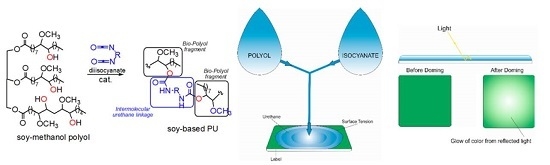
Preparation and Characterization of Soybean Oil-Based Polyurethanes for Digital Doming Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Fossil Polyol
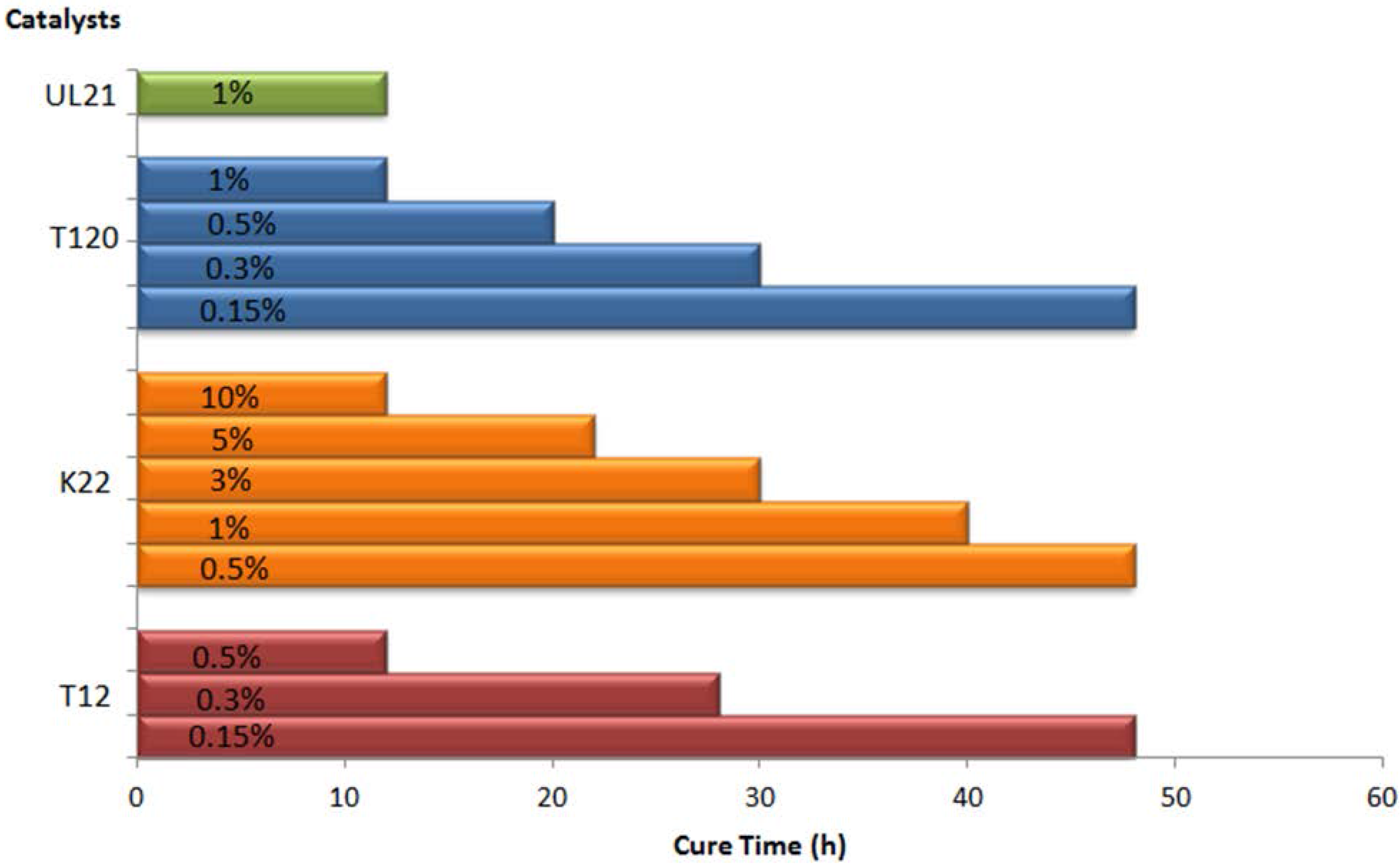
2.2. Catalyst and Loading Selection
2.3. Selection of Diisocyanate
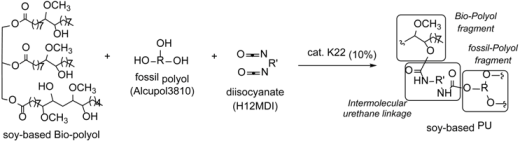
2.4. Formulations of Bio-Based Polyurethane
3. Experimental
3.1. Materials and Methods
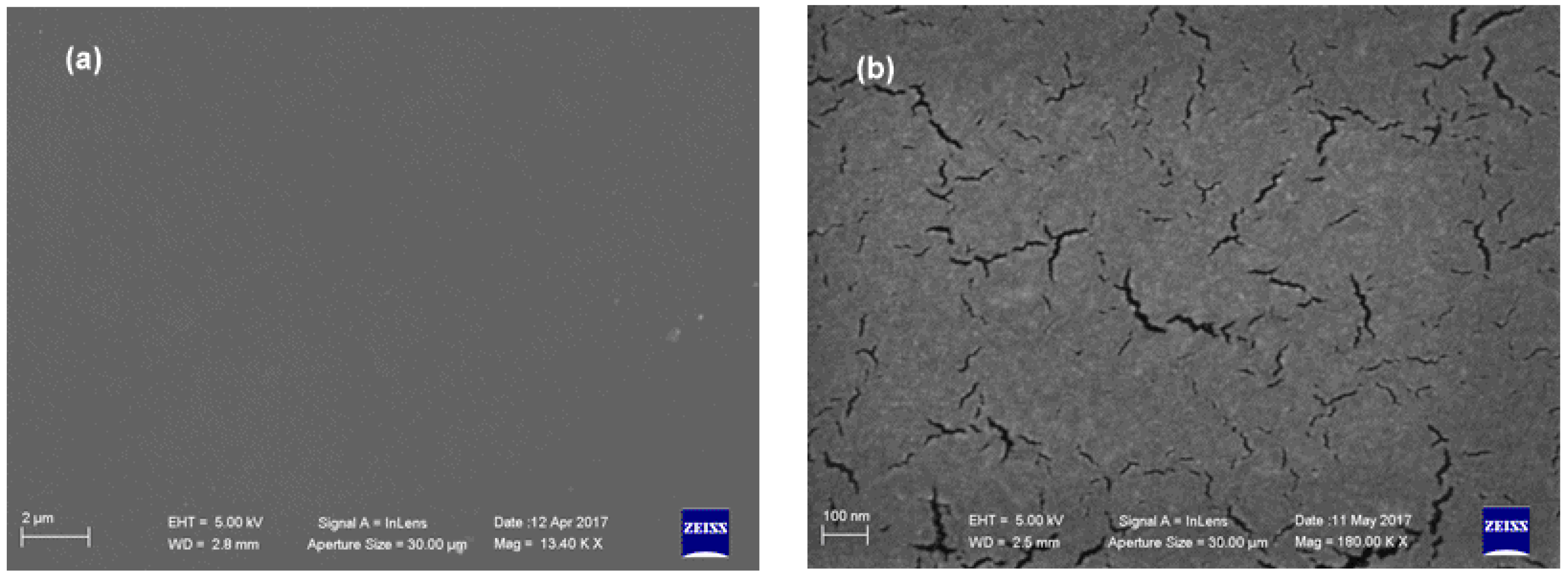
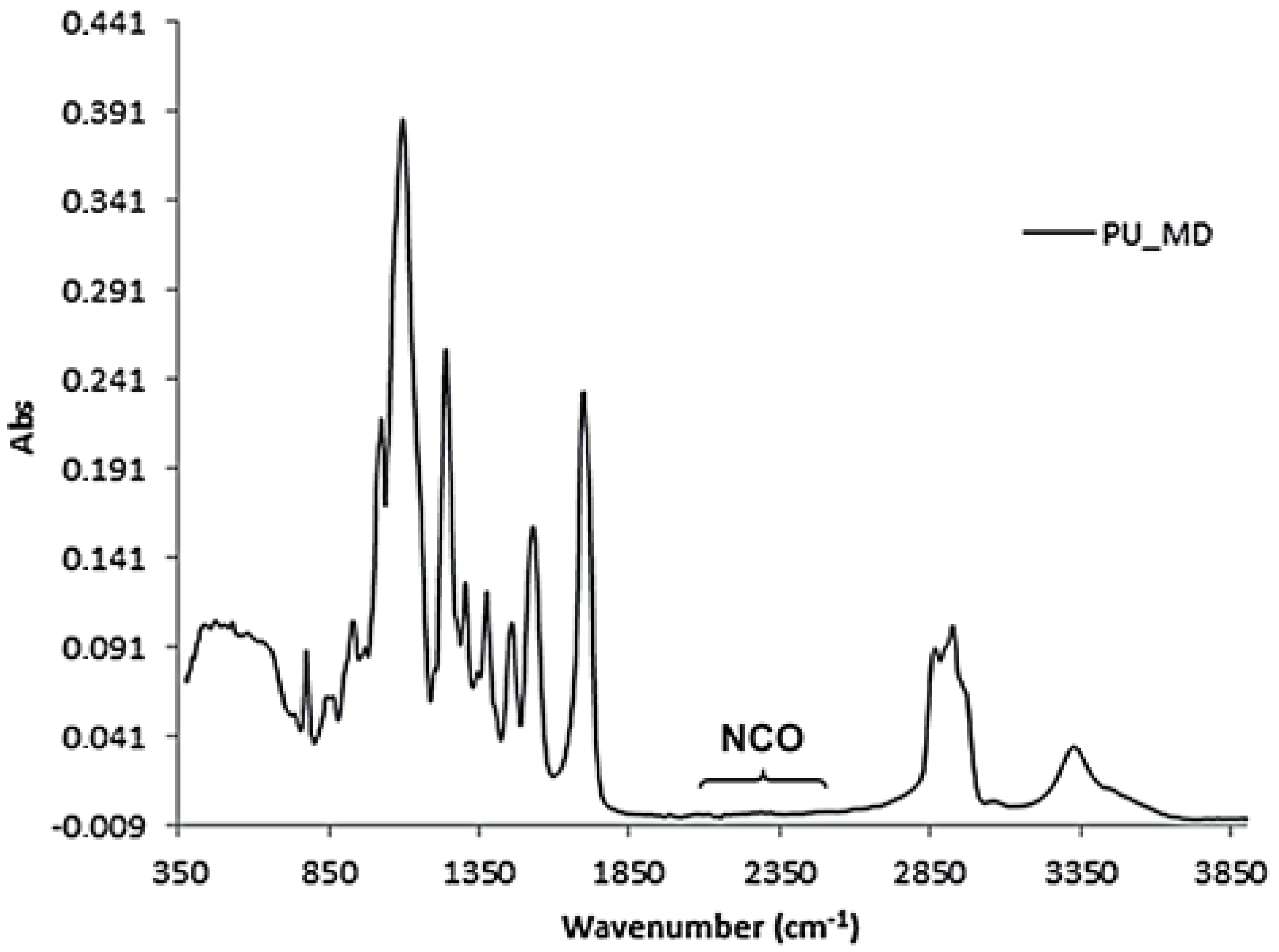
3.2. Characterization Techniques
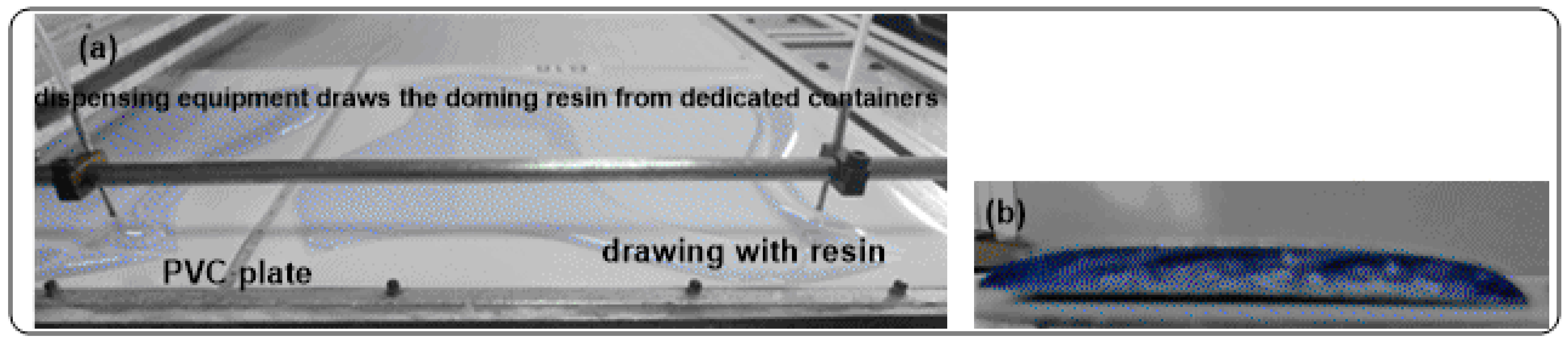
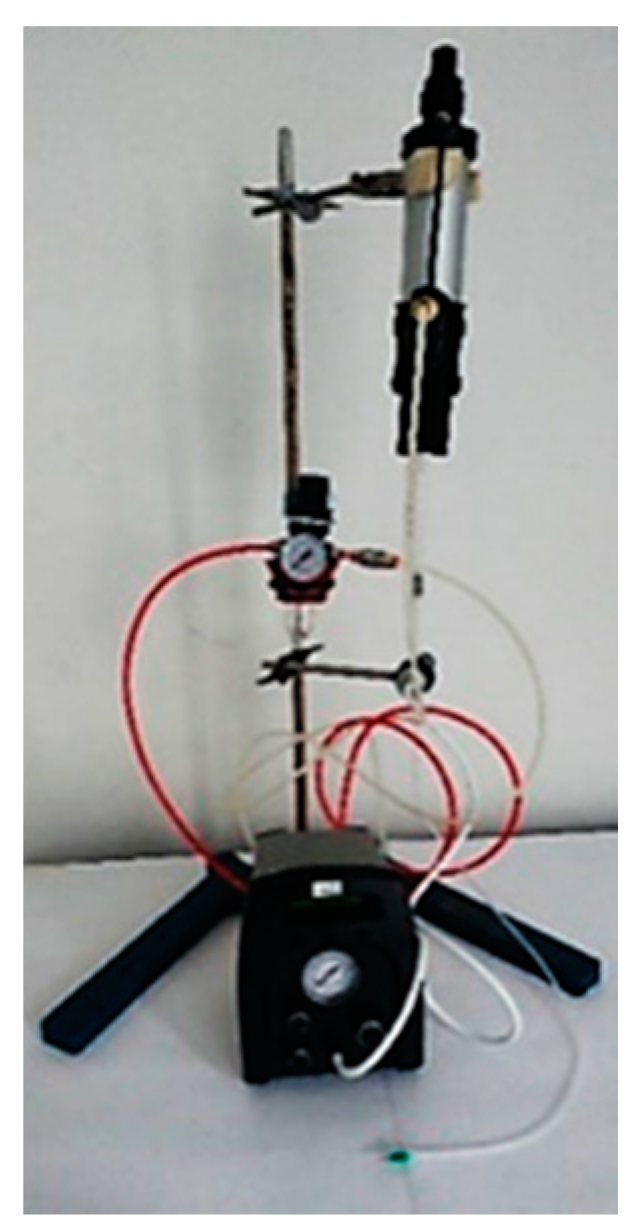
3.3. Digital Doming Polyurethane Synthesis
3.4. Bio-PU Synthesis and Determination of Bio-Carbon Percentage
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.; Luo, X.; Hu, S. Bio-Based Polyols and Polyurethanes; Springer: Cham/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015. [Google Scholar]
- Wicks, Z.W.; Jones, F.N.; Pappas, S.P. Organic Coatings Science and Technology, 2nd ed.; Wiley Interscience: Hoboken, NJ, USA, 1999; p. 180. [Google Scholar] [CrossRef]
- Stoy, D.; Freitag, W.; Beuschel, G. Resins for Coatings: Chemistry, Properties and Applications; Hanser Publishers: Munich, Germany, 1996; p. 458. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Chuang, W.-T.; Jeng, U.-S.; Hsu, S.-H. Preparation, Characterization, and Mechanism for Biodegradable and Biocompatible Polyurethane Shape Memory Elastomers. ACS Appl. Mater. Interfaces 2017, 9, 5419–5429. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnama, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Silva, A.L.; Bordado, J.C. Recent Developments in Polyurethane Catalysis: Catalytic Mechanisms Review. Catal. Rev. 2004, 46, 31–51. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Hessell, E.T. Catalysis of the isocyanate-hydroxyl reaction by non-tin catalysts. Prog. Org. Coat. 1999, 35, 19–29. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of Polyurethanes Using Organocatalysis: A Perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Website of ECHA. Available online: https://echa.europa.eu (accessed on 10 October 2016).
- Rosenfield, B.P. Raising product appeal through doming. Screen Print. 2003, 93, 36–39. [Google Scholar]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-Based Adhesives and Evaluation for Wood Composites Application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Petrovic, Z.S. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Petrovic, Z.S.; Zhang, W.; Zlatanic, A.; Lava, C.C.; Ilavsky, M. Effect of OH/NCO molar ratio on properties of soy-based polyurethane networks. J. Polym. Environ. 2002, 10, 5–12. [Google Scholar] [CrossRef]
- Zhan, C.; Kessler, M.R. Bio-based Polyurethane Foam Made from Compatible Blends of Vegetable-Oil-based Polyol and Petroleum-based Polyol. ACS Sustain. Chem. Eng. 2015, 3, 743–749. [Google Scholar] [CrossRef]
- Allauddin, S.; Somisetti, V.; Ravinder, T.; Bvsk, R.; Narayan, R.; Raju, K.V.S.N. One-pot synthesis and physicochemical properties of high functionality soy polyols and their polyurethane-Urea coatings. Ind. Crops Prod. 2016, 85, 361–371. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kuranska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Bloise, M.E.; Carbone, L.; Colafemmina, G.; D’Accolti, L.; Mazzetto, S.E.; Vasapollo, G.; Mele, G. First Example of a Lipophilic Porphyrin-Cardanol Hybrid Embedded in a Cardanol Based Micellar Nanodispersion. Molecules 2012, 12, 12252–12261. [Google Scholar] [CrossRef] [PubMed]
- Dentuto, P.L.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; D’Accolti, L.; Trevithick-Sutton, C.C.; Foote, C.S. Effect of cyclodextrins on the physicochemical properties of chlorophyll a in aqueous solution. J. Phys. Chem. B 2005, 109, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Casiello, M.; Monopoli, A.; Cotugno, P.; Sportelli, M.C.; Picca, R.A.; Cioffi, N.; Dell’Anna, M.M.; Nacci, A. Ionic liquids/ZnO nanoparticles as recyclable catalyst for polycarbonate depolymerization. J. Mol. Catal. A Chem. 2017, 426, 107–116. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Giambastiani, G.; Mangone, A.; Milella, A.; Tuci, G.; Fusco, C. Tunable Epoxidation of Single-Walled Carbon Nanotubes by Isolated Methyl (trifluoromethyl) dioxirane. Eur. J. Org. Chem. 2014, 1666–1671. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Filardi, R.; Tommasi, I.; Fusco, C. Oxidative cleavage of lactams in water using dioxiranes: An expedient and environmentally-safe route to ω-nitro acids. Tetrahedron Lett. 2013, 54, 515–517. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Fusco, C.; Curci, R. Selective Hydroxylation of Methane by Dioxiranes under Mild Conditions. Org. Lett. 2011, 13, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- Pantone, V.; Annese, C.; Fusco, C.; Fini, P.; Nacci, A.; Russo, A.; D’Accolti, L. One-Pot Conversion of Epoxidized Soybean Oil (ESO) into Soy-Based Polyurethanes by MoCl2O2 Catalysis. Molecules 2017, 22, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Botrie, A.; Deng, Y.; Foucher, D.; Cooke, J. Silylated Polyurethane Moisture Cured Doming Resins. Patent US20060251902 A1, 9 May 2005. [Google Scholar]
- Khan, M.A.; Reynolds, N.; Williams, G.; Kendall, K.N. Processing of thermoset prepregs for high-volume applications and their numerical analysis using superimposed finite elements. Compos. Struct. 2015, 131, 917–926. [Google Scholar] [CrossRef]
- Antunes, A.; Henriques, A.; Lima, F.; Ferra, J.; Jorge Martins, J.; Carvalho, L.; Magalhales, F.D. Postformable and Self-Healing Finish Foil Based on Polyurethane Impregnated Paper. Ind. Eng. Chem. Res. 2016, 55, 12376–12386. [Google Scholar] [CrossRef]
- Dörr, D.; Schirmaier, F.J.; Henning, F.; Kärger, L. A viscoelastic approach for modeling bending behavior in finite element forming simulation of continuously fiber reinforced composites. Compos. Part A Appl. Sci. Manuf. 2017, 94, 113–123. [Google Scholar] [CrossRef]
- HyeLin, K.; YoungHee, L.; JungSoo, K.; ChaCheol, P.; Hyun, P.; HoHwan, C.; HanDo, K. Preparation and properties of crosslinkable waterborne polyurethane and polyurethane-acrylic hybrid emulsions and their crosslinked polymers. J. Polym. Res. 2016, 23, 240–251. [Google Scholar] [CrossRef]
- Alves, P.; Coelho, J.F.J.; Haack, J.; Rota, A.; Bruinink, A.; Gil, M.H. Surface modification and characterization of thermoplastic polyurethane. Eur. Polym. J. 2009, 45, 1412–1419. [Google Scholar] [CrossRef]
- Several International Agency Audit the Bio Composition: USDA (United States Department of Agriculture), DIN CERTCO (Germany). Available online: https://www.vincotte.com/home (accessed on 3 September 2015).
- Christman, D.L.; Merkl, B.A. Trimerization Catalysts and Organomercury Compounds as Co-Cat Alysts for the Preparation of NonCellular Polyurethane Elastomers. U.S. Patent 4,438,248, 20 March 1984. [Google Scholar]
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K. Blocked isocyanates: From analytical and experimental considerations to non-polyurethane applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef]
- Lozada, Z.; Suppes, G.J.; Tu, Y.-C.; Hsieh, F.-H. Soy-Based Polyols from Oxirane Ring Opening by Alcoholysis Reaction. J. Appl. Polym. Sci. 2009, 113, 2552–2560. [Google Scholar] [CrossRef]
- Myers, N. Impact of curing conditions on the appearance of sealant before and after artificial weathering. In Science and Technology of Building Seals, Sealants, Glazing, and Waterproofing; ASTM Interbational: West Conshohocken, PA, USA, 1992; pp. 9–21. [Google Scholar] [CrossRef]
- ASTM D445-17a, Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- EN ISO 1675: Standard Test Method for Density. Available online: https://www.iso.org/standard/6290.html (accessed on 15 December 2016).
- EN ISO 661: Standard Method for Iodine Number. Available online: https://www.iso.org/standard/38145.html (accessed on 5 September 2016).
- ASTM D1652-11e1: Standard Test Method for Epoxy Content of Epoxy Resins; ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- ASTM D4274-16: Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols; ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]

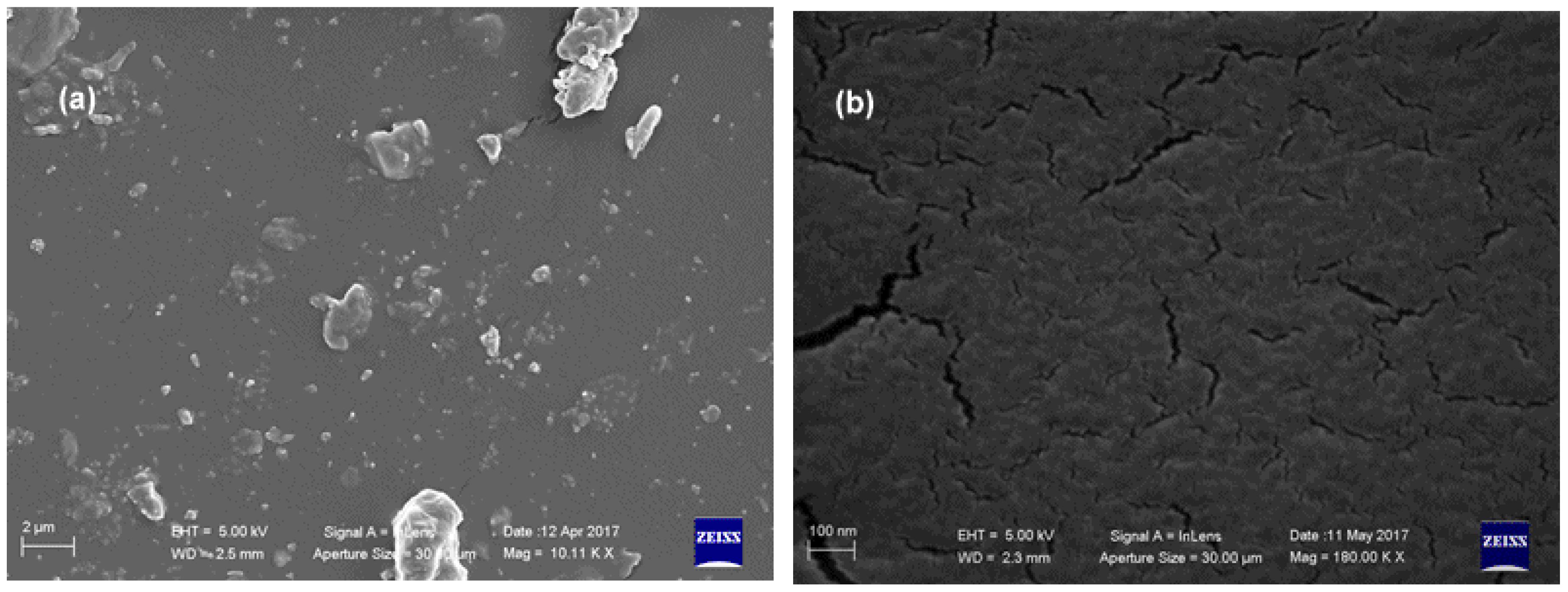
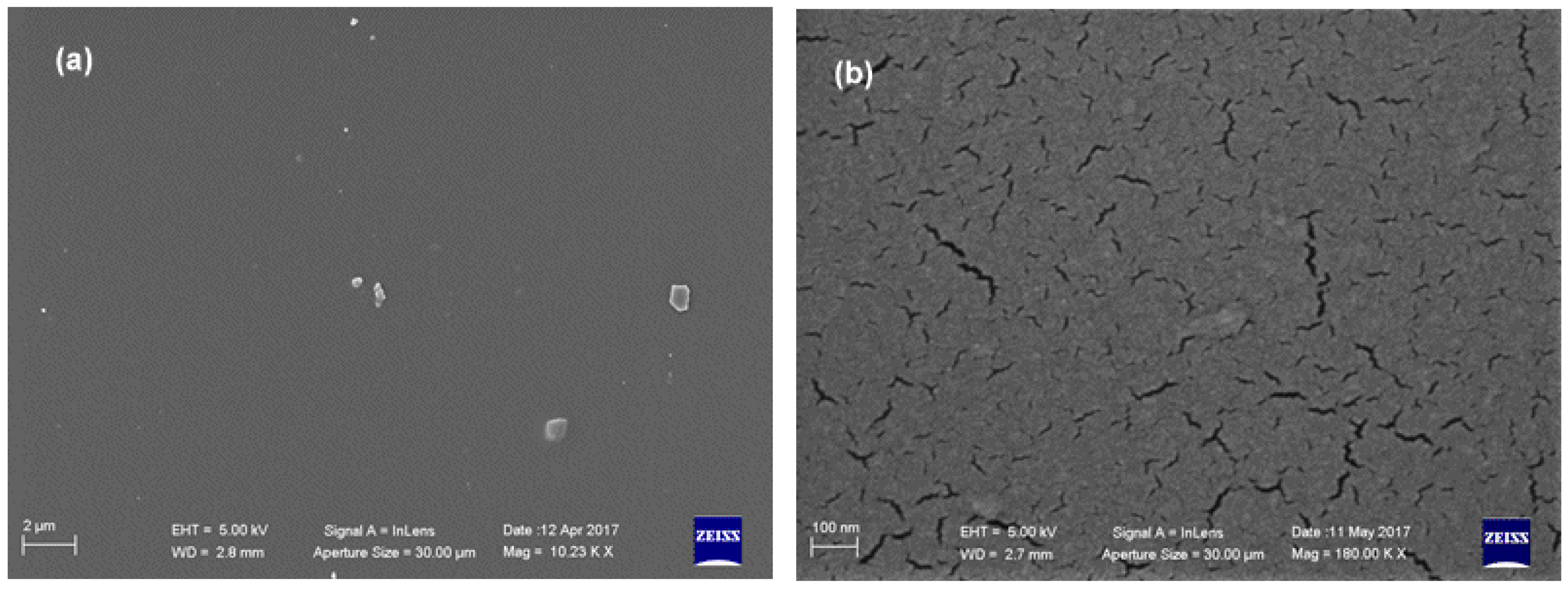

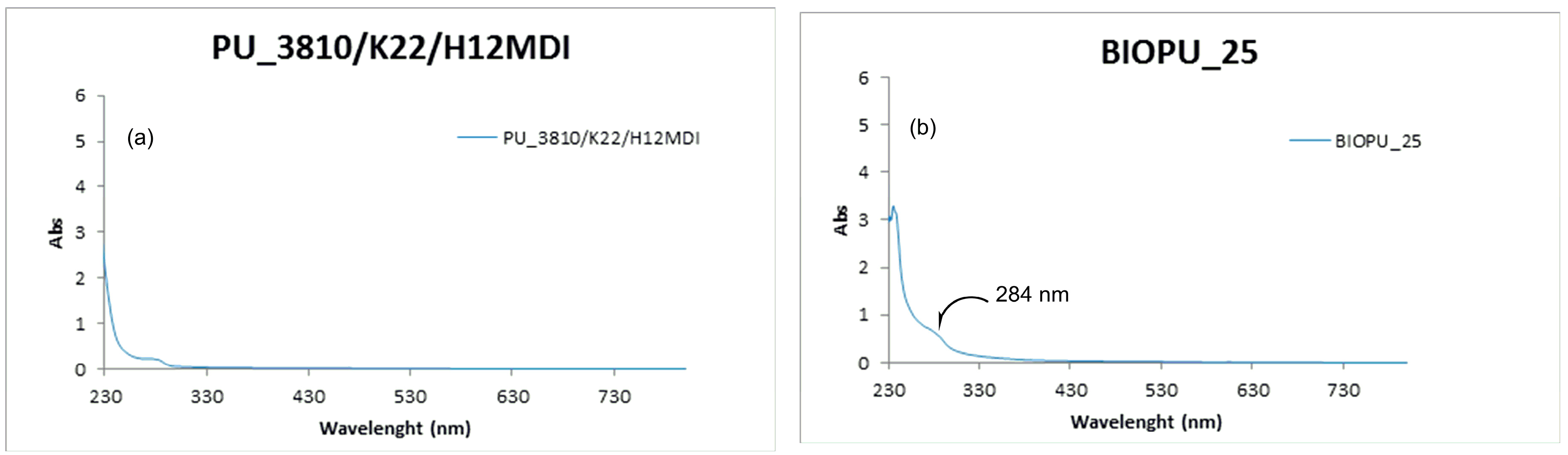
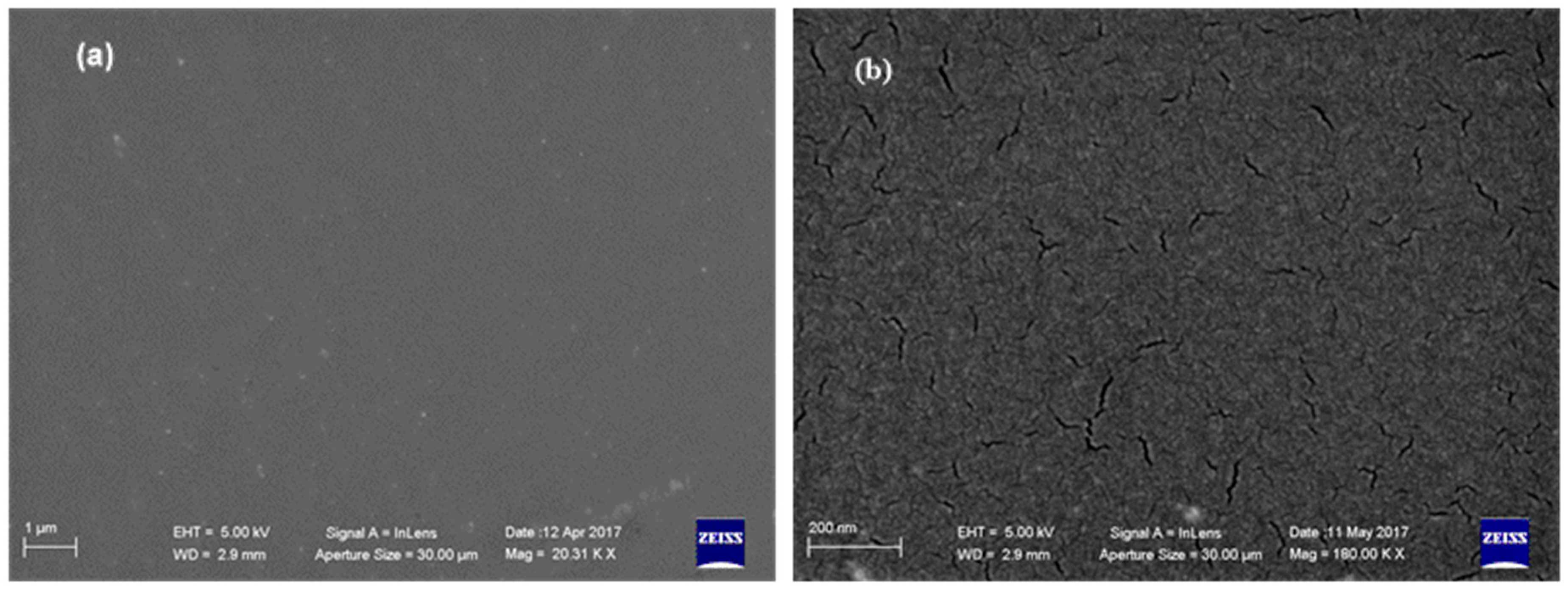
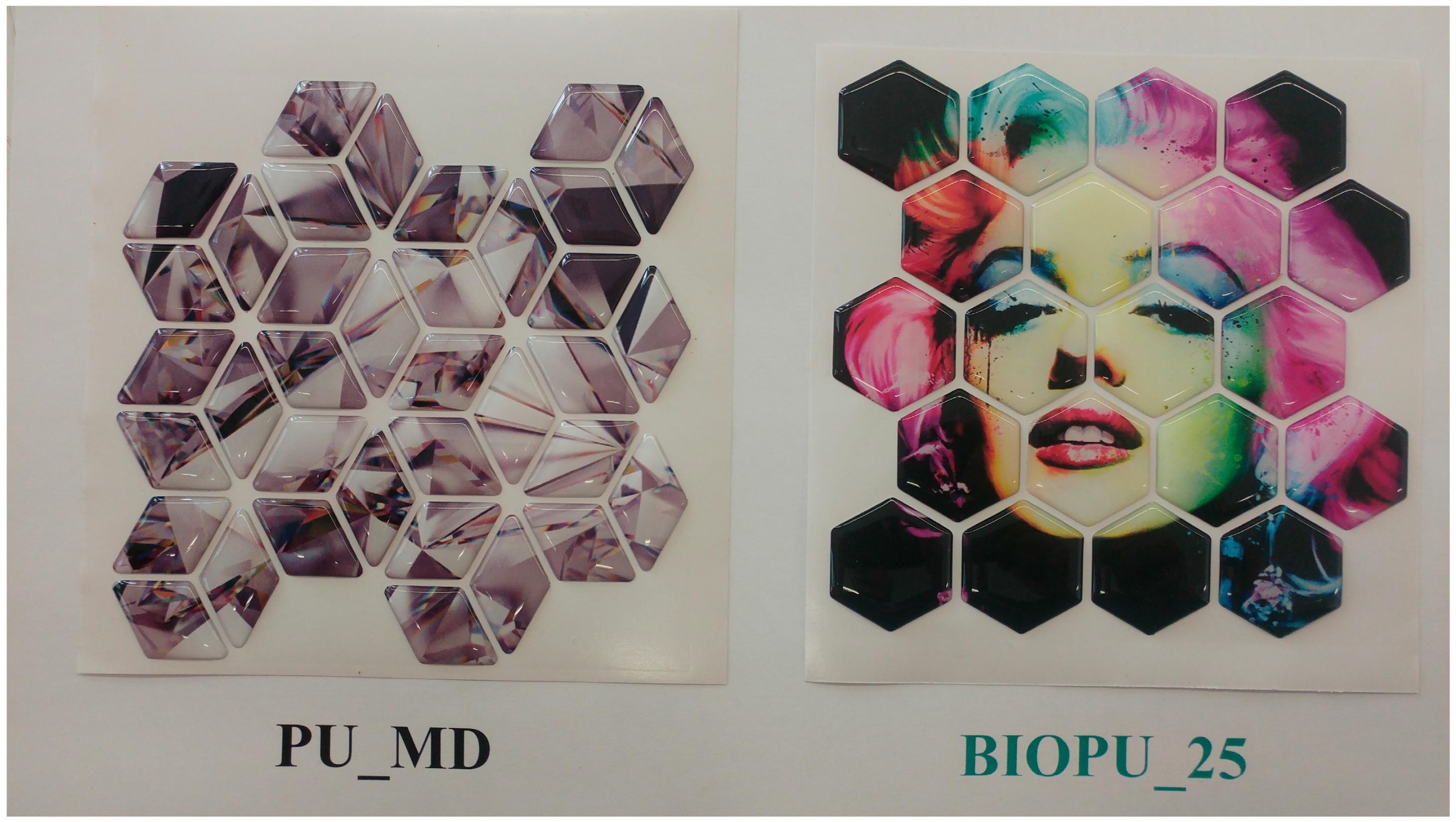
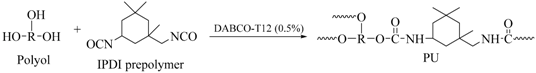 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Run | Polyol | Mn (g/mol) | n. OH (mgKOH/g) | IPDI Prep (g) | PU Products | Tg b (°C) | WCA c (°) | d (kg/dm3) |
| 1 | Domes Resin | - | 398 | 20.5 | PU_MD | 59 | 77 | 1.01 |
| 2 | Alcupol C5710 | 290 | 565 | 33.6 | PU_C5710/T12 | 50 | 71 | 1.06 |
| 3 | Alcupol R3810 | 440 | 380 | 22.2 | PU_R3810/T12 | 45 | 79 | 1.06 |
| 4 | Alcupol R2510 | 670 | 240 | 14.6 | PU_R2510/T12 | 2 | 78 | 1.12 |
| 5 | Alcupol R1610 | 1050 | 155 | 9.3 | PU_R1610/T12 | −27 | 79 | 1.49 |
| 6 | Alcupol C3531 | 4800 | 35 | 2.1 | PU_C3531/T12 | −62 | 83 | 1.38 |
| Run | Diisocyanate (g) | Product | Tg (°C) b | WCA (°) c | d (kg/dm3) |
|---|---|---|---|---|---|
| 1 | pure IPDI (17) | PU_3810/K22/IPDI | 68 | 79 | 1.11 |
| 2 | H12MDI (19.7) | PU_3810/K22/H12MDI | 65 | 77 | 1.06 |
| 3 | HMDI (12.6) | PU_3810/K22/HMDI | 19 | 72 | 0.77 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Polyols Mixture g | Diisocyanate H12MDI (g) | Kat-22 (g) (10% w/w) | % of Bio-C b | Bio-PU Products | Tg c (°C) | WCA d (°) | d (kg/dm3) | |
| Alcupol R3810 | Bio-Polyol | |||||||
| 20.2 | 4 | 19.8 | 2.42 | 11 | BIOPU_11 | 73 | 90 | 1.06 |
| 9.6 | 4 | 10.2 | 1.36 | 19 | BIOPU_19 | 62 | 75 | 1.05 |
| 9.2 | 6 | 10.4 | 1.52 | 25 | BIOPU_25 | 49 | 76 | 1.06 |
| 8 | 8 | 11.3 | 1.68 | 31 | BIOPU_31 | 48 | 82 | 1.08 |
| Domes resin | IPDI prep | PhHg decanoate | 0 | PU_MD e | 59 | 77 | 1.01 | |
| Commercial Name | n. OH (mgKOH/g) | Density (kg/dm3) | Viscosity (cP) |
|---|---|---|---|
| Alcupol C5710 | 570 | 1.05 | 700 |
| Alcupol R3810 | 380 | 1.03 | 350 |
| Alcupol R2510 | 250 | 1.02 | 260 |
| Alcupol R1610 | 160 | 1.02 | 250 |
| Alcupol C3531 | 35 | 1.05 | 800 |
| Alcupol C2831 | 28 | 1.06 | 1100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantone, V.; Laurenza, A.G.; Annese, C.; Comparelli, R.; Fracassi, F.; Fini, P.; Nacci, A.; Russo, A.; Fusco, C.; D’Accolti, L. Preparation and Characterization of Soybean Oil-Based Polyurethanes for Digital Doming Applications. Materials 2017, 10, 848. https://doi.org/10.3390/ma10080848
Pantone V, Laurenza AG, Annese C, Comparelli R, Fracassi F, Fini P, Nacci A, Russo A, Fusco C, D’Accolti L. Preparation and Characterization of Soybean Oil-Based Polyurethanes for Digital Doming Applications. Materials. 2017; 10(8):848. https://doi.org/10.3390/ma10080848
Chicago/Turabian StylePantone, Vincenzo, Amelita Grazia Laurenza, Cosimo Annese, Roberto Comparelli, Francesco Fracassi, Paola Fini, Angelo Nacci, Antonella Russo, Caterina Fusco, and Lucia D’Accolti. 2017. "Preparation and Characterization of Soybean Oil-Based Polyurethanes for Digital Doming Applications" Materials 10, no. 8: 848. https://doi.org/10.3390/ma10080848
APA StylePantone, V., Laurenza, A. G., Annese, C., Comparelli, R., Fracassi, F., Fini, P., Nacci, A., Russo, A., Fusco, C., & D’Accolti, L. (2017). Preparation and Characterization of Soybean Oil-Based Polyurethanes for Digital Doming Applications. Materials, 10(8), 848. https://doi.org/10.3390/ma10080848