Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy
Abstract
1. Introduction
2. Results and Discussion
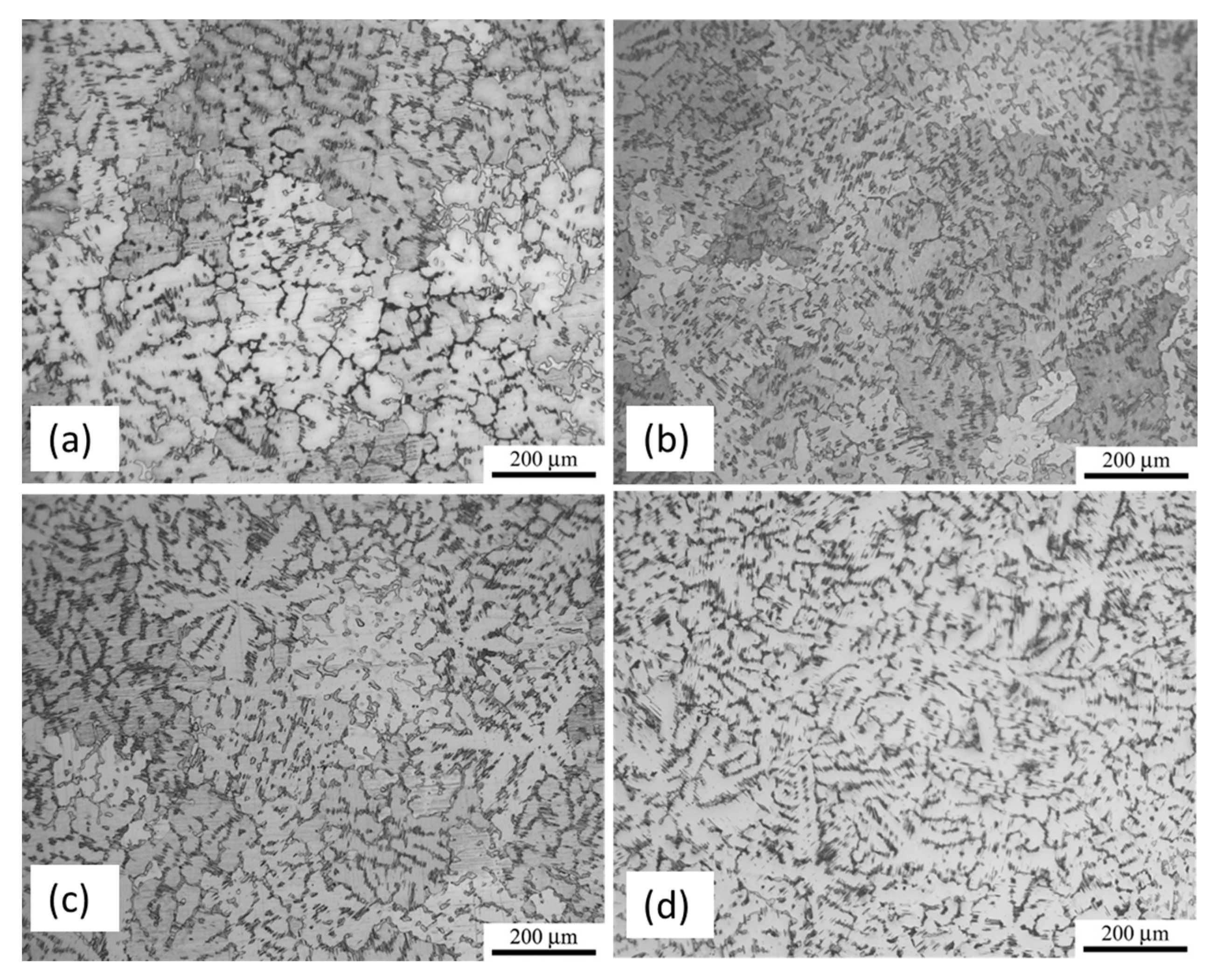
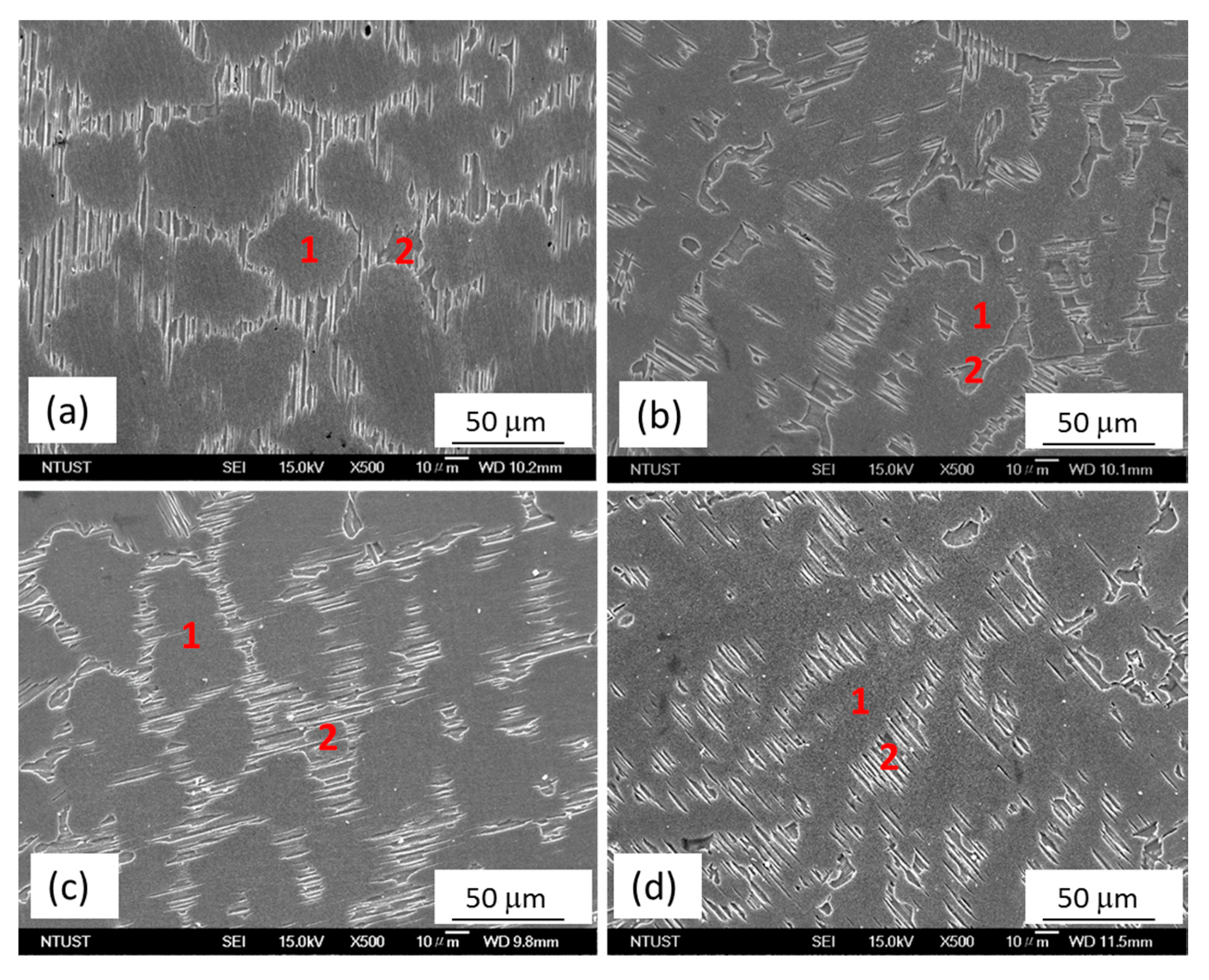
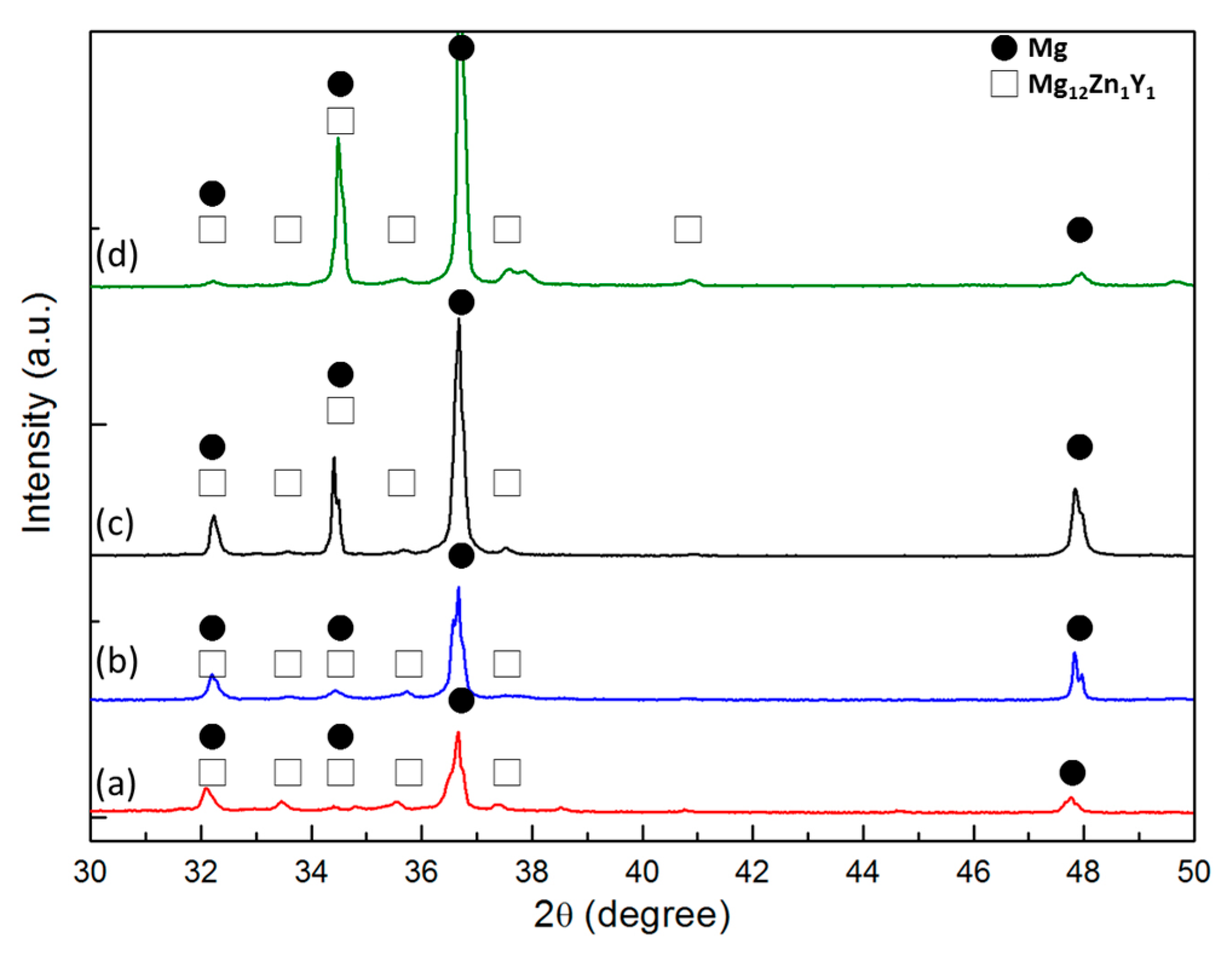
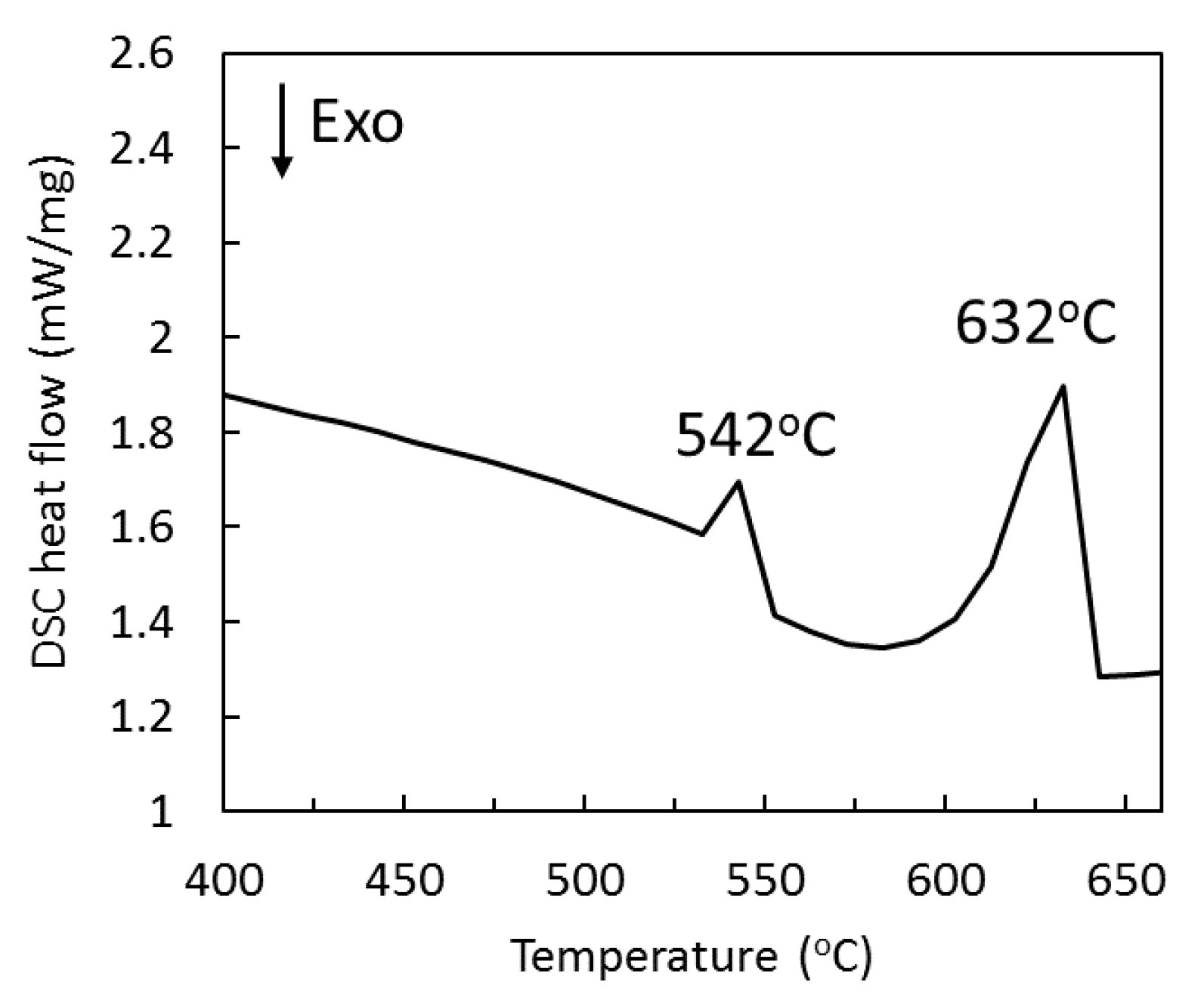
2.1. Microstructural Characterization
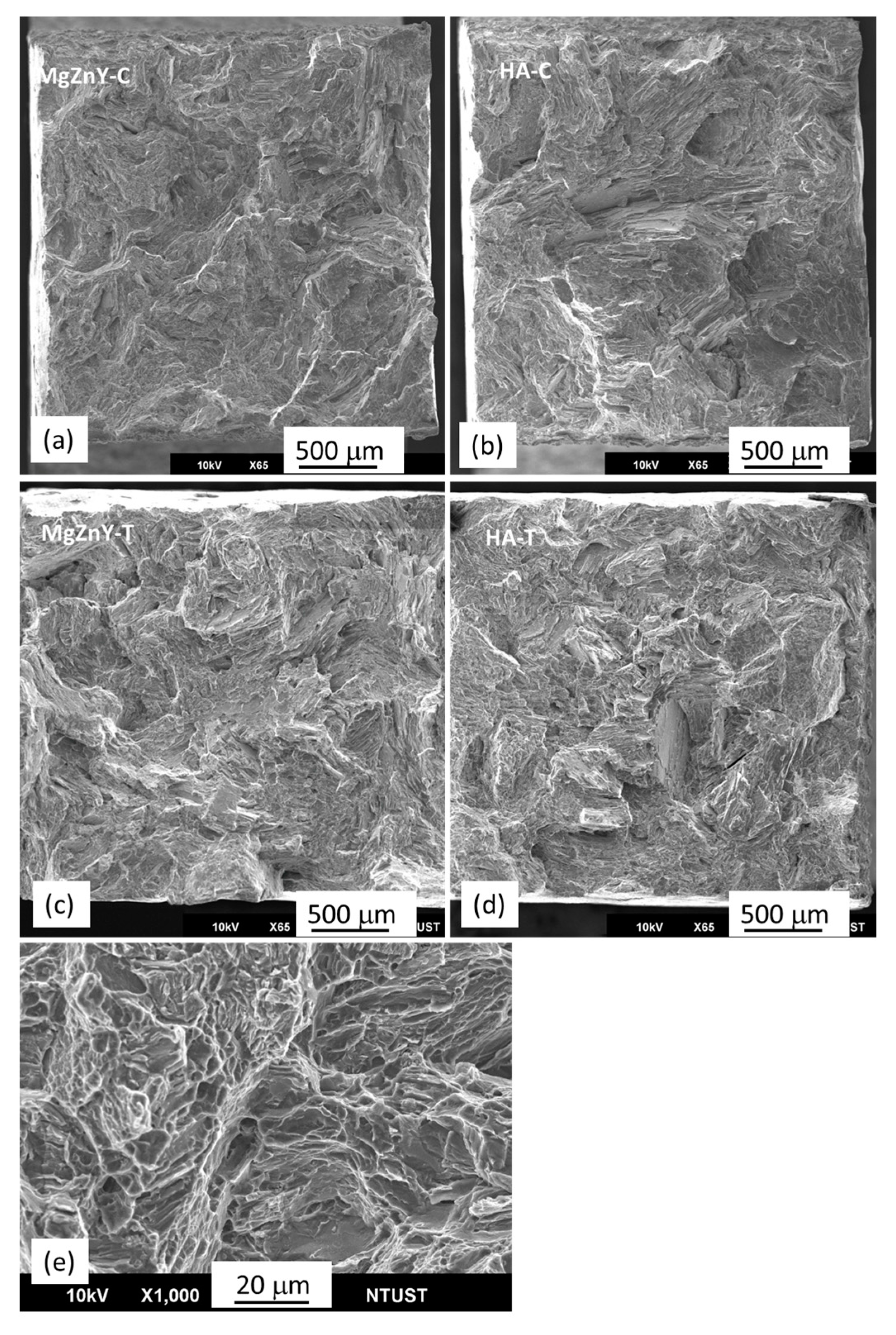
2.2. Mechanical Properties
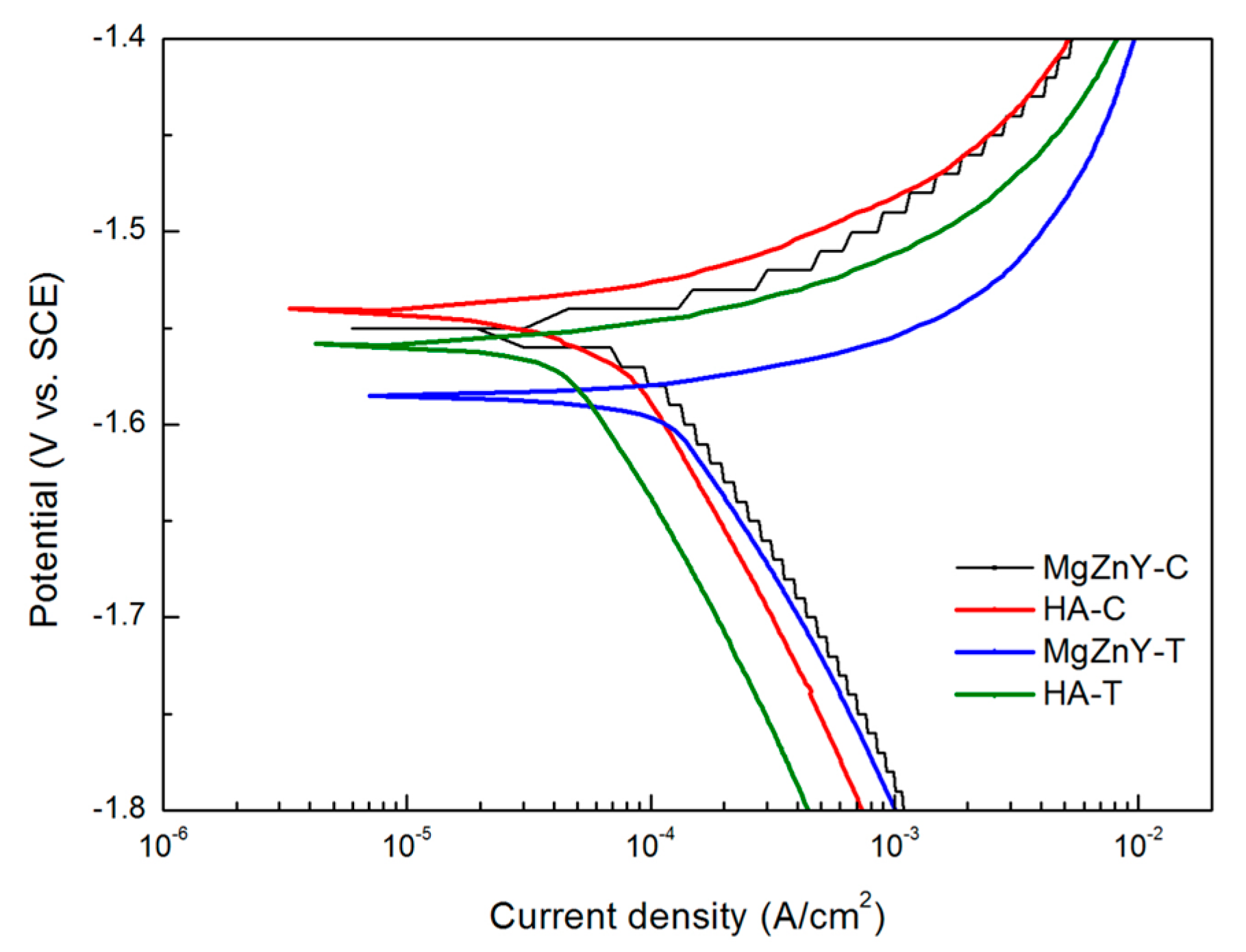
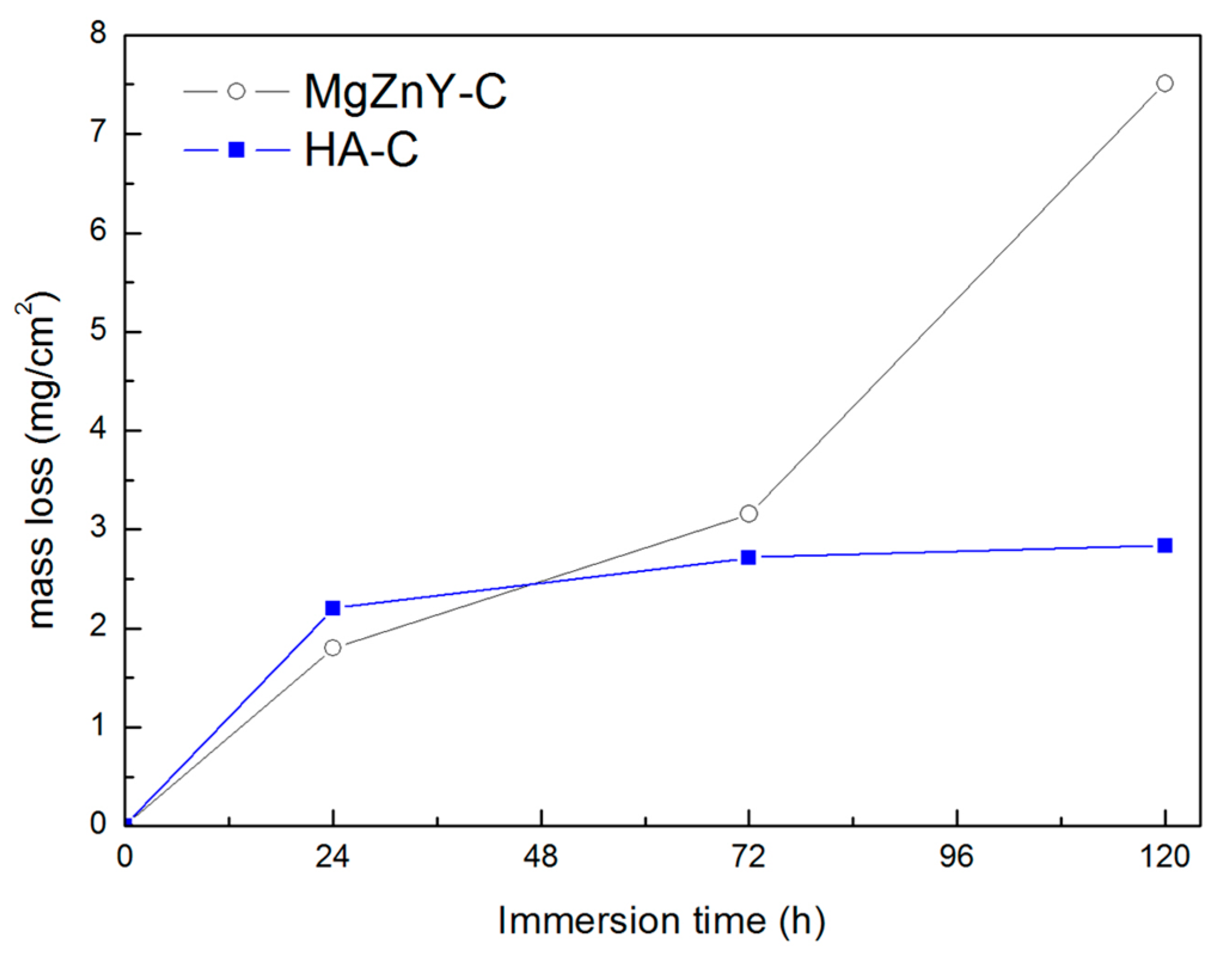
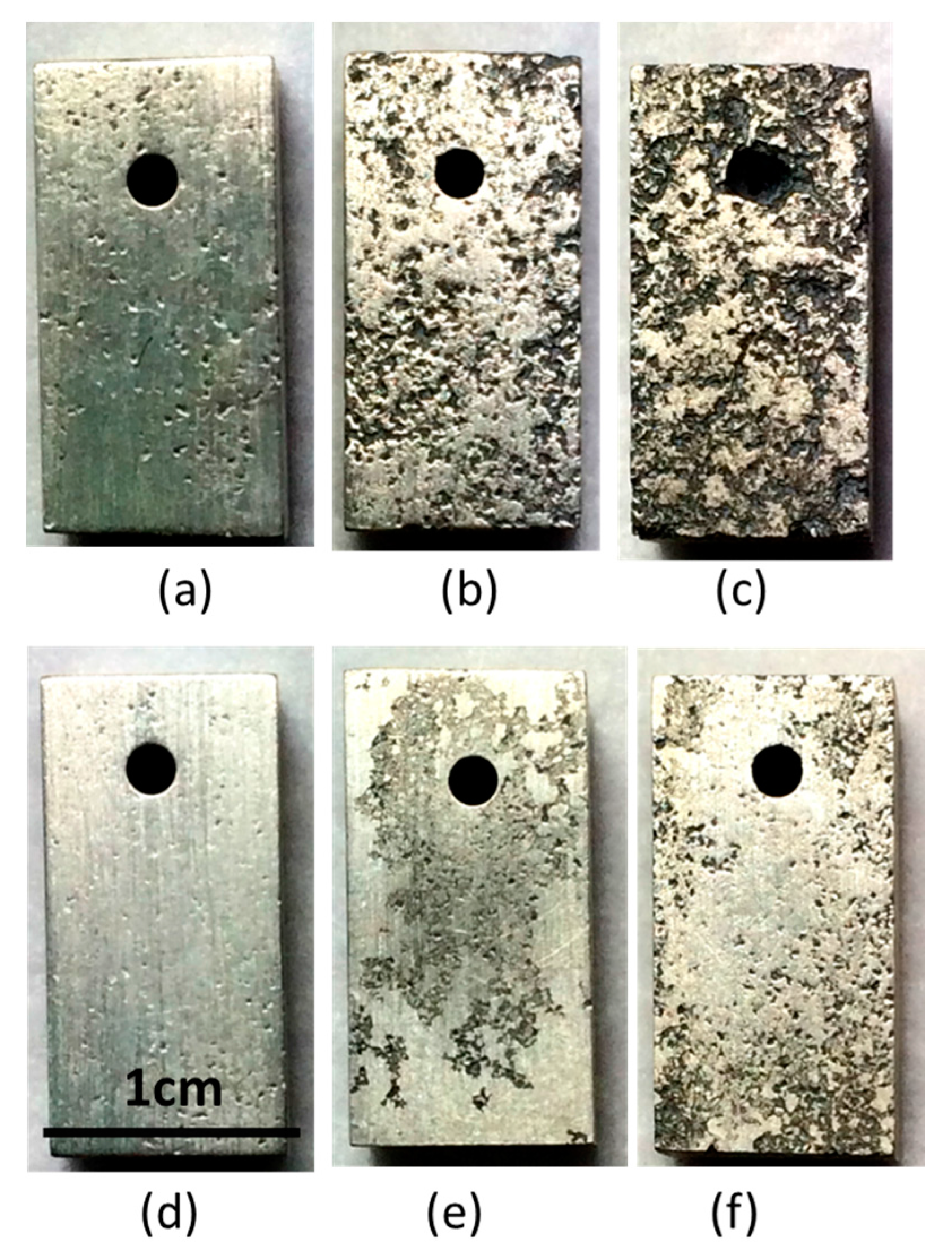
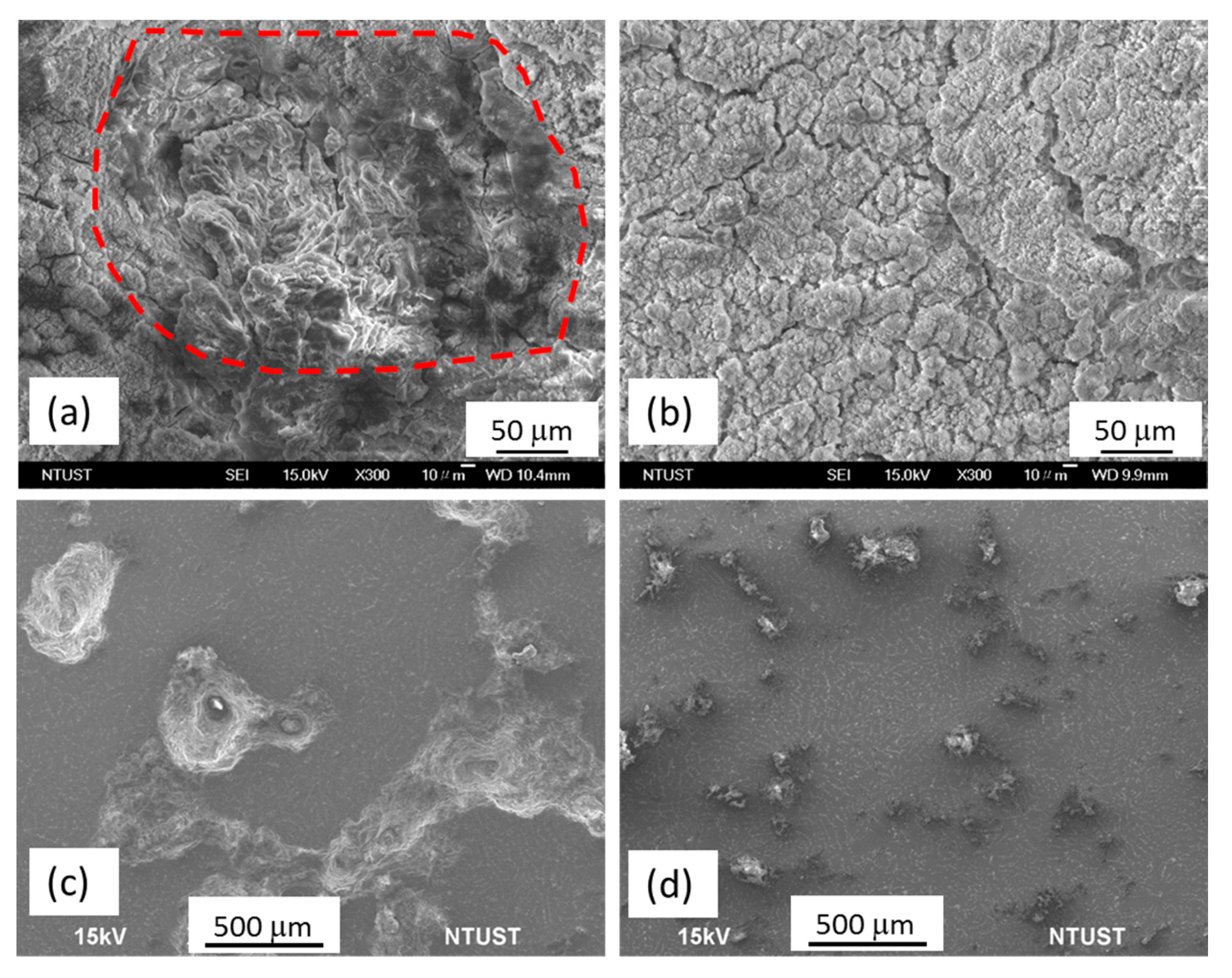
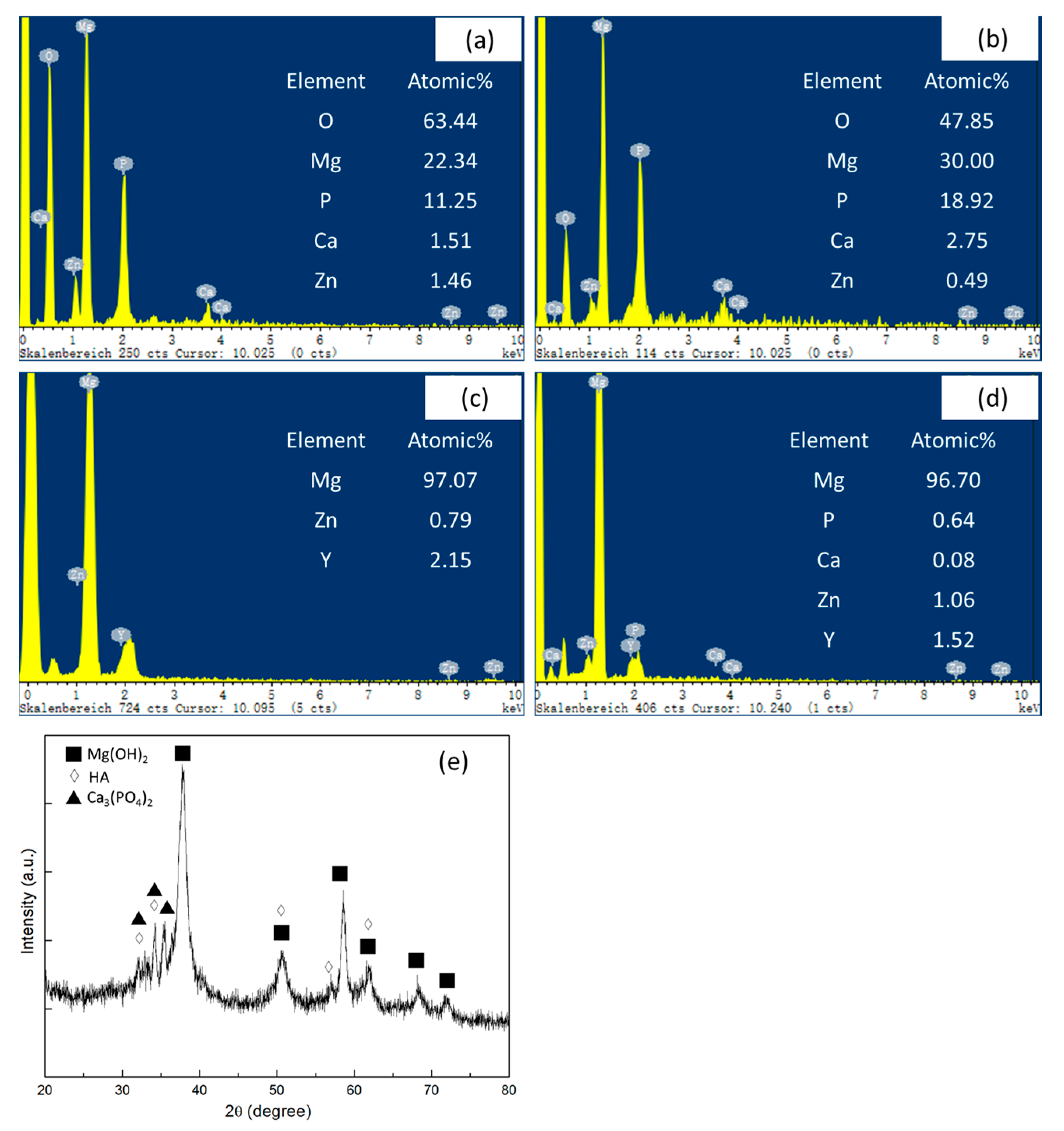
2.3. Corrosion Behavior
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, F.; Zhao, M.; Gao, C.; Feng, P.; Yang, Y.; Yang, S.; Shuai, C. Rare earth element yttrium modified Mg-Al-Zn alloy: Microstructure, degradation properties and hardness. Materials 2017, 10, 477. [Google Scholar] [CrossRef]
- Doležal, P.; Zapletal, J.; Fintová, S.; Trojanová, Z.; Greger, M.; Roupcová, P.; Podrábský, T. Influence of processing techniques on microstructure and mechanical properties of a biodegradable Mg-3Zn-2Ca alloy. Materials 2016, 9, 880. [Google Scholar] [CrossRef]
- Brar, H.S.; Wong, J.; Manuel, M.V. Investigation of the mechanical and degradation properties of Mg-Sr and Mg-Zn-Sr alloys for use as potential biodegradable implant materials. J. Mech. Behav. Biomed. 2012, 7, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Vlček, M.; Lukáč, F.; Kudrnová, H.; Smola, B.; Stulíková, I.; Luczak, M.; Szakács, G.; Hort, N.; Willumeit-Römer, R. Microhardness and in vitro corrosion of heat-treated Mg-Y-Ag biodegradable alloy. Materials 2017, 10, 55. [Google Scholar] [CrossRef]
- Erinc, M.; Sillekens, W.H.; Mannens, M.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant materials. In Magnesium Technology, Proceedings of TMS Annual Meeting, San Francisco, CA, USA, 16 February–19 February 2009; Nyberg, E.A., Agnew, S.R., Neelameggham, N.R., Pekguleryuz, M.O., Eds.; TMS: Pittsburgh, PA, USA, 2009; pp. 209–214. [Google Scholar]
- Ding, W. opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.W.; Shan, D.Y.; Han, E.H. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Mater. Lett. 2008, 62, 3276–3279. [Google Scholar] [CrossRef]
- Salman, S.A.; Kuroda, K.; Okido, M. Preparation and characterization of hydroxyapatite coating on AZ31 Mg alloy for implant applications. Bioinorg. Chem. Appl. 2013, 2013, 175756. [Google Scholar] [CrossRef] [PubMed]
- Mukhametkaliyev, T.M.; Surmeneva, M.A.; Vladescu, A.; Cotrut, C.M.; Braic, M.; Dinu, M.; Vranceanu, M.D.; Pana, I.; Mueller, M.; Surmenev, R.A. A biodegradable AZ91 magnesium alloy coated with a thin nanostructured hydroxyapatite for improving the corrosion resistance. Mater. Sci. Eng. C 2017, 75, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, L.L.; Xu, J. Biodegradable Mg-Zn-Y alloys with long-period stacking ordered structure: Optimization for mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 18, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, M.; Yang, M.; Wei, J.; Liu, D. In vitro corrosion resistance and cytocompatibility of nano-hydroxyapatite reinforced Mg-Zn-Zr composites. J. Mater. Sci. Mater. Med. 2010, 21, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, C.Q.; Zhang, Z.M. Microstructures, corrosion and mechanical properties of as-cast Mg-Zn-Y-(Gd) alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 2172–2180. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, L.L.; Xu, J. Mg-Zn-Y Alloys with long-period stacking ordered structure: In vitro assessments of biodegradation behavior. Mater. Sci. Eng. C 2013, 33, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, J.; Cheng, W.; Chen, C.; Kang, J. Corrosion behavior of Mg-Zn-Y alloy with long-period stacking ordered structures. J. Mater. Sci. Technol. 2012, 28, 1157–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Ba, Z.; Wang, Z.; Wu, Y.; Xue, Y. Effect of LPSO structure on mechanical properties and corrosion behavior of as-extruded GZ51K magnesium alloy. Mater. Lett. 2016, 163, 250–253. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Chen, F.; Wu, Y.; Wang, Z.; Wang, Q. Relation between LPSO structure and biocorrosion behavior of biodegradable GZ51K alloy. Mater. Lett. 2015, 138, 212–215. [Google Scholar] [CrossRef]
- Xu, D.; Han, E.H.; Xu, Y. Effect of long-period stacking ordered phase on microstructure, mechanical property and corrosion resistance of Mg alloys: A review. Prog. Mater. Sci. Mater. Int. 2016, 26, 117–128. [Google Scholar] [CrossRef]
- Khalil, K.A. A new-developed nanostructured Mg/HAp nanocomposite by high frequency induction heat sintering process. IOP Conf. Ser. Mater. Sci. Eng. 2012, 40, 012031. [Google Scholar] [CrossRef]
- Campo, R.D.; Savoini, B.; Muñoz, A.; Monge, M.A.; Garcés, G. Mechanical properties and corrosion behavior of Mg-HAp Composites. J. Mech. Behav. Biomed. Mater. 2014, 39, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Feyerabend, F.; Maier, P.; Fisher, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, M.; Bi, Y.; Liu, D.; Wei, J. The effect of nano-hydroxyapatite on the microstructure and properties of Mg-3Zn-0.5Zr alloy. J. Compos. Mater. 2014, 48, 825–834. [Google Scholar] [CrossRef]
- Chen, B.; Lin, D.; Zeng, X.; Liu, C. Effects of yttrium and zinc addition on the microstructure and mechanical properties of Mg-Zn-Y alloys. J. Mater. Sci. 2010, 45, 2510–2517. [Google Scholar] [CrossRef]
- Su, Z.G.; Li, R.G.; An, J.; Lu, Y. Effect of rolling temperature on the microstructures and mechanical properties of Mg97Zn1Y2 magnesium alloy. J. Mater. Eng. Perform. 2010, 19, 70–76. [Google Scholar] [CrossRef]
- Yamasaki, M.; Hashimoto, K.; Hagihara, K.; Kawamura, Y. Effect of multimodal microstructure evolution on mechanical properties of Mg-Zn-Y extruded alloy. Acta Mater. 2011, 59, 3646–3658. [Google Scholar] [CrossRef]
- Chen, B.; Lin, D.; Zeng, X.; Lu, C. Effect of solid solution treatment on microstructure and mechanical properties of Mg97Zn1Y2 alloy. J. Mater. Eng. Perform. 2013, 22, 523–527. [Google Scholar] [CrossRef]
- Oñorbe, E.; Garcés, G.; Pérez, P.; Adeva, P. Effect of the LPSO volume fraction on the microstructure and mechanical properties of Mg-Y2−x-Znx alloys. J. Mater. Sci. 2012, 47, 1085–1093. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Que, Z.; Cheng, W.; Xu, J.; Kang, J. 18R and 14H long-period stacking ordered structures in the Mg93.96Zn2Y4Sr0.04 alloy and the modification effect of Sr on X-phase. Mater. Sci. Eng. A 2012, 552, 81–88. [Google Scholar] [CrossRef]
- Wang, B.S.; Xiong, S.M.; Liu, Y.B. Tensile fracture of as-cast and hot rolled Mg-Zn-Y alloy with long-period stacking phase. Trans. Nonferrous Met. Soc. China 2010, 20, s488–s492. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Subramanian, S.; Raj, B.; Ravi, K.R. Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg-Zn-Ca bulk metallic glass for biomedical applications. Mater. Des. 2015, 86, 829–835. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Fu, H.; Cai, X.; Wang, Y.; Liu, B.; Xu, Z. Degradation behavior of Mg-based biomaterials containing different long-period stacking ordered phases. Sci. Rep. 2014, 4, 3620. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.; Zhang, J.H.; Yin, T.T.; Zhang, L.; Guo, X.Y.; Peng, Q.M.; Zhang, M.L.; Wu, R.Z. Influence of biocorrosion on microstructure and mechanical properties of deformed Mg-Y-Er-Zn biomaterial containing 18R-LPSO phase. J. Mech. Behav. Biomed. Mater. 2013, 28, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.B.; Chen, M.F.; Ye, X.Y. Fabrication and corrosion behavior of HA/Mg-Zn biocomposites. Front. Mater. Sci. China 2010, 4, 139–144. [Google Scholar] [CrossRef]


| Element | Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| MgZnY-C | MgZnY-T | HA-C | HA-T | |||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| Mg | 97.9 | 87.8 | 97.4 | 86.9 | 97.6 | 88.7 | 97.9 | 87.4 |
| Zn | 1.3 | 5.0 | 1.2 | 5.9 | 1.0 | 5.1 | 0.7 | 5.1 |
| Y | 0.8 | 7.2 | 1.4 | 7.1 | 1.4 | 6.2 | 1.4 | 7.4 |
| Sample | α-Mg (%) | LPSO (%) | Grain Size (μm) | YS (MPa) | UTS (MPa) | Elongation (%) |
|---|---|---|---|---|---|---|
| MgZnY-C | 75.1 | 24.9 | 243 ± 10 | 126 ± 13 | 172 ± 9 | 9.0 ± 0.6 |
| MgZnY-T | 77.8 | 22.2 | 254 ± 16 | 108 ± 6 | 179 ± 3 | 14.0 ± 2.4 |
| HA-C | 82.3 | 17.7 | 232 ± 19 | 117 ± 2 | 161 ± 2 | 9.2 ± 0.2 |
| HA-T | 82.2 | 17.8 | 241 ± 7 | 109 ± 9 | 163 ± 18 | 12.0 ± 5.6 |
| Sample | α-Mg | LPSO |
|---|---|---|
| MgZnY-C | 73 ± 3 | 118 ± 6 (18R) |
| MgZnY-T | 72 ± 3 | 109 ± 3 (18R + 14H) |
| HA-C | 76 ± 3 | 120 ± 5 (18R) |
| HA-T | 75 ± 4 | 107 ± 6 (18R + 14H) |
| Sample | Ecorr (V) | Icorr (μA/cm2) | Corrosion Rate (mm/year) |
|---|---|---|---|
| MgZnY-C | −1.54 | 118.63 | 2.93 |
| MgZnY-T | −1.57 | 162.67 | - |
| HA-C | −1.55 | 65.00 | 1.11 |
| HA-T | −1.58 | 76.17 | - |
| Sample | Mg97Zn1Y2 (MgZnY-C) | Mg97Zn1Y2-0.5 wt% HA (HA-C) | |||||
|---|---|---|---|---|---|---|---|
| Mg | Zn | Y | Mg | Zn | Y | n-HA | |
| Nominal composition (wt%) | 90.7 | 2.4 | 6.9 | 90.2 | 2.4 | 6.9 | 0.5 |
| Analyzed composition (wt%) | 91.7 | 1.9 | 6.4 | 90.4 | 2.1 | 7.1 | 0.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.; Lu, C.-T.; Chen, S.-H.; Ou, K.-L. Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy. Materials 2017, 10, 855. https://doi.org/10.3390/ma10080855
Chiu C, Lu C-T, Chen S-H, Ou K-L. Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy. Materials. 2017; 10(8):855. https://doi.org/10.3390/ma10080855
Chicago/Turabian StyleChiu, Chun, Chih-Te Lu, Shih-Hsun Chen, and Keng-Liang Ou. 2017. "Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy" Materials 10, no. 8: 855. https://doi.org/10.3390/ma10080855
APA StyleChiu, C., Lu, C.-T., Chen, S.-H., & Ou, K.-L. (2017). Effect of Hydroxyapatite on the Mechanical Properties and Corrosion Behavior of Mg-Zn-Y Alloy. Materials, 10(8), 855. https://doi.org/10.3390/ma10080855






