An Ultrasonication-Assisted Cobalt Hydroxide Composite with Enhanced Electrocatalytic Activity toward Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the Catalyst
2.2. Physical Characterizations
2.3. Electrochemical Measurements
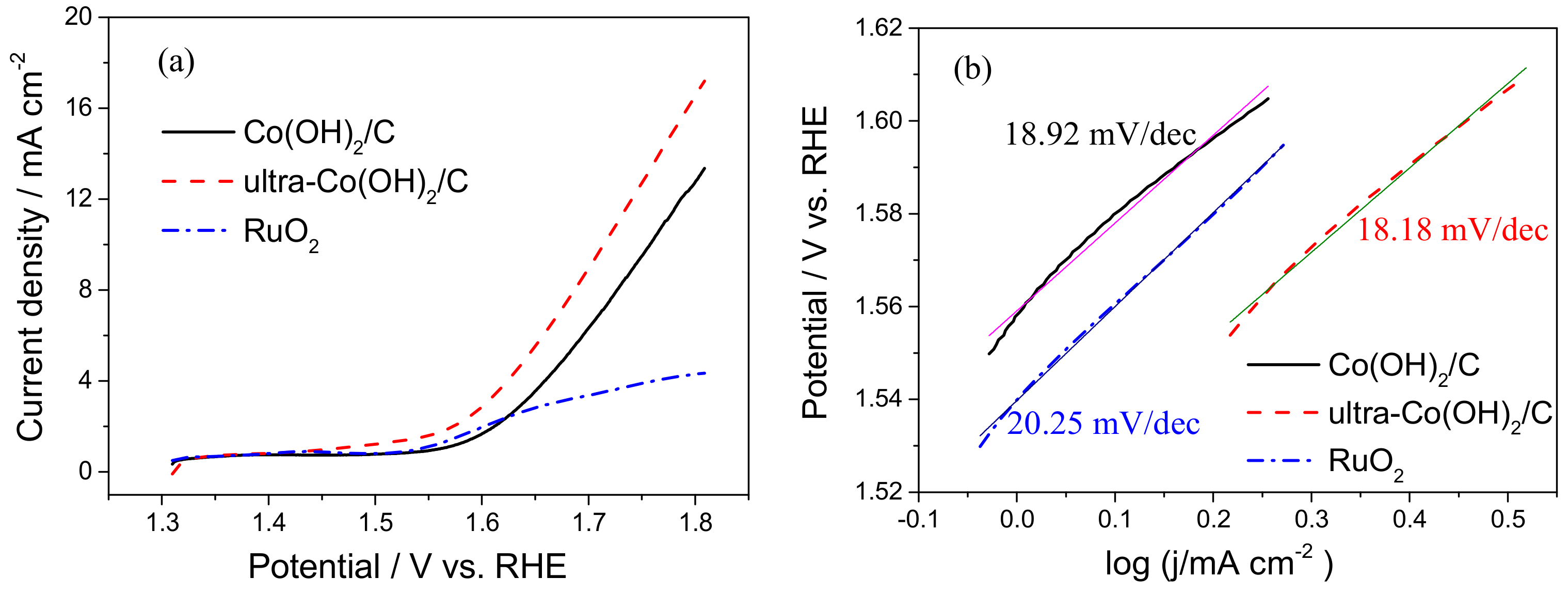
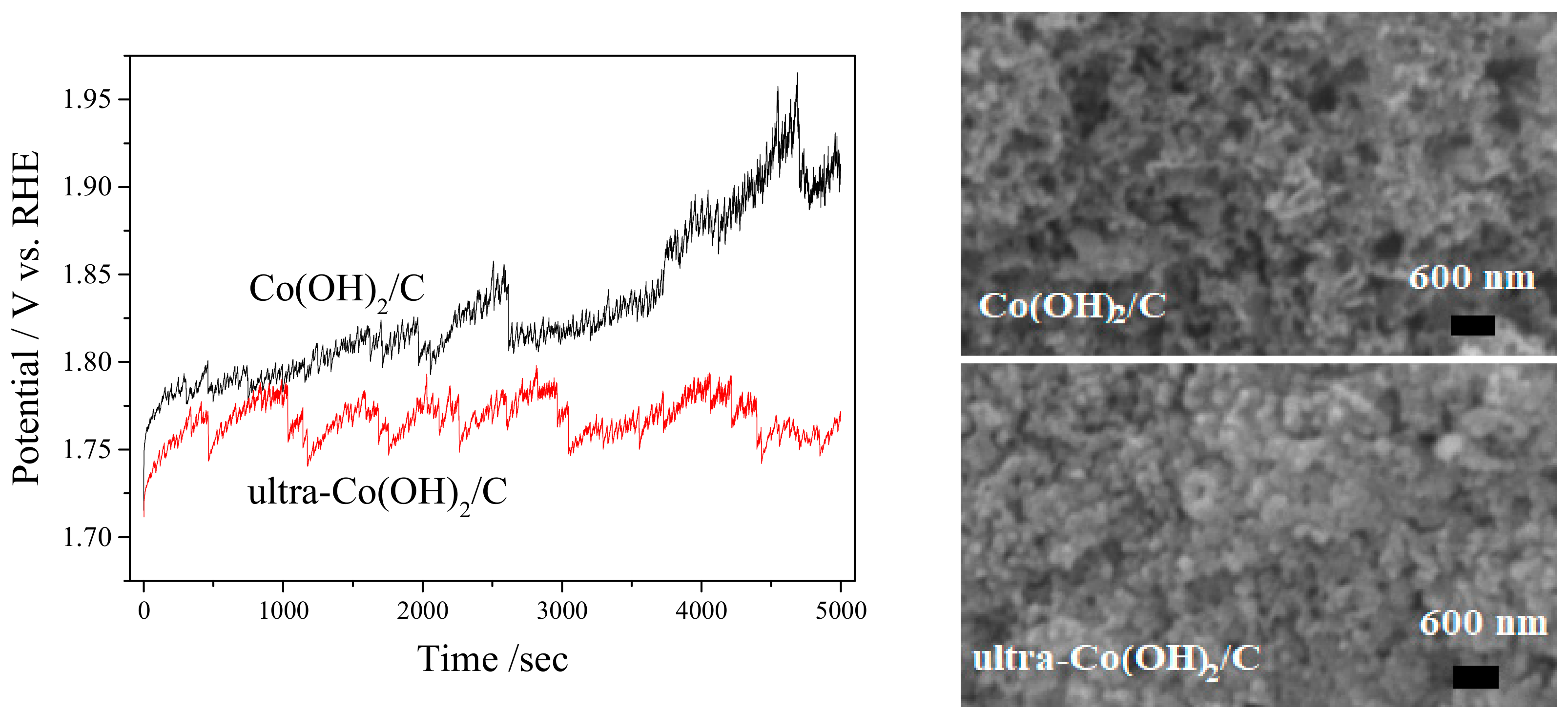
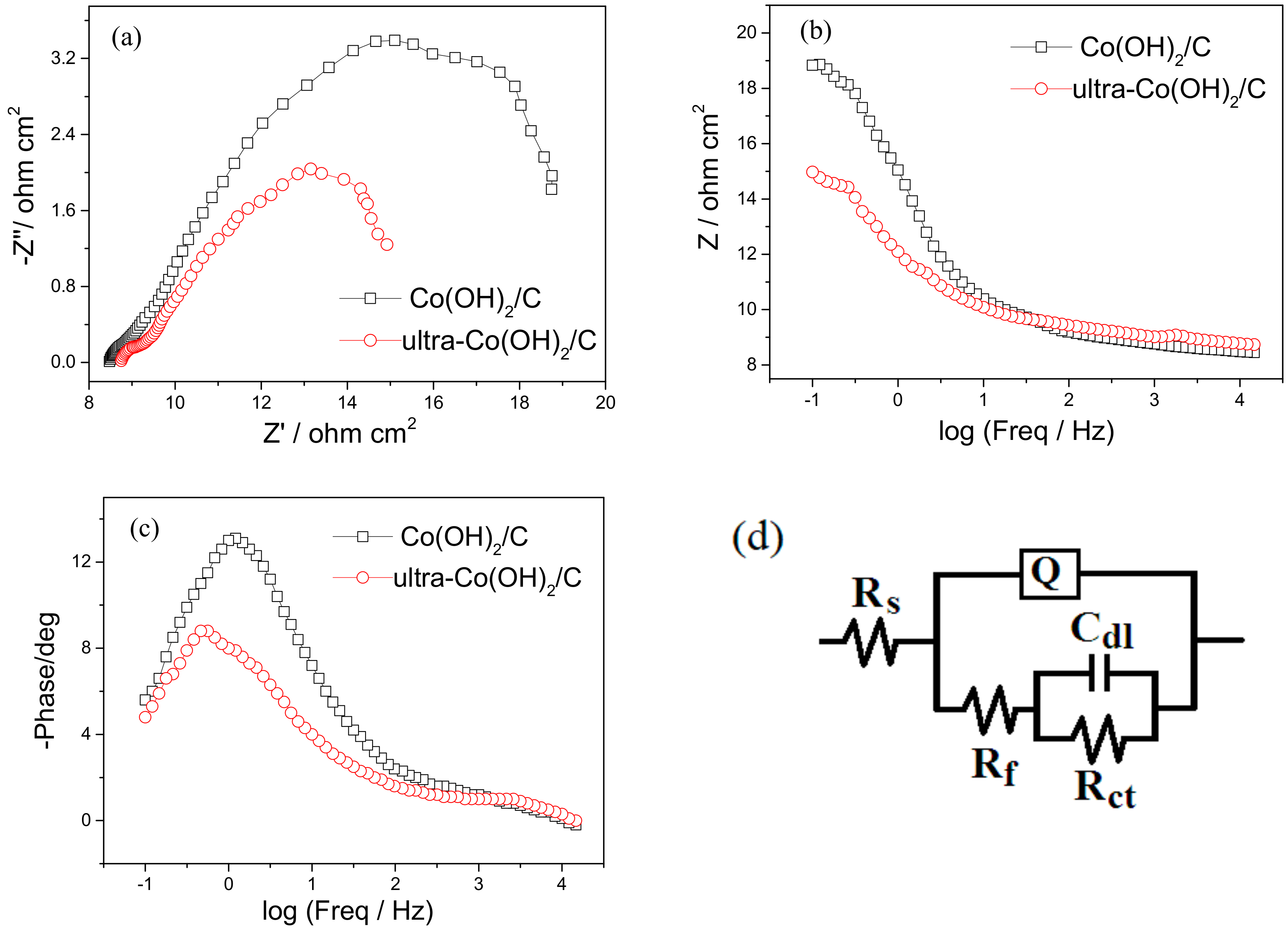
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Geng, J.; Chen, C.; Kan, E.; Liu, Y.; Wang, Q.; Geng, B. A reliable aerosol-spray-assisted approach to produce and optimize amorphous metal oxide catalysts for electrochemical water splitting. Angew. Chem. Int. Ed. 2014, 53, 7547–7551. [Google Scholar] [CrossRef] [PubMed]
- Jamesh, M.I.; Sun, X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting—A review. J. Power Sources 2018, 400, 31–68. [Google Scholar] [CrossRef]
- Liao, P.Q.; Shen, J.Q.; Zhang, J.P. Metal-organic frameworks for electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.Z.; Wang, C.C.; Lu, S.Y. In situ formation of NiO on Ni foam prepared with a novel leaven dough method as an outstanding electrocatalyst for oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 9797–9806. [Google Scholar] [CrossRef]
- Chen, L.; Dong, X.; Wang, Y.; Xia, Y. Separating hydrogen and oxygen evolution in alkaline water electrolysis using nickel hydroxide. Nat. Commun. 2016, 7, 11741–11748. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Eisenberg, R. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges. Energy Environ. Sci. 2012, 5, 6012–6021. [Google Scholar] [CrossRef]
- Farid, S.; Ren, S.; Hao, C. MOF-derived metal/carbon materials as oxygen evolution reaction catalysts. Inorg. Chem. Commun. 2018, 94, 57–74. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Liu, S.; Xu, W.; Wu, L.; Hsieh, Y.C.; Liu, P.; Zhu, Y.; Sasaki, K.; Renner, J.N.; et al. Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@IrO2 core-shell nanocatalysts. J. Electroanal. Chem. 2018, 819, 296–305. [Google Scholar] [CrossRef]
- Sung, M.; Kim, J. Oxygen evolution reaction on Pt sphere and Ir-modified Pt sphere electrodes with porous structures. Int. J. Hydrogen Energy 2018, 43, 2130–2138. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Yang, S.; Zheng, L.R.; Zhang, B.; Yang, H.G. Electrochemical etching of α-cobalt hydroxide for improvement of oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 9578–9584. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.S.; Baik, H.; Kang, K.; Lee, K. Porous β-MnO2 nanoplates derived from MnCO3 nanoplates as highly efficient electrocatalysts toward oxygen evolution reaction. RSC Adv. 2016, 6, 26535–26539. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Bediako, D.K.; Lassalle-Kaiser, B.; Surendranath, Y.; Yano, J.; Yachandra, V.K.; Nocera, D.G. Structure-activity correlations in a nickel-borate oxygen evolution catalyst. J. Am. Chem. Soc. 2012, 134, 6801–6809. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Li, T.T.; Zheng, Y.Q. Co3O4 nanosheet arrays treated by defect engineering for enhanced electrocatalytic water oxidation. Int. J. Hydrogen Energy 2018, 43, 2009–2017. [Google Scholar] [CrossRef]
- Xie, T.; Min, J.; Liu, J.; Chen, J.; Fu, D.; Zhang, R.; Zhu, K.; Lei, M. Synthesis of mesoporous Co3O4 nanosheet-assembled hollow spheres towards efficient electrocatalytic oxygen evolution. J. Alloys Compd. 2018, 754, 72–77. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, Z.; Tian, Z.; Ma, Y.; Qu, Y. Ultrathin porous Co3O4 nanoplates as highly efficient oxygen evolution catalysts. J. Mater. Chem. A 2015, 3, 8107–8114. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Wang, T.; Wang, C. Enhanced electrocatalytic oxygen evolution of α-Co(OH)2 nanosheets on carbon nanotube/polyimide films. Nanoscale 2016, 8, 9667–9675. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, J.; Luo, Z.; Wang, J.; Li, C.; Li, Z. MOF-mediated synthesis of monodisperse Co(OH)2 flower-like nanosheets for enhanced oxygen evolution reaction. Electrochim. Acta 2018, 273, 327–334. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616–6623. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhao, W.; Wu, L.; Li, X.; Zheng, X.; Ye, Q.; Xu, X.; Wang, F. New insights into graphite paper as electrocatalytic substrate for oxygen evolution reaction. Appl. Surf. Sci. 2017, 396, 1146–1154. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhavale, V.M.; Kurungot, S. Surface-tuned Co3O4 nanoparticles dispersed on nitrogen-doped graphene as an efficient cathode electrocatalyst for mechanical rechargeable zinc-Air battery application. ACS Appl. Mater. Interfaces 2015, 7, 21138–21149. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C. Highly efficient and robust oxygen evolution catalysts achieved by anchoring nanocrystalline cobalt oxides onto mildly oxidized multiwalled carbon nanotubes. J. Mater. Chem. A 2013, 1, 12053–12059. [Google Scholar] [CrossRef]
- Leng, X.; Zeng, Q.C.; Wu, K.H.; Gentlea, I.R.; Wang, D.W. Reduction-induced surface amorphization enhances the oxygen evolution activity in Co3O4. RSC Adv. 2015, 5, 27823–27828. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Li, K.; Li, L.; Wei, J.; Feng, L.; Fu, Q. Morphology-controlled fabrication of Co3O4 nanostructures and their comparative catalytic activity for oxygen evolution reaction. J. Alloys Compd. 2016, 680, 146–154. [Google Scholar] [CrossRef]
- Xie, J.; Fang, C.; Zou, J.; Lu, H.; Tian, C.; Han, C.; Zhao, D. In situ ultrasonic formation of AgBr/Ag2CO3 nanosheets composite with enhanced visible-driven photocatalytic performance. Mater. Lett. 2016, 170, 62–66. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Fang, D.; Xu, J.; Liang, J.; Zhou, L. Microwave-ultrasound assisted synthesis of β-FeOOH and its catalytic property in a photo-Fenton-like process. Ultrason. Sonochem. 2015, 27, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Guo, C.; Xie, C.; Xiong, Z. An Ultrasonication-Assisted Cobalt Hydroxide Composite with Enhanced Electrocatalytic Activity toward Oxygen Evolution Reaction. Materials 2018, 11, 1912. https://doi.org/10.3390/ma11101912
Si Y, Guo C, Xie C, Xiong Z. An Ultrasonication-Assisted Cobalt Hydroxide Composite with Enhanced Electrocatalytic Activity toward Oxygen Evolution Reaction. Materials. 2018; 11(10):1912. https://doi.org/10.3390/ma11101912
Chicago/Turabian StyleSi, Yujun, Chaozhong Guo, Chenglong Xie, and Zhongping Xiong. 2018. "An Ultrasonication-Assisted Cobalt Hydroxide Composite with Enhanced Electrocatalytic Activity toward Oxygen Evolution Reaction" Materials 11, no. 10: 1912. https://doi.org/10.3390/ma11101912
APA StyleSi, Y., Guo, C., Xie, C., & Xiong, Z. (2018). An Ultrasonication-Assisted Cobalt Hydroxide Composite with Enhanced Electrocatalytic Activity toward Oxygen Evolution Reaction. Materials, 11(10), 1912. https://doi.org/10.3390/ma11101912



