Comparison of Medium Manganese Steel and Q345 Steel on Corrosion Behavior in a 3.5 wt % NaCl Solution
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of the Tested Steels
2.2. Cyclic Wet/Dry Accelerated Corrosion Test
2.3. Morphology Observation and Composition Analysis
3. Results and Discussion
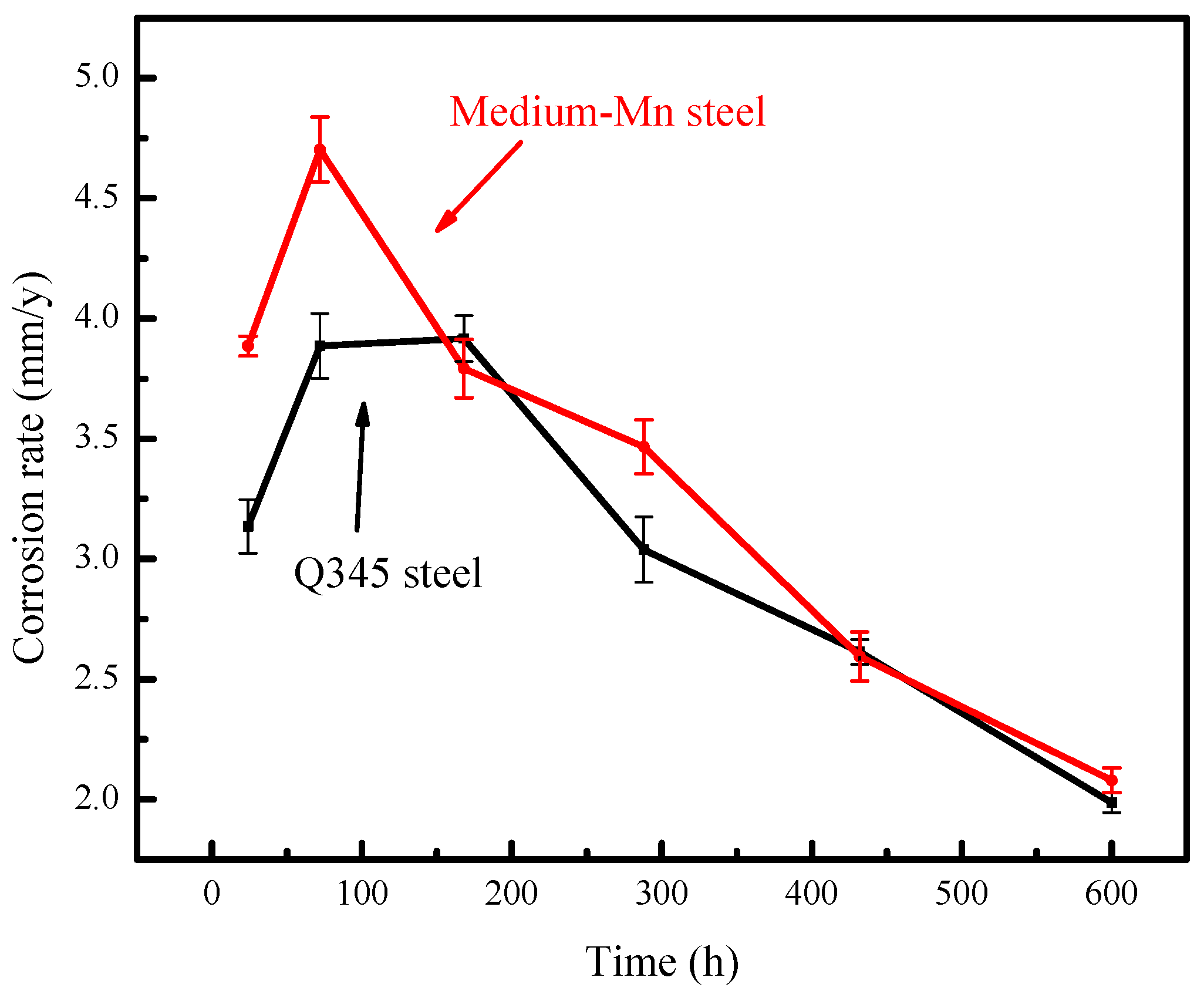
3.1. Corrosion Rate
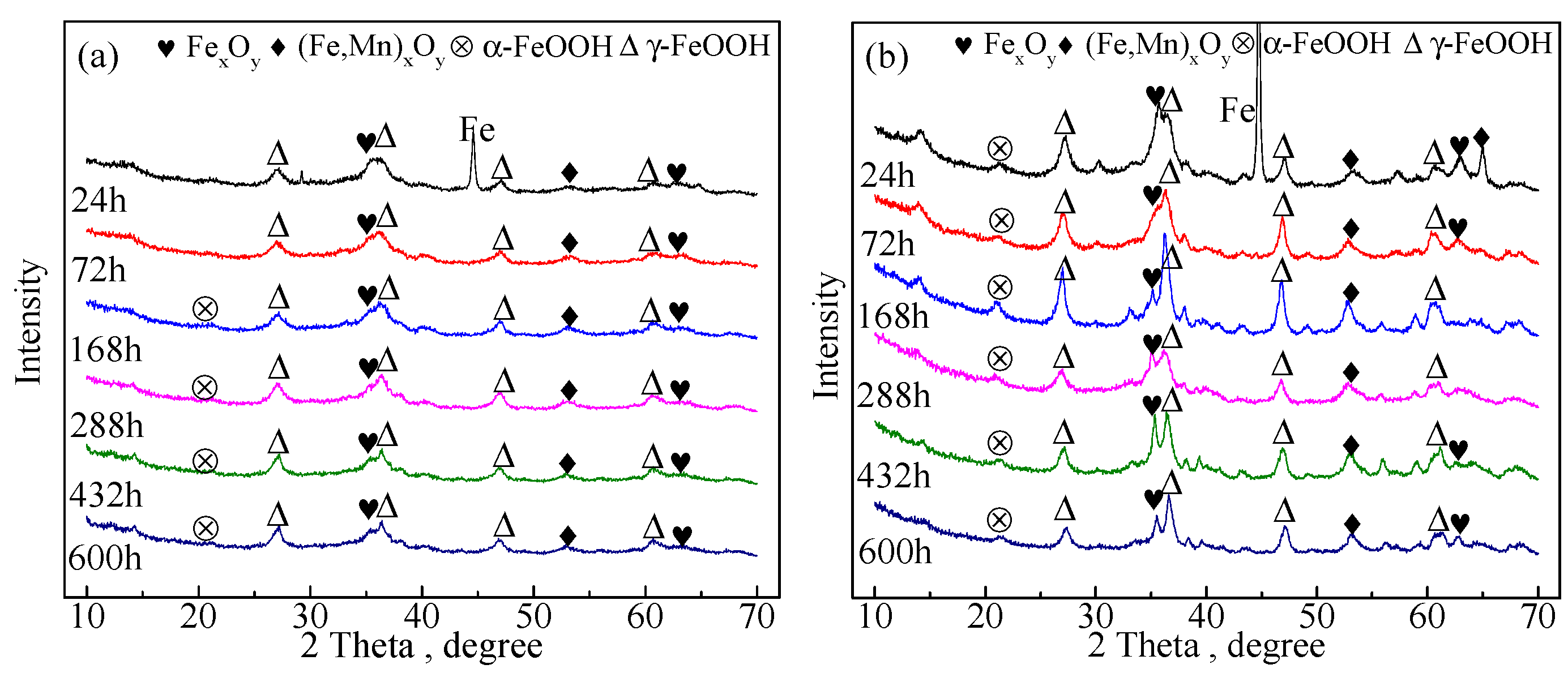
3.2. X-ray Diffraction
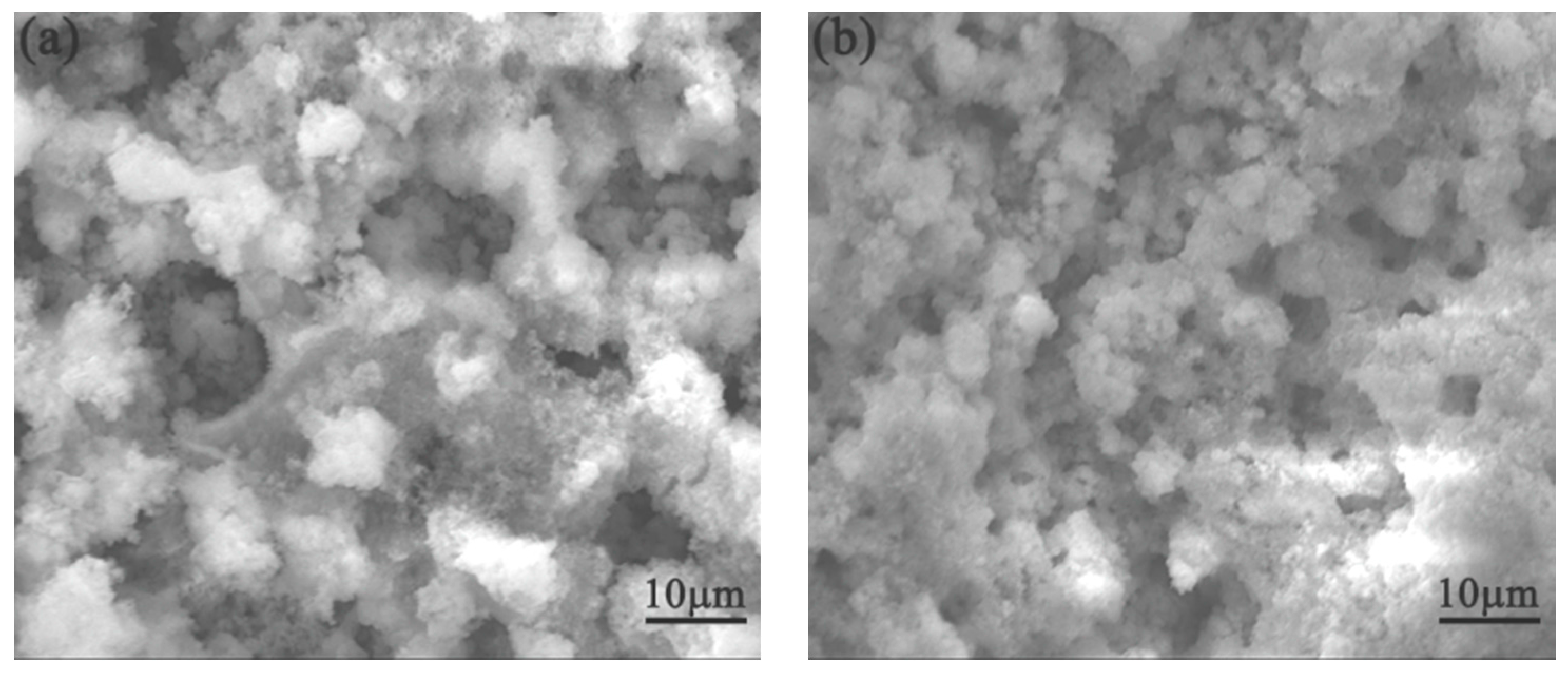
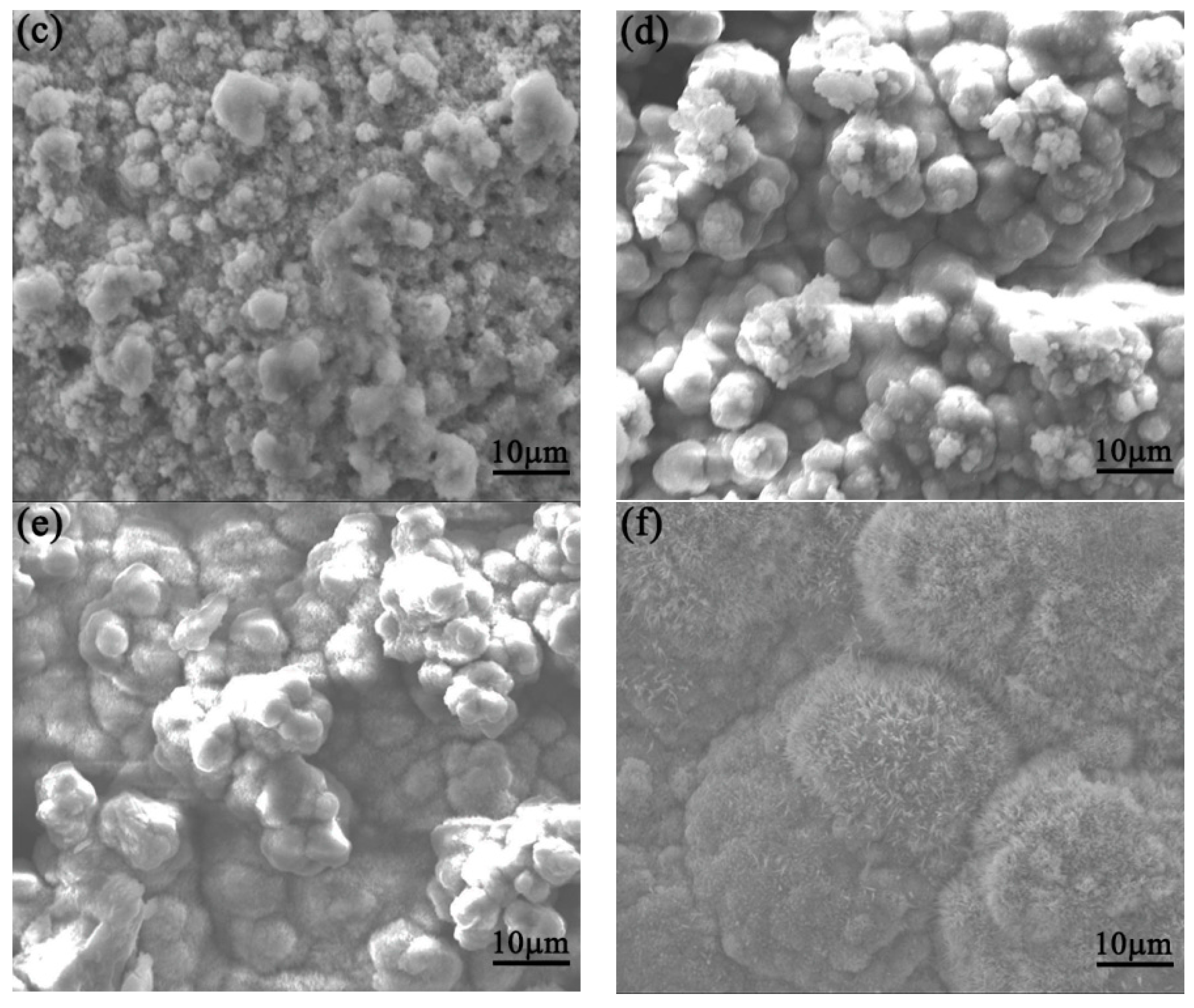
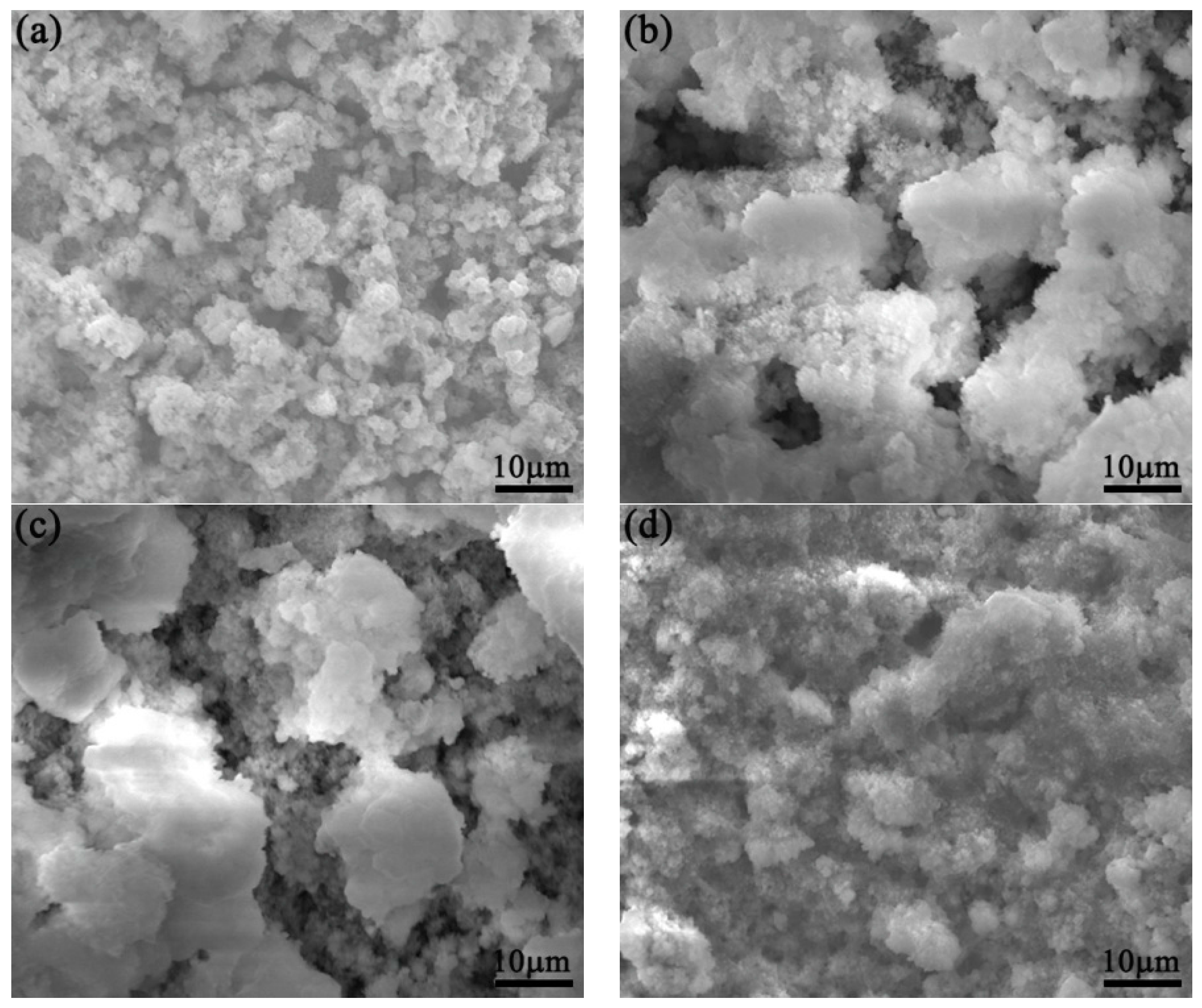
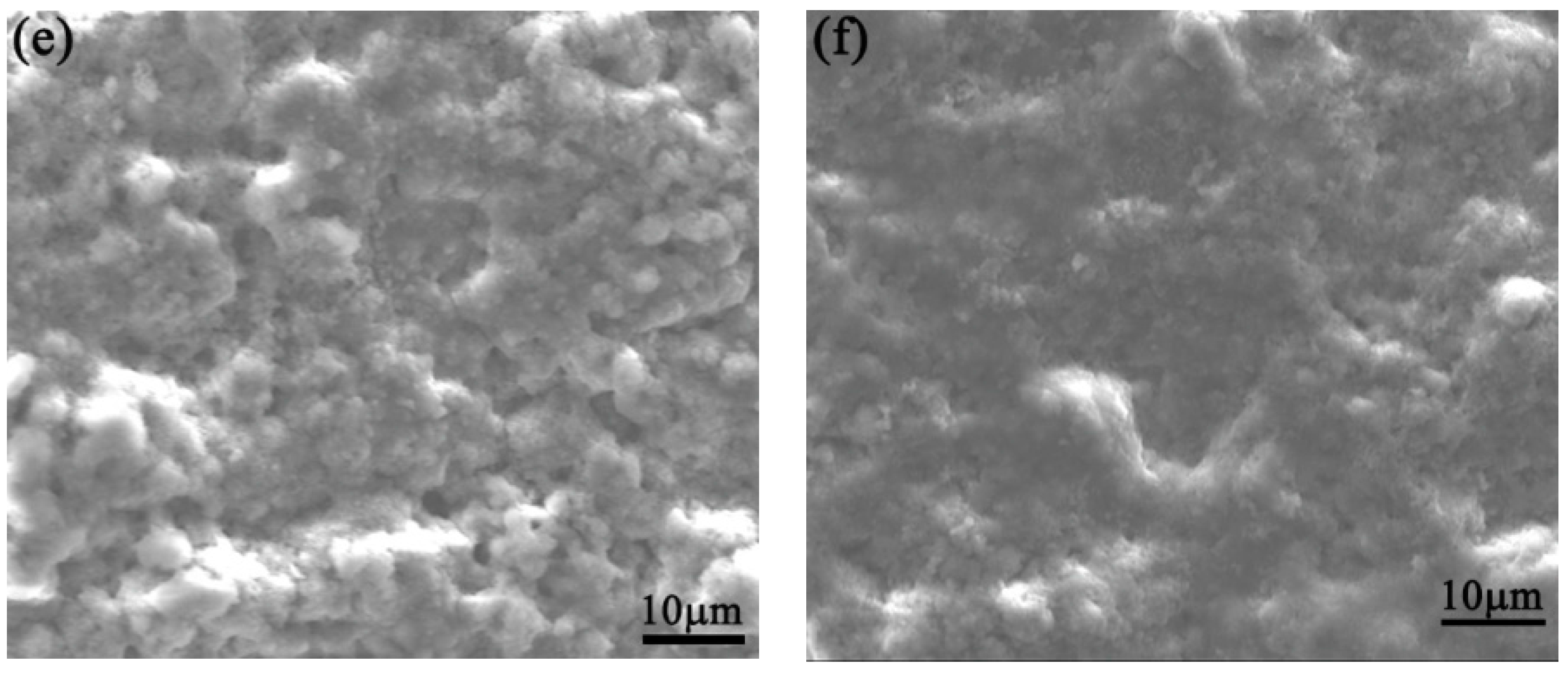
3.3. SEM Morphology
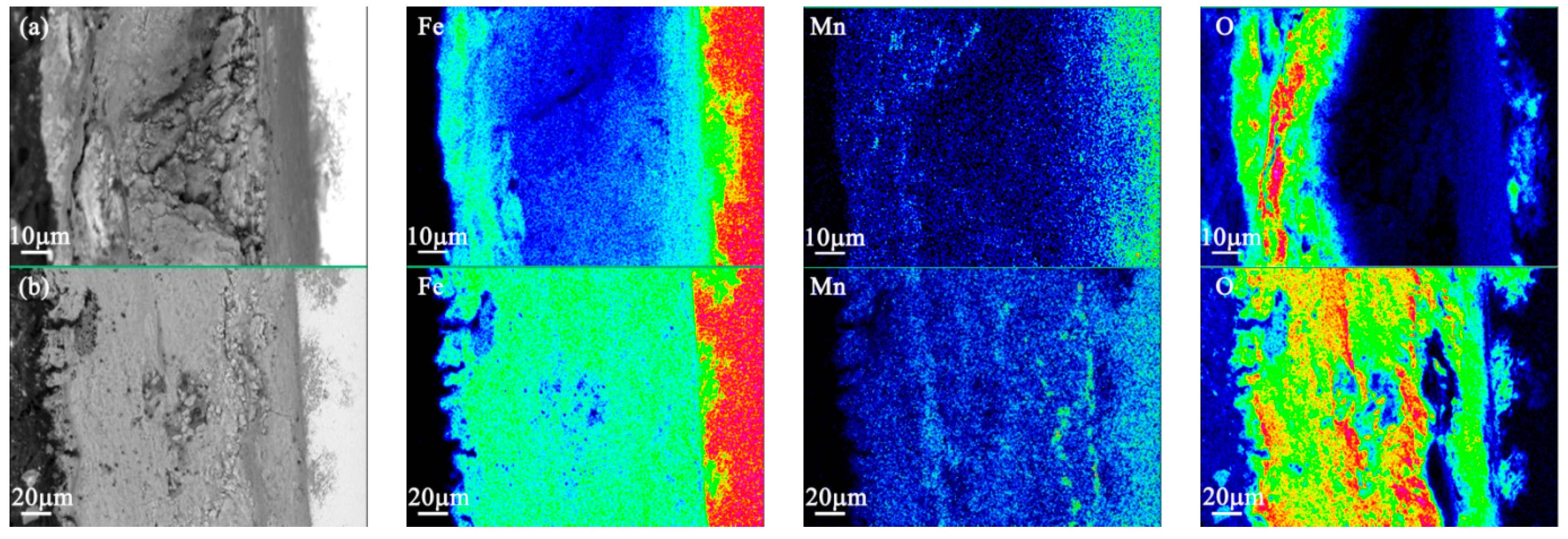
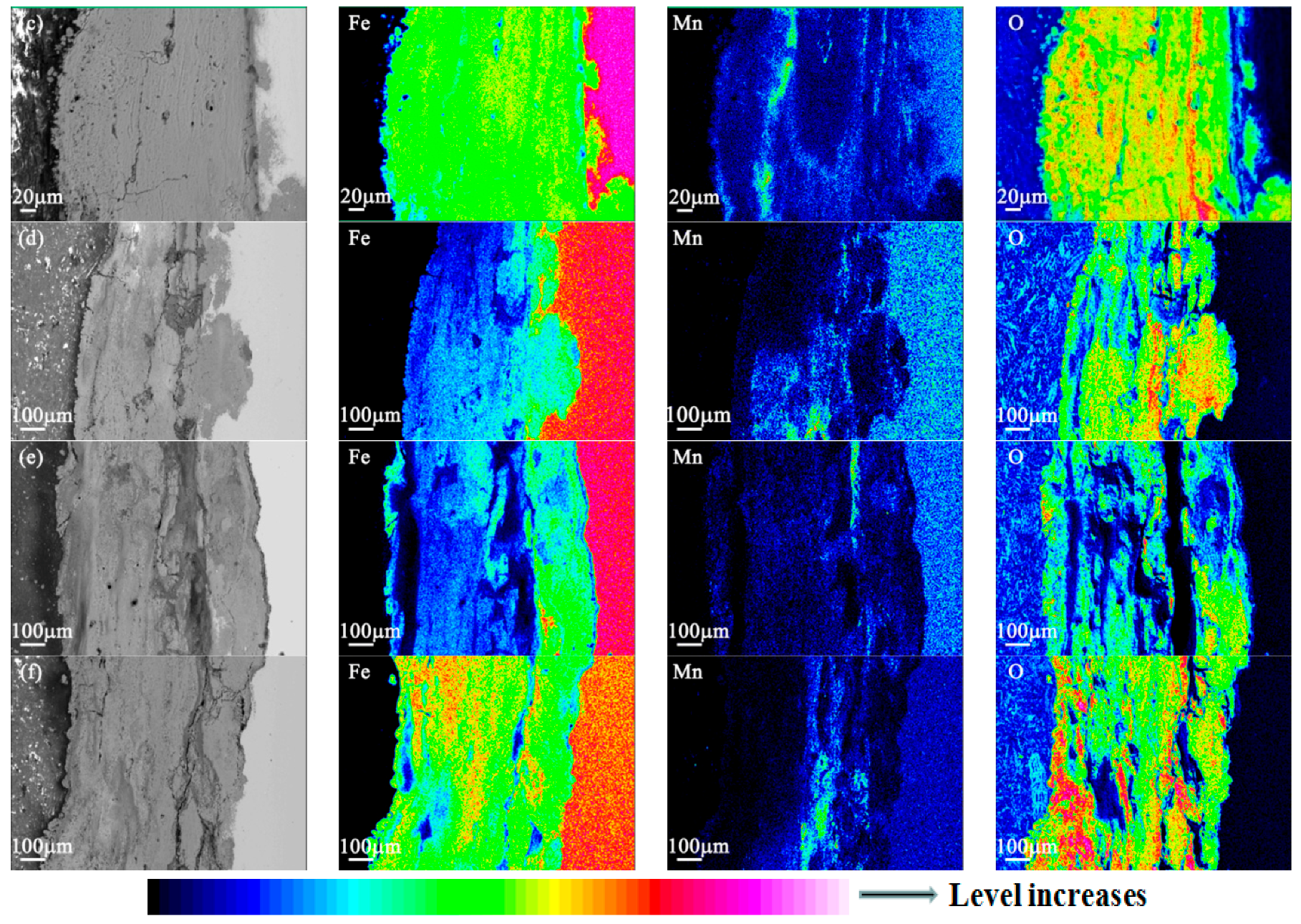
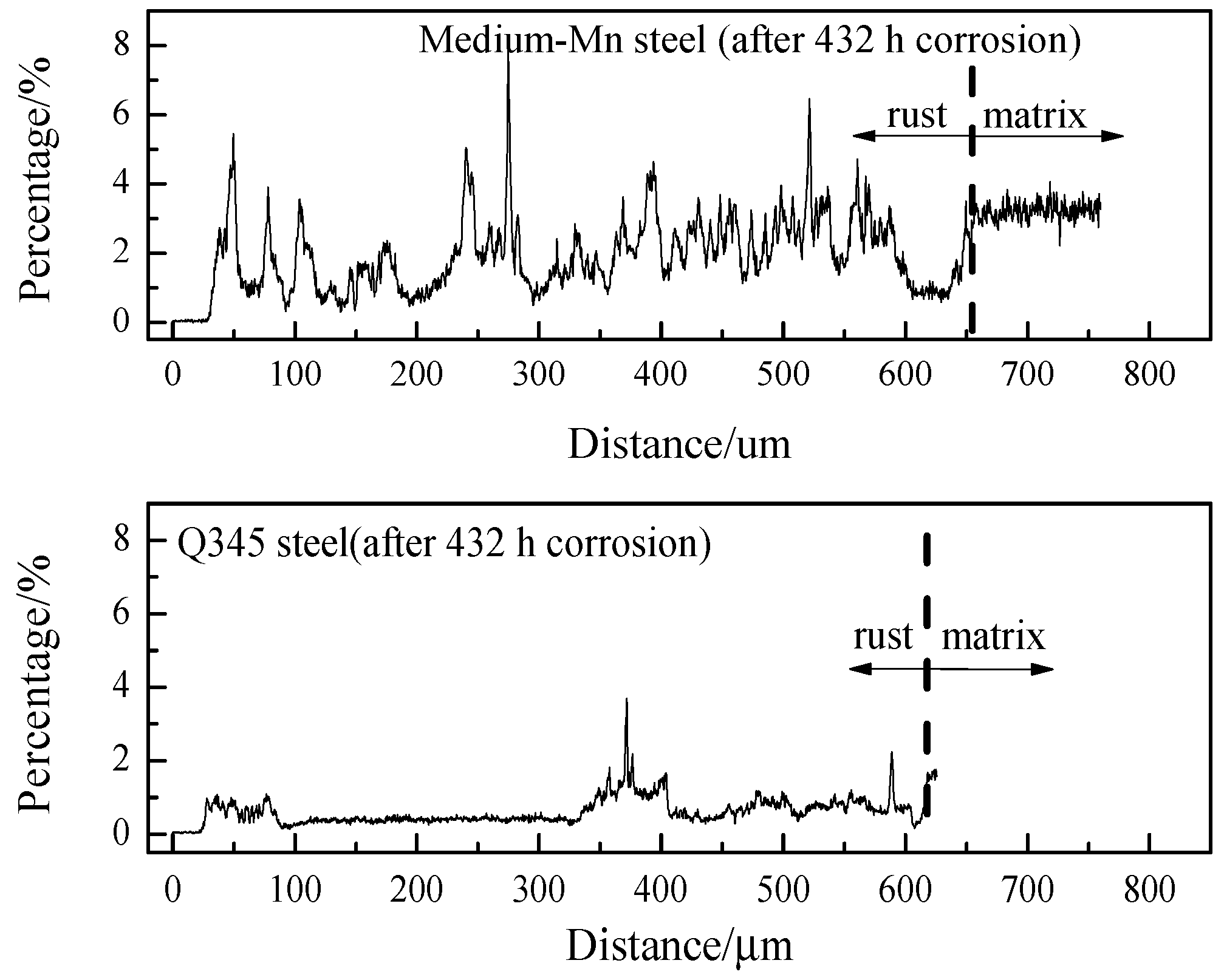
3.4. EPMA Results
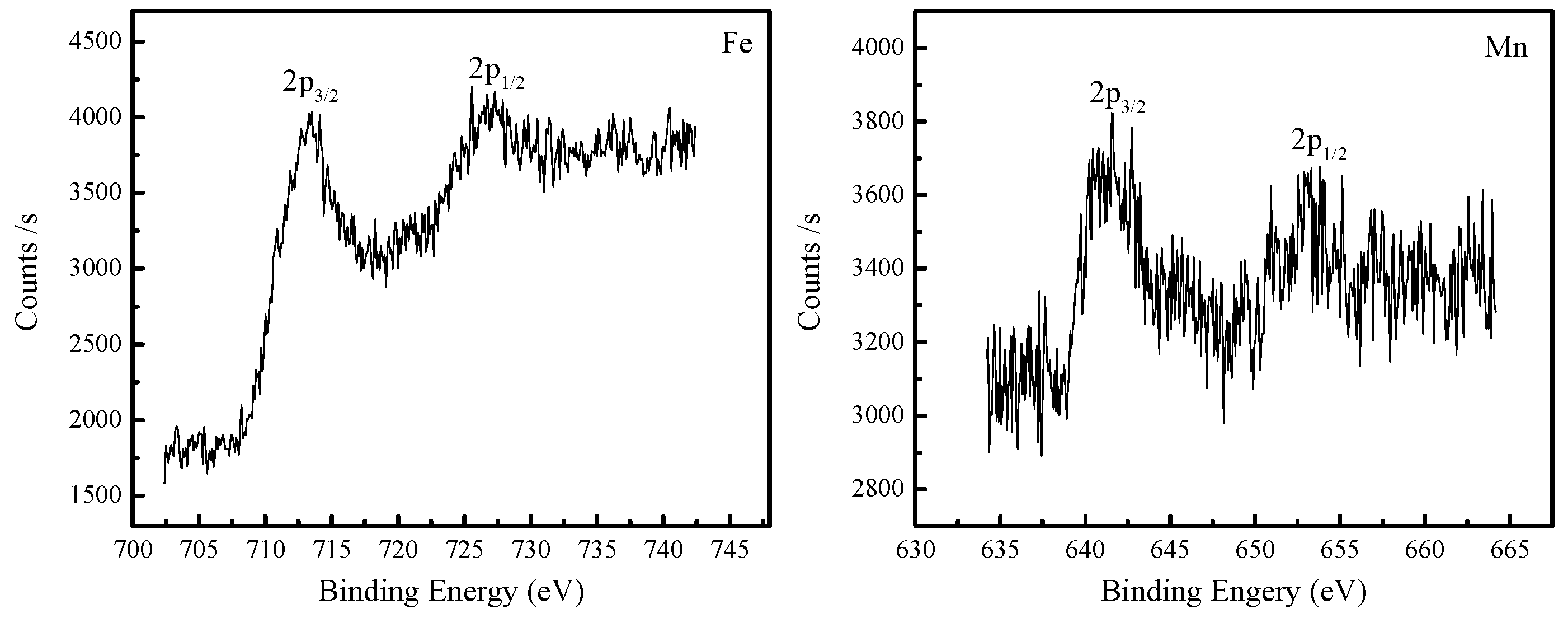
3.5. X-ray Photoelectron Spectroscopy
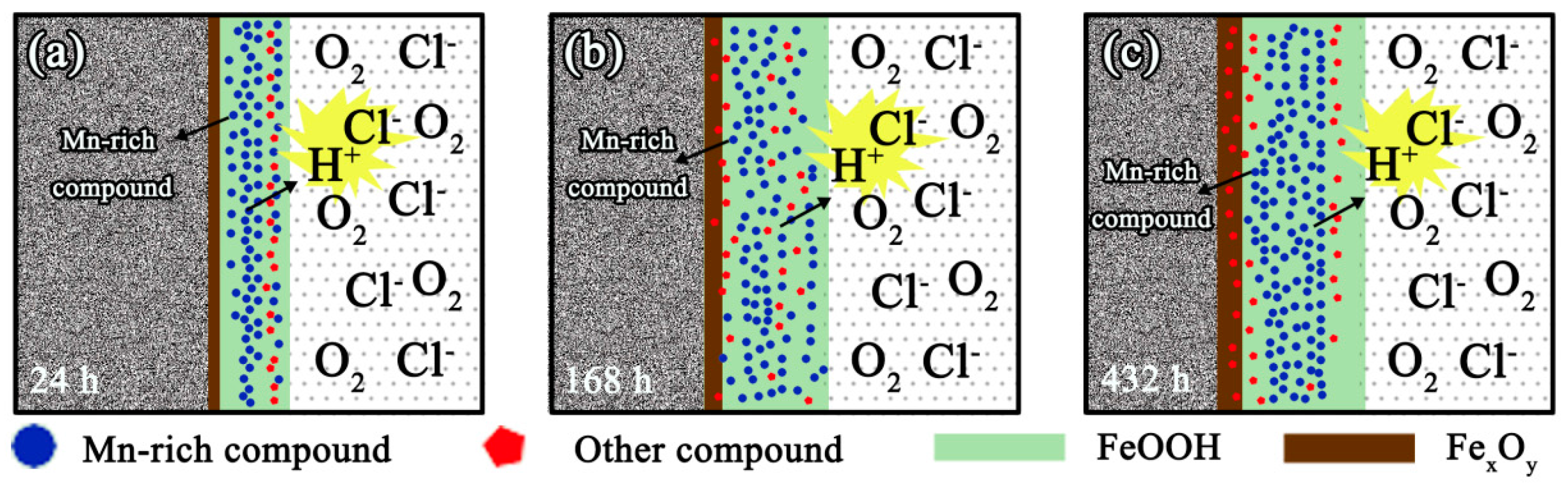
3.6. Corrosion Process of the Tested Medium-Mn Steel
4. Conclusions
- (1)
- Through a comparison of medium-manganese steel and Q345 steel on their corrosion behavior in a 3.5 wt % NaCl solution, the medium-Mn steel exhibited lower corrosion resistance due to the adverse effect of Mn. Mn-rich compounds presented adverse effects during the corrosion process.
- (2)
- The two tested steels had similar corrosion behavior. Moreover, the rust morphologies of the Q345 steel were much denser than that of the medium-Mn steel. The corrosion rate was regulated because of the existence of the anti-corrosion elements in the medium manganese steel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwon, K.H.; Yi, I.-C.; Ha, Y.; Um, K.-K.; Choi, J.-K.; Hono, K.; Oh-ishi, K.; Kim, N.J. Origin of intergranular fracture in martensitic 8Mn steel at cryogenic temperatures. Scr. Mater. 2013, 69, 420–423. [Google Scholar] [CrossRef]
- Wang, M.-M.; Tasan, C.C.; Ponge, D.; Dippel, A.-Ch.; Raabe, D. Nanolaminate transformation-induced plasticity–twinning-induced plasticity steel with dynamic strain partitioning and enhanced damage resistance. Acta Mater. 2015, 85, 216–228. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Liu, H.; Sun, G.S.; Xie, H.; Yi, H.L.; Misra, R.D.K. Structure–mechanical property relationship in a low-C medium-Mn ultrahigh strength heavy plate steel with austenite-martensite submicro-laminate structure. Mater. Sci. Eng. A 2015, 647, 144–151. [Google Scholar] [CrossRef]
- Murata, T. Weathering steel. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Boyd, W.K. Corrosion of Metals in the Atmosphere; Metals and Ceramics Information Center: Columbus, OH, USA, 1974. [Google Scholar]
- Wang, X.-N.; Zhang, S.-H.; Zhou, J.; Zhang, M.; Chen, C.-J.; Misra, R.D.K. Effect of heat input on microstructure and properties of hybrid fiber laser-arc weld joints of the 800 MPa hot-rolled Nb-Ti-Mo microalloyed steels. Opt. Lasers Eng. 2017, 91, 86–96. [Google Scholar] [CrossRef]
- Itagaki, M.; Nozue, R.; Watanabe, K.; Katayama, H.; Noda, K. Electrochemical impedance of thin rust film of low-alloy steels. Corros. Sci. 2004, 46, 1301–1310. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Sun, G.S.; Xie, H.; Misra, R.D.K. The determining role of reversed austenite in enhancing toughness of a novel ultra-low carbon medium manganese high strength steel. Scr. Mater. 2015, 104, 87–90. [Google Scholar] [CrossRef]
- ISO. Corrosion of Metals and Alloys—Alternate Immersion Test in Salt Solution; ISO 11130:2010(E); International Organization for Standardization (ISO): Geneva, Switzerland, 2010; pp. 1–14. [Google Scholar]
- Liu, Z.G.; Gao, X.H.; Li, J.P.; Du, L.X.; Yu, C.; Li, P.; Bai, X.L. Corrosion behaviour of low-alloy martensite steel exposed to vapour-saturated CO2 and CO2-saturated brine conditions. Electrochim. Acta 2016, 213, 842–855. [Google Scholar] [CrossRef]
- Liu, Z.G.; Gao, X.H.; Chi, Y.; Du, L.X.; Li, J.P.; Hao, P.J. Corrosion behaviour of low-alloy pipeline steel with 1% Cr under CO2 condition. Acta. Metall. Sin. (Engl. Lett.) 2015, 28, 739–747. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- De la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of rust surfaces formed on mild steel exposed to marine atmospheres using XRD and SEM/Micro-Raman techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Li, B.J.; Cao, H.Q.; Shao, J.; Qu, M.Z. Enhanced anode performances of the Fe3O4–Carbon–rGO three dimensional composite in lithium ion batteries. Chem. Commun. 2011, 47, 10374–10376. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.J.; Cai, Y.M.; Lu, F.; Wei, F.Y.; Wang, X.Y.; Wang, S.B. Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J. Hazard. Mater. 2014, 270, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kassim, J.; Baird, T.; Fryer, J.R. Electron microscope studies of iron corrosion products in water at room temperature. Corros. Sci. 1982, 22, 147–158. [Google Scholar] [CrossRef]
- Hœrlé, S.; Mazaudier, F.; Dillmann, Ph.; Santarini, G. Advances in understanding atmospheric corrosion of iron. II. Mechanistic modeling of wet–dry cycles. Corros. Sci. 2004, 46, 1431–1465. [Google Scholar]
- Singh, A.K.; Ericsson, T.; Häggström, L.; Gullman, J. Mössbauer and X-ray diffraction phase analysis of rusts from atmospheric test sites with different environments in Sweden. Corros. Sci. 1985, 25, 931–945. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for iron at 25–300 °C. Corros. Sci. 1996, 38, 2121–2135. [Google Scholar] [CrossRef]
- Marcus, Y. Ion Properties; Marcel Dekker, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Su, G.Q.; Gao, X.H.; Du, L.X.; Zhang, D.Z.; Hu, J.; Liu, Z.G. Influence of Mn on the corrosion behaviour of medium manganese steels in simulated seawater environment. Int. J. Electrochem. Sci. 2016, 11, 9447–9461. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Mohammadi, F.; Alfantazi, A. An electrochemical investigation on the effect of the chloride content on CO2 corrosion of API-X100 steel. Corros. Sci. 2012, 64, 37–43. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. On the theory of CO2 corrosion reactions—Investigating their interrelation with the corrosion products and API-X100 steel microstructure. Corros. Sci. 2014, 85, 380–393. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.X.; Dong, J.H.; Ke, W. Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments. Corros. Sci. 2011, 53, 4187–4192. [Google Scholar] [CrossRef]
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, U. The NBS tables of chemical thermodynamic properties. J. Phys. Chem. Ref. Data 1982, 11, 154–194. [Google Scholar]
- Akhtar, M.J.; Younas, M. Structural and transport properties of nanocrystalline MnFe2O4 synthesized by co-precipitation method. Solid State Sci. 2012, 1536–1542. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Mazarío, E.; Mayoral, A.; Salas, E.; Menéndez, N.; Herrasti, P.; Sánchez-Marcos, J. Synthesis and characterization of manganese ferrite nanoparticles obtained by electrochemical/chemical method. Mater. Des. 2016, 111, 646–650. [Google Scholar] [CrossRef]
- Kimura, M.; Kihira, H.; Ohta, N.; Hashimoto, M.; Senuma, T. Control of Fe(O,OH)6 nano-network structures of rust for high atmospheric-corrosion resistance. Corros. Sci. 2005, 47, 2499–2509. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tzeng, H.J.; Wei, L.I.; Wang, L.H.; Oung, J.C.; Shih, H.C. Corrosion resistance and mechanical properties of low-alloy steels under atmospheric conditions. Corros. Sci. 2005, 47, 1001–1021. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Nishimura, T.; Kodama, T. Clarification of chemical state for alloying elements in iron rust using a binary-phase potential-pH diagram and physical analyses. Corros. Sci. 2003, 45, 1073–1084. [Google Scholar] [CrossRef]
| Samples | C | Si | Mn | P | S | Al | Mo | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Medium-Mn Steel | 0.04 | 0.2 | 5.5 | 0.005 | 0.003 | 0.03 | 0.22 | 0.30 | 0.39 | Bal. |
| Q345 Steel | 0.17 | 0.32 | 1.38 | 0.019 | 0.009 | 0.04 | - | - | - | Bal. |
| Species | ΔG° (298.15 K) (J·mol−1) | Source | Species | ΔG° (298.15 K) (J·mol−1) | Source |
|---|---|---|---|---|---|
| H2 | 0 | [25] | O2 | 0 | [25] |
| H+ | 0 | [25] | H2O | −237,191 | [20] |
| Mn2+ | −228,100 | [20] | Mn(OH)3− | −744,200 | [25] |
| MnFe2O4 | −1,121,790 | [25] | Mn3O4 | −1,283,200 | [25] |
| Fe | 0 | [25] | Fe2+ | −78,900 | [25] |
| FeO | −256,354 | [25] | Fe2O3 | −740,986 | [25] |
| FeOOH | −485,300 | [19] | Fe3O4 | −1,015,450 | [25] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, G.; Gao, X. Comparison of Medium Manganese Steel and Q345 Steel on Corrosion Behavior in a 3.5 wt % NaCl Solution. Materials 2017, 10, 938. https://doi.org/10.3390/ma10080938
Su G, Gao X. Comparison of Medium Manganese Steel and Q345 Steel on Corrosion Behavior in a 3.5 wt % NaCl Solution. Materials. 2017; 10(8):938. https://doi.org/10.3390/ma10080938
Chicago/Turabian StyleSu, Guanqiao, and Xiuhua Gao. 2017. "Comparison of Medium Manganese Steel and Q345 Steel on Corrosion Behavior in a 3.5 wt % NaCl Solution" Materials 10, no. 8: 938. https://doi.org/10.3390/ma10080938
APA StyleSu, G., & Gao, X. (2017). Comparison of Medium Manganese Steel and Q345 Steel on Corrosion Behavior in a 3.5 wt % NaCl Solution. Materials, 10(8), 938. https://doi.org/10.3390/ma10080938




