Effect of Electric Current Pulse on Microstructure and Corrosion Resistance of Hypereutectic High Chromium Cast Iron
Abstract
:1. Introduction
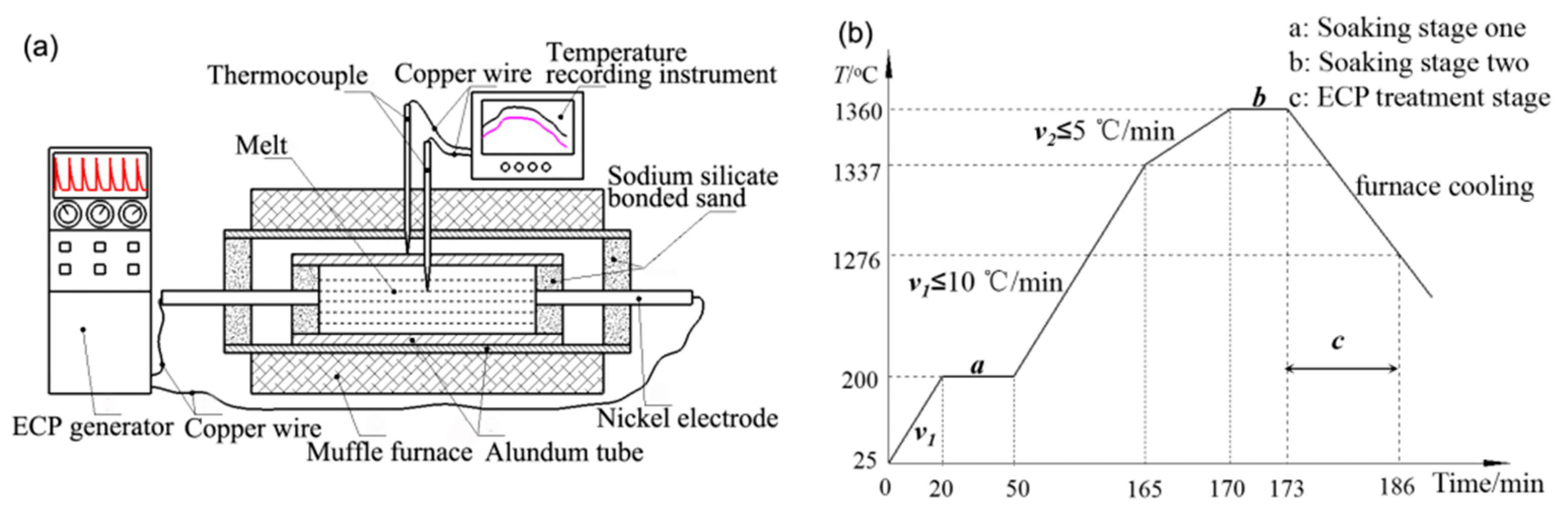
2. Materials and Methods
3. Results and Discussion
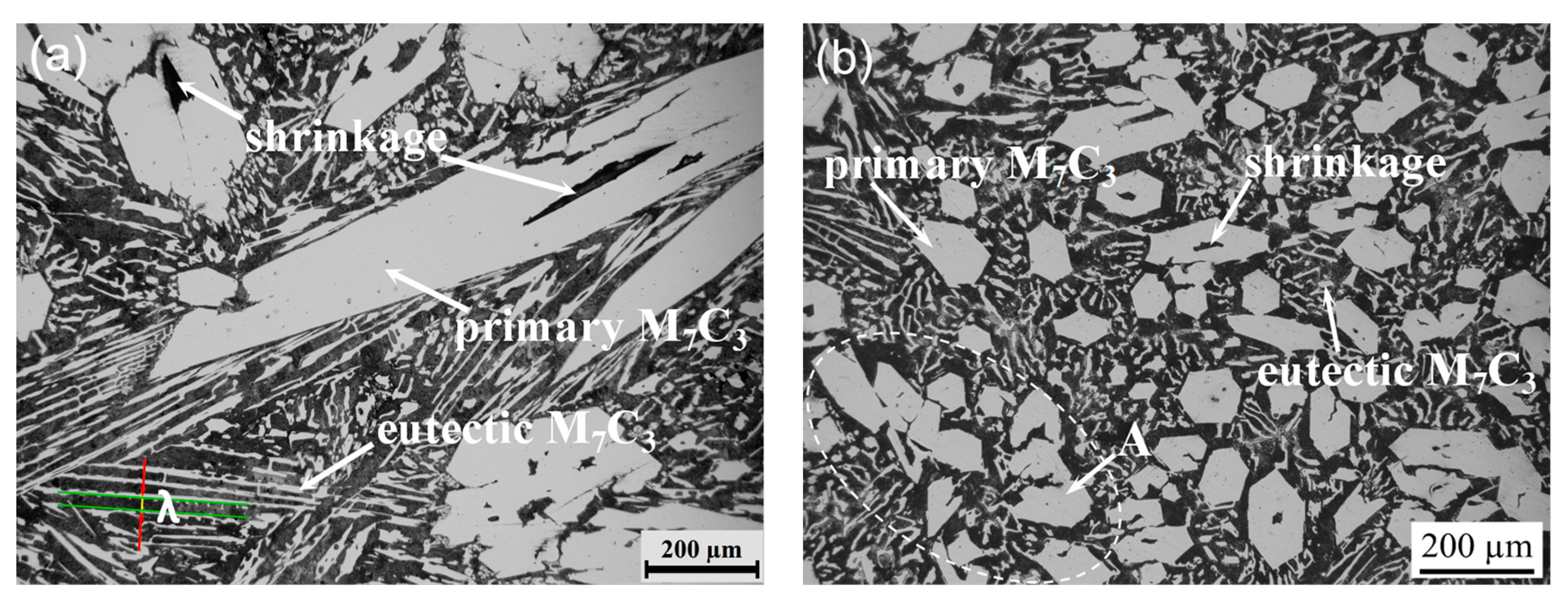
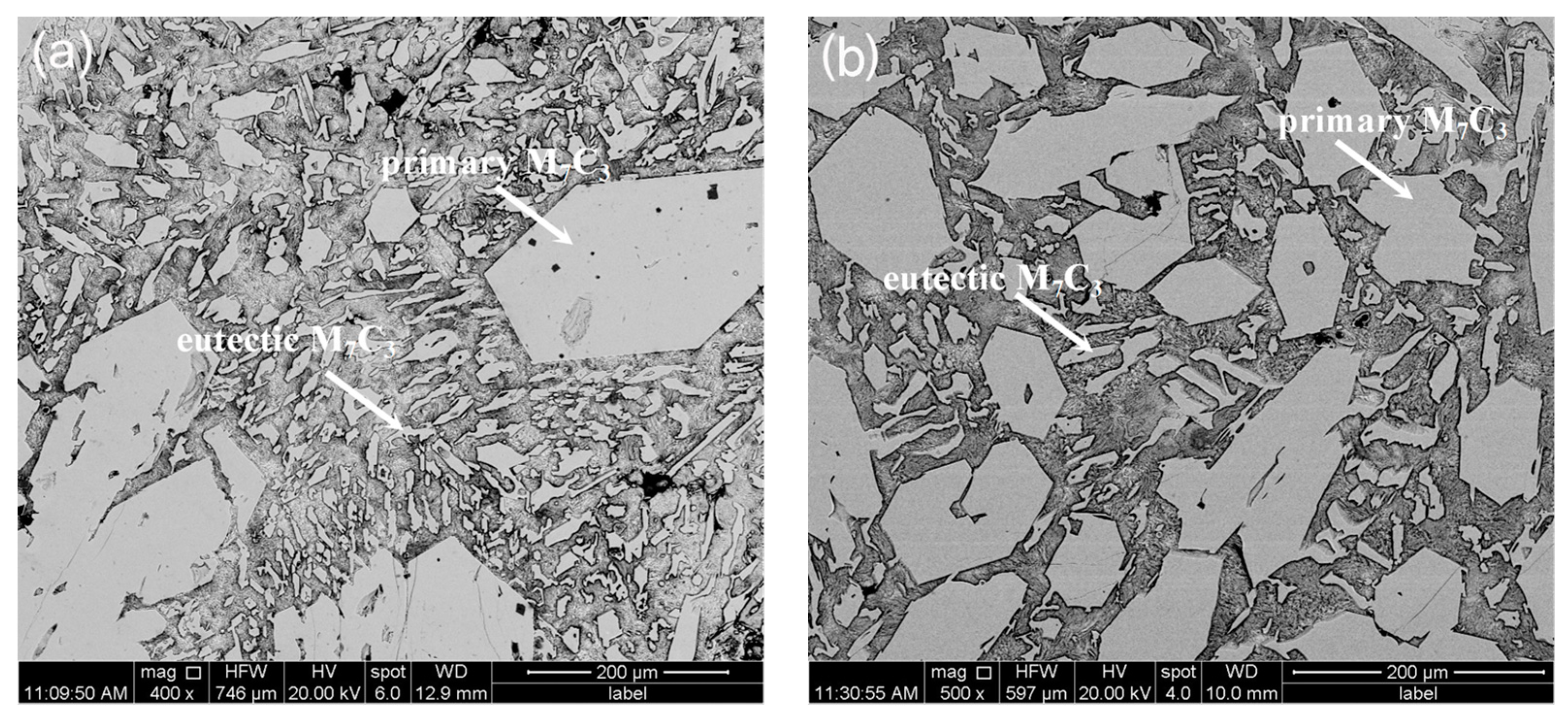
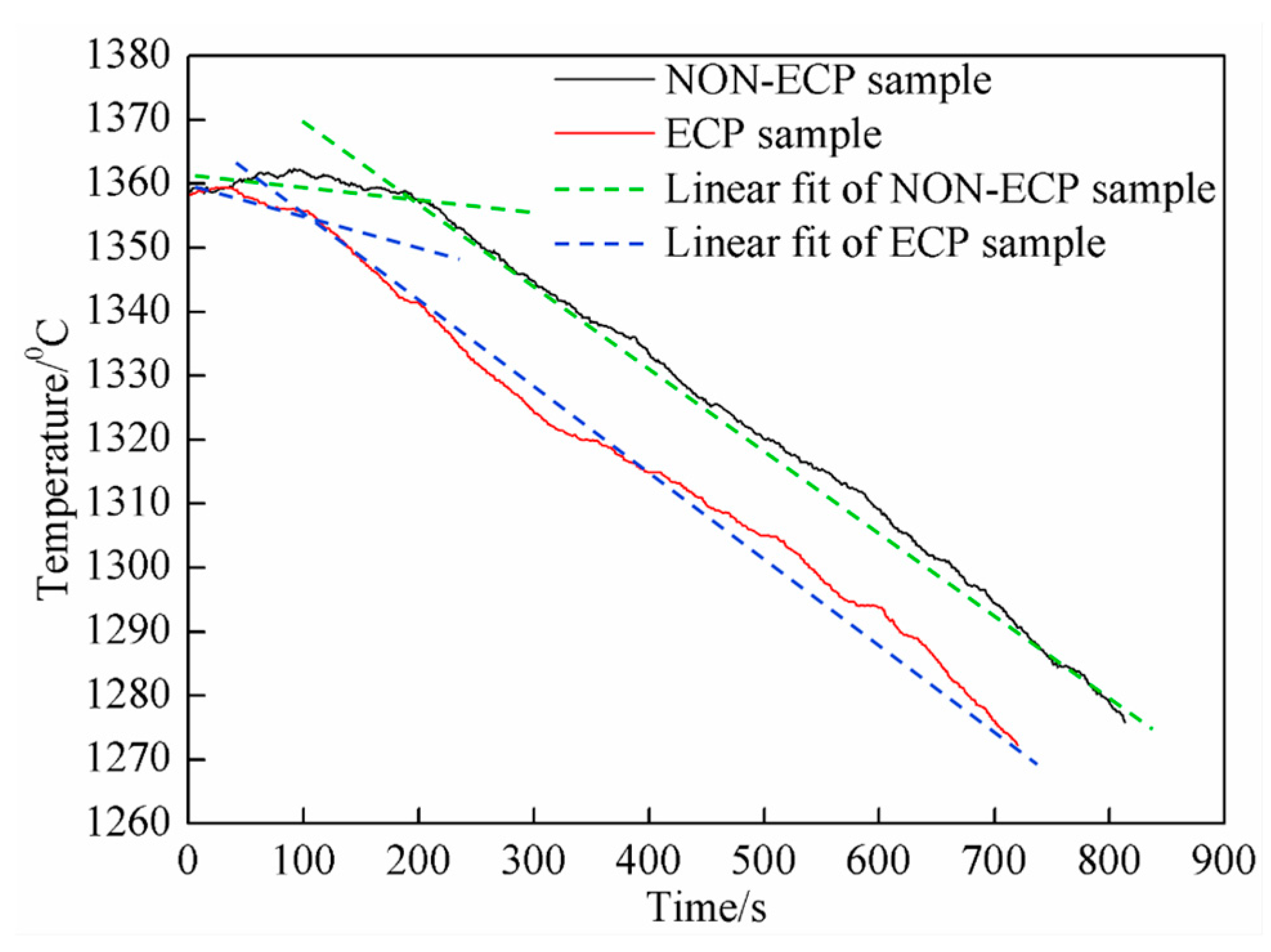
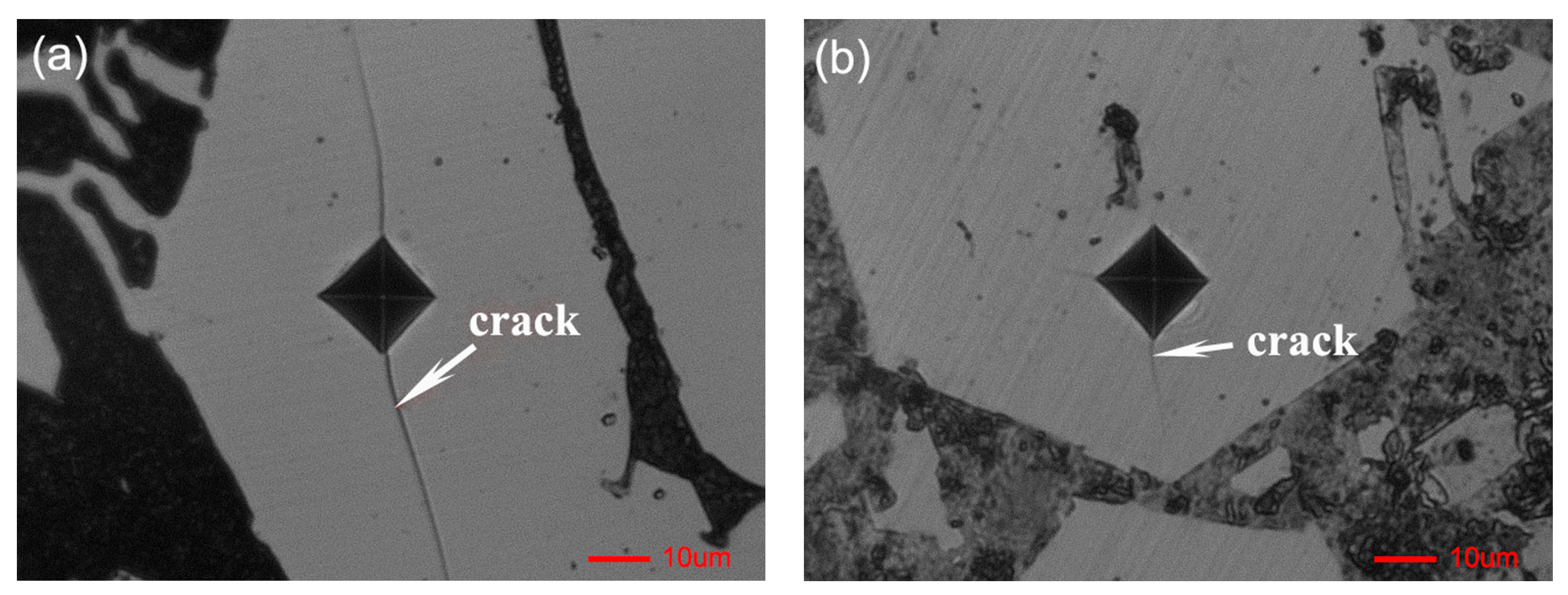
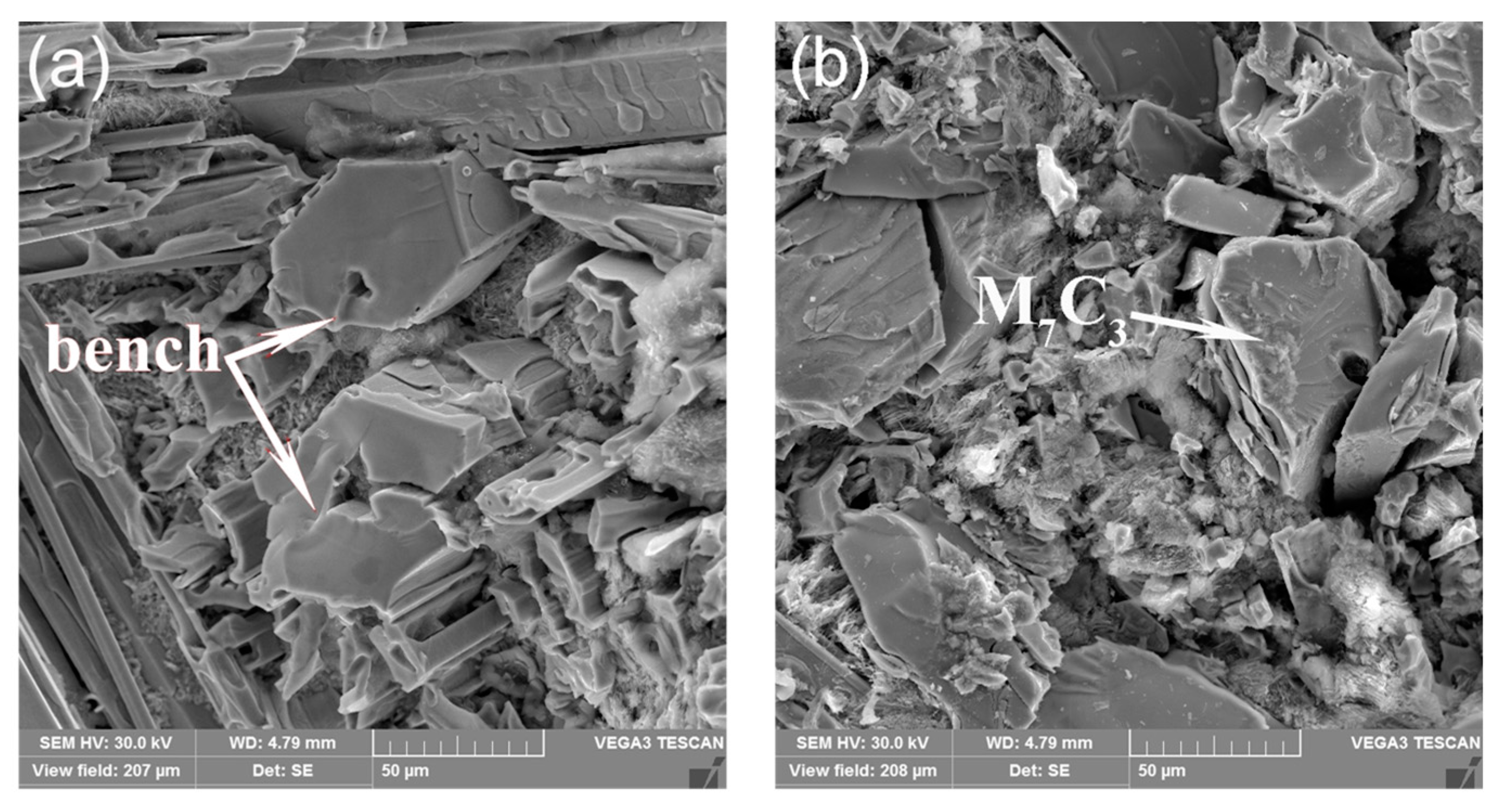
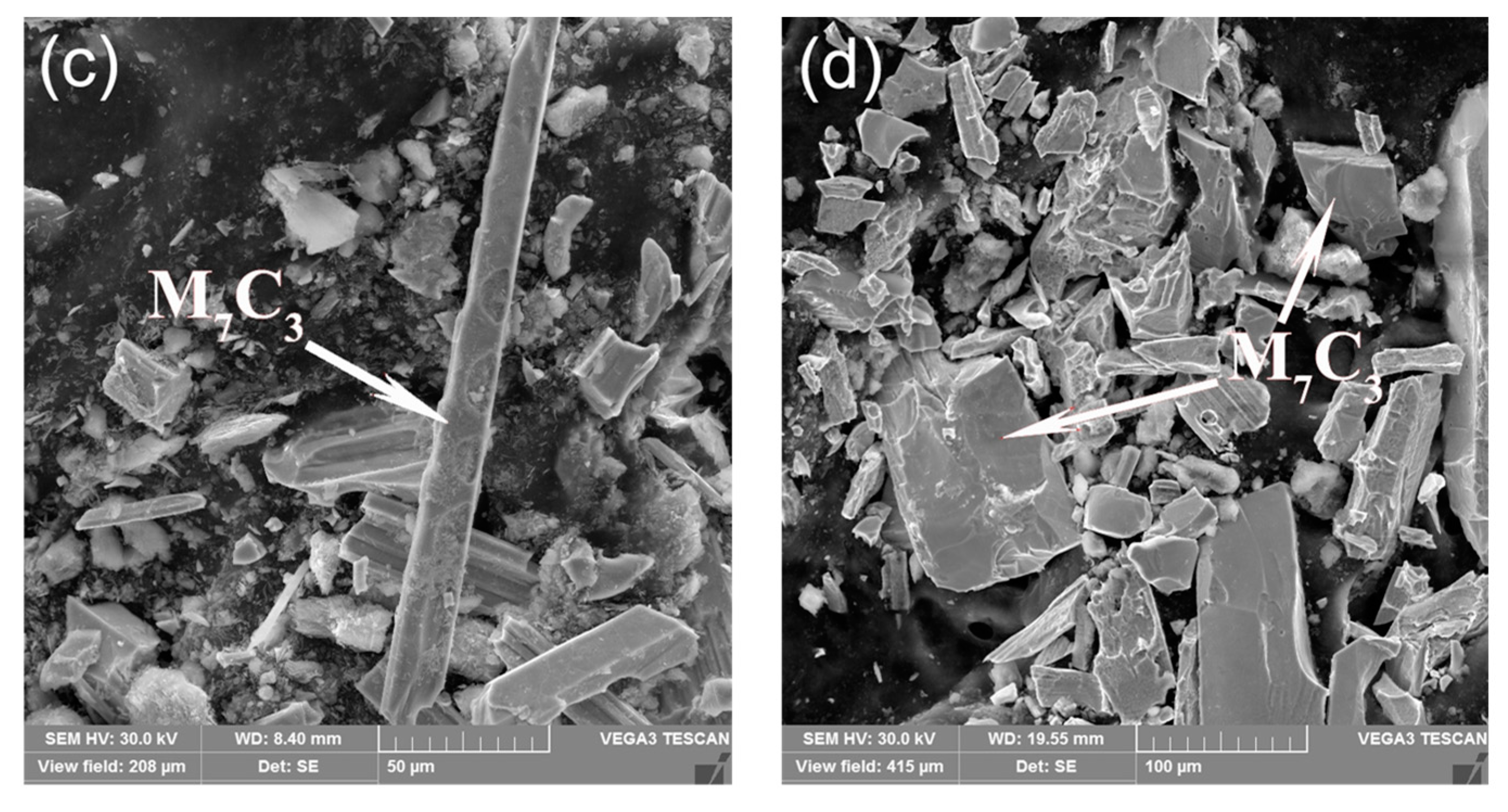
3.1. Morphological Properties
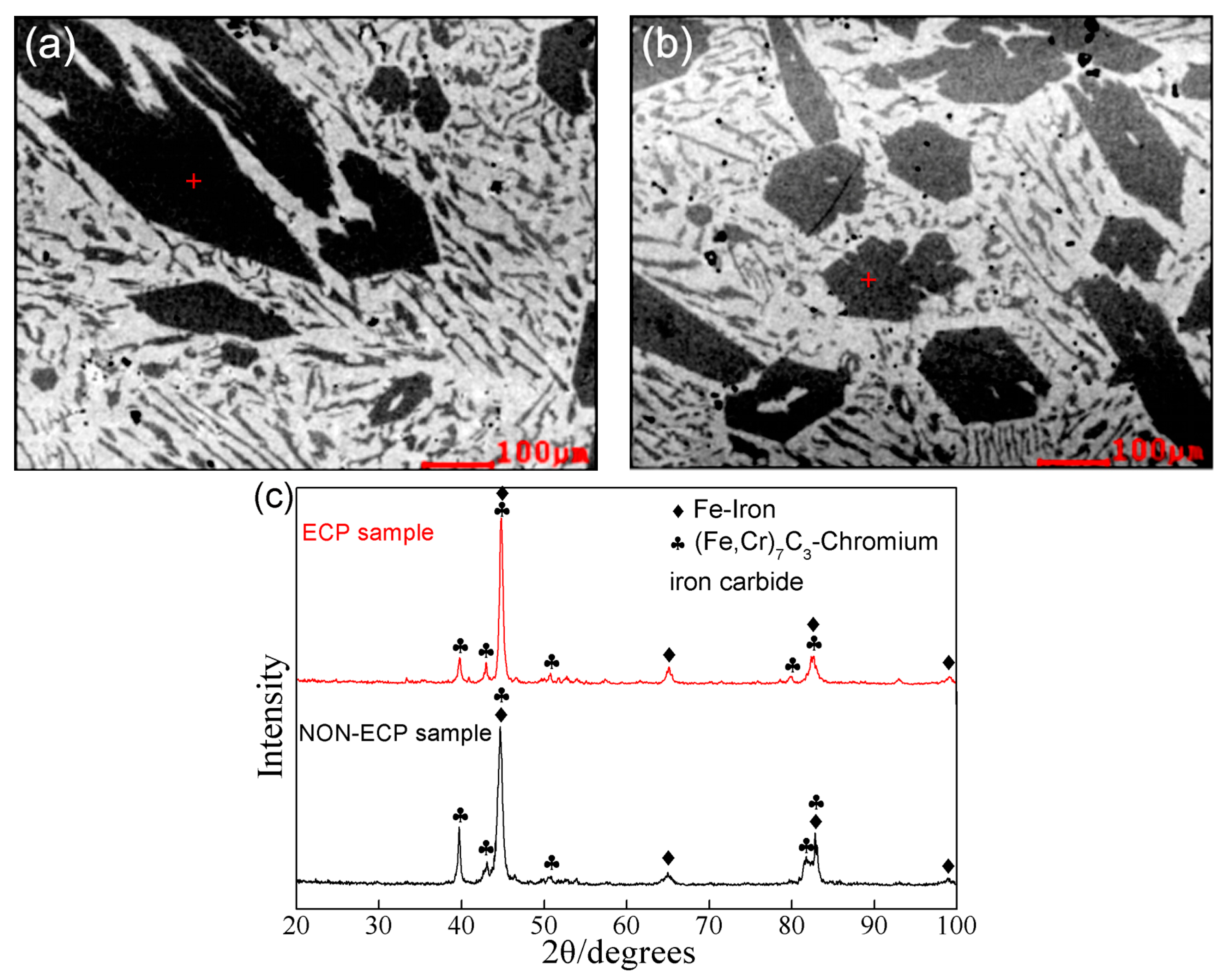
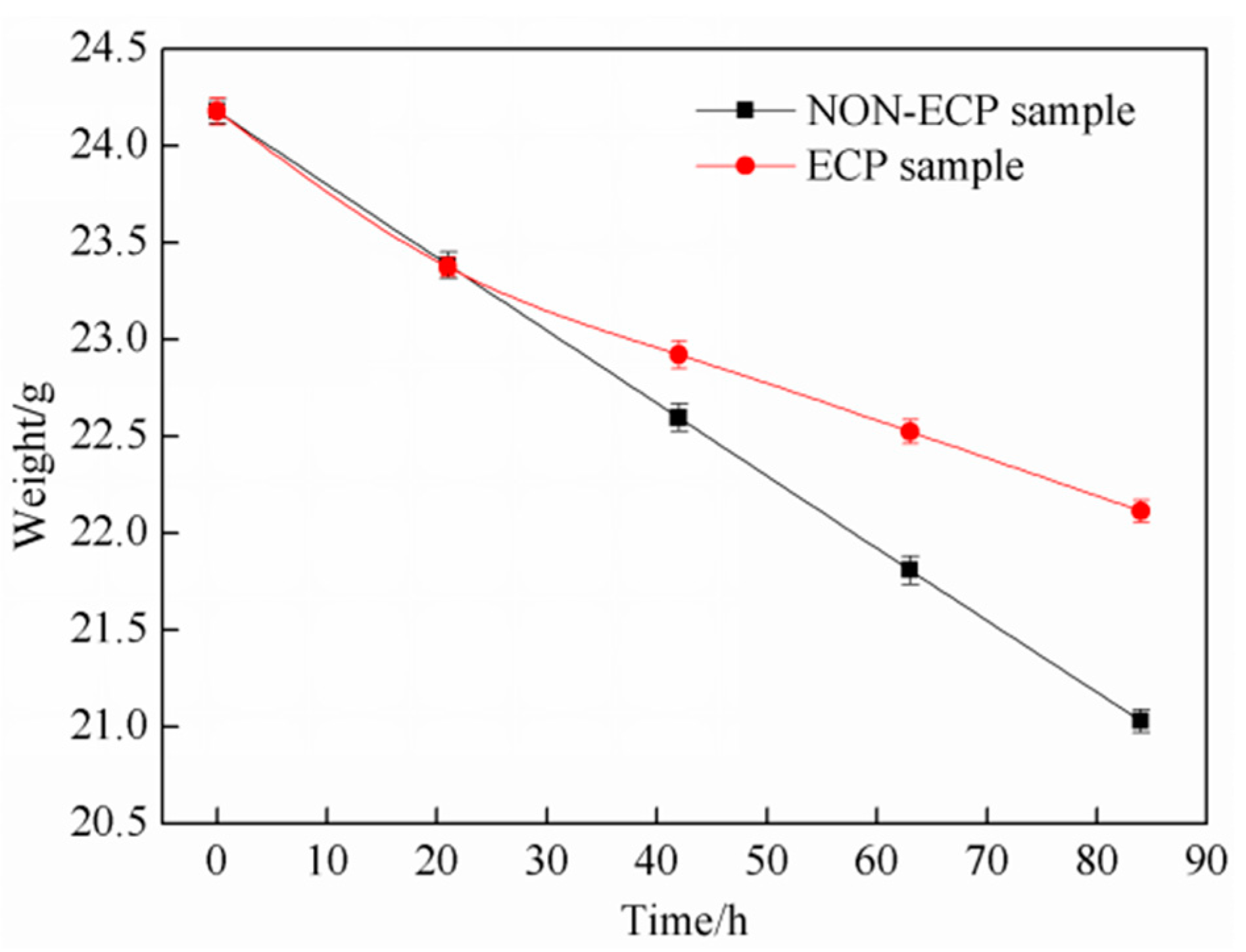
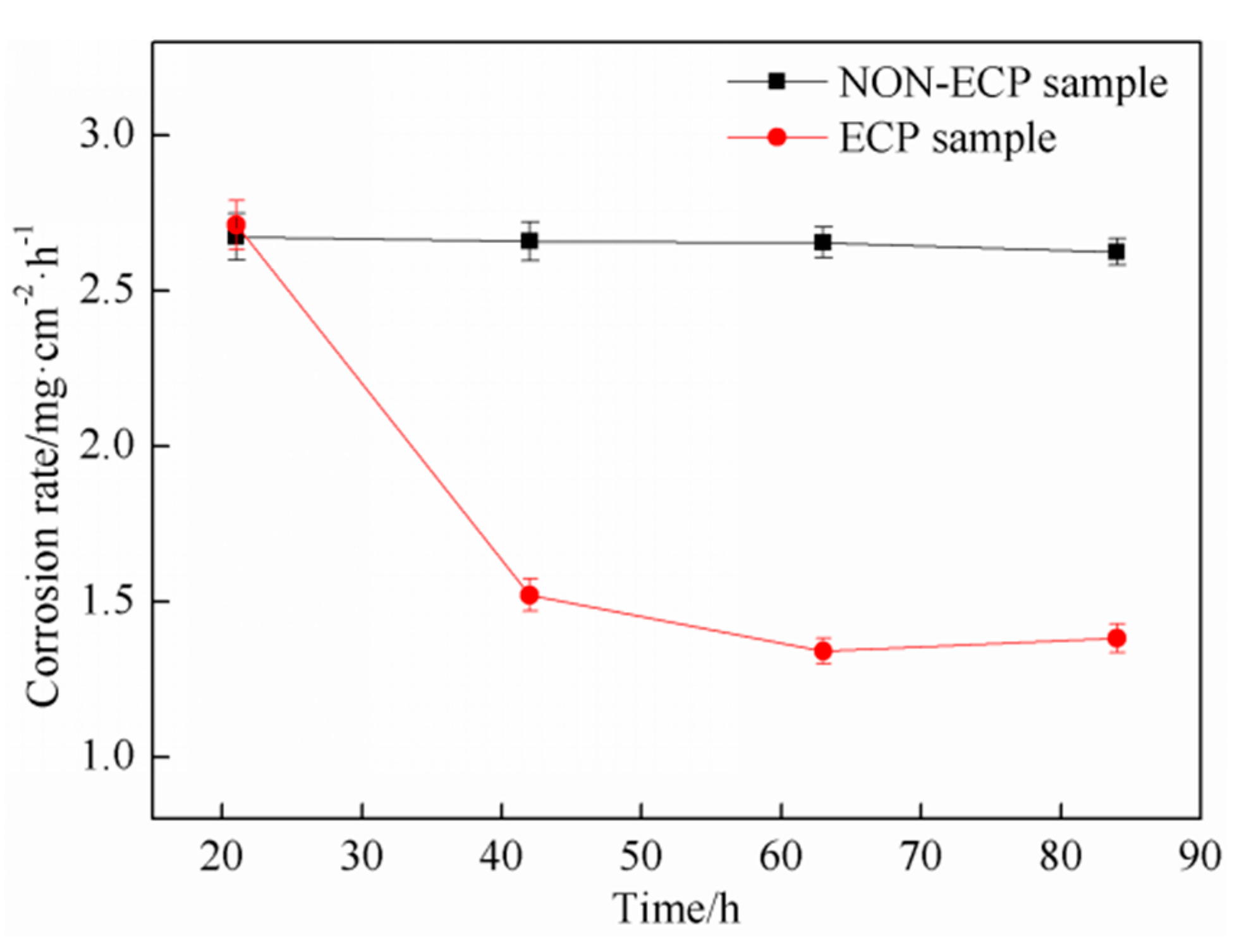
3.2. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gasan, H.; Erturk, F. Effects of a destabilization heat treatment on the microstructure and abrasive wear behavior of high-chromium white cast iron investigated using different characterization techniques. Metall. Mater. Trans. A 2013, 44, 4993–5005. [Google Scholar] [CrossRef]
- Lai, J.P.; Pan, Q.L.; Wang, Z.B.; Cui, H.R.; Wang, X.D.; Gao, Z.Z. Effects of destabilization temperature on the microstructure and mechanical properties of high chromium cast iron. J. Mater. Eng. Perform. 2017, 26, 1–9. [Google Scholar] [CrossRef]
- Zumelzu, E.; Cabezas, C.; Opitz, O.; Quiroz, E.; Goyos, L.; Parada, A. Microstructural characteristics and corrosion behaviour of high-chromium cast iron alloys in sugar media. Prot. Met. 2003, 39, 183–188. [Google Scholar] [CrossRef]
- Neville, A.; Reza, F.; Chiovelli, S.; Revega, T. Characterization and corrosion behavior of high-chromium white cast irons. Metall. Mater. Trans. A 2006, 37, 2339–2347. [Google Scholar] [CrossRef]
- Qu, Y.; Xing, J.; Zhi, X.; Peng, J.; Fu, H. Effect of cerium on the as-cast microstructure of a hypereutectic high chromium cast iron. Mater. Lett. 2008, 62, 3024–3027. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D.; Pearce, J.T.H. XRD and electron microscope study of an as-cast 26.6% chromium white iron microstructure. Mater. Chem. Phys. 2004, 85, 32–40. [Google Scholar] [CrossRef]
- Sarkar, T.; Mukherjee, M.; Pal, T.K. Effect of Cu addition on microstructure and hardness of As-Cast and heat-treated high-Cr cast iron. Trans. Indian Inst. Met. 2018, 71, 1455–1461. [Google Scholar] [CrossRef]
- Chen, L.; Stahl, J.E.; Zhao, W.; Zhou, J. Assessment on abrasiveness of high chromium cast iron material on the wear performance of PCBN cutting tools in dry machining. J. Mater. Process. Technol. 2018, 255, 110–120. [Google Scholar] [CrossRef]
- Chung, R.J.; Tang, X.; Li, D.Y.; Hinckley, B.; Dolman, K. Abnormal erosion-slurry velocity relationship of high chromium cast iron with high carbon concentrations. Wear 2011, 271, 1454–1461. [Google Scholar] [CrossRef]
- Zhi, X.H.; Liu, J.Z.; Xing, J.; Ma, S. Effect of cerium modification on microstructure and properties of hypereutectic high chromium cast iron. Mater. Sci. Eng. A 2014, 603, 98–103. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Liu, H.H. The precipitation and transformation of secondary carbides in a high chromium cast iron. Mater. Charact. 2006, 56, 73–78. [Google Scholar] [CrossRef]
- Huang, Z.F.; Xing, J.D.; Zhang, A.F. Microstructure and property of hypereutectic high chromium cast Iron prepared by slope cooling body-centrifugal casting method. J. Mater. Sci. Technol. 2006, 26, 775–778. [Google Scholar]
- Cortés-Carrillo, E.; Bedolla-Jacuinde, A.; Mejía, I.; Zepeda, C.M.; Zuno-Silva, J.; Guerra-Lopez, F.V. Effects of tungsten on the microstructure and on the abrasive wear behavior of a high-chromium white iron. Wear 2017, 376–377, 77–85. [Google Scholar] [CrossRef]
- Jena, P.S.M.; Sahu, J.K.; Rai, R.K.; Das, S.K.; Singh, R.K. Influence of duplex ferritic-austenitic matrix on two body abrasive wear behaviour of high chromium white cast iron. Wear 2018, 406–407, 140–148. [Google Scholar] [CrossRef]
- Babutskii, A.I. Effect of electric current pulse treatment on the corrosion rate and strength of specimens made of 45 steel. Strength Mater. 2010, 42, 432–438. [Google Scholar] [CrossRef]
- Dost, S.; Liu, Y.C.; Haas, J.; Roszmann, J.; Grenier, S.; Audet, N. Effect of applied electric current on impurity transport in zone refining. J. Cryst. Growth. 2007, 307, 211–218. [Google Scholar] [CrossRef]
- Jung, J.; Yang, J.; Morita, Y.; Toku, Y. Effect of pulsed electric current on the growth behavior of fatigue crack in Al alloy. Procedia Struct. Integr. 2016, 2, 2989–2993. [Google Scholar] [CrossRef]
- Lu, Z.; Li, L.; Nan, W.; Jiang, X.; Zhai, Q. Soft Magnetic Materials I: Effect of electric current pulse on grain boundary of grain oriented silicon steel during primary recrystallization annealing. In Energy Technology 2015; Jha, A., Wang, C., Eds.; Springer: Cham, Germany, 2016; pp. 255–262. ISBN 9783319486024. [Google Scholar]
- Li, Q.C.; Li, R.X.; Yue, X.D.; Chang, G.W.; Zhai, Q.J. Efect of eleetropulsing on the formation of graphite during solid-state graphitization of spherical graphite iron. Mater. Chem. Phys. 2008, 12, 402–406. [Google Scholar] [CrossRef]
- Liao, X.; Zhai, Q.; Luo, J.; Chen, W.; Gong, Y. Refining mechanism of the electric current pulse on the solidification structure of pure aluminum. Acta Mater. 2007, 55, 3103–3109. [Google Scholar] [CrossRef]
- Wang, J.Z.; Jin-Gang, Q.I.; Zhao, Z.F.; Guo, H.S.; Zhao, T. Effects of electric pulse modification on liquid structure of Al–5%Cu alloy. Trans. Nonferr. Met. Soc. China 2013, 23, 2792–2796. [Google Scholar] [CrossRef]
- Oh, S.T.; Tajima, K.I.; Ando, M.; Ohji, T. Strengthening of porous alumina by pulse electric current sintering and nanocomposite processing. J. Am. Ceram. Soc. 2000, 83, 1314–1316. [Google Scholar] [CrossRef]
- Lv, H.Y. Research on the Solidification Microstructures of Low Superheat Hypereutectic High Chromium Cast Iron Melt under Electric Current Pulse Treatment. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2015. [Google Scholar]
- Shtansky, D.V.; Nakai, K.; Ohmori, Y. Crystallography and interface boundary structure of pearlite with M7C3 carbide lamellae. Acta Mater. 1999, 47, 1105–1115. [Google Scholar] [CrossRef]
- Hao, S.J. Wear-Resistant High Chromium Cast Iron; China Coal Industry Publishing House: Beijing, China, 1993; ISBN 9787502008222. [Google Scholar]
- Gu, J.; Xiao, P.; Song, J.; Li, Z.; Lu, R. Sintering of a hypoeutectic high chromium cast iron as well as its microstructure and properties. J. Alloys Compd. 2017, 740, 485–491. [Google Scholar] [CrossRef]
- Barnak, J.P.; Sprecher, A.F.; Conrad, H. Colony (grain) size reduction in eutectic Pb-Sn castings by electroplusing. Scr. Metall. Mater. 1995, 32, 879–884. [Google Scholar] [CrossRef]
- Räbiger, D.; Zhang, Y.; Galindo, V.; Franke, S.; Willers, B.; Eckert, S. The relevance of melt convection to grain refinement in Al–Si alloys solidified under the impact of electric currents. Acta Mater. 2014, 79, 327–338. [Google Scholar] [CrossRef]
- Wang, R.Z.; Qi, J.G.; Wang, B.; Zhang, W.; Wang, J.Z. Solidification behavior and crystal growth mechanism of aluminum under electric pulse. J. Mater. Process. Technol. 2016, 237, 235–242. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Hu, Z.L.; Jie, W.Q. Solidification Technology; China Machine Press: Beijing, China, 1998; ISBN 9787111064909. [Google Scholar]
- Misra, A.K. A novel solidification technique of metals and alloys: Under the influence of applied potential. Metall. Trans. A 1985, 16, 1354–1355. [Google Scholar] [CrossRef]
| Element | C | Cr | Si | Mn | Ni | Fe |
|---|---|---|---|---|---|---|
| Content | 3.94 | 20.30 | 2.10 | 0.60 | 0.30 | 72.76 |
| Element | C (at%) | Cr (at%) | Fe (at%) | C (wt%) | Cr (wt%) | Fe (wt%) |
|---|---|---|---|---|---|---|
| non-ECP sample | 31.27 | 40.22 | 28.51 | 7.17 | 52.44 | 40.39 |
| ECP sample | 30.39 | 38.03 | 31.58 | 7.45 | 49.63 | 42.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Zhou, R.; Li, L.; Ni, H.; Zhu, J.; Feng, T. Effect of Electric Current Pulse on Microstructure and Corrosion Resistance of Hypereutectic High Chromium Cast Iron. Materials 2018, 11, 2220. https://doi.org/10.3390/ma11112220
Lv H, Zhou R, Li L, Ni H, Zhu J, Feng T. Effect of Electric Current Pulse on Microstructure and Corrosion Resistance of Hypereutectic High Chromium Cast Iron. Materials. 2018; 11(11):2220. https://doi.org/10.3390/ma11112220
Chicago/Turabian StyleLv, Haiyang, Rongfeng Zhou, Lu Li, Haitao Ni, Jiang Zhu, and Tong Feng. 2018. "Effect of Electric Current Pulse on Microstructure and Corrosion Resistance of Hypereutectic High Chromium Cast Iron" Materials 11, no. 11: 2220. https://doi.org/10.3390/ma11112220
APA StyleLv, H., Zhou, R., Li, L., Ni, H., Zhu, J., & Feng, T. (2018). Effect of Electric Current Pulse on Microstructure and Corrosion Resistance of Hypereutectic High Chromium Cast Iron. Materials, 11(11), 2220. https://doi.org/10.3390/ma11112220




