Effect of Core-shell Ceria/Poly(Vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties (2): Increasing the Refractive Index
Abstract
:1. Introduction
2. Materials and Methods
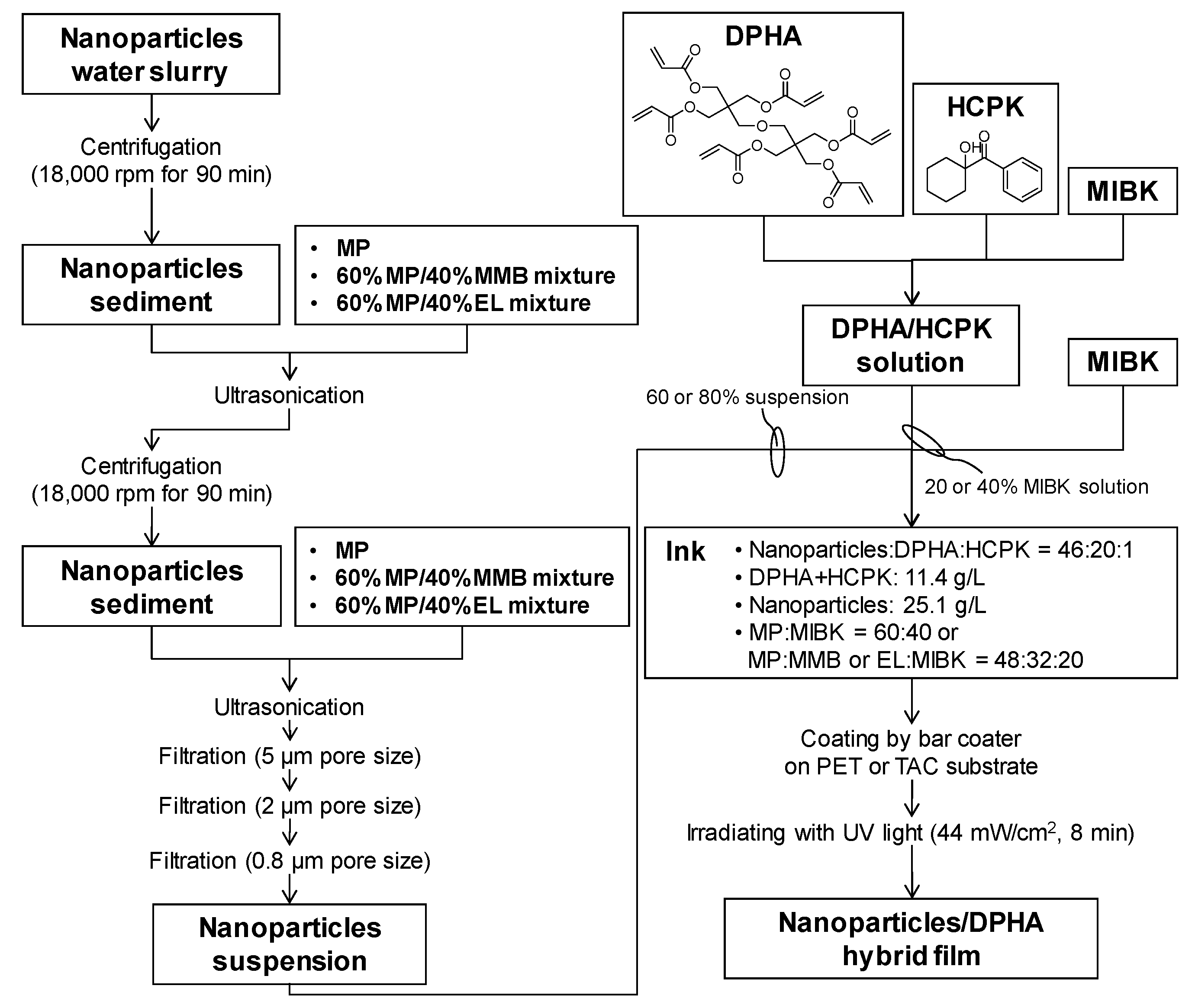
2.1. Dispersibility of Core-Shell Ceria-PVP Nanoparticles in Various Solvents
2.2. Synthesis of Core-Shell Ceria-PVP Nanoparticle/DPHA Hybrid Films
2.3. Analysis of Hybrid Films
3. Results and Discussion
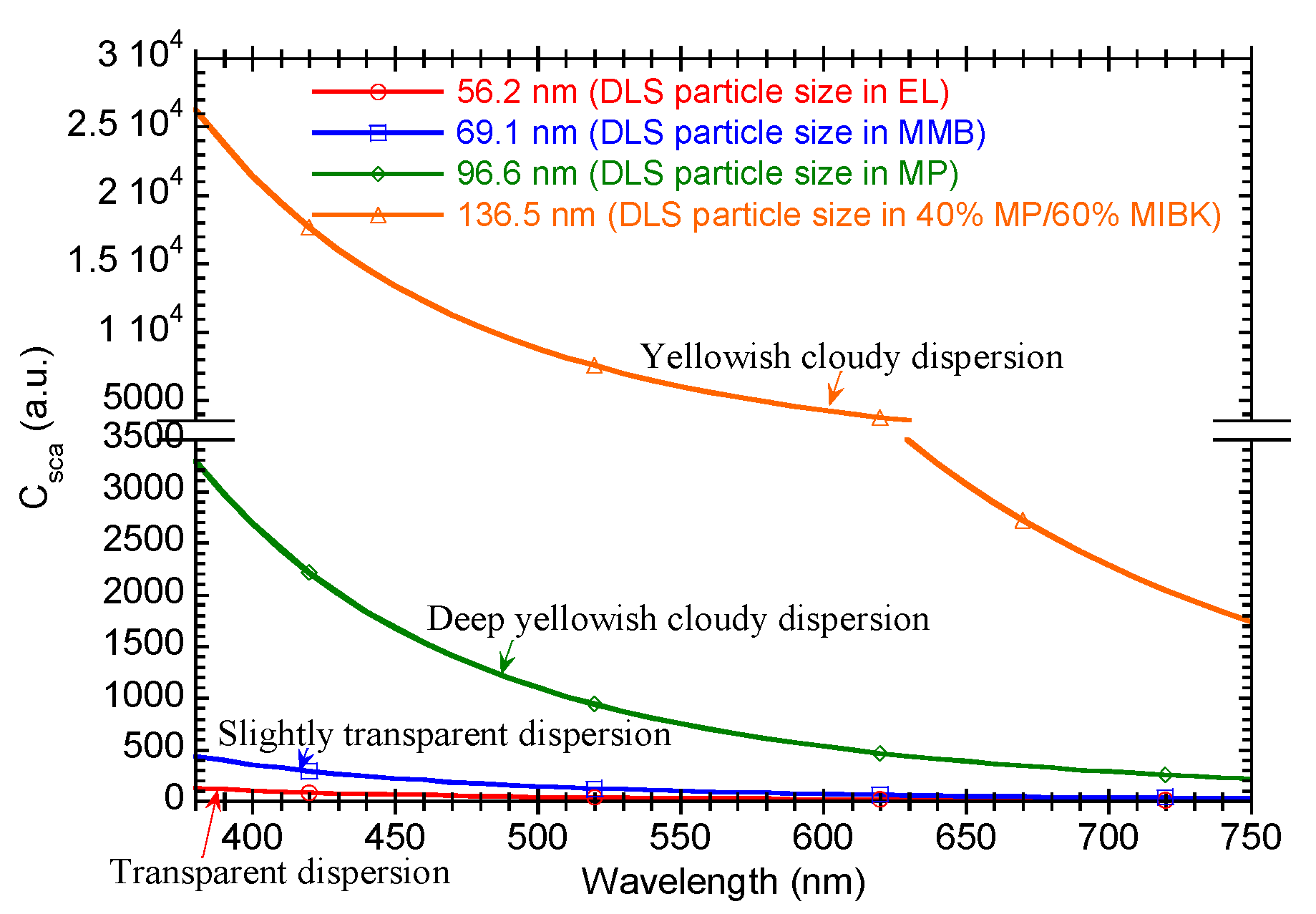
3.1. Dispersibility of Core-Shell Ceria-PVP Nanoparticles in an MP/MIBK Solvent Mixture
3.2. Dispersibility of Core-Shell Ceria-PVP Nanoparticles in Protic Solvents
3.3. Relationship between the Condition of Dispersant and the DLS Particle Size
3.4. Dispersibility of Core-Shell Ceria-PVP Nanoparticles in MP, MMB, EL, and MIBK Solvent Mixtures
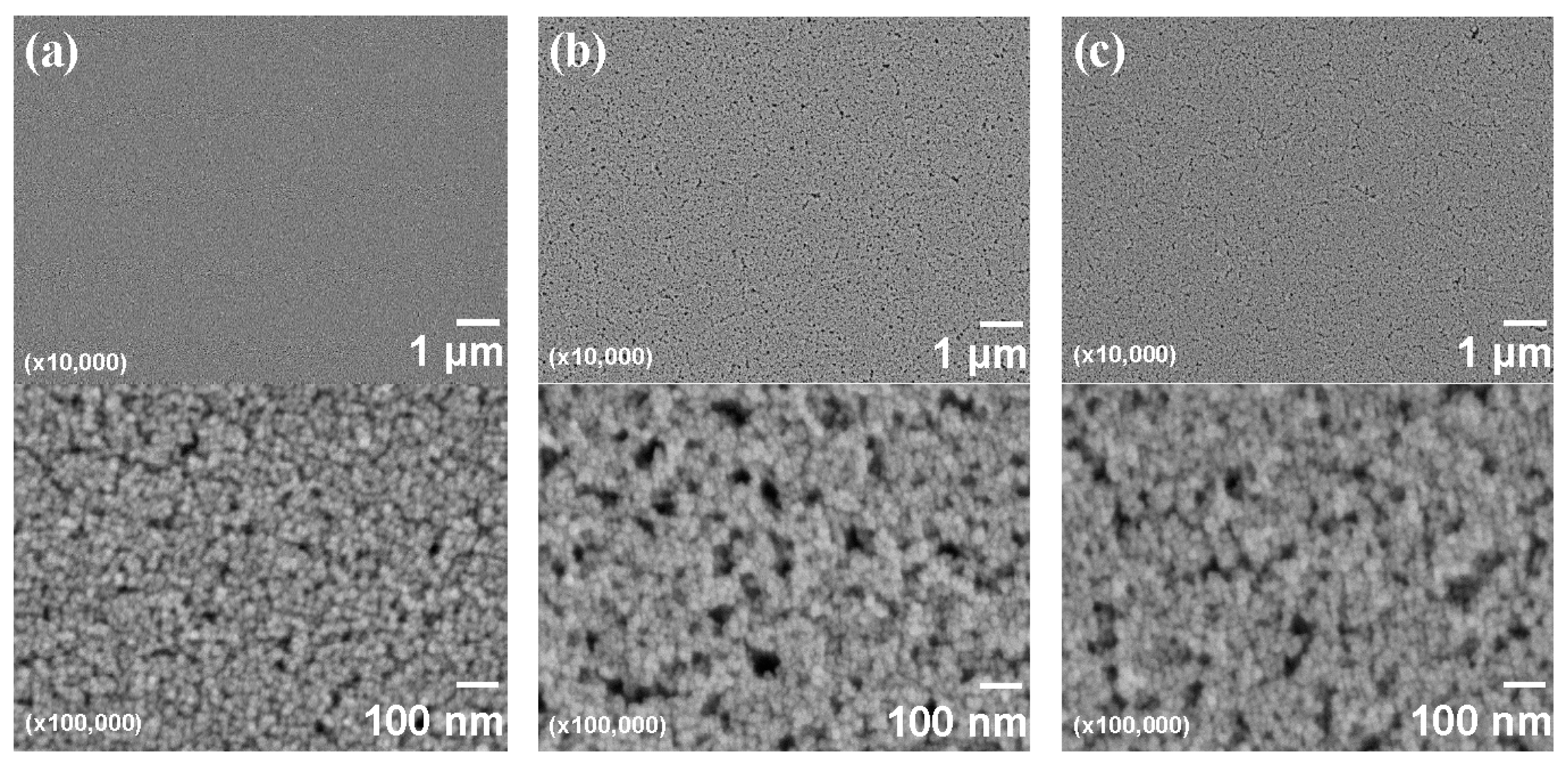
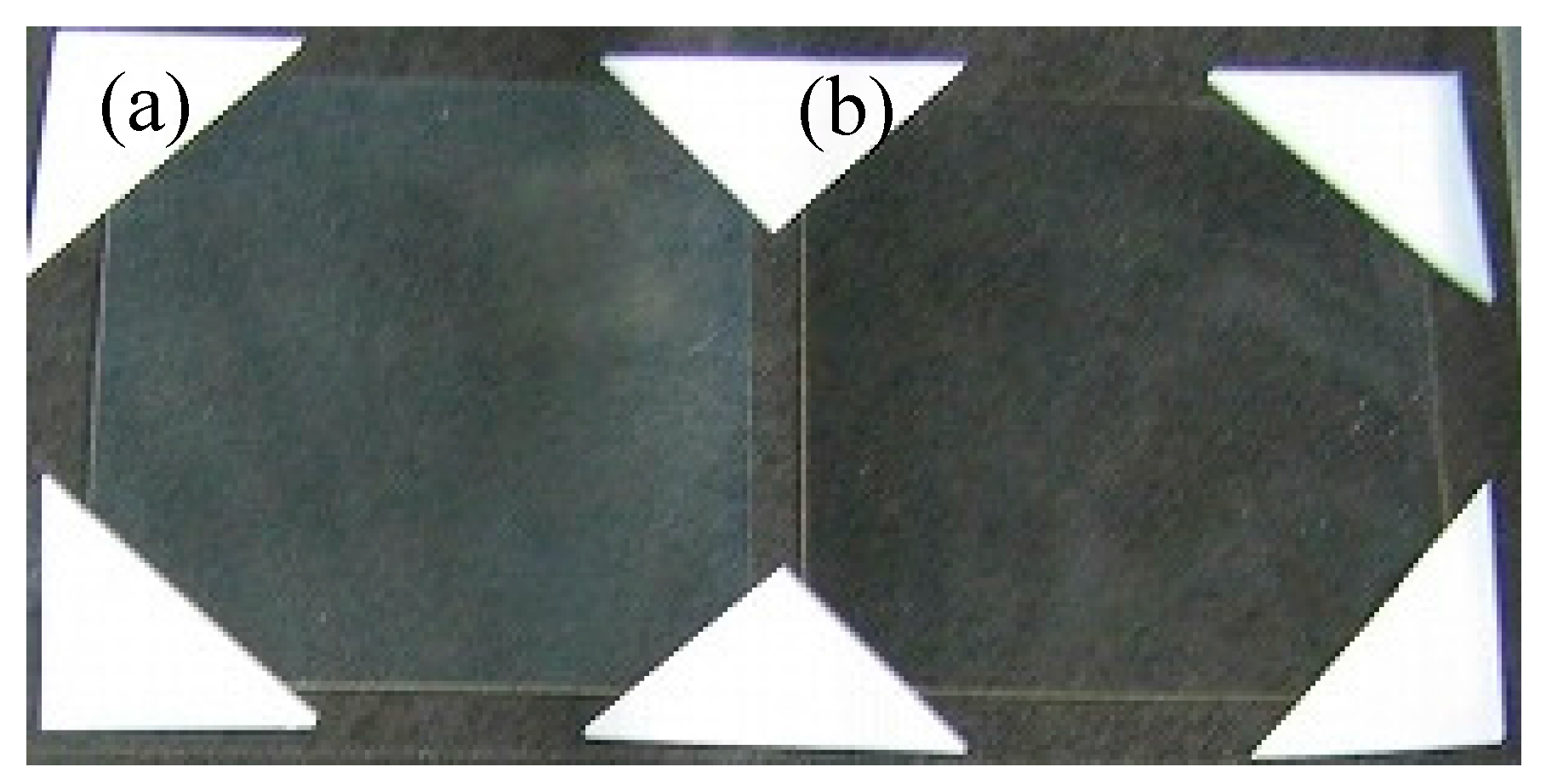
3.5. Evaluation of Core-Shell Ceria-PVP Nanoparticle/DPHA Hybrid Films
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Munzert, P.; Schulz, U.; Kaiser, N.; Schönberger, W.; Fahland, M. Thin film growth on nanostructured polymer webs for anti-reflection purposes. Surf. Coat. Technol. 2011, 205 (Suppl. 2), S498–S501. [Google Scholar] [CrossRef]
- Chang, T.-L.; Cheng, K.-Y.; Chou, T.-H.; Su, C.-C.; Yang, H.-P.; Luo, S.-W. Hybrid-polymer nanostructures forming an anti-reflection film using two-beam interference and ultraviolet nanoimprint lithography. Microelectron. Eng. 2009, 86, 874–877. [Google Scholar] [CrossRef]
- Moiseev, S.G. Composite medium with silver nanoparticles as an anti-reflection optical coating. Appl. Phys. A 2011, 103, 619–622. [Google Scholar] [CrossRef]
- Chen, C.-H.; Li, S.-Y.; Chiang, A.S.T.; Wu, A.T.; Sun, Y.S. Scratch-resistant zeolite anti-reflective coating on glass for solar applications. Sol. Energy Mater. Sol. Cells 2011, 95, 1694–1700. [Google Scholar] [CrossRef]
- Chhajed, S.; Poxson, D.J.; Yan, X.; Cho, J.; Schubert, E.F.; Welser, R.E.; Sood, A.K.; Kim, J.K. Nanostructured multilayer tailored-refractive-index antireflection coating for glass with broadband and omnidirectional characteristics. Appl. Phys. Express 2011, 4, 052503. [Google Scholar] [CrossRef]
- Nishino, G.; Sugimoto, H.; Nakanishi, E. Preparation and properties of acrylic melamine hard coating. J. Appl. Polym. Sci. 2012, 123, 307–315. [Google Scholar] [CrossRef]
- Higashihara, T.; Ueda, M. Recent Progress in High Refractive Index Polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Sangermano, M.; Voit, B.; Sordo, F.; Eichhorn, K.-J.; Rizza, G. High refractive index transparent coatings obtained via UV/thermal dual-cure process. Polymer 2008, 49, 2018–2022. [Google Scholar] [CrossRef]
- Itoh, T.; Uchida, T.; Izu, N.; Matsubara, I.; Shin, W. Effect of Core-shell Ceria/Poly(vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties. Materials 2013, 6, 2119–2129. [Google Scholar] [CrossRef]
- Sangermano, M.; Gross, S.; Pracella, L.; Priola, A.; Rizza, G. Hybrid organic-inorganic nanostructured acrylic films based on methacylate modified zircon ium oxocluster. Macromol. Chem. Phys. 2007, 208, 1730–1736. [Google Scholar] [CrossRef]
- Sarwar, M.I.; Zulfiqar, S.; Ahmad, Z. Polyamide-silica nanocomposites: Mechanical, morphological and thermomechanical investigations. Polym. Int. 2008, 57, 292–296. [Google Scholar] [CrossRef]
- Zeng, X.-F.; Kong, X.-R.; Ge, J.-L.; Liu, H.-T.; Gao, C.; Shen, Z.-G.; Chen, J.-F. Effective solution mixing method to fabricate highly transparent and optical functional organic-inorganic nanocomposite film. Ind. Eng. Chem. Res. 2011, 50, 3253–3258. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hwang, F.-H.; Hsieh, C.-Y.; Chen, C.-C.; Cheng, L.-P. Preparation and characterization of polymer/zirconia nanocomposite antistatic coatings on plastic substrates. J. Coat. Technol. Res. 2013, 10, 73–78. [Google Scholar] [CrossRef]
- Chang, C.-C.; Oyang, T.-Y.; Chen, Y.-C.; Hwang, F.-H.; Cheng, L.-P. Preparation of hydrophobic nanosilica-filled polyacrylate hard coatings on plastic substrates. J. Coat. Technol. Res. 2014, 11, 381–386. [Google Scholar] [CrossRef]
- Mataki, H.; Yamaki, S.; Fukui, T. Nanostructured Organic/Inorganic Composites as Transparent Materials for Optical Components. Jpn. J. Appl. Phys. 2004, 43, 5819–5823. [Google Scholar] [CrossRef]
- Shilpa, K.N.; Nithin, K.S.; Sachhidananda, S.; Madhukar, B.S.; Hatna, S. Visibly transparent PVA/sodium doped dysprosia (Na2Dy2O4) nano composite films, with high refractive index: An optical study. J. Alloys Compd. 2017, 694, 884–891. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Li, R.; Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T. Synthesis and UV-shielding properties of ZnO- and CaO-doped CeO2 via soft solution chemical process. Solid State Ion. 2002, 151, 235–241. [Google Scholar] [CrossRef]
- Krishna, M.G.; Hartridge, A.; Bhattacharya, A.K. Temperature and ionic size dependence of the properties of ceria based optionic thin films. Mater. Sci. Eng. B 1998, 55, 14–20. [Google Scholar] [CrossRef]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Hirano, M.; Fukuda, Y.; Iwata, H.; Hotta, Y.; Inagaki, M. Preparation and Spherical agglomeration of crystalline Cerium (IV) oxide nanoparticles by thermal hydrolysis. J. Am. Ceram. Soc. 2000, 83, 1287–1289. [Google Scholar] [CrossRef]
- Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S. Morphology-Controllable synthesis of mesoporous CeO2 Nano- and microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- Izu, N.; Matsubara, I.; Itoh, T.; Shin, W.; Nishibori, M. Controlled synthesis of monodispersed cerium oxide nanoparticle sols applicable to preparing ordered self-assemblies. Bull. Chem. Soc. Jpn. 2008, 81, 761–766. [Google Scholar] [CrossRef]
- Izu, N.; Uchida, T.; Matsubara, I.; Itoh, T.; Shin, W.; Nishibori, M. Formation mechanism of monodispersed spherical core-shell ceria/polymer hybrid nanoparticles. Mater. Res. Bull. 2011, 46, 1168–1176. [Google Scholar] [CrossRef]
- Izu, N.; Itoh, T.; Nishibori, M.; Shin, W.; Matsubara, I. Physicochemical properties and microstructures of core-shell type cerium oxide/organic polymer nanospheres. J. Ceram. Soc. Jpn. 2009, 117, 773–776. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, J.-S.; Choi, S.C. Synthesis of nano-sized ceria powders by two-emulsion method using sodium hydroxide. Mater. Lett. 2005, 59, 395–398. [Google Scholar] [CrossRef]
- Itoh, T.; Izu, N.; Matsubara, I.; Shin, W.; Nishibori, M. 13C CP/MAS NMR Study of Cross-linked Poly(vinylpyrrolidone) on surface of cerium oxide nanoparticles. Chem. Lett. 2008, 37, 1116–1117. [Google Scholar] [CrossRef]
- Elim, H.I.; Cai, B.; Kurata, Y.; Sugihara, O.; Kaino, T.; Adschiri, T.; Chu, A.-L.; Kambe, N. Refractive index control and rayleigh scattering properties of transparent TiO2 nanohybrid polymer. J. Phys. Chem. B 2009, 113, 10143–10148. [Google Scholar] [CrossRef] [PubMed]
- Grulke, E.A. Solubility Parameter Values in Polymer Handbook, 3rd ed.; Brandrup, J., Immergut, E.H., Eds.; John Wiley & Sons: New York, NY, USA, 1989; pp. 519–559. [Google Scholar]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Hoy, K.L. New values of solubility parameters from vapor pressure data. J. Paint Technol. 1970, 42, 76–78. [Google Scholar]
| Name | Structural Formula | Viscosityat 25 °C (cP) | Boiling Point (°C) | Saturated Vapor Pressure at 20 °C (kPa) |
|---|---|---|---|---|
| Cyclohexanol | 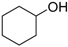 | 4.6 | 161 | 0.13 |
| Diacetone alcohol |  | 3.05 | 168 | 0.21 (26.7 °C) |
| Methyl lactate |  | 2.88 | 145 | 0.36 |
| Ethyl lactate (EL) | 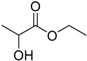 | 2.58 | 155 | 0.36 |
| Butyl lactate | 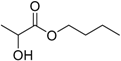 | 3.35 | 188 | 0.05 |
| 3-Methoxy-3-methyl-1-butanol (MMB) | 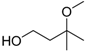 | 6.12 | 174 | 0.5 |
| 3-Methoxy-1-butanol | 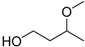 | No data | 161 | No data |
| 1-Methoxy-2-propanol (MP) | 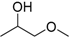 | 1.78 | 120 | 0.89 |
| Methyl i-butyl ketone (MIBK) | 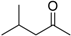 | 0.62 | 114-117 | 2.1 |
| Ratio of Solvent (vol%) | Dispersion Behavior | DLS (nm) | |
|---|---|---|---|
| MP | MIBK | ||
| 40 | 60 | Yellowish cloudy dispersion with slight precipitation | 136.5 |
| 100 | 0 | Deep yellowish cloudy dispersion with slight precipitation | 96.6 |
| Name | Dispersion Behavior | DLS (nm) |
|---|---|---|
| Cyclohexanol | No dispersion | – |
| Diacetone alcohol | No dispersion | – |
| Methyl lactate | White cloudy dispersion | – |
| Ethyl lactate (EL) | Transparent dispersion | 56.2 |
| Butyl lactate | White cloudy dispersion | – |
| 3-Methoxy-3-methyl-1-butanol (MMB) | Slightly transparent dispersion with slightly precipitation | 69.1 |
| 3-Methoxy-1-butanol | White cloudy dispersion | – |
| 1-Methoxy-2-propanol (MP) | Deep yellowish cloudy dispersion with slightly precipitation | 96.6 |
| Ratio of Solvent (vol%) | Dispersion Behavior | DLS (nm) | |
|---|---|---|---|
| MP | MMB | ||
| 20 | 80 | Yellowish cloudy dispersion with large amount of precipitation | 83.5 |
| 40 | 60 | Slightly transparent dispersion with slight precipitation | 47.0 |
| 60 | 40 | Slightly transparent dispersion with slight precipitation | 46.9 |
| 80 | 20 | Yellowish cloudy dispersion with large amount of precipitation | 75.6 |
| Ratio of Solvent (vol%) | Dispersion Behavior | DLS (nm) | ||
|---|---|---|---|---|
| MP | MMB | MIBK | ||
| 48 | 32 | 20 | Slightly transparent dispersion with slight precipitation | 66.6 |
| 36 | 24 | 40 | White cloudy dispersion | – |
| 24 | 16 | 60 | White cloudy dispersion | – |
| 32 | 48 | 20 | Slightly transparent dispersion with slight precipitation | – |
| 24 | 36 | 40 | White cloudy dispersion | – |
| 16 | 24 | 60 | White cloudy dispersion | – |
| Ratio of Solvent (vol%) | Dispersion Behavior | DLS (nm) | |
|---|---|---|---|
| MP | EL | ||
| 20 | 80 | Slightly transparent dispersion with slight precipitation | 47.8 |
| 40 | 60 | Slightly transparent dispersion | 60.3 |
| 60 | 40 | Slightly transparent dispersion | 56.4 |
| 80 | 20 | Yellowish cloudy dispersion with large amount of precipitation | 76.2 |
| Ratio of Solvent (vol%) | Dispersion Behavior | DLS (nm) | ||
|---|---|---|---|---|
| MP | EL | MIBK | ||
| 48 | 32 | 20 | Slightly transparent dispersion with slight precipitation | – |
| 36 | 24 | 40 | White cloudy dispersion | – |
| 24 | 16 | 60 | White cloudy dispersion | – |
| 32 | 48 | 20 | Slightly transparent dispersion with slight precipitation | – |
| 24 | 36 | 40 | White cloudy dispersion | – |
| 16 | 24 | 60 | White cloudy dispersion | – |
| Solvent of Dispersant in Ink | Refractive Index (λ = 550 nm) | |
|---|---|---|
| PET Substrate | TAC Substrate | |
| 48% MP/32% MMB/20% MIBK | 1.694 | 1.689 |
| 48% MP/32% EL/20% MIBK | 1.656 | – |
| 60% MP/40% MIBK | 1.636 | 1.586 |
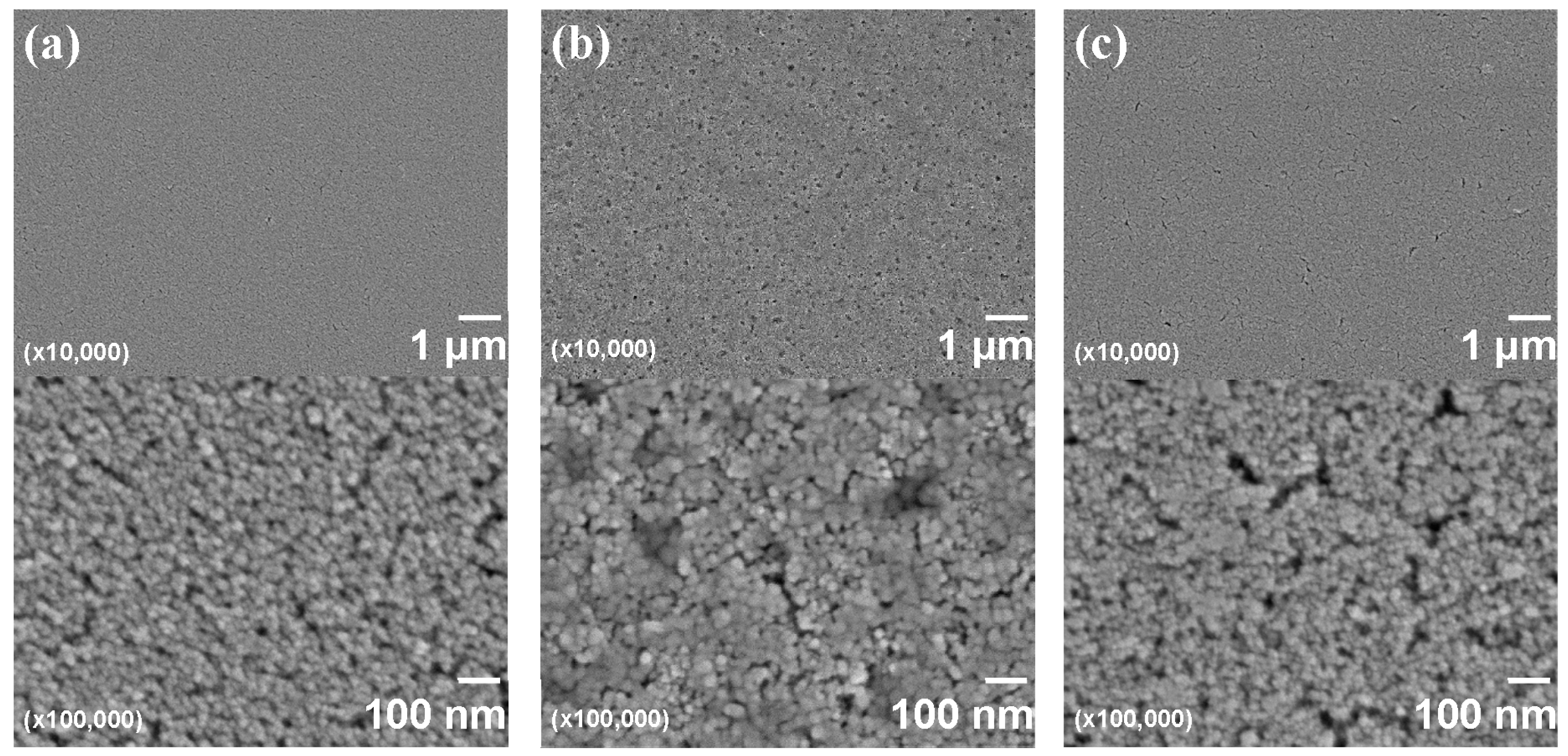
| Item | Figure 6a | Figure 6b |
|---|---|---|
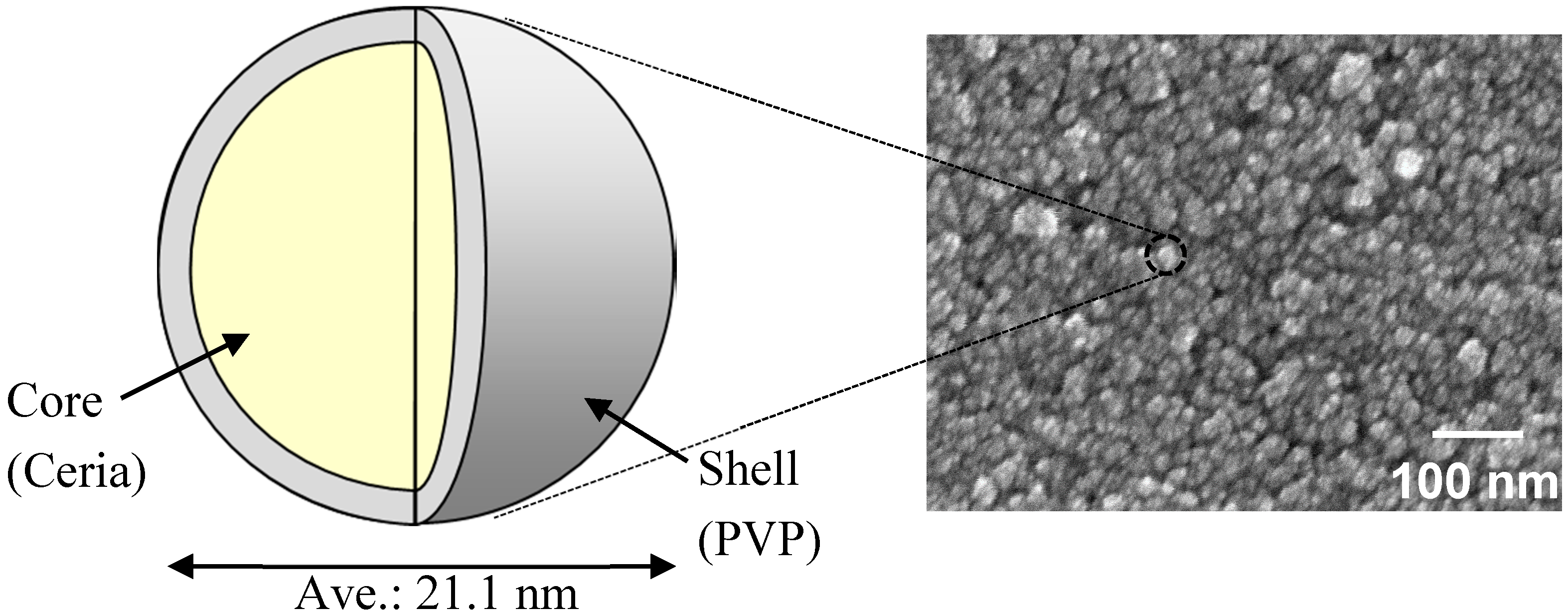
| Particle size of the nanoparticles and its coefficient of variation (CV) by FE-SEM | 21.1 nm 15.0% | 21.1 nm 15.0% |
| DLS particle size of the nanoparticles in solvents | 137.9 nm (in 60% MP/40% MIBK) | 46.9 nm (in 48% MP/32% MMB/20% MIBK) |
| Film thickness | 198 nm | 325 nm |
| Refractive index (λ = 550 nm) | 1.636 | 1.694 |
| Haze; Hfilm | 1.98 | 0.37 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itoh, T.; Uchida, T.; Izu, N.; Shin, W. Effect of Core-shell Ceria/Poly(Vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties (2): Increasing the Refractive Index. Materials 2017, 10, 710. https://doi.org/10.3390/ma10070710
Itoh T, Uchida T, Izu N, Shin W. Effect of Core-shell Ceria/Poly(Vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties (2): Increasing the Refractive Index. Materials. 2017; 10(7):710. https://doi.org/10.3390/ma10070710
Chicago/Turabian StyleItoh, Toshio, Toshio Uchida, Noriya Izu, and Woosuck Shin. 2017. "Effect of Core-shell Ceria/Poly(Vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties (2): Increasing the Refractive Index" Materials 10, no. 7: 710. https://doi.org/10.3390/ma10070710
APA StyleItoh, T., Uchida, T., Izu, N., & Shin, W. (2017). Effect of Core-shell Ceria/Poly(Vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties (2): Increasing the Refractive Index. Materials, 10(7), 710. https://doi.org/10.3390/ma10070710






