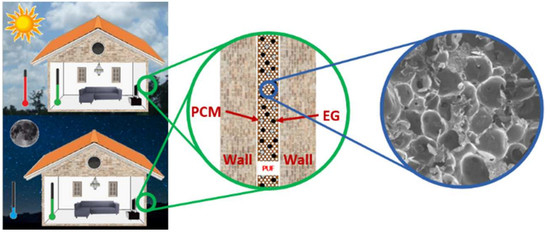
Thermal Energy Storage and Mechanical Performance of Crude Glycerol Polyurethane Composite Foams Containing Phase Change Materials and Expandable Graphite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization of Crude Glycerol
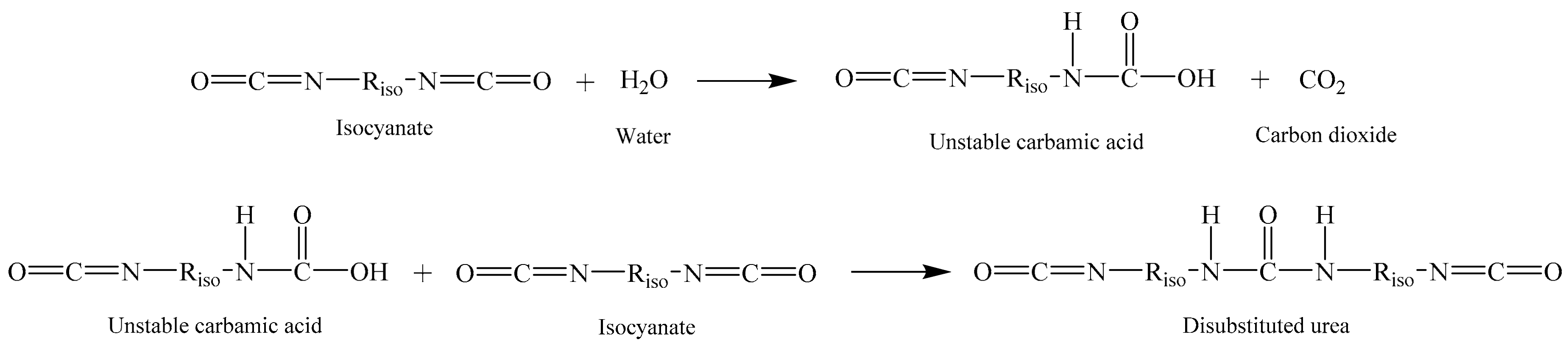
2.3. Production of PUFs
2.4. Characterization of PUFs
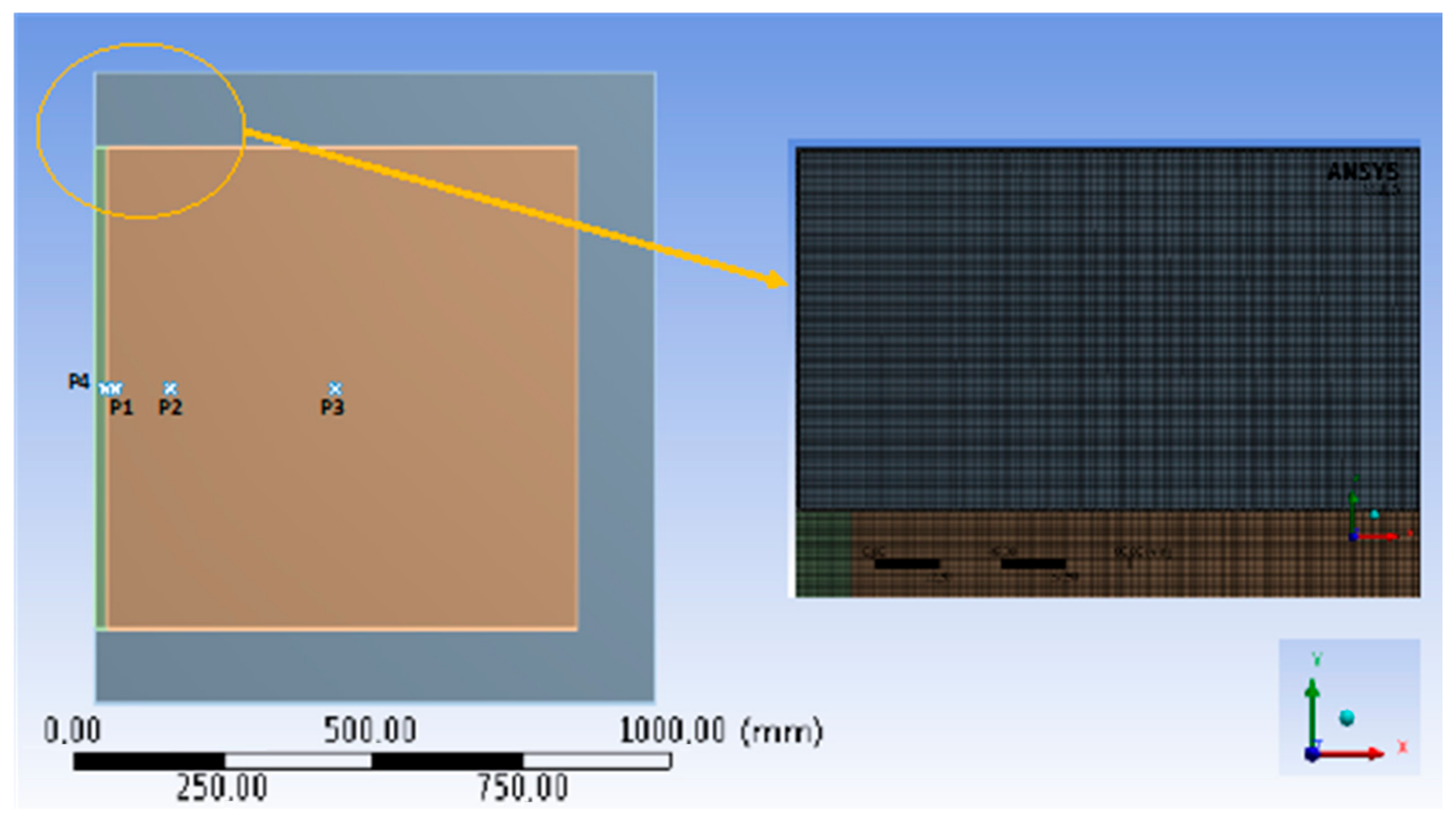
2.5. Numerical Simulation
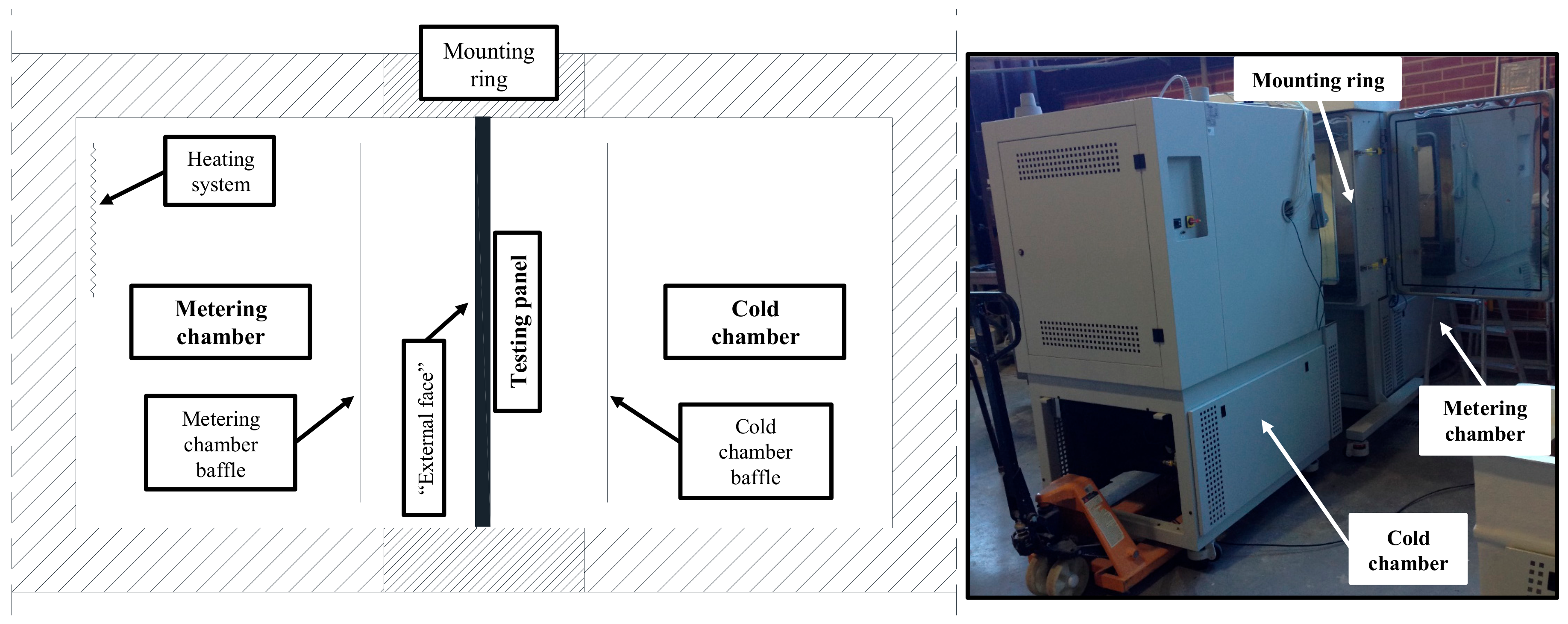
2.6. Numerical Validation of the PUF Panels
2.7. Numerical Testing
3. Results and Discussion
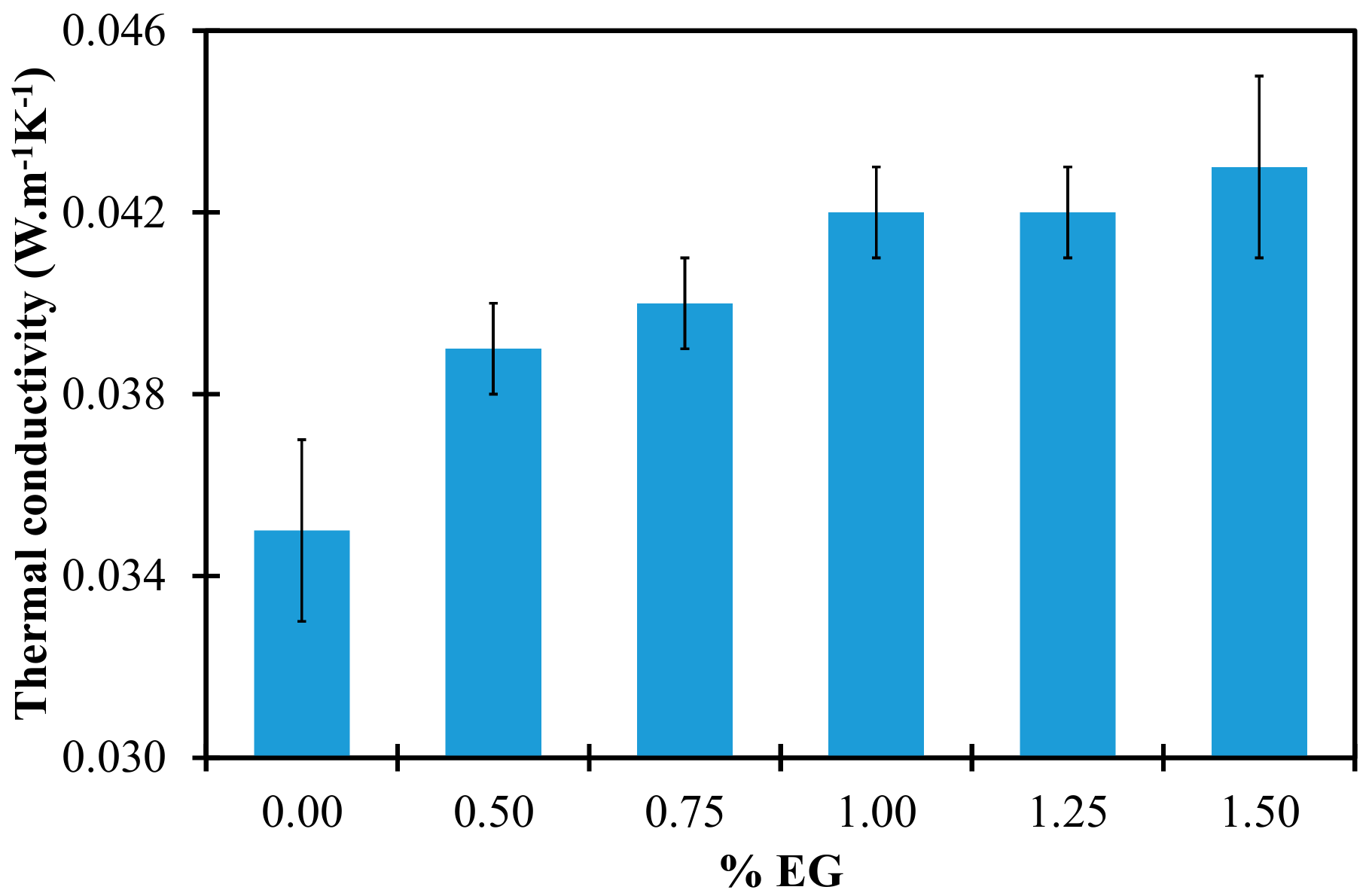
3.1 Thermal Conductivity
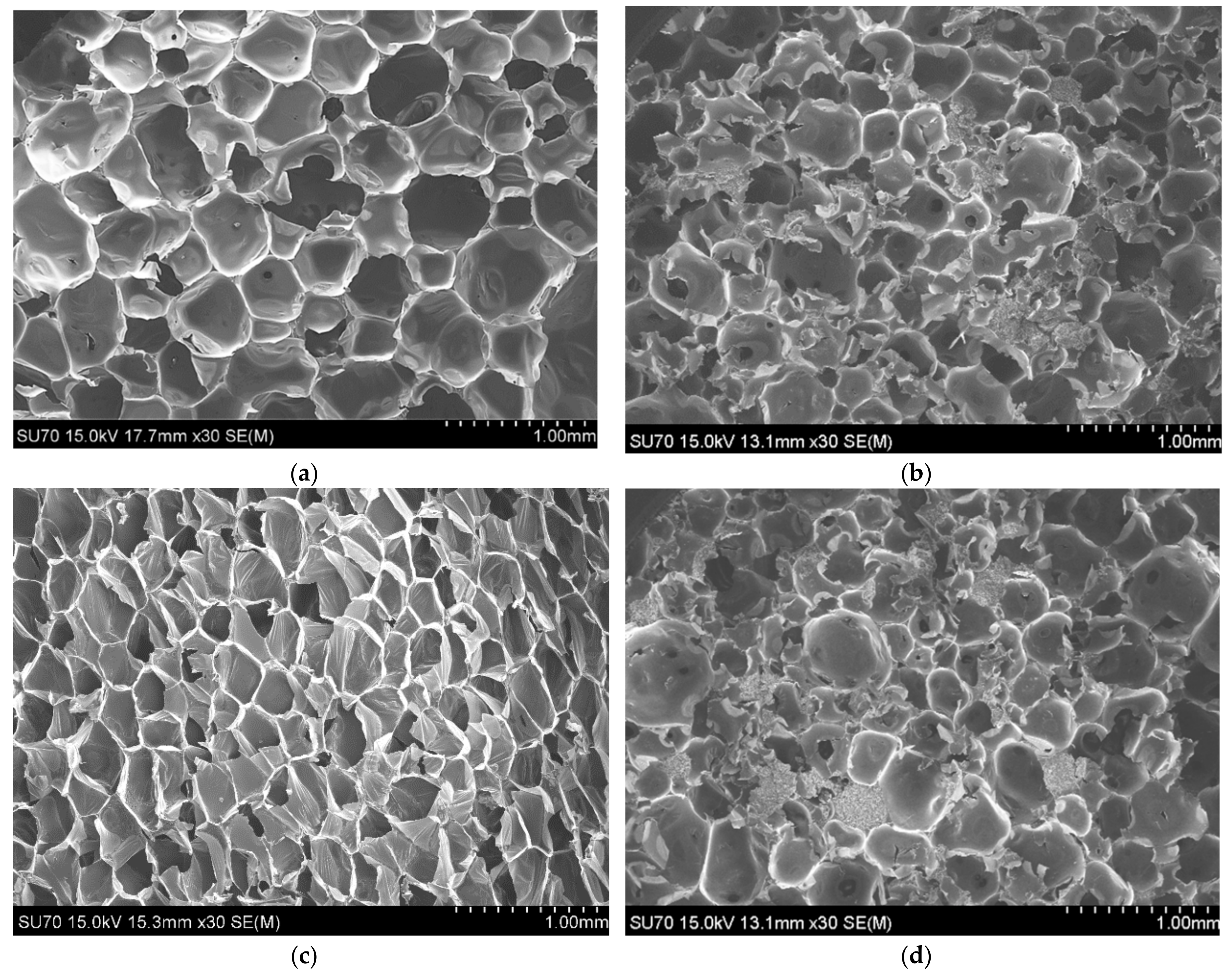
3.2 Morphology
3.3. Density
3.4. Mechanical Properties
3.5. Thermogravimetric Analysis
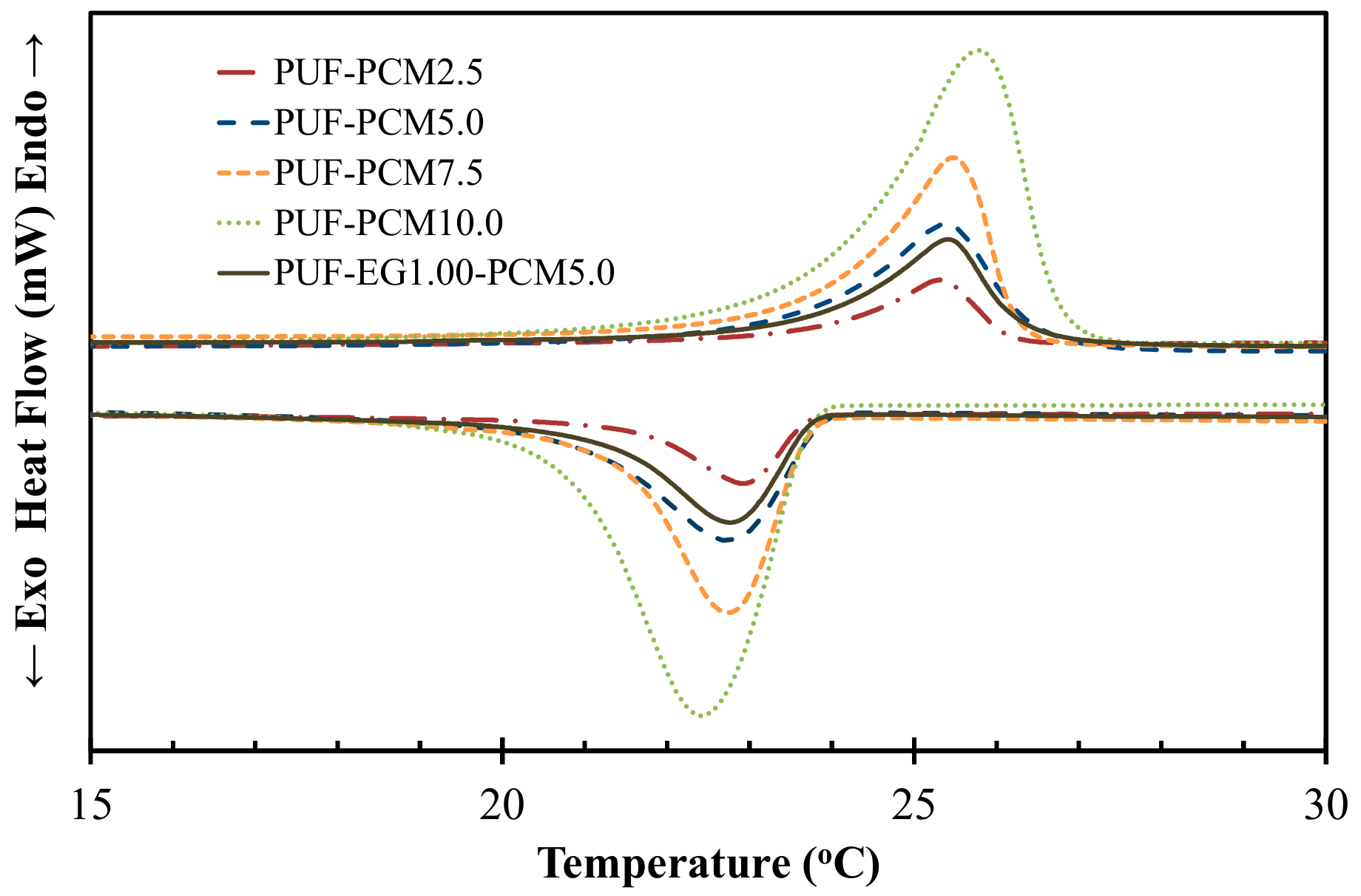
3.6. Differential Scanning Calorimetry Analysis
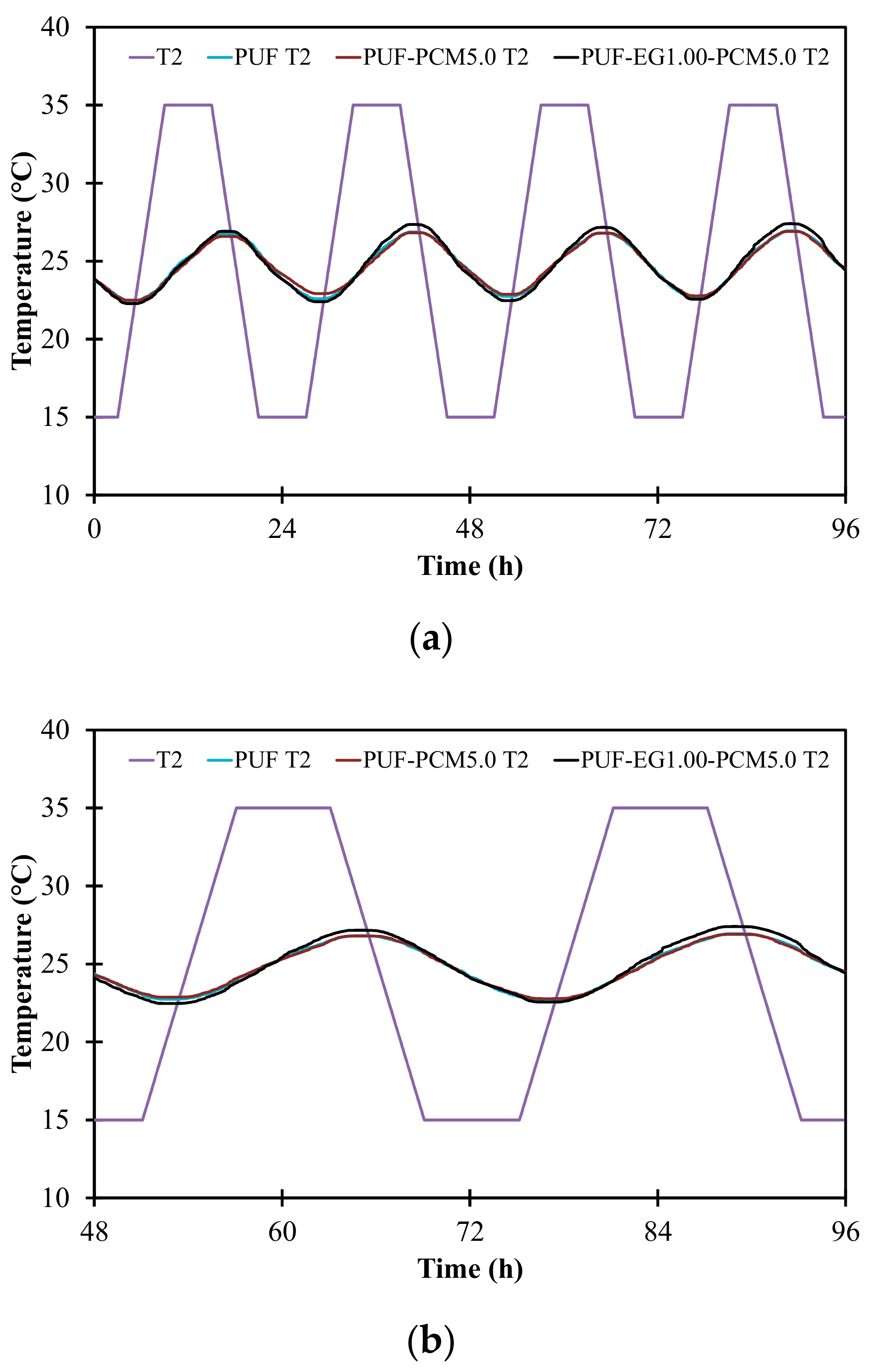
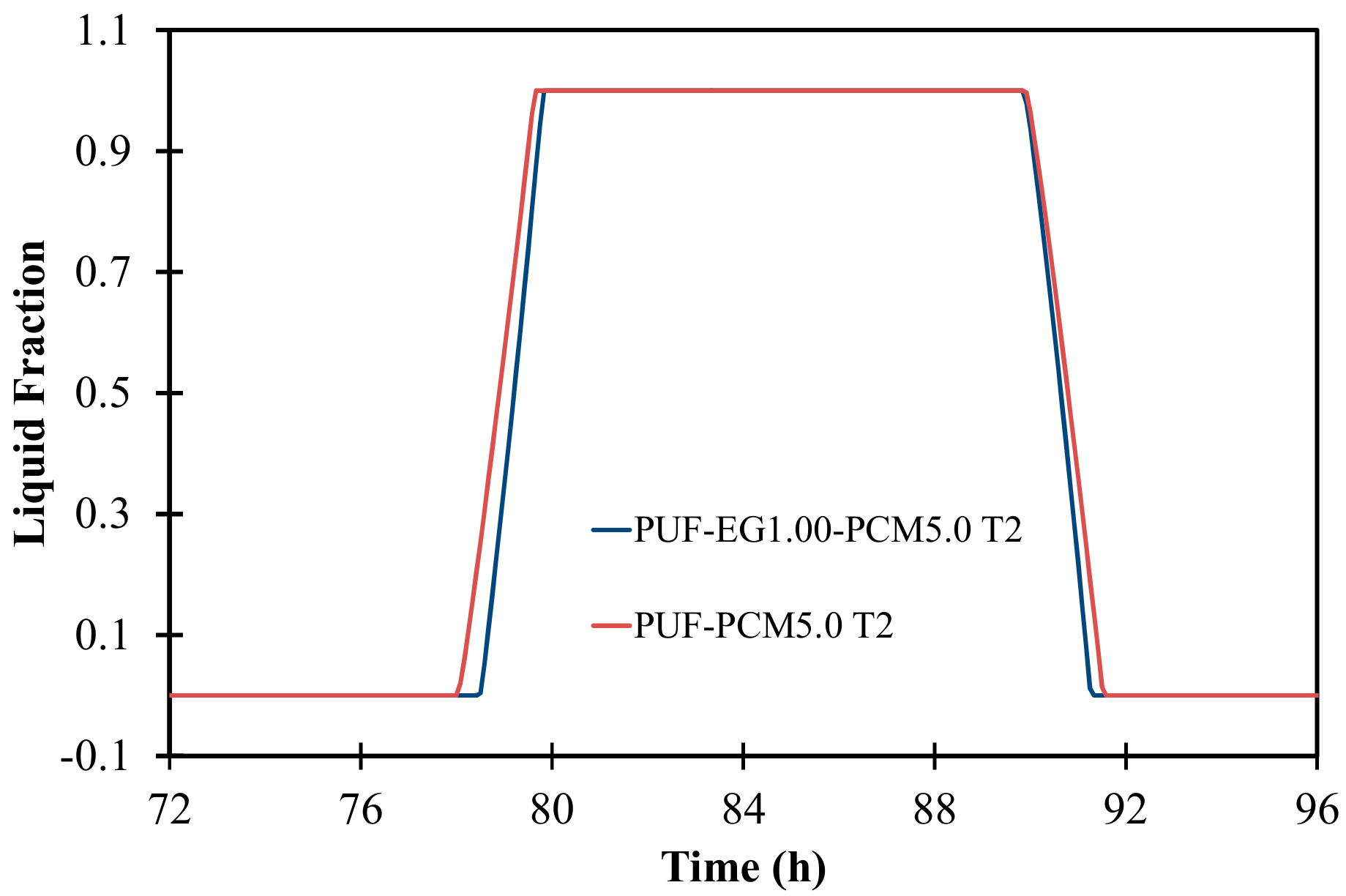
3.7. Numerical Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, L.X.; Ren, L. Synthesis of polyurethane microPCMs containing n-octadecane by interfacial polycondensation: Influence of styrene-maleic anhydride as a surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 268–275. [Google Scholar] [CrossRef]
- Xi, P.; Xia, L.; Fei, P.; Zhang, D.; Cheng, B. Preparation and performance of a novel thermoplastics polyurethane solid–solid phase change materials for energy storage. Sol. Energy Mater. Sol. Cells 2012, 102, 36–43. [Google Scholar] [CrossRef]
- Su, J.C.; Liu, P.S. A novel solid–solid phase change heat storage material with polyurethane block copolymer structure. Energy Convers. Manag. 2006, 47, 3185–3191. [Google Scholar] [CrossRef]
- Alkan, C.; Günther, E.; Hiebler, S.; Ensari, Ö.F.; Kahraman, D. Polyurethanes as solid–solid phase change materials for thermal energy storage. Sol. Energy 2012, 86, 1761–1769. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim. Acta 2007, 454, 90–98. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Thermal insulation capability of PEG-containing polyurethane foams. Thermochim. Acta 2008, 475, 15–21. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Khanlarkhani, M.; Mojtaba Sadrameli, S. Simulation of energy storage system with phase change material (PCM). Energy Build. 2012, 49, 419–422. [Google Scholar] [CrossRef]
- Ke, G.Z.; Xie, H.F.; Ruan, R.P.; Yu, W.D. Preparation and performance of porous phase change polyethylene glycol/polyurethane membrane. Energy Convers. Manag. 2010, 51, 2294–2298. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, P. Hyperbranched polyurethane as novel solid–solid phase change material for thermal energy storage. Eur. Polym. J. 2006, 42, 2931–2939. [Google Scholar] [CrossRef]
- Xi, P.; Duan, Y.; Fei, P.; Xia, L.; Liu, R.; Cheng, B. Synthesis and thermal energy storage properties of the polyurethane solid–solid phase change materials with a novel tetrahydroxy compound. Eur. Polym. J. 2012, 48, 1295–1303. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A poly(ethylene glycol)-based smart phase change material. Sol. Energy Mater. Sol. Cells 2008, 92, 1260–1268. [Google Scholar] [CrossRef]
- Amaral, C.; Vicente, R.; Marques, P.A.A.P.; Barros-Timmons, A. Phase change materials and carbon nanostructures for thermal energy storage: A literature review. Renew. Sustain. Energy Rev. 2017, 79, 1212–1228. [Google Scholar] [CrossRef]
- Aydın, A.A.; Okutan, H. Polyurethane rigid foam composites incorporated with fatty acid ester-based phase change material. Energy Convers. Manag. 2013, 68, 74–81. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- El Hasnaoui, M.; Triki, A.; Graça, M.P.F.; Achour, M.E.; Costa, L.C.; Arous, M. Electrical conductivity studies on carbon black loaded ethylene butylacrylate polymer composites. J. Non-Cryst. Solids 2012, 358, 2810–2815. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Dittrich, B.; Schartel, B.; Roso, M.; Modesti, M. Expandable graphite in polyurethane foams: The effect of expansion volume and intercalants on flame retardancy. J. Appl. Polym. Sci. 2017, 134, 45173. [Google Scholar] [CrossRef]
- Soares, N.; Samagaio, A.; Vicente, R.; Costa, J. Numerical Simulation of a PCM Shutter for Buildings Space Heating During the Winter. World Renew. Energy Congr. 2011, 1797–1804. [Google Scholar]
- Abdel Hakima, A.A.; Nassara, M.; Aisha Emamb, M.S. Preparation and characterization of rigid polyurethane foam prepared from sugar-cane bagasse polyol. Mater. Chem. Phys. 2011, 129, 301–307. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.V.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Spent Coffee Grounds as a Renewable Source for Ecopolyols Production. J. Chem. Technol. Biotechnol. 2014, 64, 250–275. [Google Scholar]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Rigid polyurethane foams derived from cork liquefied at atmospheric pressure. Polym. Int. 2014, 64, 250–257. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Pascoal Neto, C.; Ferreira, A. Ecopolyol Production from Industrial Cork Powder via Acid Liquefaction Using Polyhydric Alcohols. ACS Sustain. Chem. Eng. 2014, 2, 846–854. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Niu, M.; Zhao, G.; Alma, M.H. Polycondensation reaction and its mechanism during lignocellulosic liquefaction by an acid catalyst: A review. For. Stud. China 2011, 13, 71–79. [Google Scholar] [CrossRef]
- Belgacem, M.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 273–288. [Google Scholar]
- Gama, N.V.; Silva, R.; Costa, M.; Barros-Timmons, A.; Ferreira, A. Statistical evaluation of the effect of formulation on the properties of crude glycerol polyurethane foams. Polym. Test. 2016, 56, 200–206. [Google Scholar] [CrossRef]
- Ferreira, A.; Gama, N.V.; Soares, B.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P. Method for Production of Rigid Polyurethane Foams Using Unrefined Crude Glycerol; INPI—Instituto Nacional da Propriedade Industrial: Lisboa, Portugal, 2014.
- Li, C.; Luo, X.; Li, T.; Tong, X.; Li, Y. Polyurethane foams based on crude glycerol-derived biopolyols: One-pot preparation of biopolyols with branched fatty acid ester chains and its effects on foam formation and properties. Polymer 2014, 55, 6529–6538. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.; Silva, R.; Ferreira, A.; Barros-Timmons, A. Effect of unrefined crude glycerol composition on the properties of polyurethane foams. J. Cell. Plast. 2017, 54, 633–649. [Google Scholar] [CrossRef]
- Gama, N.; Silva, R.; Carvalho, A.P.O.; Ferreira, A.; Barros-Timmons, A. Sound absorption properties of polyurethane foams derived from crude glycerol and liquefied coffee grounds polyol. Polym. Test. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Singh, S.N. Blowing Agents for Polyurethane Foams; Rapra Technology: Shawbury, UK, 2002; Volume 12, ISBN 1859573215. [Google Scholar]
- Amaral, C.; Vicente, R.; Ferreira, V.M.; Silva, T. Polyurethane foams with microencapsulated phase change material: Comparative analysis of thermal conductivity characterization approaches. Energy Build. 2017, 153, 392–402. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of expandable graphite on the properties of intumescent flame-retardant polyurethane foam. J. Appl. Polym. Sci. 2008, 110, 2586–2594. [Google Scholar] [CrossRef]
- Kwon, O.; Yang, S.; Kim, D.; Park, J. Characterization of Polyurethane Foam Prepared by Using Starch as Polyol. J. Appl. Polym. Sci. 2006, 103, 1544–1553. [Google Scholar] [CrossRef]
- Valentini, M.; Piana, F.; Pionteck, J.; Lamastra, F.R.; Nanni, F. Electromagnetic properties and performance of exfoliated graphite (EG)—Thermoplastic polyurethane (TPU) nanocomposites at microwaves. Compos. Sci. Technol. 2015, 114, 26–33. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A. Improvement on fire behaviour of water blown PIR–PUR foams: Use of an halogen-free flame retardant. Eur. Polym. J. 2003, 39, 263–268. [Google Scholar] [CrossRef]
- Doroudiani, S.; Kortschot, M.T. Polystyrene foams. II. Structure-impact properties relationships. J. Appl. Polym. Sci. 2003, 90, 1421–1426. [Google Scholar] [CrossRef]
- Lim, H. Effects of silicon surfactant in rigid polyurethane foams. eXPRESS Polym. Lett. 2008, 2, 194–200. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Foaming and Blowing Agents; ChemTec Publishing: Toronto, ON, Canada, 2017. [Google Scholar]
- Li, Y.; Zou, J.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Luo, W. Effect of expandable graphite particle size on the flame retardant, mechanical, and thermal properties of water-blown semi-rigid polyurethane foam. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gama, N.; Costa, L.C.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Insights into the physical properties of biobased polyurethane/expanded graphite composite foams. Compos. Sci. Technol. 2017, 138, 24–31. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]


| Sample a | CG | Isocyanate (PHP—wt/wt) | Catalyst (PHP—wt/wt) | Surfactant (PHP—wt/wt) | Blowing Agent (PHP—wt/wt) | EG (%—wt/wt) | PCM (%—wt/wt) |
|---|---|---|---|---|---|---|---|
| PUF | 100 | 209 | 3 | 4 | 4.4 | 0.0 | 0.0 |
| PUF-EG0.50 | 100 | 209 | 3 | 4 | 4.4 | 1.4 | 0.0 |
| PUF-EG0.75 | 100 | 209 | 3 | 4 | 4.4 | 2.0 | 0.0 |
| PUF-EG1.00 | 100 | 209 | 3 | 4 | 4.4 | 2.7 | 0.0 |
| PUF-EG1.25 | 100 | 209 | 3 | 4 | 4.4 | 3.4 | 0.0 |
| PUF-EG1.50 | 100 | 209 | 3 | 4 | 4.4 | 4.1 | 0.0 |
| PUF-PCM2.5 | 100 | 209 | 3 | 4 | 4.4 | 0.0 | 6.9 |
| PUF-PCM5.0 | 100 | 209 | 3 | 4 | 4.4 | 0.0 | 14.2 |
| PUF-PCM7.5 | 100 | 209 | 3 | 4 | 4.4 | 0.0 | 21.9 |
| PUF-PCM10.0 | 100 | 209 | 3 | 4 | 4.4 | 0.0 | 29.9 |
| PUF-EG1.00-PCM5.0 | 100 | 209 | 3 | 4 | 4.4 | 2.9 | 14.3 |
| Sample | T1 (°C) | T2 (°C) | T3 (°C) | Enthalpy (J·g−1) | % PCM |
|---|---|---|---|---|---|
| PCM | 23.87 | 26.16 | 27.51 | 103.3891 | 100 |
| PUF-PCM2.5 | 23.95 | 25.31 | 26.16 | 2.5989 ± 0.1415 | 2.5 ± 0.1 |
| PUF-PCM5.0 | 23.57 | 25.34 | 26.48 | 5.2420 ± 0.1266 | 5.1 ± 0.1 |
| PUF-PCM7.5 | 23.77 | 25.41 | 26.13 | 7.6177 ± 0.2090 | 7.4 ± 0.2 |
| PUF-PCM10.0 | 24.11 | 25.78 | 26.74 | 10.3811 ± 0.3448 | 10.0 ± 0.3 |
| PUF-EG1.00-PCM5.0 | 24.29 | 25.91 | 26.80 | 5.1499 ± 0.4821 | 5.0 ± 0.5 |
| Sample | Density (kg·m−3) | Thermal Conductivity (W·m−1·K−1) | Young Modulus (kPa) | Toughness (J·m−3) | Compressive Stress σ10% (kPa) |
|---|---|---|---|---|---|
| PUF | 43.8 ± 1.4 | 0.035 ± 0.002 | 643 ± 27 | 15560 ± 1371 | 55 ± 2 |
| PUF-EG0.50 | 74.0 ± 3.7 | 0.039 ± 0.001 | 1334 ± 43 | 29313 ± 1344 | 95 ± 4 |
| PUF-EG0.75 | 84.2 ± 2.6 | 0.040 ± 0.001 | 1541 ± 59 | 34500 ± 2027 | 114 ± 7 |
| PUF-EG1.00 | 92.9 ± 3.9 | 0.042 ± 0.001 | 1739 ± 74 | 40640 ± 1754 | 144 ± 8 |
| PUF-EG1.25 | 84.8 ± 4.6 | 0.042 ± 0.001 | 1812 ± 66 | 42775 ± 2814 | 152 ± 8 |
| PUF-EG1.50 | 99.3 ± 5.0 | 0.043 ± 0.002 | 1875 ± 89 | 45075 ± 4738 | 162 ± 8 |
| PUF-PCM2.5 | 81.8 ± 2.9 | 0.036 ± 0.001 | 1729 ± 57 | 40280 ± 2132 | 144 ± 8 |
| PUF-PCM5.0 | 91.9 ± 4.0 | 0.037 ± 0.002 | 2079 ± 91 | 47467 ± 2605 | 167 ± 11 |
| PUF-PCM7.5 | 103.0 ± 6.0 | 0.038 ± 0.003 | 2312 ± 85 | 51960 ± 7412 | 175 ± 12 |
| PUF-PCM10.0 | 118.2 ± 7.9 | 0.039 ± 0.001 | 2771 ± 117 | 59983 ± 3068 | 193 ± 12 |
| PUF-EG1.00-PCM5.0 | 115.4 ± 5.8 | 0.044 ± 0.002 | 2765 ± 113 | 58856 ± 2163 | 193 ± 11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, N.V.; Amaral, C.; Silva, T.; Vicente, R.; Coutinho, J.A.P.; Barros-Timmons, A.; Ferreira, A. Thermal Energy Storage and Mechanical Performance of Crude Glycerol Polyurethane Composite Foams Containing Phase Change Materials and Expandable Graphite. Materials 2018, 11, 1896. https://doi.org/10.3390/ma11101896
Gama NV, Amaral C, Silva T, Vicente R, Coutinho JAP, Barros-Timmons A, Ferreira A. Thermal Energy Storage and Mechanical Performance of Crude Glycerol Polyurethane Composite Foams Containing Phase Change Materials and Expandable Graphite. Materials. 2018; 11(10):1896. https://doi.org/10.3390/ma11101896
Chicago/Turabian StyleGama, Nuno Vasco, Cláudia Amaral, Tiago Silva, Romeu Vicente, João Araújo Pereira Coutinho, Ana Barros-Timmons, and Artur Ferreira. 2018. "Thermal Energy Storage and Mechanical Performance of Crude Glycerol Polyurethane Composite Foams Containing Phase Change Materials and Expandable Graphite" Materials 11, no. 10: 1896. https://doi.org/10.3390/ma11101896
APA StyleGama, N. V., Amaral, C., Silva, T., Vicente, R., Coutinho, J. A. P., Barros-Timmons, A., & Ferreira, A. (2018). Thermal Energy Storage and Mechanical Performance of Crude Glycerol Polyurethane Composite Foams Containing Phase Change Materials and Expandable Graphite. Materials, 11(10), 1896. https://doi.org/10.3390/ma11101896