Activation of a Raw Clay by Mechanochemical Process—Effects of Various Parameters on the Process Efficiency and Cementitious Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
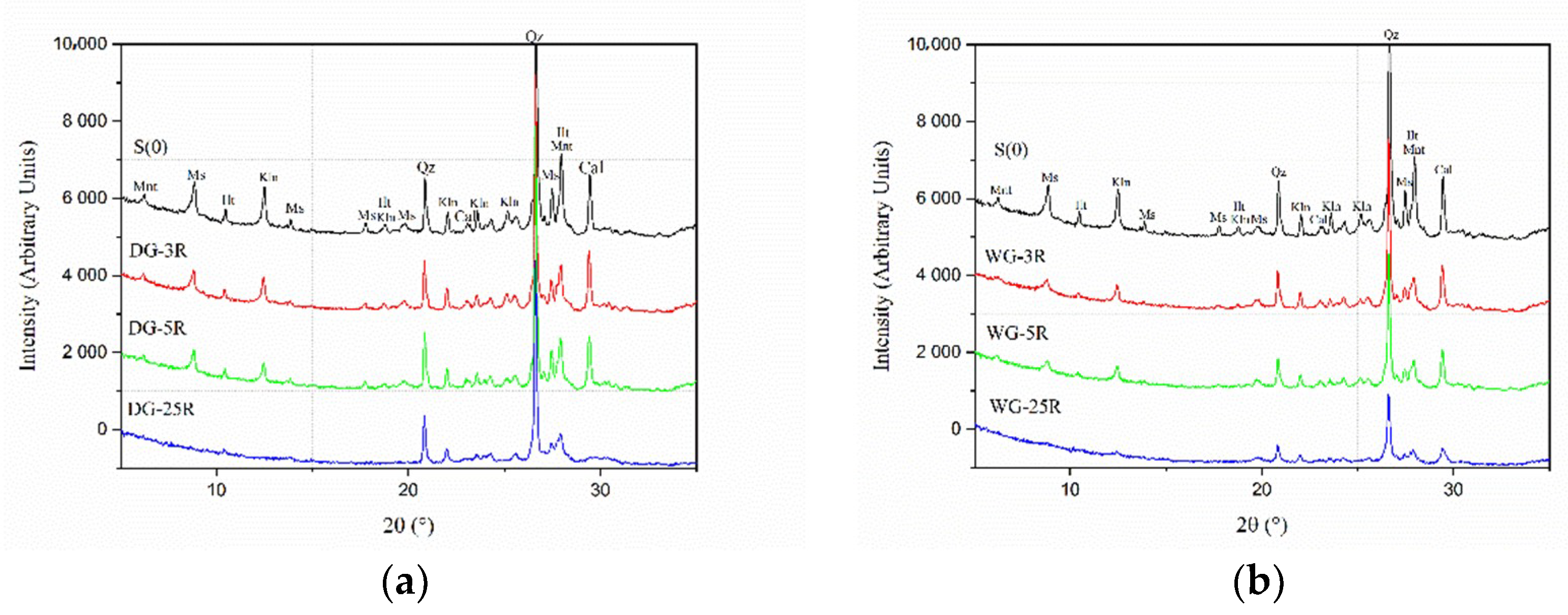
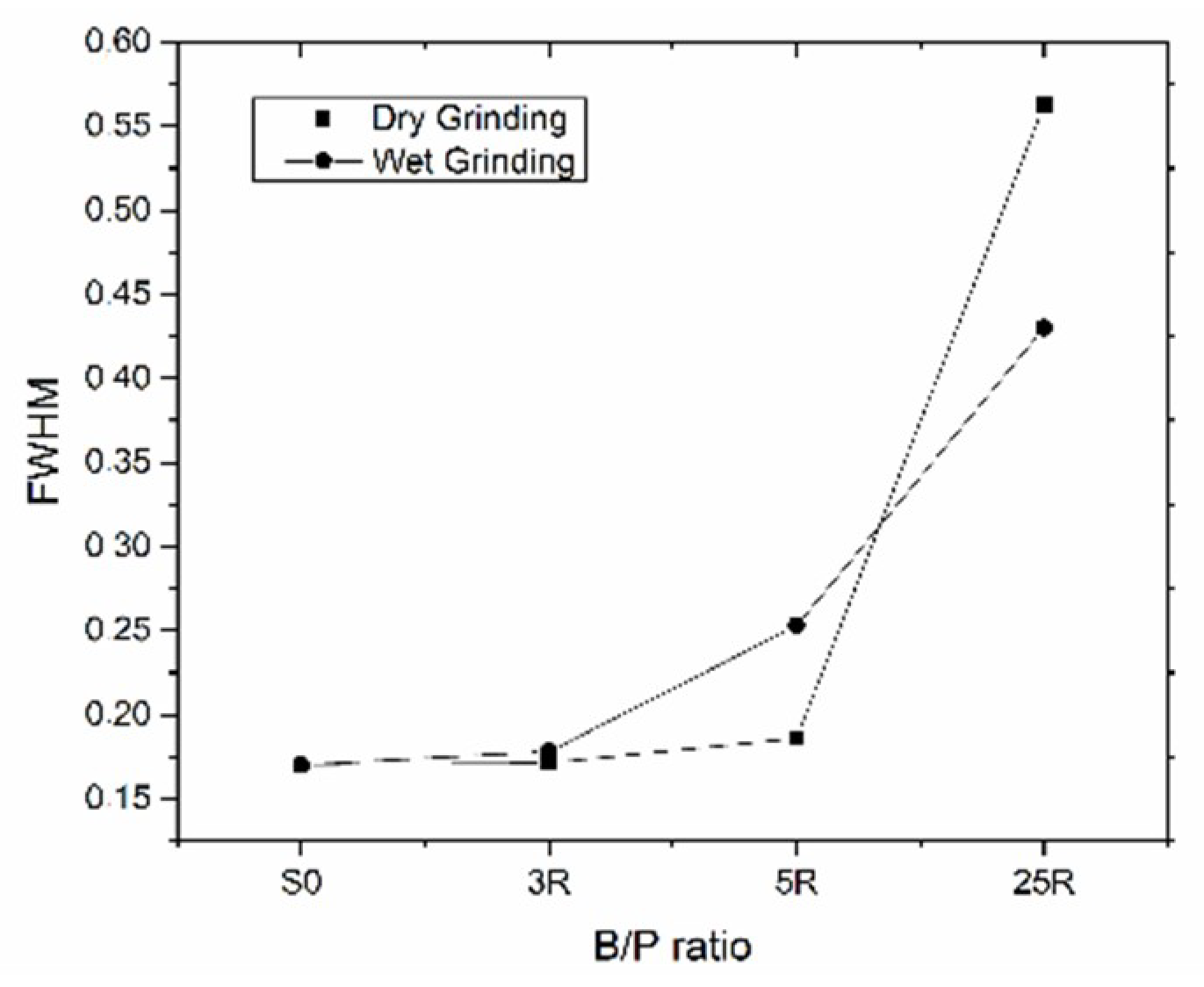
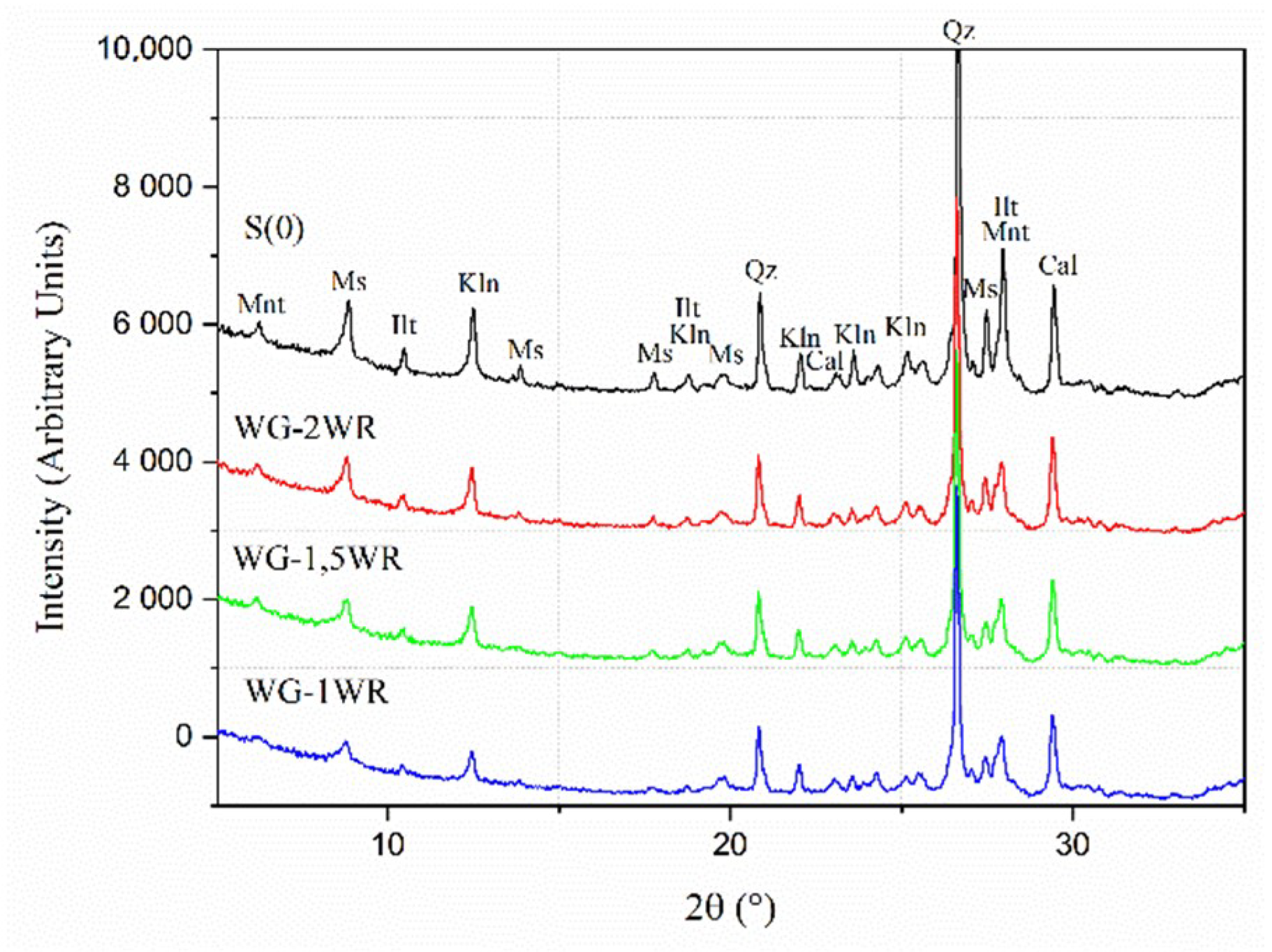
3.1. Grinding Media
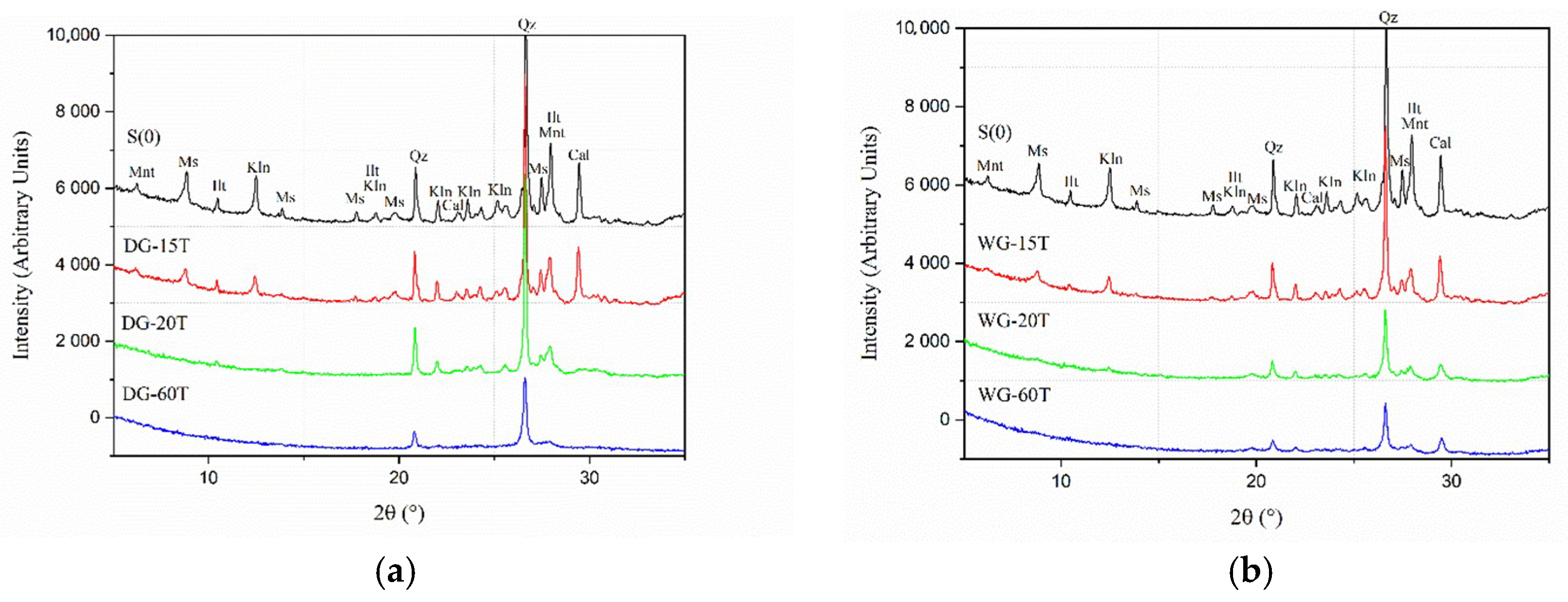
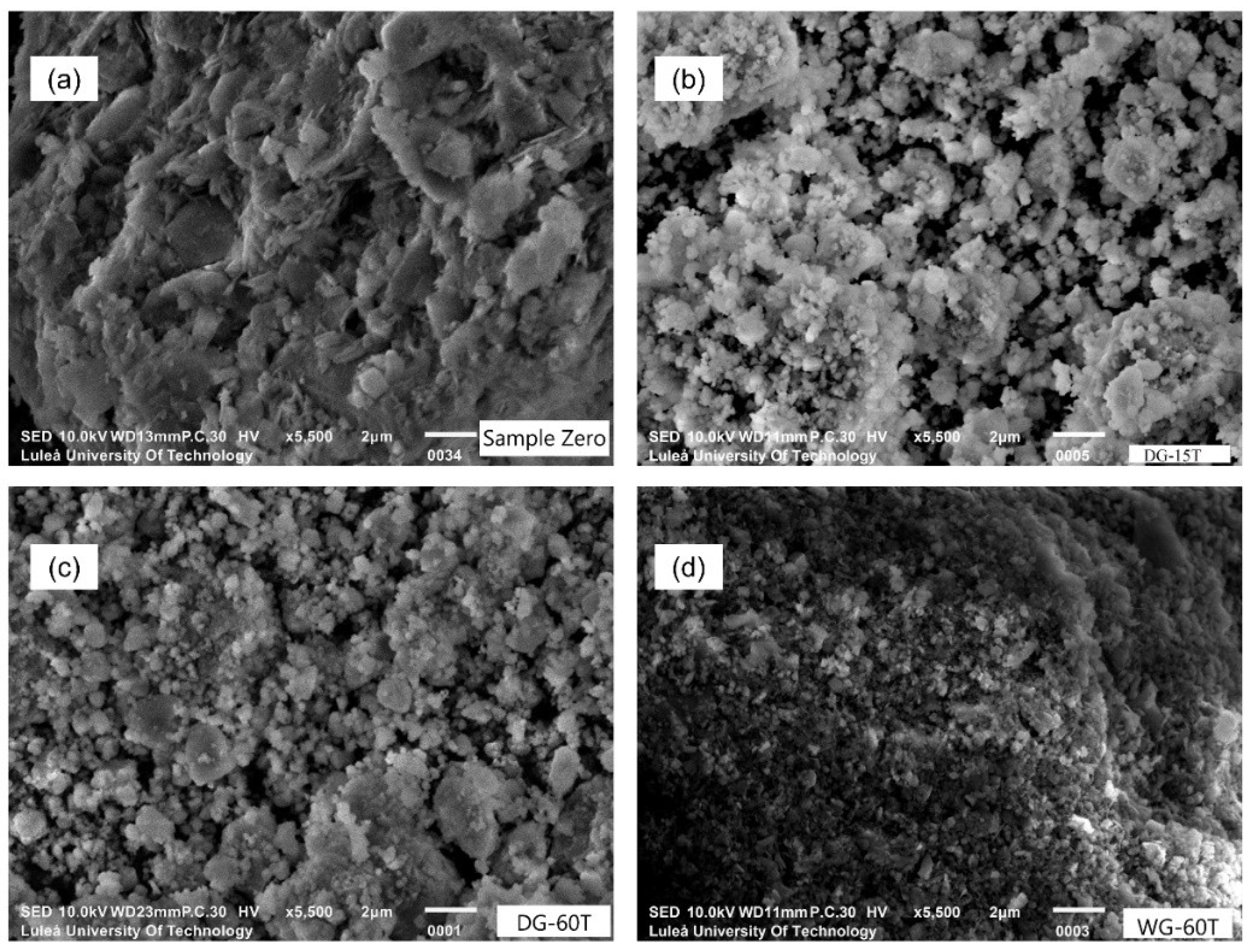
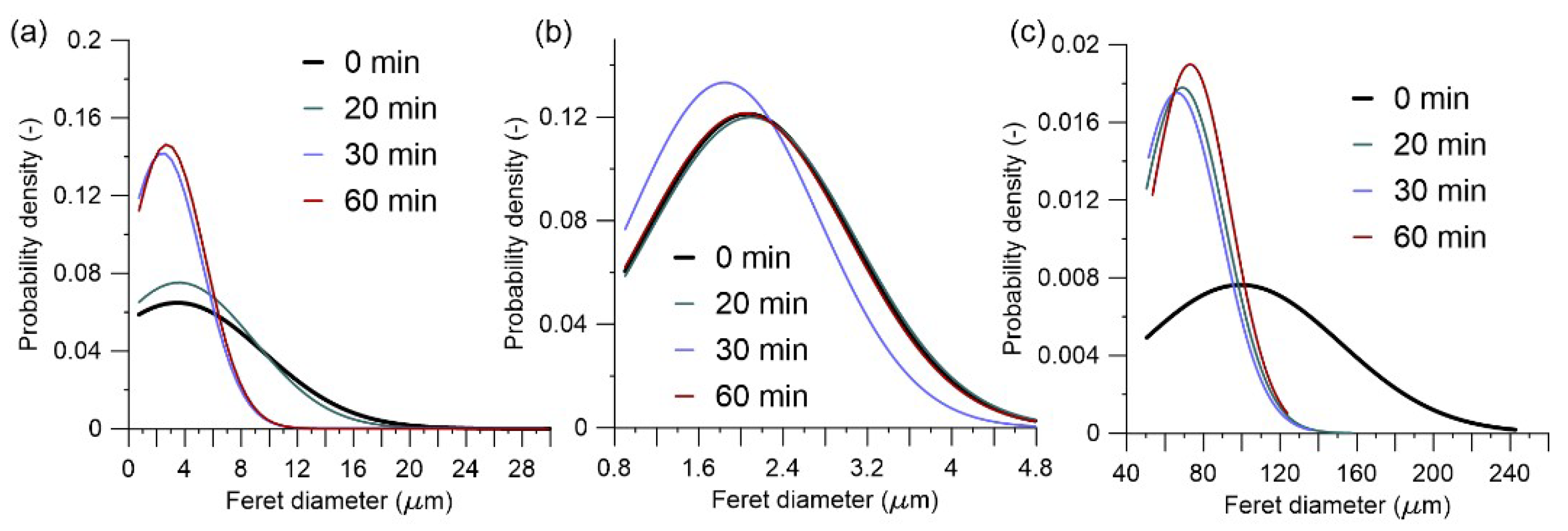
3.2. Grinding Duration
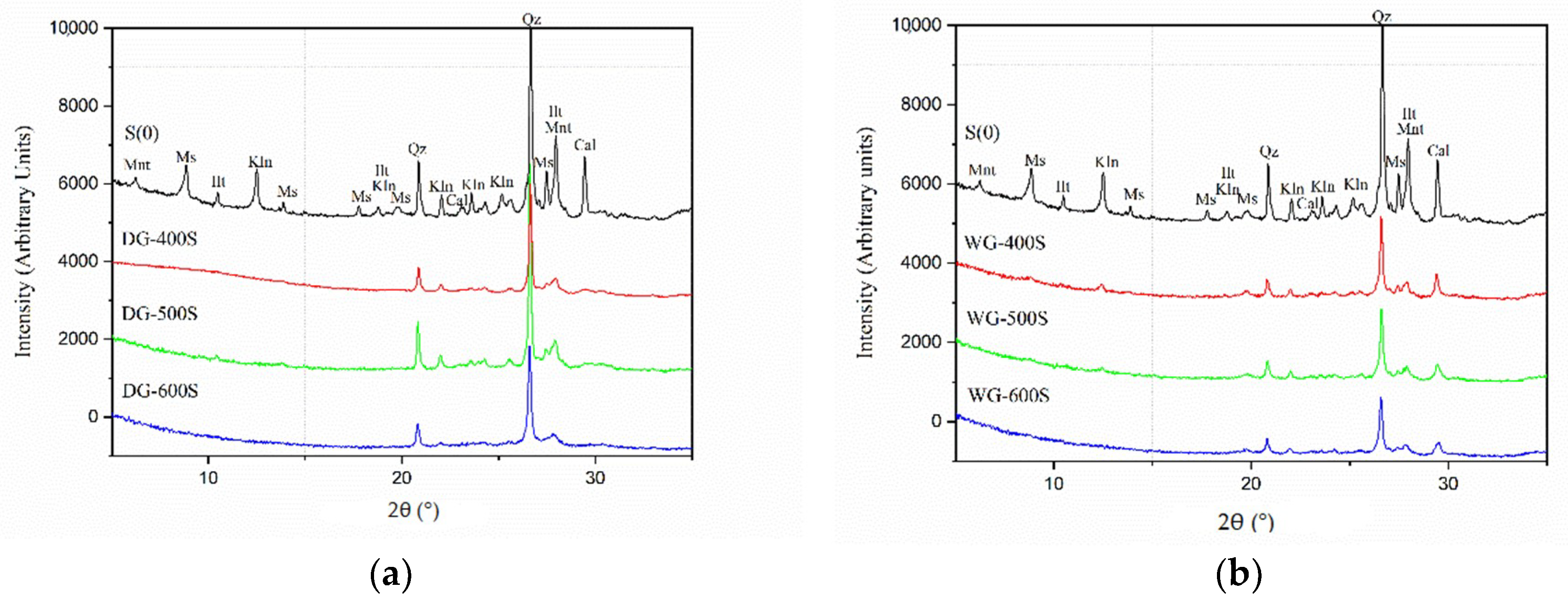
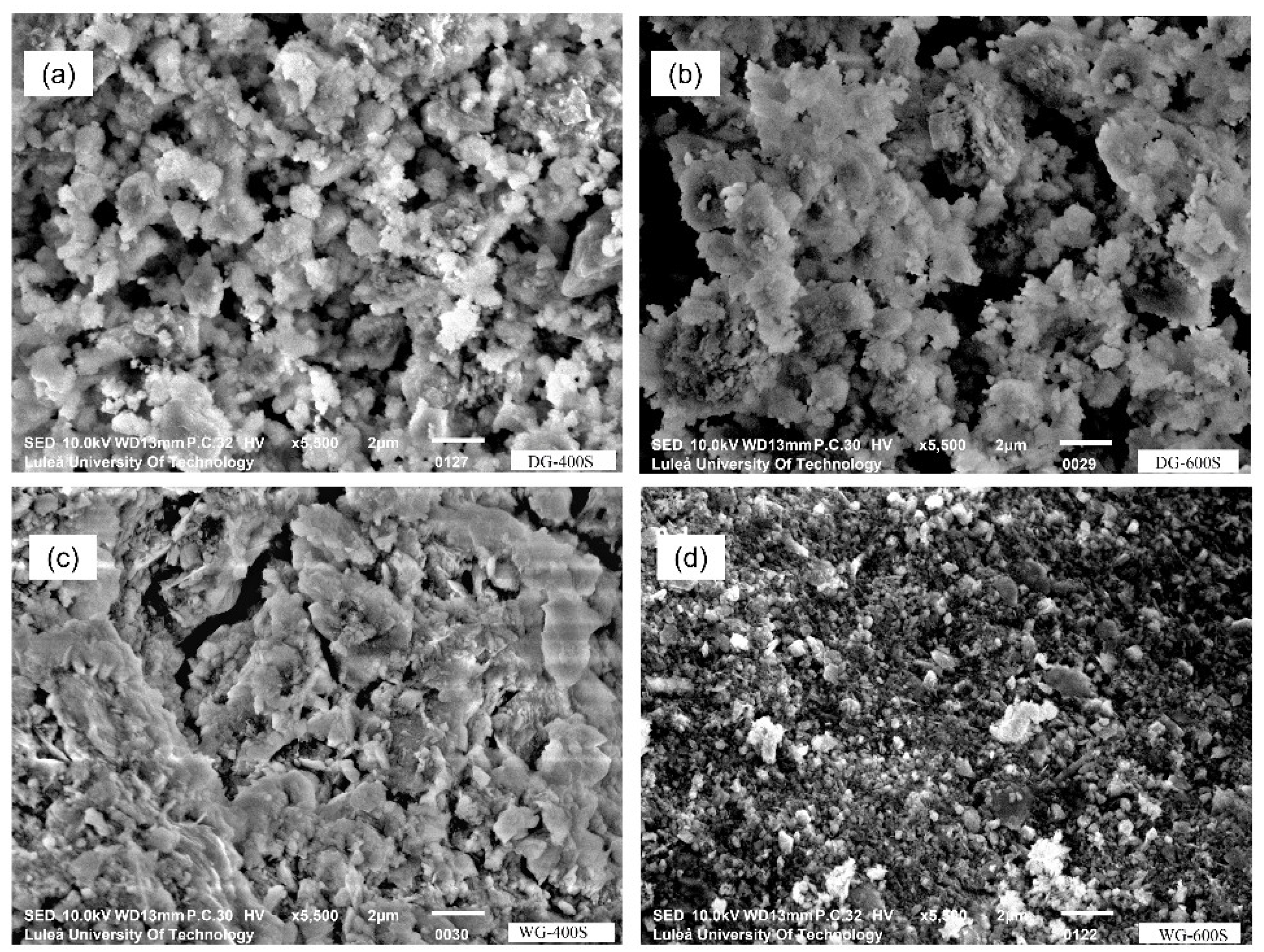
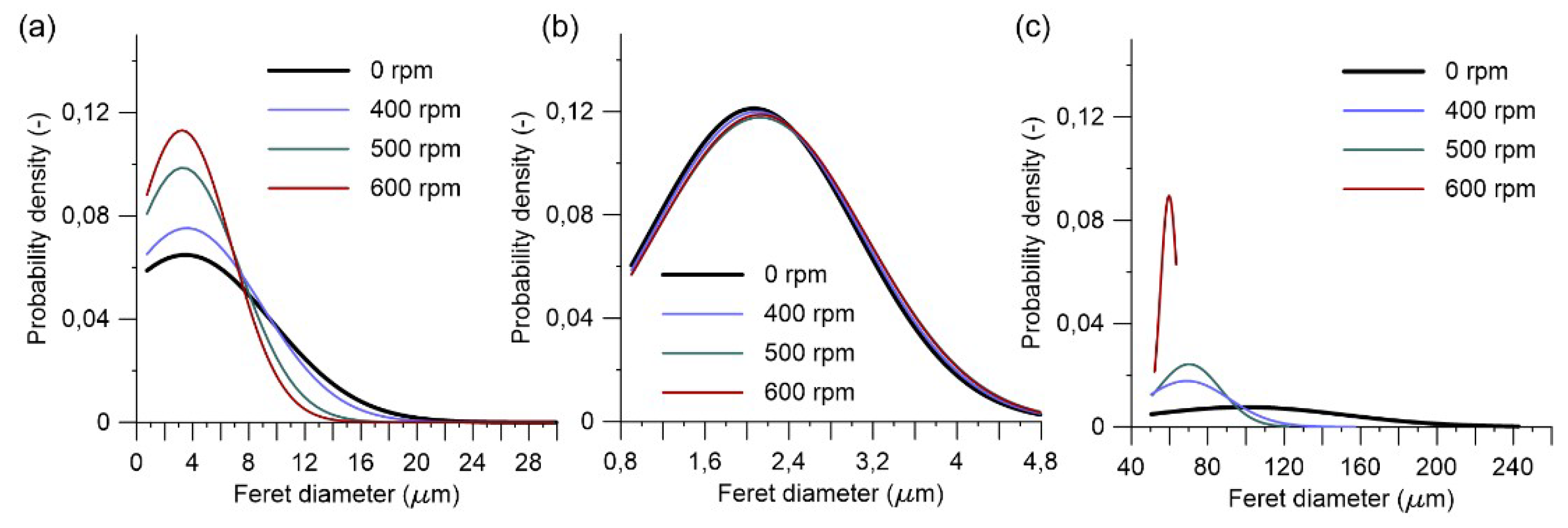
3.3. Grinding Speed
3.4. Alkali Activation of Processed Material—Preliminary Tests
4. Conclusions
- The effectiveness of the mechanochemical activation in the early stage of grinding (within the Rittinger’s zone), is very sensitive to changes of the ball to powder ratio, the amount of the grinding medium, the water to powder ratio and the speed regime of the equipment.
- Dry grinding process promoted more extensive amorphization when using a higher number of grinding balls versus the amount of the processed clay powder.
- Dry grinding was more effective in amorphization of kaolinite than wet grinding process.
- Effectiveness of the wet grinding increased with a higher water to processed powder ratio but required longer processing times in comparison with the dry grinding process.
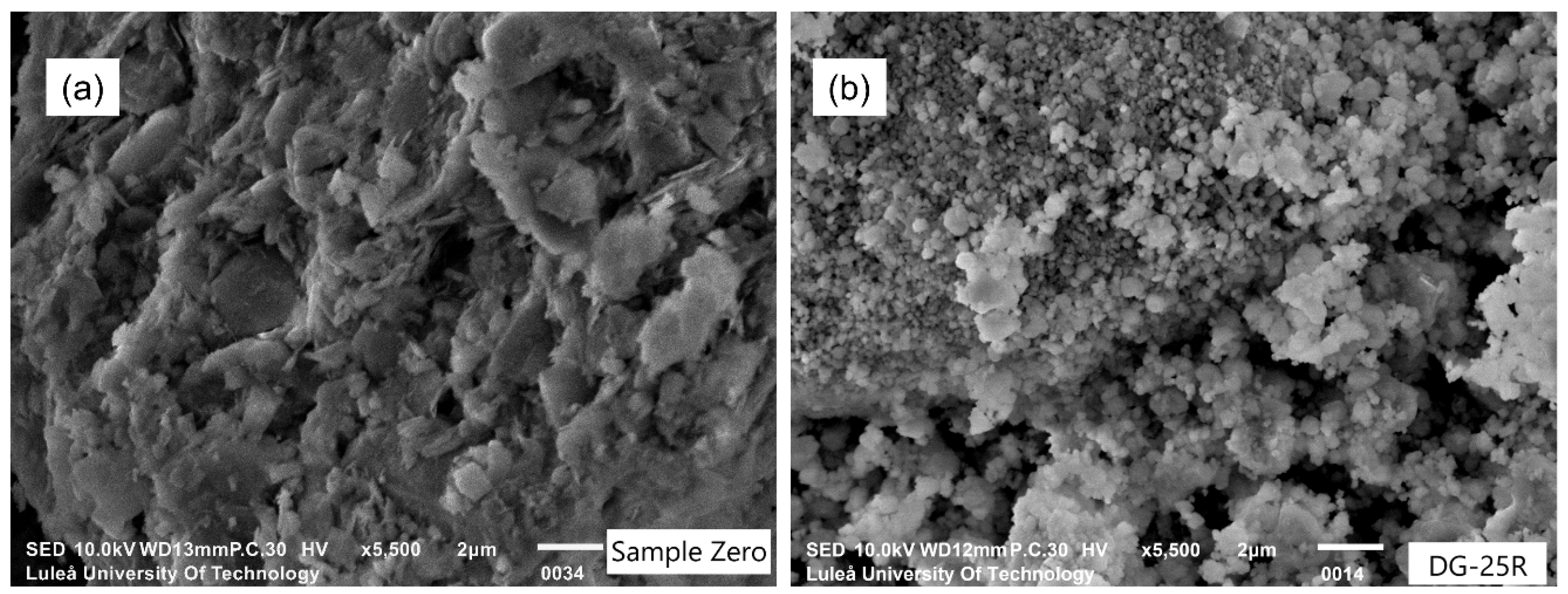
- Longer grinding enabled a more extensive amorphization in both wet and dry processes, produced finer particles with spherulitic morphology, but also tended to increase agglomeration when outside of the Rittinger zone.
- Higher rotational speed enhanced amorphization efficiency of clay minerals in both wet and dry processes. The trend was similar in the case of quartz but only when the wet grinding was used.
- The most efficient amorphization of the studied Swedish raw clay was achieved when using the following process parameters: B/P ratio equal to 25, speed 500 rpm and the grinding time of 20 min. This process also caused less agglomeration, caking effect and wearing.
- The processed raw clay, using the defined parameters, should enable its usage as pozzolanic material and as cementitious binder for production of concrete.
- Preliminary strength tests validated the concept that the MCA can enhance the reactivity of the raw clay to a degree enabling its utilization as a binder in alkali-activated systems for e.g., production of mortars or concretes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snow, R.H.; Allen, T.; Ennis, B.J.; Litster, J.D. Size Reduction and Size Enlargement. In Perry’s Chemical Engineers’ Handbook, 7th ed.; Perry, R.H., Green, D.W., Maloney, J.O., Eds.; McGraw-Hill: New York, NY, USA, 1973; ISBN 0-07-049841-5. [Google Scholar]
- Zhang, Q.; Kano, J.; Saito, F. Fine grinding of materials in dry systems and mechanochemistry. Handb. Powder Technol. 2007, 12, 509–528. [Google Scholar] [CrossRef]
- Beke, B. Grindability of materials. In The Process of Fine Grinding; Springer: Dordrecht, The Netherlands, 1981; pp. 35–45. ISBN 978-04-009-8260-4. [Google Scholar]
- Juhász, A.Z.; Opoczky, L. Mechanical Activation of Minerals by Grinding Pulverizing and Morphology of Particles; Ellis Horwood Limited Publishers: Chichester, UK, 1990; ISBN 0-7458-0015-7. [Google Scholar]
- Hasa, D.; Jones, W. Screening for new pharmaceutical solid forms using mechanochemistry: A practical guide. Adv. Drug Deliv. Rev. 2017, 117, 147–161. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, V.V.; Pavlov, S.V.; Goldberg, E.L. Interrelation between fine grinding and mechanical activation. Int. J. Miner. Process. 1996, 44, 181–185. [Google Scholar] [CrossRef]
- Baláž, P. From minerals to nanoparticles. In Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin, Germany, 2008; pp. 177–256. ISBN 978-3-540-74854-0. [Google Scholar]
- Tkačova, K.; Boldyrev, V.V.; Lyakhov, N. Energy dissipation during mechanical treatment of inorganic non-metallic substances. Proc. Indian Nat. Sci. Acad. 1989, 55, 778–789. [Google Scholar]
- Tkácová, K. Mechanical Activation of Minerals; Veda: Sydney, Australia, 1989; ISBN 80-224-0007-6. [Google Scholar]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Aglietti, E.F.; Porto Lopez, J.M.; Pereira, E. Mechanochemical effects in kaolinite grinding. I. Textural and physicochemical aspects. Int. J. Miner. Process. 1986, 16, 125–133. [Google Scholar] [CrossRef]
- Ashrafizadeh, H.; Ashrafizaadeh, M. Influence of processing parameters on grinding mechanism in planetary mill by employing discrete element method. Adv. Powder Technol. 2012, 23, 708–716. [Google Scholar] [CrossRef]
- Wahl, M.; Bröckel, U.; Brendel, L.; Feise, H.J.; Weigl, B.; Röck, M.; Schwedes, J. Understanding powder caking: Predicting caking strength from individual particle contacts. Powder Technol. 2008, 188, 147–152. [Google Scholar] [CrossRef]
- Takahashi, H. Wet grinding on kaolin minerals. Bull. Chem. Soc. Jpn. 1959, 32, 381–387. [Google Scholar] [CrossRef]
- Ambroise, J.; Maximilien, S.; Pera, J. Properties of metakaolin blended cements. Adv. Cem. Based Mater. 1994, 1, 161–168. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177. [Google Scholar] [CrossRef]
- Balczár, I.; Korim, T.; Kovács, A.; Makó, É. Mechanochemical and thermal activation of kaolin for manufacturing geopolymer mortars—Comparative study. Ceram. Int. 2016, 42, 15367–15375. [Google Scholar] [CrossRef]
- Pérez Rodríguez, J.L.; Sánchez del Villar, L.M.S.; Sánchez-Soto, P.J. Effects of dry grinding on pyrophyllite. Clay Miner. 1988, 23, 399–410. [Google Scholar]
- Xia, M.; Jiang, Y.; Zhao, L.; Li, F.; Xue, B.; Sun, M.; Liu, D.; Zhang, X. Wet grinding of montmorillonite and its effect on the properties of mesoporous montmorillonite. Colloid Surf. A 2010, 356, 1–9. [Google Scholar] [CrossRef]
- Yang, H.; Yang, W.; Hu, Y.; Du, C.; Tang, A. Effect of mechanochemical processing on illite particles. Part. Part. Syst. Charact. 2005, 22, 207–211. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Potential use of Argentine kaolinitic clays as pozzolanic material. Appl. Clay Sci. 2014, 101, 468–476. [Google Scholar] [CrossRef]
- Ilić, B.; Radonjanin, V.; Malešev, M.; Zdujić, M.; Mitrović, A. Effects of mechanical and thermal activation on pozzolanic activity of kaolin containing mica. Appl. Clay Sci. 2016, 123, 173–181. [Google Scholar] [CrossRef]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Makó, É.; Frost, R.L.; Kristóf, J.; Horváth, E. The effect of quartz content on the mechanochemical activation of kaolinite. J. Colloid Interface Sci. 2001, 244, 359–364. [Google Scholar] [CrossRef]
- Kristof, E.; Juhasz, A.Z.; Vassanyi, I. The effect of mechanical treatment on the crystal structure and thermal behavior of kaolinite. Clays Clay Miner. 1993, 41, 608–612. [Google Scholar] [CrossRef]
- Frost, R.L.; Mako, E.; Kristóf, J.; Horváth, E.; Kloprogge, J.T. Modification of kaolinite surfaces by mechanochemical treatment. Langmuir 2001, 17, 4731–4738. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, L.; Ren, X.; Xu, B.; Zhang, H.; Ma, F. The characteristics of mechanical grinding on kaolinite structure and thermal behavior. Energy Procedia 2012, 16, 1237–1240. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Muslim, F.; Guessasma, S.; Bennabi, A.; Guillin, J. Structural and thermal behavior of proclay kaolinite using high energy ball milling process. Powder Technol. 2015, 271, 228–237. [Google Scholar] [CrossRef]
- Prokof’ev, V.Y.; Gordina, N. Comminution and mechanochemical activation in oxide ceramics technology. Glass Ceram. 2012, 69, 65–70. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kimata, M.; Shimane, M.; Shoji, T.; Tsuruta, M. The effect of liquid additives on dry ultrafine grinding of quartz. Powder Technol. 2001, 114, 145–151. [Google Scholar] [CrossRef]
- Da C Andrade, E.N.; Randall, R.F.Y.; Makin, M.J. The Rehbinder effect. Proc. Phys. Soc. Sect. B 1950, 63, 990. [Google Scholar] [CrossRef]
- Klimpel, R.R.; Austin, L.G. Chemical additives for wet grinding of minerals. Powder Technol. 1982, 31, 239–253. [Google Scholar] [CrossRef]
- Westwood, A.R.C. Tewksbury lecture: Control and application of environment-sensitive fracture processes. J. Mater. Sci. 1974, 9, 1871–1895. [Google Scholar] [CrossRef]

| Component | Content (wt.%) |
|---|---|
| SiO2 | 52.6 |
| Al2O3 | 15.1 |
| Fe2O3 | 6.9 |
| CaO | 6.41 |
| K2O | 3.78 |
| MgO | 2.51 |
| Na2O | 1.68 |
| TiO2 | 0.696 |
| LOI | 7.5 |
| Water/Powder | Balls/Powder | Speed (rpm) | Grinding Duration (min) | Sample ID. |
|---|---|---|---|---|
| - | 3 | 500 | 20 | DG-3R |
| 5 | DG-5R | |||
| 25 | DG-25R | |||
| - | 25 | 500 | 15 | DG-15T |
| 20 | DG-20T | |||
| 60 | DG-60T | |||
| - | 25 | 400 | 20 | DG-400S |
| 500 | DG-500S | |||
| 600 | DG-600S | |||
| 1 | 3 | 500 | 20 | WG-3R |
| 5 | WG-5R | |||
| 25 | WG-25R | |||
| 1 | 3 | 500 | 15 | WG-1WR |
| 1.5 | WG-1,5WR | |||
| 2 | WG-2WR | |||
| 1 | 25 | 500 | 20 | WG-20T |
| 30 | WG-30T | |||
| 60 | WG-60T | |||
| 1 | 25 | 400 | 20 | WG-400S |
| 500 | WG-500S | |||
| 600 | WG-600S |
| Mix Identification | |||
|---|---|---|---|
| Untreated Clay (S0) | DG-3R | DG-25R | |
| Binder content wt. (g) | 30 | 30 | 30 |
| Water/Binder (ratio) | 0.5 | 0.5 | 0.5 |
| Aggregate content wt. (g) | 30 | 30 | 30 |
| Wt.% alkali activator solution | 10 | 10 | 10 |
| Activator Modulus Ms (SiO2/Na2O) | 1 | 1 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tole, I.; Habermehl-Cwirzen, K.; Rajczakowska, M.; Cwirzen, A. Activation of a Raw Clay by Mechanochemical Process—Effects of Various Parameters on the Process Efficiency and Cementitious Properties. Materials 2018, 11, 1860. https://doi.org/10.3390/ma11101860
Tole I, Habermehl-Cwirzen K, Rajczakowska M, Cwirzen A. Activation of a Raw Clay by Mechanochemical Process—Effects of Various Parameters on the Process Efficiency and Cementitious Properties. Materials. 2018; 11(10):1860. https://doi.org/10.3390/ma11101860
Chicago/Turabian StyleTole, Ilda, Karin Habermehl-Cwirzen, Magdalena Rajczakowska, and Andrzej Cwirzen. 2018. "Activation of a Raw Clay by Mechanochemical Process—Effects of Various Parameters on the Process Efficiency and Cementitious Properties" Materials 11, no. 10: 1860. https://doi.org/10.3390/ma11101860
APA StyleTole, I., Habermehl-Cwirzen, K., Rajczakowska, M., & Cwirzen, A. (2018). Activation of a Raw Clay by Mechanochemical Process—Effects of Various Parameters on the Process Efficiency and Cementitious Properties. Materials, 11(10), 1860. https://doi.org/10.3390/ma11101860







