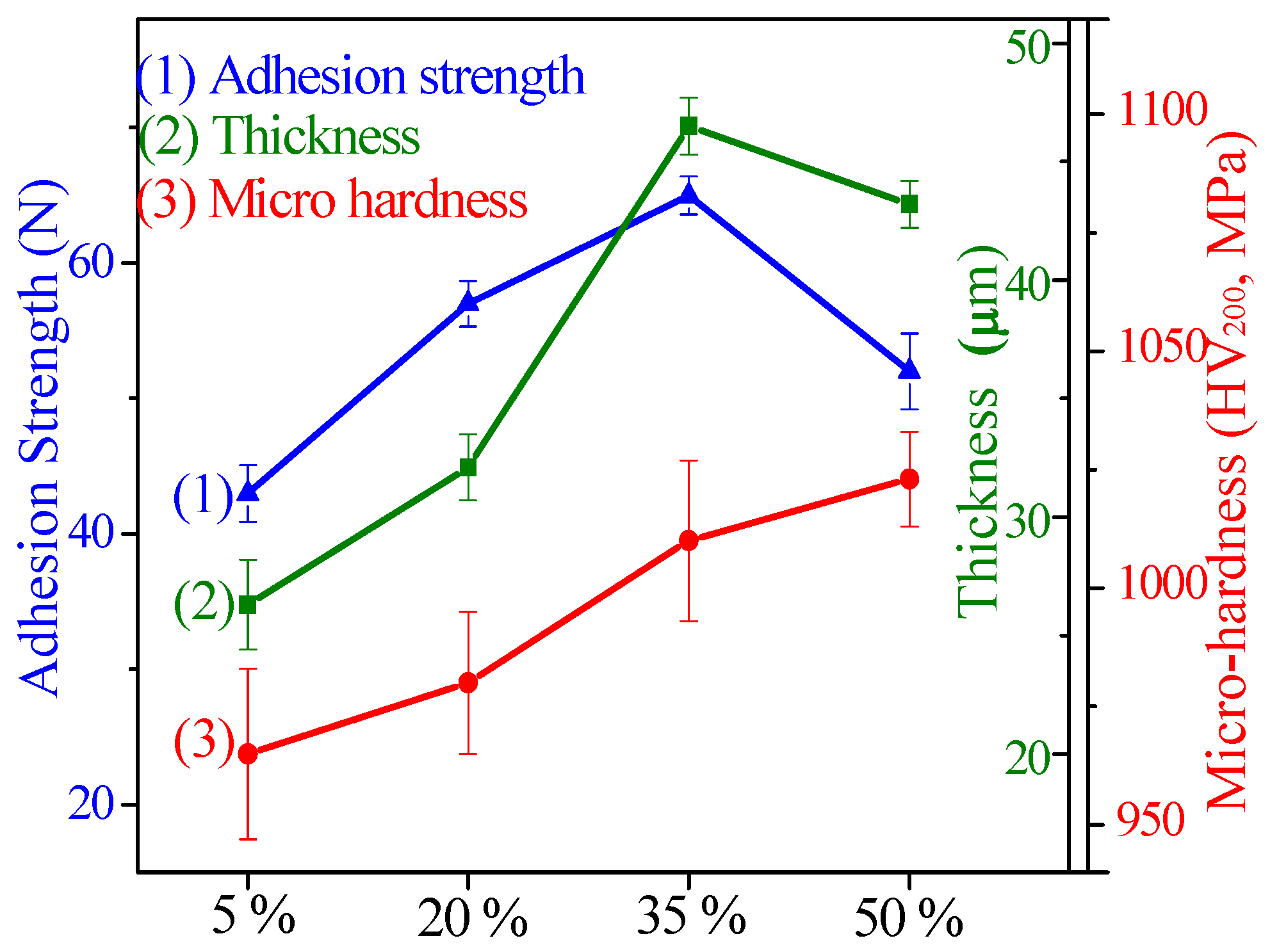
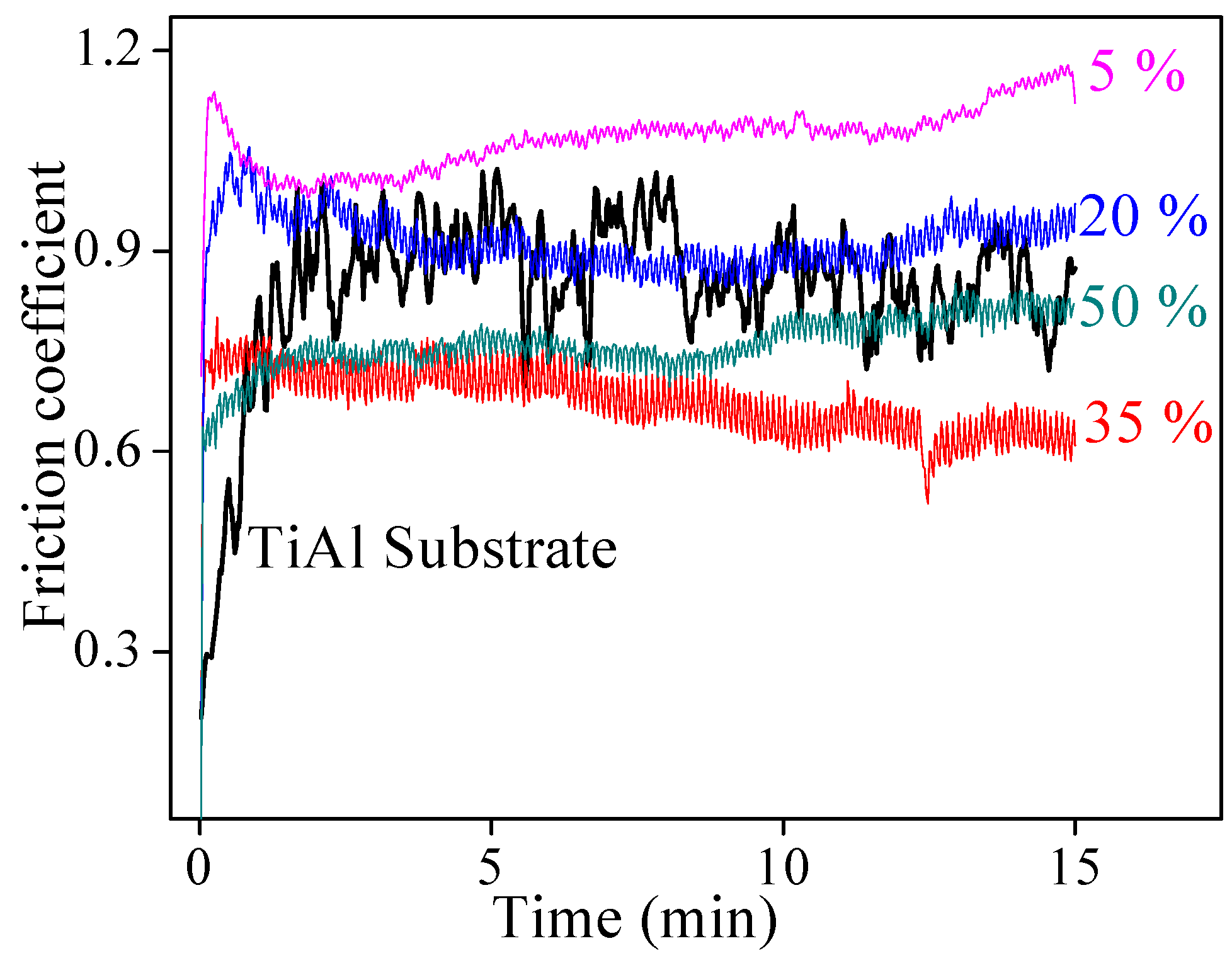
3.3. The Mechanical Properties and Wear-Resistance of CPED Coatings
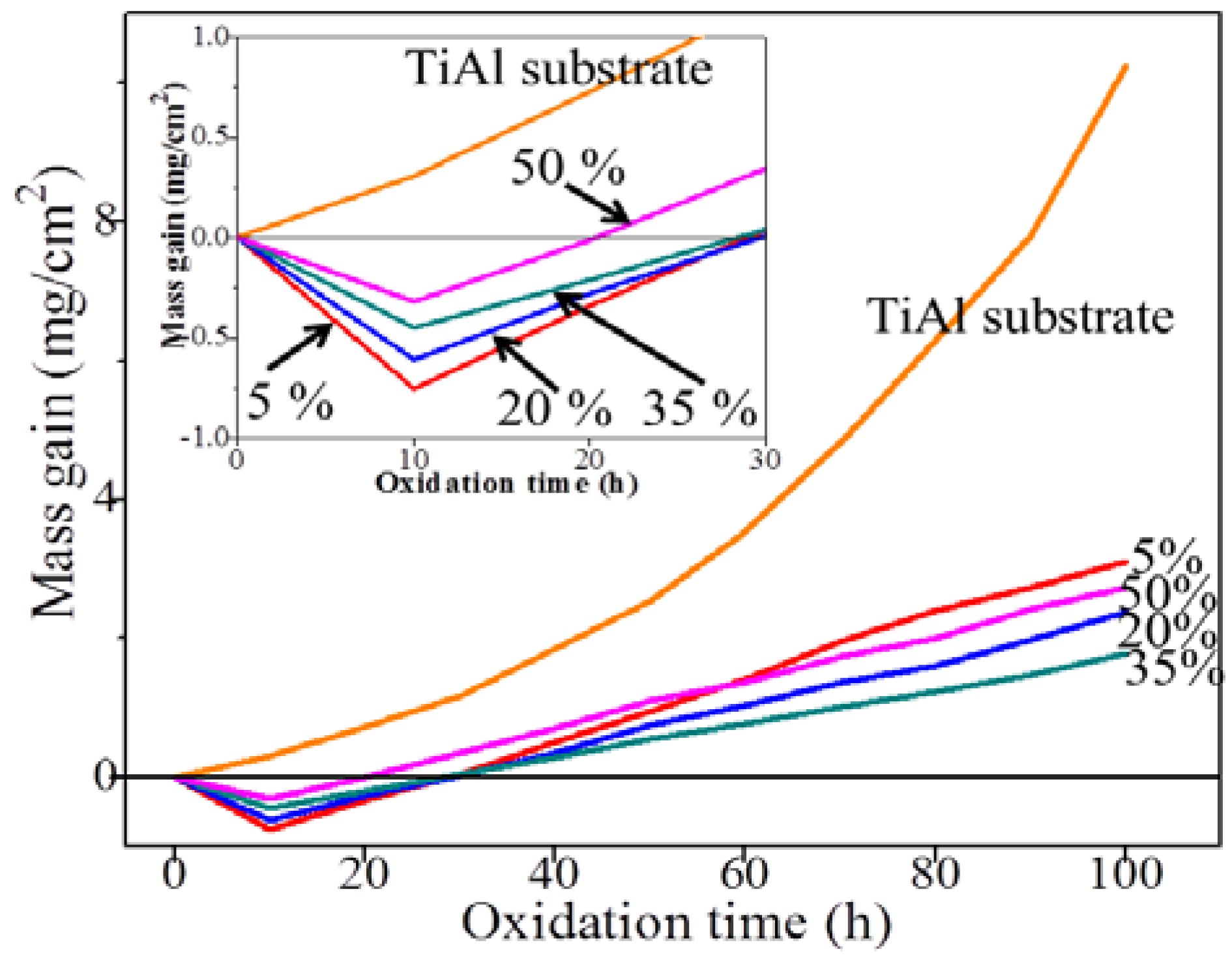
3.4. Heat-Resistance of TiAl Alloy Substrate and CPED Coatings
3.5. The Analysis of Mechanism of Duty Cycle
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ–TiAl based alloys–A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Narksitipan, S.; Thongtem, T.; Nallan, M.M.; Thongtem, S. Surface modification of γ–TiAl alloys by acetylene plasma deposition. Appl. Surf. Sci. 2006, 252, 8510–8513. [Google Scholar] [CrossRef]
- Boonruang, C.; Thongtem, S. Surface modification of TiAl alloy via current heating technique. Appl. Surf. Sci. 2009, 256, 484–488. [Google Scholar] [CrossRef]
- Hashinokuchi, M.; Tode, M.; Yoshigoe, A.; Teraoka, Y.; Okada, M. Oxidation of TiAl surface with hyperthermal oxygen molecular beams. Appl. Surf. Sci. 2013, 276, 276–283. [Google Scholar] [CrossRef]
- Deng, S.; Wang, P.; He, Y. Influence of adding glass beads in cathode region on the kinetics of cathode plasma electrolytic depositing ZrO2 coating. Surf. Coat. Technol. 2015, 279, 92–100. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D. Study on energy consumption of Al2O3 coating prepared by cathode plasma electrolytic deposition. Ceram. Int. 2018, 44, 657–662. [Google Scholar] [CrossRef]
- Bahadori, E.; Javadpour, S.; Shariat, M.H.; Mahzoon, F. Preparation and properties of ceramic Al2O3 coating as TBCs on MCrAly layer applied on Inconel alloy by cathodic plasma electrolytic deposition. Surf. Coat. Technol. 2013, 228, S611–S614. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Liu, X.; Yao, Z. Influence of treating frequency on microstructure and properties of Al2O3 coating on 304 stainless steel by cathodic plasma electrolytic deposition. Appl. Surf. Sci. 2009, 255, 8836–8840. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Yang, W.; Cai, K.; Chen, Y. Al2O3–ZrO2 ceramic coatings fabricated on WE43 magnesium alloy by cathodic plasma electrolytic deposition. Mater. Lett. 2012, 70, 16–18. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Ma, Z.; Gao, P. The growth mechanism of CPED coating with zirconia sol addition on an Al–12Si alloy. J. Alloys Compd. 2018, 740, 735–742. [Google Scholar] [CrossRef]
- Jin, X.; Wang, B.; Xue, W.; Du, J.; Wu, X.; Wu, J. Characterization of wear–resistant coatings on 304 stainless steel fabricated by cathodic plasma electrolytic oxidation. Surf. Coat. Technol. 2013, 236, 22–28. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Wang, B.; Jin, X.; Du, J.; Li, Y. Characterization of carburized layer on T8 steel fabricated by cathodic plasma electrolysis. Surf. Coat. Technol. 2014, 245, 9–15. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Song, Y.; Liu, Z.; Qin, D. Structure and properties of Al2O3 coatings formed on Ni Ti alloy by cathodic plasma electrolytic deposition. Surf. Coat. Technol. 2016, 285, 128–133. [Google Scholar] [CrossRef]
- Shaoqing, W.; Faqin, X.; Xiangqing, W. Mechanism of Al2O3 coating by cathodic plasma electrolytic deposition on TiAl alloy in Al(NO3)3 ethanol–water electrolytes. Mater. Chem. Phys. 2017, 202, 114–119. [Google Scholar]
- Cheng, Y.; Wang, T.; Li, S.; Cheng, Y.; Cao, J.; Xie, H. The effects of anion deposition and negative pulse on the behaviours of plasma electrolytic oxidation (PEO)—A systematic study of the PEO of a Zirlo alloy in aluminate electrolytes. Electrochim. Acta 2017, 225, 47–68. [Google Scholar] [CrossRef]
- Erfanifar, E.; Aliofkhazraei, M.; Nabavi, H.F.; Sharifi, H.; Rouhaghdam, A.S. Growth kinetics and morphology of plasma electrolytic oxidation coating on aluminum. Mater. Chem. Phys. 2017, 185, 162–175. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, X.; Liang, Y.; Hao, G.; Zhang, H.; Lin, J. Favorable deposition of γ-Al2O3 coatings by cathode plasma electrolysis for high-temperature application of Ti-45Al-8.5Nb alloys. Surf. Coat. Technol. 2018, 333, 187–194. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Kainer, K.U.; Zheludkevich, M.L. Investigation of the formation mechanisms of plasma electrolytic oxidation coatings on Mg alloy AM50 using particles. Electrochim. Acta 2016, 196, 680–691. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xie, F.; Wu, X.; An, J. Effect of Duty Cycle on Properties of Al2O3 Ceramic Coatings Fabricated on TiAl Alloy via Cathodic Plasma Electrolytic Deposition. Materials 2018, 11, 1962. https://doi.org/10.3390/ma11101962
Wang S, Xie F, Wu X, An J. Effect of Duty Cycle on Properties of Al2O3 Ceramic Coatings Fabricated on TiAl Alloy via Cathodic Plasma Electrolytic Deposition. Materials. 2018; 11(10):1962. https://doi.org/10.3390/ma11101962
Chicago/Turabian StyleWang, Shaoqing, Faqin Xie, Xiangqing Wu, and Jixiang An. 2018. "Effect of Duty Cycle on Properties of Al2O3 Ceramic Coatings Fabricated on TiAl Alloy via Cathodic Plasma Electrolytic Deposition" Materials 11, no. 10: 1962. https://doi.org/10.3390/ma11101962
APA StyleWang, S., Xie, F., Wu, X., & An, J. (2018). Effect of Duty Cycle on Properties of Al2O3 Ceramic Coatings Fabricated on TiAl Alloy via Cathodic Plasma Electrolytic Deposition. Materials, 11(10), 1962. https://doi.org/10.3390/ma11101962



