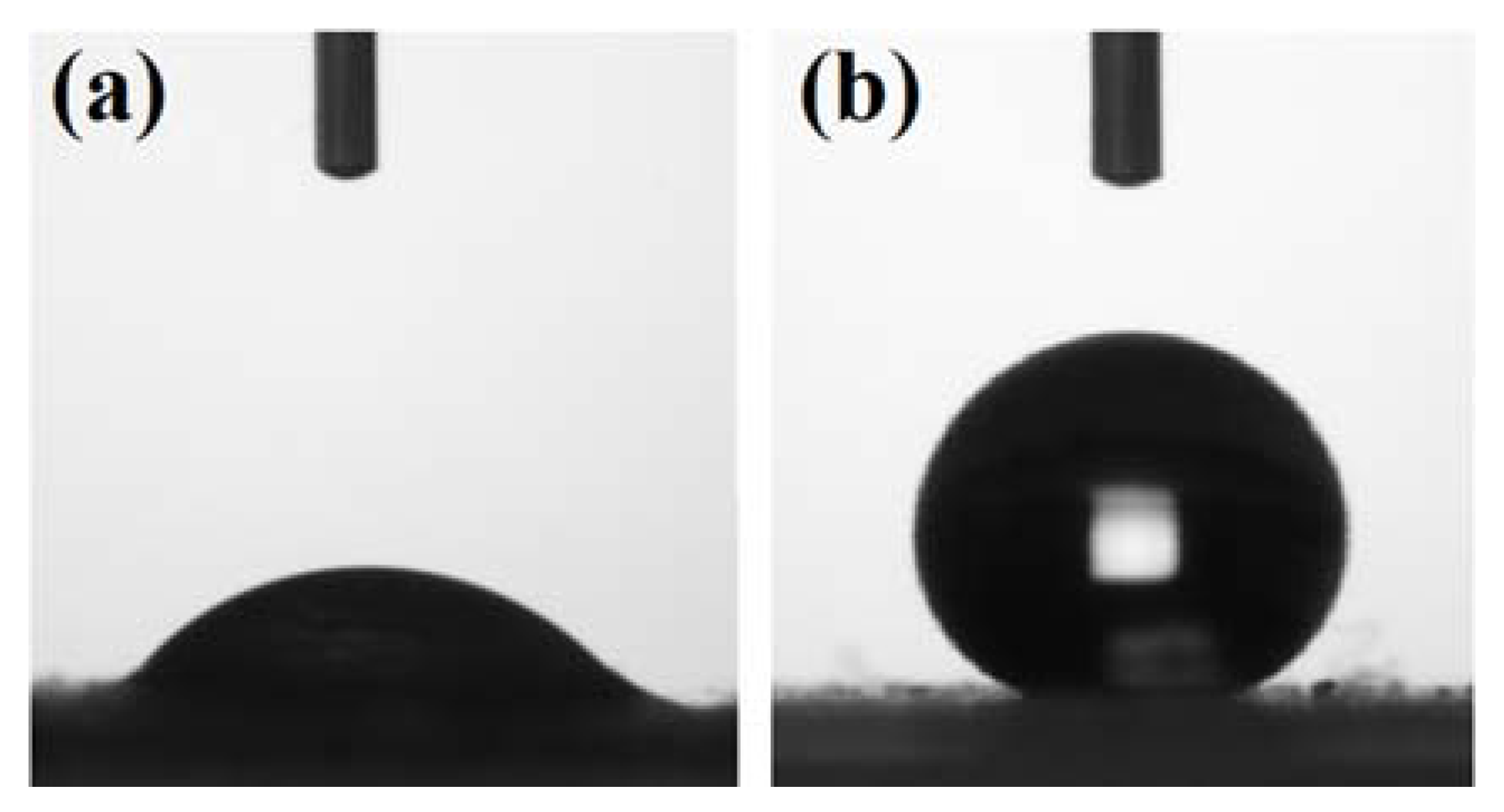
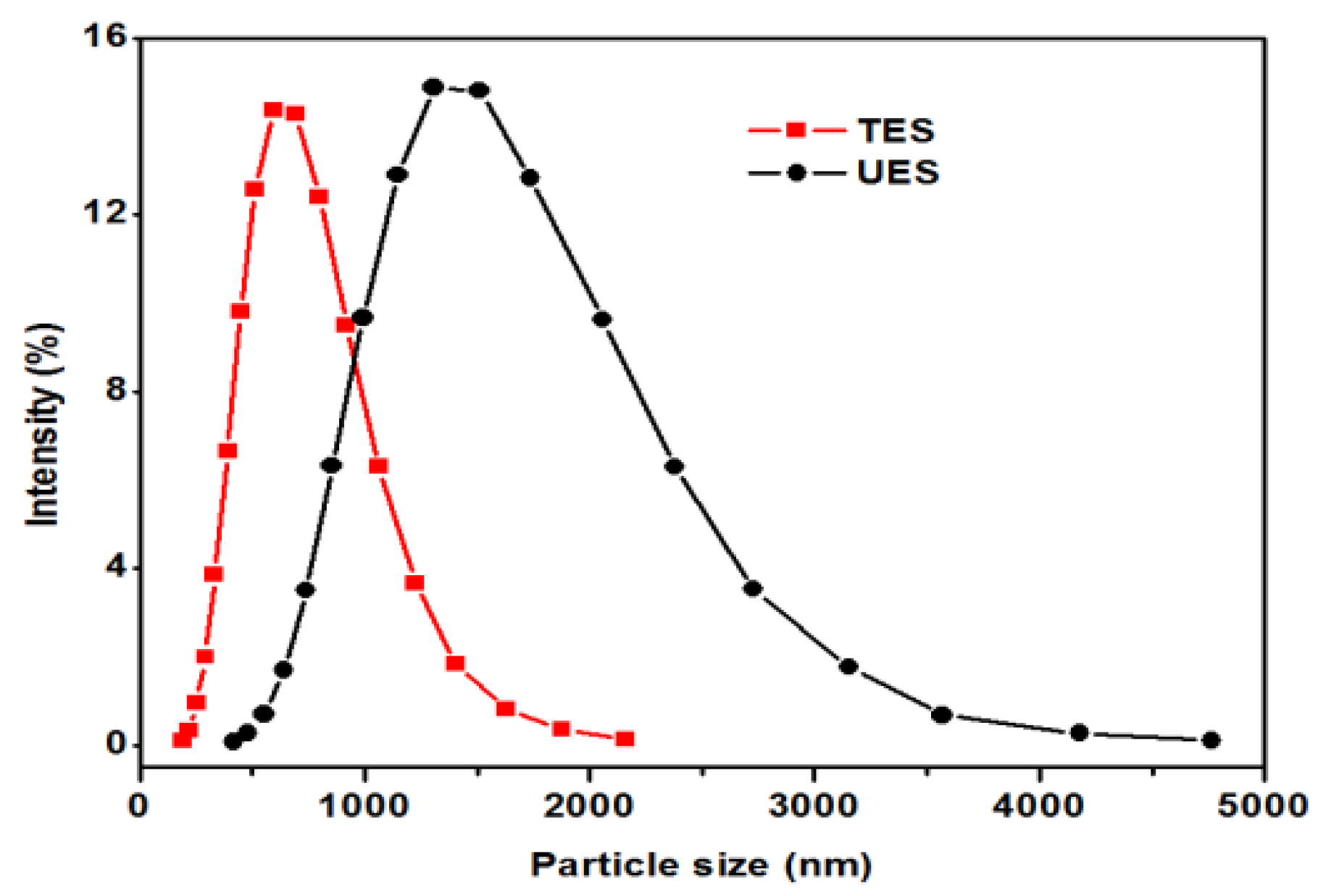
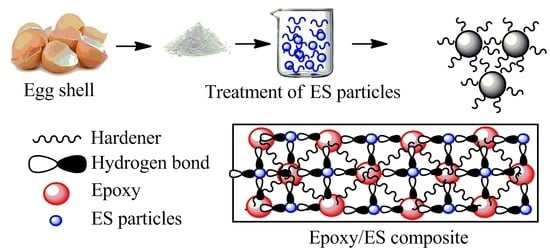
The epoxy/UES and epoxy/TES composites were prepared on 15 and 20 wt % loading of particles. The effects of particles loading on the epoxy matrix were studied by evaluating the chemical structure, thermomechanical, mechanical, and thermal properties.
3.2.1. FTIR
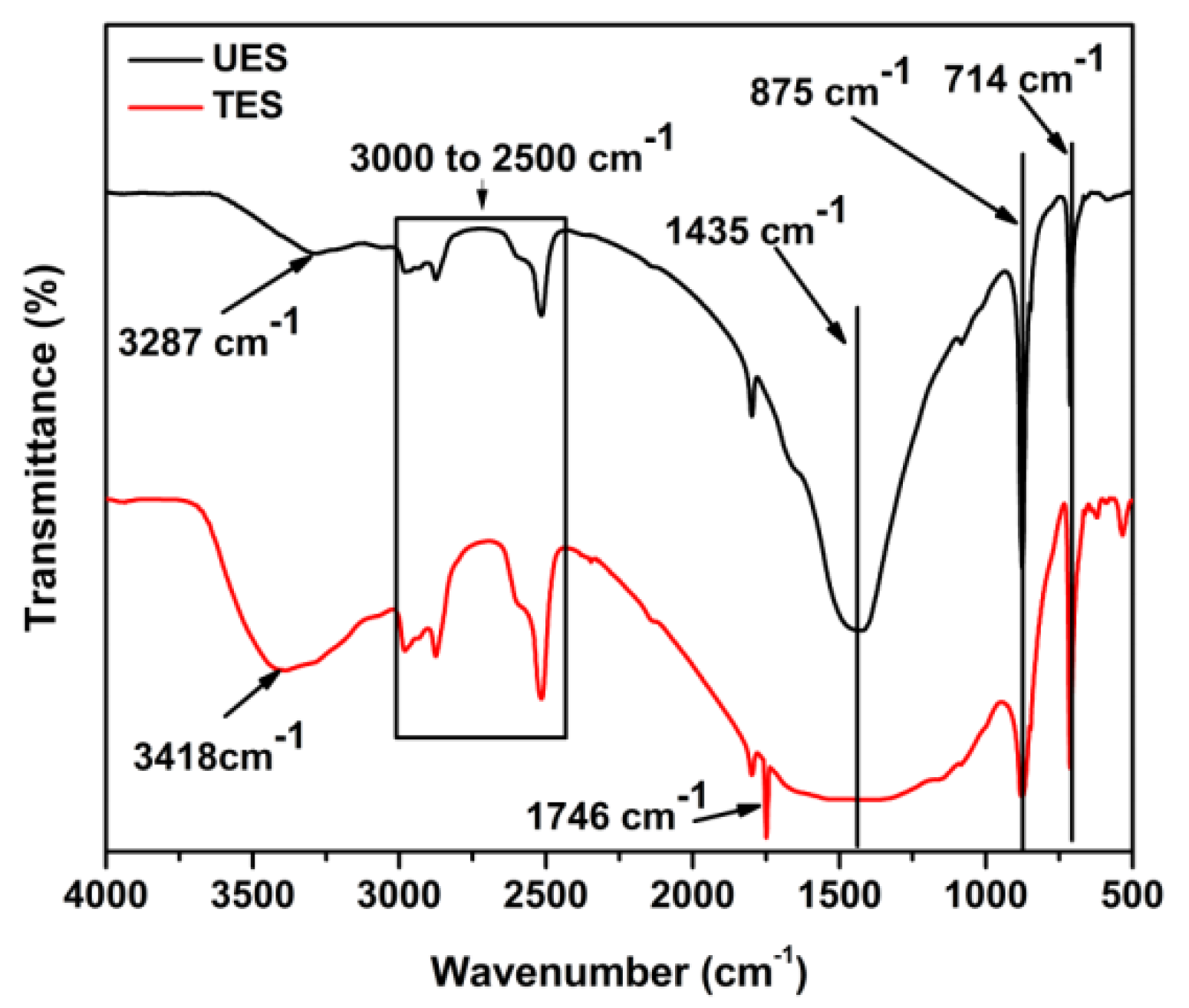
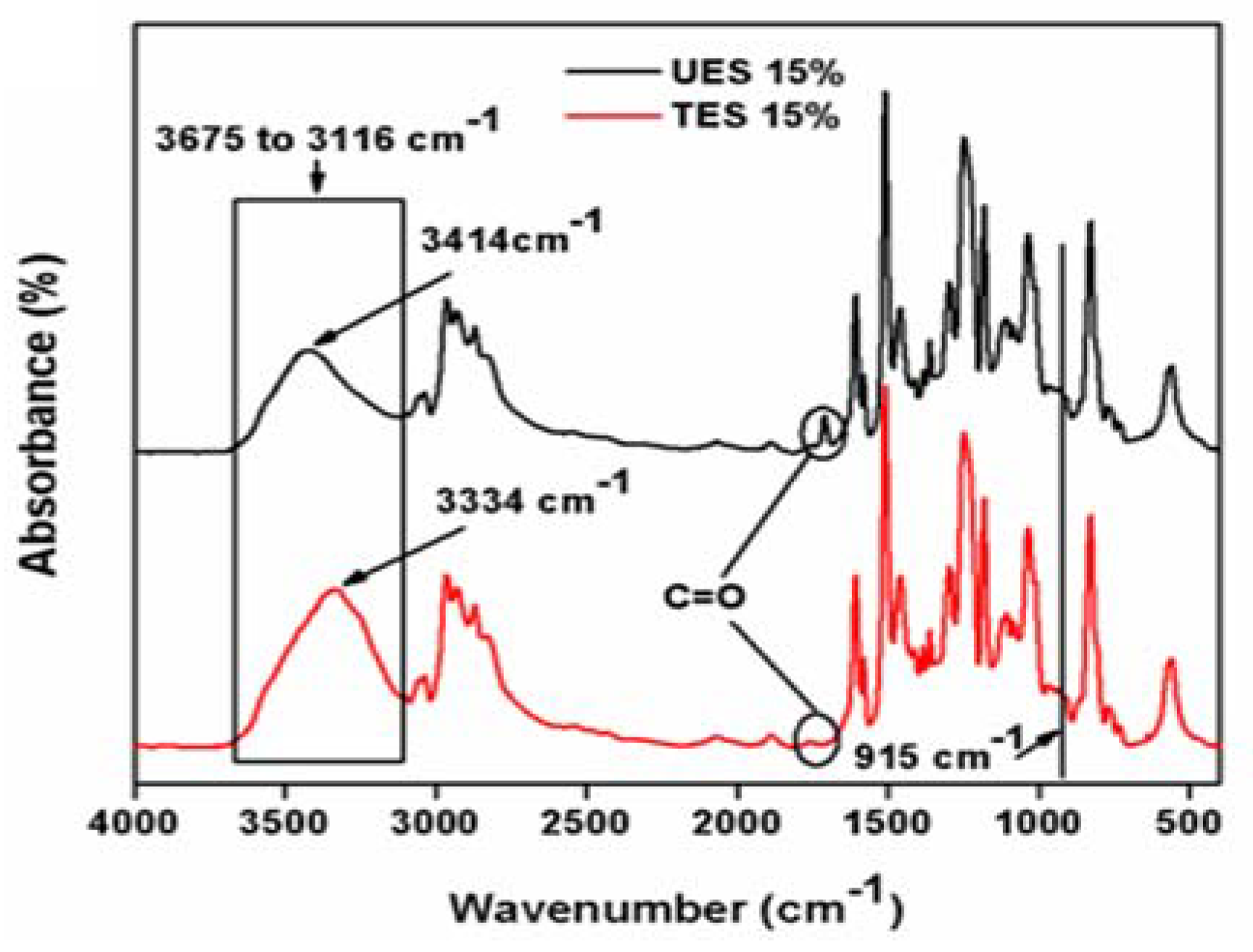
The chemical structure of UES and TES filled epoxy composites were examined by FTIR and plotted as
Figure 7.
It is important to highlight that there are two main parameters to describe the changes in the chemical structure for UES and TES particles reinforced composites; i.e., shifting in wavelength and intensity variation. The wavelength shift represents either hydrogenation or dehydrogenation if shifted to lower or higher wavelength, respectively [
38]. The intensity variation represents the bonds formation or elimination [
39]. The spectra of both composites represent characteristics peaks of epoxy. The oxirane group peak located at 915 cm
−1 corresponds to C–O stretching. The absence of this peak represents that composites were completely cured at given curing conditions. The –OH stretching vibrations are located in the region at 3675 to 3116 cm
−1. As the reaction progresses, this region between 3675 to 3116 cm
−1 develops in the structure. The carbonyls stretch (C=O) due to ES particles can be seen at 1710 cm
−1 and (–OH) peak at 3414 cm
−1 in UES composites. In TES composites (–OH), the stretching peak is shifted to lower wave number 3334 cm
−1 while no peak is detected for carbonyl (C=O) stretch. The absence of carbonyl peak reflects the dissociation of formed surface carbonyls with epoxy and is also reported [
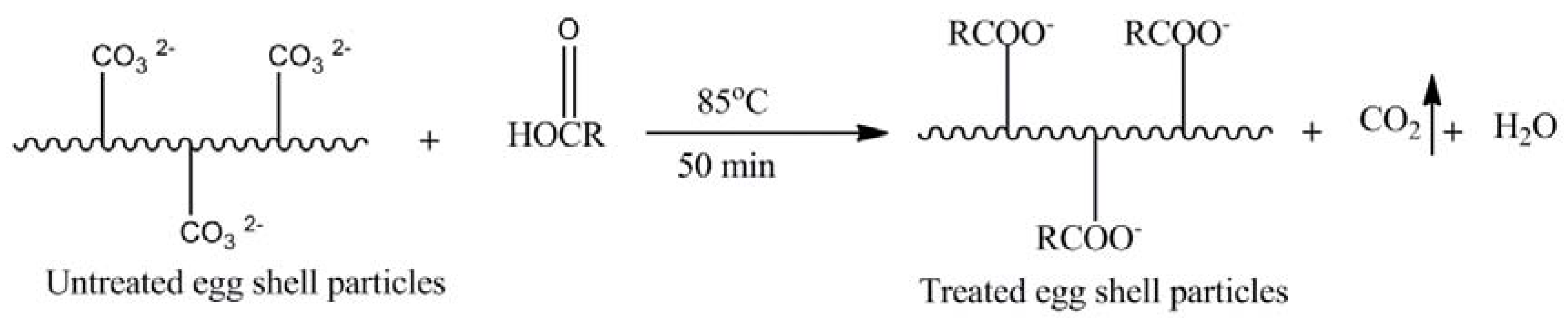
40]. The area of hydroxyl (–OH) region intensity is also broadened for TES composites. This observation clearly reflects that TES particles are attached with increased hydrogen bonding with epoxy due to greater functionality of TES particles. The proposed chemical structure is shown in
Scheme 2.
3.2.2. Particles–Matrix Interaction
The interaction of UES and TES particles with epoxy matrix was examined by studying the variation in storage modulus (
E′), loss modulus (
E″), and damping factor (
tan δ) with respect to temperature on a dynamic mechanical analysis (DMA). The plots for particles filled epoxy composites are shown in
Figure 8 and the values are tabulated in
Table 5.
The study of
E′,
Figure 8A, shows that the
E′ values steady fall along with temperature increase. Two distinct regions, glassy and rubbery, were observed in all composites. In the glassy region, the high values of
E′ are due to highly immobile, close and tightly packed matrix components. The UES and TES particles filled composite has increased values of
E′ values in comparison to neat epoxy due to stiffer nature of particles. In the rubbery region, the 15 wt % UES particles filled composites showed a little higher modulus value due to the interaction of protein contained by the UES particles with epoxy, and they formed a chemically cross-link with epoxy [
41]. The TES particles filled epoxy composites showed higher stiffness (
E′ at 50 °C) values, the stiffness for 15 and 20 wt % composite were 23 and 25% higher than the neat matrix value, respectively. In addition to this, a distinct increase in the rubbery region was also observed. This can be dedicated to the increased porosity, sufficient removal of bio-organic impurities, and reduced TES particles size after SA treatment, along with the chemical cross-linking of alkyl chains with epoxy. This behavior also confirms the improved cross-link points of TES particles in epoxy. The cross-link density was calculated by following equation [
42] and values are already summarized in
Table 5.
where
E′ is the storage modulus in rubbery plateau (i.e.,
Tg + 30 °C),
R is the universal gas constant (8.314472 J/K mol), and
T (K) is the temperature in a rubbery plateau.
The TES particles filled composite exhibit higher number of cross-link density confirming the improved stiffness of the composite. We can observe that, at 15 wt % UES loading the cross-link density of composite was improved than the neat epoxy, but on 20 wt % UES loading the cross-link density value decreases to a greater extent, this shows agglomeration of particles in the composites. On the other hand, the 20 wt % TES filled composites showed an increase cross-link density value which is in consistent with E′ results.
The
tan δ plot of pure epoxy, UES and TES particles filled epoxy composites with respect to temperature are shown in
Figure 8B. The neat epoxy showed increased
tan δ height value as compared to UES and TES particles filled composites; this represents the weak interfaces and frictional damping behavior. The deformation energy is dissolute mostly at the interface of composite materials. If the particle amount, geometry and matrix are identical, the
tan δ value can be used to study the interfacial properties of composites. The higher
tan δ height means the composite has a tendency to dissipate more energy—i.e., poor interfacial adhesion results in higher damping values. Moreover, the broadening of
tan δ is related to molecular relaxations at the interfacial region. The introduction of ES particles reduces the height and increase the broadening of
tan δ values, the composite showed increase of molecular interaction and relaxations at the interfacial regions. The smallest and broadening damping curves were observed for TES particles filled composites as compared to the UES particles filled composites [
43]. This confirmed that TES particles filled composites have higher load bearing capacity than the UES particles filled composites. Moreover, a positive shift in loss modulus values to higher temperature of TES filled composites composite also represents more energy retention in contrast to UES filled composites and pure epoxy as shown in
Figure 8C, which is in consistent with the
tan δ results.
The effectiveness coefficient (
C) was also calculated to cross check the adhesion effectiveness of particles and matrix in the composites. It is the ratio of composite storage modulus (
E′) in glassy and rubbery region in relation to the neat resin, and calculated by using the equation [
44]
Here E′ represents storage modulus, while subscripts g and r represent glassy and rubbery regions, respectively.
The
C for UES and TES particles filled composites was calculated at three different temperatures—i.e., 100, 120, and 140 °C—and values are shown in
Table 5. The glassy region of composites and neat matrix was taken at 40 °C. The lower value of
C represents more effectiveness for particle–matrix adhesion [
45]. From the calculated values for
C, we can conclude that the TES particles filled composite show lower values at all temperatures, which also cross verified the improved particle–matrix adhesion for TES particles than the UES particles.
3.2.3. Tensile Properties
The tensile stress–strain behavior of epoxy filled particle composites is shown in
Figure 9 and the corresponding values are tabulated in
Table 6.
The UES particles filled epoxy composites showed semi ductile behavior, while TES particles filled epoxy composites showed increased ductility. It has been observed that on the macro size particles reinforcement ultimate strength of the composites was lower than the neat matrix [
46]. On the other hand, the effective surface modification can change the behavior depending upon the bonding and size alteration. The tensile strength values at 15 and 20 wt % were recorded as 68.31 ± 1.95 and 66.44 ± 1.84 MPa, respectively, while the elongation was recorded as 5.87 ± 0.09 and 6.67 ± 0.11% for 15 and 20 wt % UES particles loading, respectively, the values for neat epoxy were 74.91 ± 2.21 MPa and 8.84 ± 0.15%. The highest elongation value (11.31 ± 0.18%) was recorded for 15 wt % TES filled epoxy composite, on 20 wt % TES loading the value reduced and recorded as 10.76 ± 0.12%. This enhancement in the elongation values can be explained by the reduced amount of protein and increased carbonyl groups due to SA treatment, improved dispersion of TES particle, reduced crystal size and increased hydrogen bonding as earlier discussed in FTIR. This employs the reduced brittleness (
B) of the epoxy composites, the
B was calculated by using the equation [
47].
Here
is elongation at break and
E′ is storage modulus by DMA. The calculated
B values, summarized in
Table 6, shows that the loading of TES particles filled epoxy composites have very lower brittleness as compared to UES particles filled epoxy composites and pure epoxy. These results are consistent with the toughness value, which confirms that TES filled epoxy composites have increased load bearing capacity with improved bending.