Extractant Immobilization in Alginate Capsules (Matrix- and Mononuclear-Type): Application to Pb(II) Sorption from HCl Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Sorbents
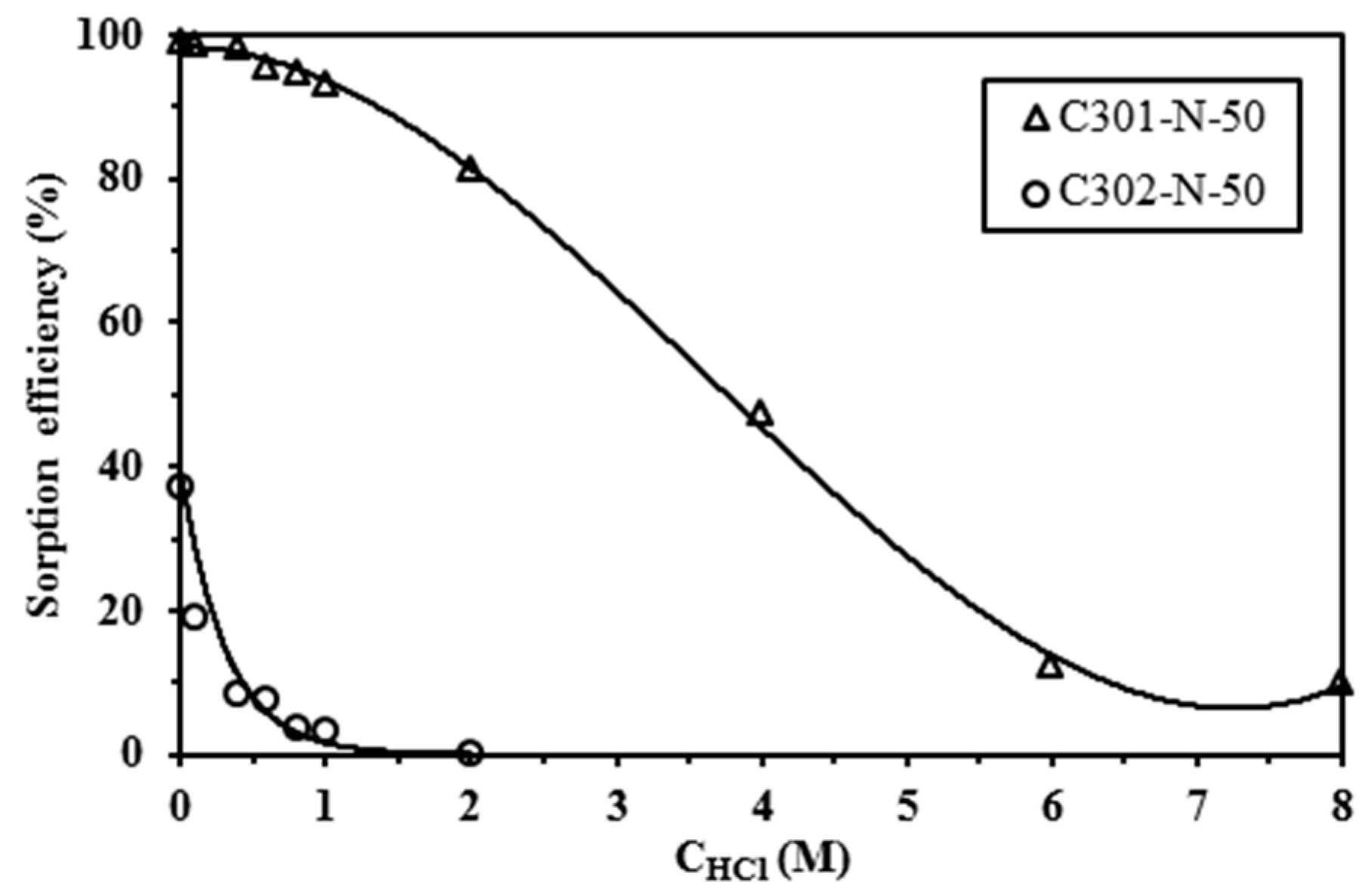
2.2. Influence of HCl Concentration on Pb(II) Sorption
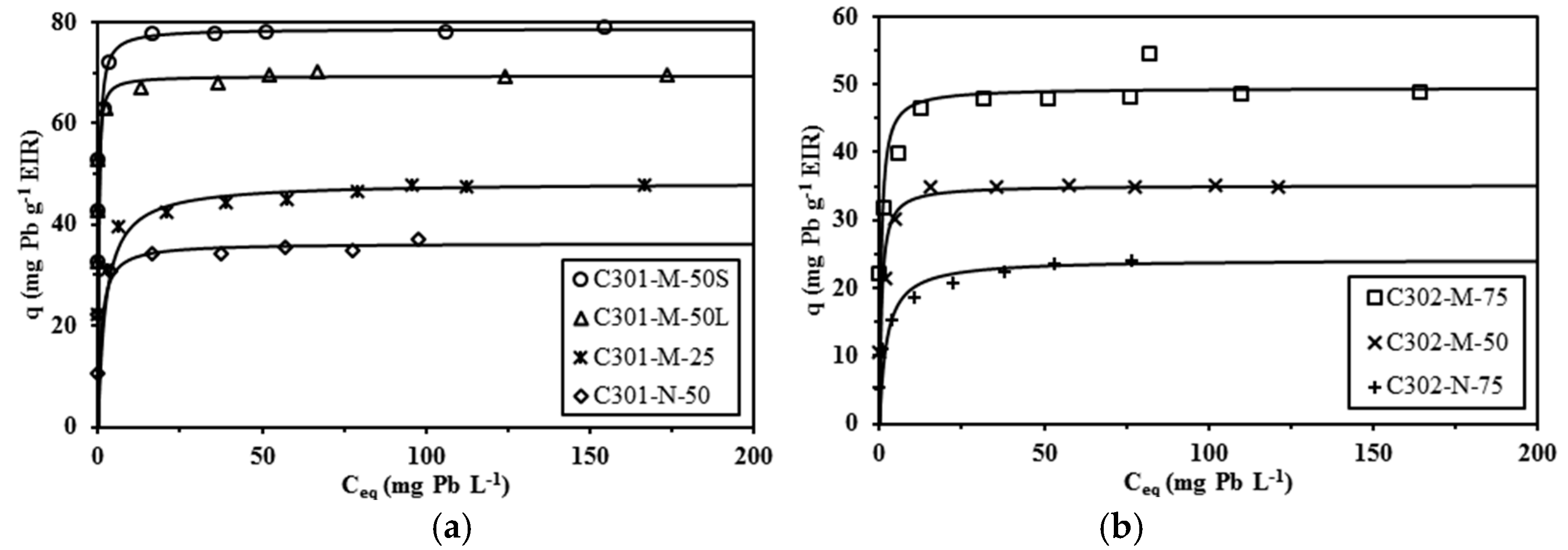
2.3. Sorption Isotherms
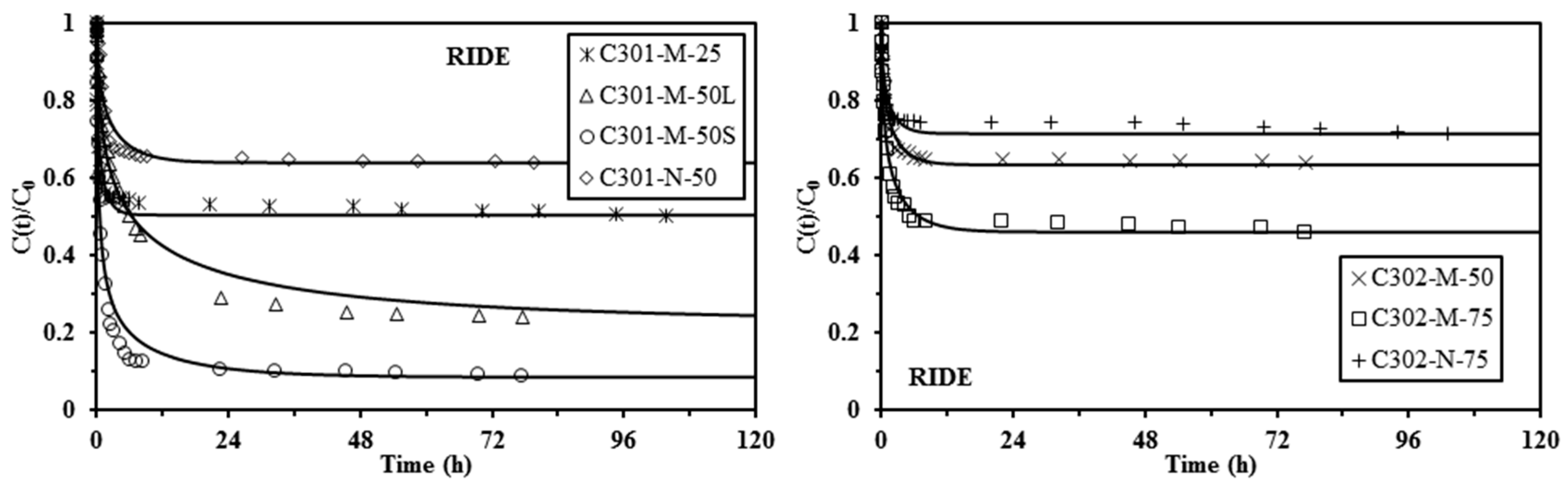
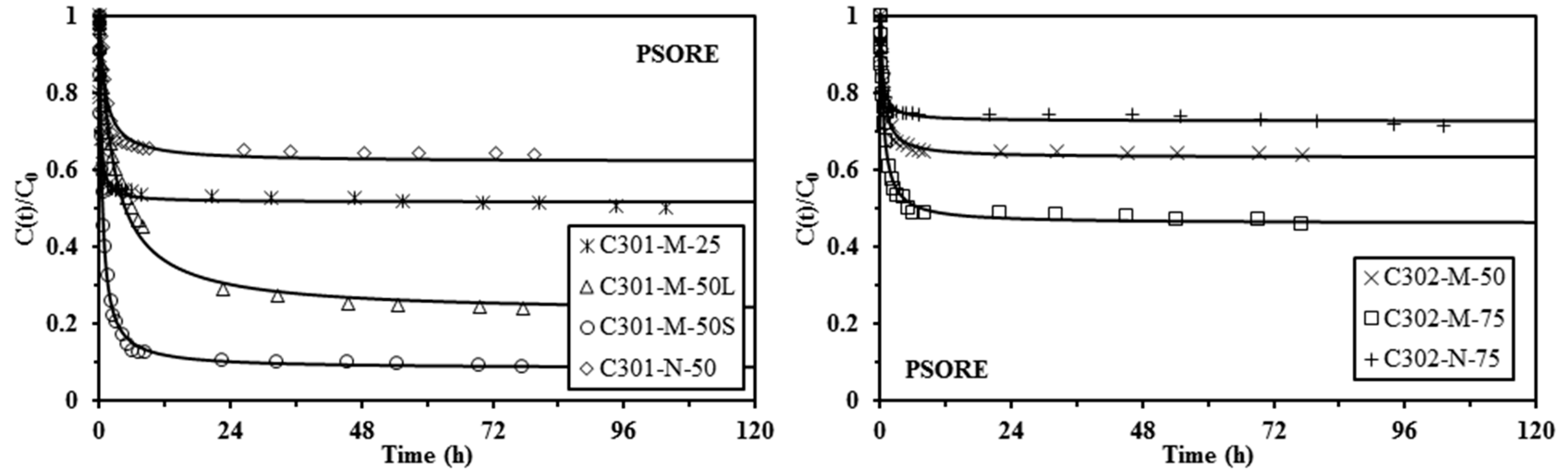
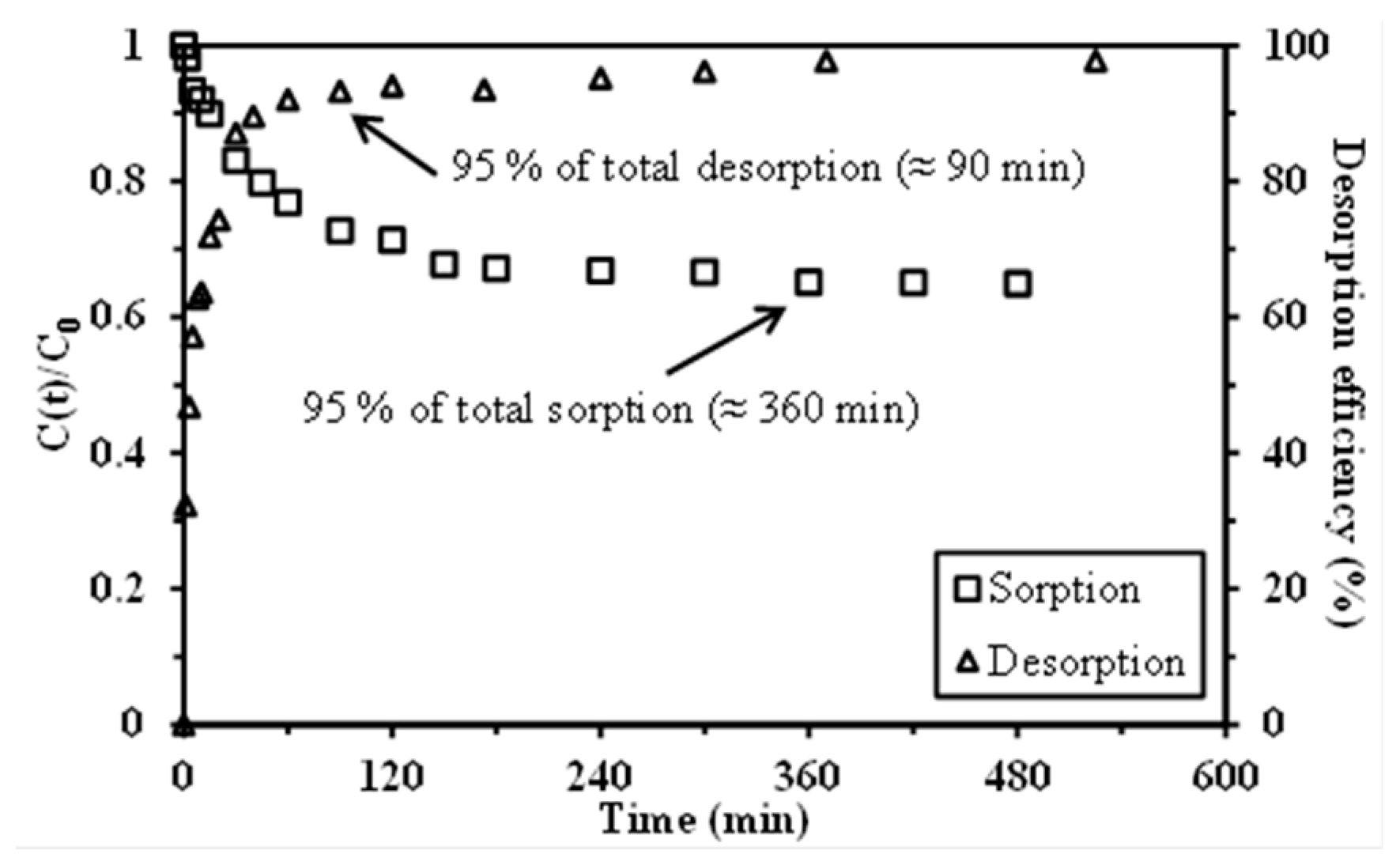
2.4. Uptake Kinetics
2.5. Metal Desorption and Sorbent Recycling
3. Materials and Methods
3.1. Materials
3.2. Encapsulation Procedures
3.3. Characterization of Encapsulated Materials
3.4. Sorption Procedures
3.5. Sorption Modeling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Facon, S.; Cote, G.; Bauer, D. Solvent extraction of antimony(III), bismuth(III), lead(II) and tin(IV) with bis(2,4,4-trimethylpentyl)phosphinodithioic acid (Cyanex 301). Solvent Extr. Ion Exch. 1991, 9, 717–734. [Google Scholar] [CrossRef]
- Menoyo, B.; Benito, R.; Elizalde, M.P. Extraction of lead(II) by Cyanex 302 in toluene, effect of Cyanex 301 on the extraction by Cyanex 302. Solvent Extr. Ion Exch. 1996, 14, 69–88. [Google Scholar] [CrossRef]
- Fujinaga, K.; Nagura, H.; Yamasaki, R.; Kokusen, H.; Komatsu, Y.; Seike, Y.; Okumura, M. The selective liquid-liquid extraction of cadmium(II) and lead(II) with 2-pyridinealdoxime. Solv. Extr. Res. Dev. Jpn. 2006, 13, 175–184. [Google Scholar]
- Regel-Rosocka, M.; Staszak, K.; Wieszczycka, K.; Masalska, A. Removal of cobalt(II) and zinc(II) from sulphate solutions by means of extraction with sodium bis(2,4,4-trimethylpentyl)phosphinate (Na-Cyanex 272). Clean Technol. Environ. Policy 2016, 18, 1961–1970. [Google Scholar] [CrossRef]
- Zhou, Y.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Extraction of metal ions with task specific ionic liquids: Influence of a coordinating anion. Sep. Sci. Technol. 2015, 50, 38–44. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Cuhorka, J.; Mikulasek, P. Analysis of lead(II) retention from single salt and binary aqueous solutions by a polyamide nanofiltration membrane: Experimental results and modelling. J. Membr. Sci. 2013, 436, 132–144. [Google Scholar] [CrossRef]
- Hajdu, I.; Bodnar, M.; Csikos, Z.; Wei, S.; Daroczi, L.; Kovacs, B.; Gyori, Z.; Tamas, J.; Borbely, J. Combined nano-membrane technology for removal of lead ions. J. Membr. Sci. 2012, 409, 44–53. [Google Scholar] [CrossRef]
- Misra, R.K.; Jain, S.K.; Khatri, P.K. Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr(VI), Cd(II), Ni(II) and Pb(II) from their aqueous solutions. J. Hazard. Mater. 2011, 185, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Vergili, I.; Soltobaeva, G.; Kaya, Y.; Gonder, Z.B.; Cavus, S.; Gurdag, G. Study of the removal of Pb(II) using a weak acidic cation resin: Kinetics, thermodynamics, equilibrium, and breakthrough curves. Ind. Eng. Chem. Res. 2013, 52, 9227–9238. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Li, Y.; Zhang, Y.; Ma, X.; Ye, Z. Study on adsorption mechanism of Pb(II) and Cu(II) in aqueous solution using PS-EDTA resin. Chem. Eng. J. 2010, 163, 364–372. [Google Scholar] [CrossRef]
- Xiong, C.-H.; Yao, C.-P. Adsorption behavior of gel-type weak acid resin (110-H) for Pb(2+). Trans. Nonferrous Met. Soc. China 2008, 18, 1290–1294. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Wang, C.; Wei, Y. A novel polyvinyltetrazole-grafted resin with high capacity for adsorption of Pb(II), Cu(II) and Cr(III) ions from aqueous solutions. J. Mater. Chem. A 2014, 2, 10444–10453. [Google Scholar] [CrossRef]
- Tharanitharan, V.; Srinivasan, K. Removal of Pb(II) from aqueous solutions by using dioctyl sodium sulphosuccinate-EDTA modified Amberlite XAD-7HP resin. Indian J. Chem. Technol. 2009, 16, 417–425. [Google Scholar]
- Tharanitharan, V.; Srinivasan, K. Kinetic and equilibrium studies of removal of pb(ii) and cd(ii) ions from aqueous solution by modified duolite XAD-761 resins. Asian J. Chem. 2010, 22, 3036–3046. [Google Scholar]
- Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Hsieh, Y.-L.; Wei, Q. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef]
- Allouche, F.-N.; Mameri, N.; Guibal, E. Pb(II) biosorption on Posidonia oceanica biomass. Chem. Eng. J. 2011, 168, 1174–1184. [Google Scholar] [CrossRef]
- Martin-Lara, M.A.; Blazquez, G.; Ronda, A.; Rodriguez, I.L.; Calero, M. Multiple biosorption-desorption cycles in a fixed-bed column for Pb(II) removal by acid-treated olive stone. J. Ind. Eng. Chem. 2012, 18, 1006–1012. [Google Scholar] [CrossRef]
- Yipmantin, A.; Maldonado, H.J.; Ly, M.; Taulemesse, J.M.; Guibal, E. Pb(II) and Cd(II) biosorption on Chondracanthus chamissoi (a red alga). J. Hazard. Mater. 2011, 185, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraja, G.; Krishnaiah, N.; Subbaiah, M.V.; Krishnaiah, A. Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf. B 2014, 114, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Cai, J.C.; Zhang, Z.H.; Liu, L.J.; Yang, G.L. Investigation of removal of Pb(II) and Hg(II) by a novel cross-linked chitosan-poly(aspartic acid) chelating resin containing disulfide bond. Colloid Polym. Sci. 2014, 292, 2157–2172. [Google Scholar] [CrossRef]
- Wang, L.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, R.; Li, P. Studies on adsorption behavior of Pb(II) onto a thiourea-modified chitosan resin with Pb(II) as template. Carbohydr. Polym. 2010, 81, 305–310. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, E.O.; Alayande, O.S.; Msagati, T.A.M. Synthesis and characterization of biopolymeric chitosan derived from land snail shells and its potential for Pb2+ removal from aqueous solution. Materials 2015, 8, 8630–8640. [Google Scholar] [CrossRef]
- Mohammad, M.; Yaakob, Z.; Abdullah, S.R.S. Carbon derived from Jatropha seed hull as a potential green adsorbent for cadmium (II) removal from wastewater. Materials 2013, 6, 4462–4478. [Google Scholar] [CrossRef]
- Rahman, M.M.; Adil, M.; Yusof, A.M.; Kamaruzzaman, Y.B.; Ansary, R.H. Removal of heavy metal ions with acid activated carbons derived from oil palm and coconut shells. Materials 2014, 7, 3634–3650. [Google Scholar] [CrossRef]
- Ricco, R.; Konstas, K.; Styles, M.J.; Richardson, J.J.; Babarao, R.; Suzuki, K.; Scopece, P.; Falcaro, P. Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. A 2015, 3, 19822–19831. [Google Scholar] [CrossRef]
- Su, Q.; Pan, B.; Pan, B.; Zhang, Q.; Zhang, W.; Lv, L.; Wang, X.; Wu, J.; Zhang, Q. Fabrication of polymer-supported nanosized hydrous manganese dioxide (HMO) for enhanced lead removal from waters. Sci. Total Environ. 2009, 407, 5471–5477. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. Preparation and characterization of functionalized silica spheres for removal of Cu(II), Pb(II), Cr(VI) and Cd(II) from aqueous solutions. RSC Adv. 2015, 5, 28624–28632. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Nowicki, M.; Wisniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gonzalez, M.P.; Saucedo, I.; Navarro, R.; Avila, M.; Guibal, E. Selective separation of Fe(III), Cd(II), and Ni(II) from dilute solutions using solvent-impregnated resins. Ind. Eng. Chem. Res. 2001, 40, 6004–6013. [Google Scholar] [CrossRef]
- Arias, A.; Saucedo, I.; Navarro, R.; Gallardo, V.; Martinez, M.; Guibal, E. Cadmium(II) recovery from hydrochloric acid solutions using Amberlite XAD-7 impregnated with a tetraalkyl phosphonium ionic liquid. React. Funct. Polym. 2011, 71, 1059–1070. [Google Scholar] [CrossRef]
- Navarro, R.; Saucedo, I.; Avila, M.; Gonzalez, M.P.; Garcia, S.; Guibal, E. Zinc(II) extraction from hydrochloric acid solutions using Amberlite XAD-7 impregnated with Cyanex 921 (tri-octyl phosphine oxide). Solvent Extr. Ion Exch. 2007, 25, 273–297. [Google Scholar] [CrossRef]
- Navarro, R.; Saucedo, I.; Nunez, A.; Avila, M.; Guibal, E. Cadmium extraction from hydrochloric acid solutions using Amberlite XAD-7 impregnated with Cyanex 921 (tri-octyl phosphine oxide). React. Funct. Polym. 2008, 68, 557–571. [Google Scholar] [CrossRef]
- Draa, M.T.; Belaid, T.; Benamor, M. Extraction of Pb(II) by XAD7 impregnated resins with organophosphorus extractants (DEHPA, IONQUEST 801, CYANEX 272). Sep. Purif. Technol. 2004, 40, 77–86. [Google Scholar] [CrossRef]
- Hosseini-Bandegharaei, A.; Karimzadeh, M.; Sarwghadi, M.; Heydarbeigi, A.; Hosseini, S.H.; Nedaie, M.; Shoghi, H. Use of a selective extractant-impregnated resin for removal of Pb(II) ion from waters and wastewaters: Kinetics, equilibrium and thermodynamic study. Chem. Eng. Res. Des. 2014, 92, 581–591. [Google Scholar] [CrossRef]
- Huynh, H.T.; Tanaka, M. Removal of Bi, Cd, Co, Cu, Fe, Ni, Pb, and Zn from an aqueous nitrate medium with bis(2-ethylhexyl)phosphoric acid impregnated kapok fiber. Ind. Eng. Chem. Res. 2003, 42, 4050–4054. [Google Scholar] [CrossRef]
- Nghiem Van, N.; Lee, J.-C.; Hai Trung, H.; Jeong, J. Extraction and separation of cadmium from the chloride solution of E-waste using Cyanex 923 impregnated Amberlite XAD-7HP resin. Mater. Trans. 2015, 56, 1294–1301. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowska, J.; Kozlowski, C.; Trochimczuk, A. Sorption of Pb(II), Cd(II) and Zn(II) performed with the use of carboxyphenylresorcinarene-impregnated Amberlite XAD-4 resin. Desalin. Water Treat. 2014, 52, 314–323. [Google Scholar] [CrossRef]
- Navarro, R.; Ruiz, P.; Saucedo, I.; Guibal, E. Bismuth(III) recovery from hydrochloric acid solutions using Amberlite XAD-7 impregnated with a tetraalkylphosphonium ionic liquid. Sep. Purif. Technol. 2014, 135, 268–277. [Google Scholar] [CrossRef]
- Navarro, R.; Alba, J.; Saucedo, I.; Guibal, E. Hg(II) removal from HCl Solutions using a tetraalkylphosphonium ionic liquid impregnated onto Amberlite XAD-7. J. Appl. Polym. Sci. 2014, 131, 547–557. [Google Scholar] [CrossRef]
- Mimura, H.; Hoshi, H.; Akiba, K.; Onodera, Y. Separation of americium from europium by biopolymer microcapsules enclosing Cyanex 301 extractant. J. Radioanal. Nucl. Chem. 2001, 247, 375–379. [Google Scholar] [CrossRef]
- Mimura, H.; Ohta, H.; Akiba, K.; Onodera, Y. Selective uptake and recovery of palladium by biopolymer microcapsules enclosing Cyanex 302 extractant. J. Nucl. Sci. Technol. 2001, 38, 342–348. [Google Scholar] [CrossRef]
- Mimura, H.; Ohta, H.; Hoshi, H.; Akiba, K.; Onodera, Y. Uptake properties of palladium for biopolymer microcapsules enclosing Cyanex 302 extractant. Sep. Sci. Technol. 2001, 36, 31–44. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Jouannin, C. Immobilization of extractants in biopolymer capsules for the synthesis of new resins: A focus on the encapsulation of tetraalkyl phosphonium ionic liquids. J. Mater. Chem. 2009, 19, 8515–8527. [Google Scholar] [CrossRef]
- Mimura, H.; Outokesh, M.; Niibori, Y.; Tanaka, K. Preparation of biopolymer microcapsules and their uptake properties for Cd2+ ions. In Waste Management in Japan; Itoh, H., Ed.; WIT Press: Southampton, UK, 2004; pp. 99–108. [Google Scholar]
- Ngomsik, A.-F.; Bee, A.; Siaugue, J.-M.; Talbot, D.; Cabuil, V.; Cote, G. Co(II) removal by magnetic alginate beads containing Cyanex 272 (R). J. Hazard. Mater. 2009, 166, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Ocio, A.; Mijangos, F.; Elizalde, M. Copper and cadmium extraction from highly concentrated phosphoric acid solutions using calcium alginate gels enclosing bis(2,4,4-trimethylpentyl)thiophosphinic acid. J. Chem. Technol. Biotechnol. 2006, 81, 1409–1418. [Google Scholar] [CrossRef]
- Outokesh, M.; Mimura, H.; Niibori, Y.; Tanaka, K. Equilibrium and kinetics of silver uptake by multinuclear alginate microcapsules comprising an ion exchanger matrix and Cyanex 302 organophosphinic acid extractant. Ind. Eng. Chem. Res. 2006, 45, 3633–3643. [Google Scholar] [CrossRef]
- Outokesh, M.; Mimura, H.; Niibori, Y.; Tanaka, K. Preparation of stable alginate microcapsules coated with chitosan or polyethyleneimine for extraction of heavy metal ions. J. Microencapsul. 2006, 23, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Outokesh, M.; Niibori, Y.; Mimura, H.; Ahmadi, S.J. Comparison of the batch and breakthrough properties of stable and plain alginate microcapsules with a chelating resin and an ion exchanger in Ag+ adsorption. Ind. Eng. Chem. Res. 2008, 47, 6742–6752. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K.; Burmistrzak, P. Removal of cadmium(II) ions from chloride solutions by Cyanex 301 and Cyanex 302. Sep. Sci. Technol. 2011, 46, 1495–1502. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B. Solvent-extraction of copper by Cyanex-272, Cyanex-302 and Cyanex-301. Hydrometallurgy 1995, 37, 129–147. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Kovalev, V.; Khotimchenko, Y. Comparative equilibrium studies of sorption of Pb(II) ions by sodium and calcium alginate. J. Environ. Sci. China 2008, 20, 827–831. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Argekar, A.P.; Shetty, A.K. Extraction of lead(II) with cyanex 302 and its spectrophotometric determination with PAR. Talanta 1998, 45, 909–915. [Google Scholar] [CrossRef]
- Juang, R.-S.; Ju, C.-Y. Kinetics of sorption of Cu(II)-ethylenediaminetetraacetic acid chelated anions onto cross-linked, polyaminated chitosan beads. Ind. Eng. Chem. Res. 1998, 37, 3463–3469. [Google Scholar] [CrossRef]
- Abate, G.; Masini, J.C. Complexation of Cd(II) and Pb(II) with humic acids studied by anodic stripping voltammetry using differential equilibrium functions and discrete site models. Org. Geochem. 2002, 33, 1171–1182. [Google Scholar] [CrossRef]
- Navarro, R.; Saucedo, I.; Gonzalez, C.; Guibal, E. Amberlite XAD-7 impregnated with Cyphos IL-101 (tetraalkylphosphonium ionic liquid) for Pd(II) recovery from HCl solutions. Chem. Eng. J. 2012, 185, 226–235. [Google Scholar] [CrossRef]
- Navarro, R.; Saucedo, I.; Lira, M.A.; Guibal, E. Gold(III) recovery from HCl solutions using Amberlite XAD-7 impregnated with an ionic liquid (Cyphos IL-101). Sep. Sci. Technol. 2010, 45, 1950–1962. [Google Scholar] [CrossRef]
- Navarro, R.; Lira, M.A.; Saucedo, I.; Alatorre, A.; Guibal, E. Amberlite XAD-1180 impregnation with Cyphos IL101 for the selective recovery of precious metals from HCl solutions. Gold Bull. 2017, 50, 7–23. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B.; Ferguson, T.L. An assessment of the long-term stabilities of Cyanex 302 and Cyanex 301 in sulfuric acid and nitric acids. Solvent Extr. Ion Exch. 1993, 11, 783–796. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural regime identification in ionotropic alginate gels: Influence of the cation nature and alginate structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Newton, MA, USA, 1994; p. 243. [Google Scholar]
- Krys, P.; Testa, F.; Trochimczuk, A.; Pin, C.; Taulemesse, J.-M.; Vincent, T.; Guibal, E. Encapsulation of ammonium molybdophosphate and zirconium phosphate in alginate matrix for the sorption of rubidium(I). J. Colloid Interface Sci. 2013, 409, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E.; Campos Gavilan, K.; Bunio, P.; Vincent, T.; Trochimczuk, A. Cyphos IL 101 (tetradecyl(trihexyl)phosphonium chloride) immobilized in biopolymer capsules for Hg(II) recovery from HCl solutions. Sep. Sci. Technol. 2008, 43, 2406–2433. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lin, H.-C. Metal sorption with extractant-impregnated macroporous resins. 1. Particle diffusion kinetics. J. Chem. Technol. Biotechnol. 1995, 62, 132–140. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lin, H.-C. Metal sorption with extractant-impregnated macroporous resins. 2. Chemical reaction and particle diffusion kinetics. J. Chem. Technol. Biotechnol. 1995, 62, 141–147. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]

| Extractant | Mode | Extractant Concentration * (% v/v) | Size | Cyanex Content qHA (mg·g−1) | Cyanex Content qHA (µmol·g−1) | Capsule Size (µm) |
|---|---|---|---|---|---|---|
| C301 | M | 25 | - | 144.0 ± 2.5 | 446 ± 8 | 623 ± 14 |
| C301 | M | 50 | L | 338.9 ± 10.8 | 1051 ± 33 | 1846 ± 39 |
| C301 | M | 50 | S | 290.2 ± 4.7 | 900 ± 15 | 724 ± 19 |
| C301 | N | 50 | - | 93.9 ± 4.5 | 291 ± 14 | 1110 ± 31 |
| C302 | M | 50 | - | 314.5 ± 9.0 | 1026 ± 29 | 1430 ± 46 |
| C302 | M | 75 | - | 480.9 ± 25.3 | 1569 ± 8 | 1378 ± 19 |
| C302 | N | 50 | - | 55.8 ± 2.1 | 182 ± 7 | 876 ± 25 |
| C302 | N | 75 | - | 203.8 ± 5.0 | 665 ± 16 | 1034 ± 54 |
| Sorbent | Cyanex Content qHA (µmol·g−1) | qm (mg·Pb·g−1) | qm (µmol·Pb·g−1) | b (L·mg−1) | R2 | Cyanex/Pb(II) (mol/mol) |
|---|---|---|---|---|---|---|
| C301-M-25 | 446 ± 8 | 48.3 | 233 | 0.445 | 0.999 | 1.91 |
| C301-M-50L | 1051 ± 33 | 69.3 | 334 | 5.96 | 0.999 | 3.14 |
| C301-M-50S | 900 ± 15 | 78.6 | 379 | 3.44 | 0.999 | 2.37 |
| C301-N-50 | 291 ± 14 | 36.3 | 175 | 1.06 | 0.998 | 1.66 |
| C302-M-50 | 1026 ± 29 | 35.1 | 169 | 1.58 | 0.999 | 6.06 |
| C302-M-75 | 1569 ± 8 | 49.5 | 239 | 1.83 | 0.997 | 6.57 |
| C302-N-75 | 665 ± 16 | 24.2 | 117 | 0.47 | 0.998 | 5.69 |
| Sorbent | Cyanex Content qHA (µmol·g−1) | De × 1011 (m2·min−1) | MSR |
|---|---|---|---|
| C301-M-25 | 446 ± 8 | 7.95 | 0.077 |
| C301-M-50L | 1051 ± 33 | 1.60 | 0.036 |
| C301-M-50S | 900 ± 15 | 0.27 | 0.078 |
| C301-N-50 | 291 ± 14 | 9.26 | 0.233 |
| C302-M-50 | 1026 ± 29 | 2.40 | 0.049 |
| C302-M-75 | 1569 ± 8 | 7.13 | 0.050 |
| C302-N-75 | 665 ± 16 | 3.20 | 0.108 |
| Sorbent | qHA | qe,exp | PFORE | PSORE | ||||
|---|---|---|---|---|---|---|---|---|
| qe,1 | k1 × 102 | MSR | qe,2 | k2 × 103 | MSR | |||
| C301-M-25 | 446 | 42.94 | 40.13 | 5.83 | 2.67 | 41.88 | 2.05 | 0.804 |
| C301-M-50L | 1051 | 64.76 | 61.60 | 0.42 | 25.0 | 65.03 | 0.10 | 8.016 |
| C301-M-50S | 900 | 76.88 | 73.06 | 2.26 | 17.0 | 76.99 | 0.45 | 1.333 |
| C301-N-50 | 291 | 29.65 | 28.94 | 1.15 | 0.50 | 31.17 | 0.53 | 2.956 |
| C302-M-50 | 1026 | 30.62 | 29.25 | 1.89 | 1.31 | 30.77 | 0.99 | 0.666 |
| C302-M-75 | 1569 | 45.63 | 43.25 | 1.81 | 4.74 | 45.57 | 0.62 | 1.111 |
| C302-N-75 | 665 | 24.69 | 22.46 | 3.10 | 1.06 | 23.60 | 1.96 | 0.831 |
| Sorbent | Desorption Efficiency (%) | ||
|---|---|---|---|
| 0.1 M HNO3 | 1 M HNO3 | 1 M Thiourea/1 M HCl | |
| C301-M-25 | 56.1 | >99.9 | 88.2 |
| C302-M-50 | 39.0 | >99.9 | 83.1 |
| C302-N-75 | 78.0 | >99.9 | 85.9 |
| Sorbent | Cycle # 1 | Cycle # 2 | Cycle # 3 | |||
|---|---|---|---|---|---|---|
| Sorption | Desorption | Sorption | Desorption | Sorption | Desorption | |
| C301-M-25 | 35.5 | 97.7 | 2.9 | 94.6 | 2.1 | 93.5 |
| C302-M-50 | 55.8 | 99.5 | 4.6 | 90.5 | 5.8 | 70.9 |
| C302-N-75 | 33.2 | 98.6 | 4.9 | 6.6 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba, J.; Navarro, R.; Saucedo, I.; Vincent, T.; Guibal, E. Extractant Immobilization in Alginate Capsules (Matrix- and Mononuclear-Type): Application to Pb(II) Sorption from HCl Solutions. Materials 2017, 10, 634. https://doi.org/10.3390/ma10060634
Alba J, Navarro R, Saucedo I, Vincent T, Guibal E. Extractant Immobilization in Alginate Capsules (Matrix- and Mononuclear-Type): Application to Pb(II) Sorption from HCl Solutions. Materials. 2017; 10(6):634. https://doi.org/10.3390/ma10060634
Chicago/Turabian StyleAlba, Janette, Ricardo Navarro, Imelda Saucedo, Thierry Vincent, and Eric Guibal. 2017. "Extractant Immobilization in Alginate Capsules (Matrix- and Mononuclear-Type): Application to Pb(II) Sorption from HCl Solutions" Materials 10, no. 6: 634. https://doi.org/10.3390/ma10060634
APA StyleAlba, J., Navarro, R., Saucedo, I., Vincent, T., & Guibal, E. (2017). Extractant Immobilization in Alginate Capsules (Matrix- and Mononuclear-Type): Application to Pb(II) Sorption from HCl Solutions. Materials, 10(6), 634. https://doi.org/10.3390/ma10060634







