Co3O4@CoS Core-Shell Nanosheets on Carbon Cloth for High Performance Supercapacitor Electrodes
Abstract
:1. Introduction
2. Experimental
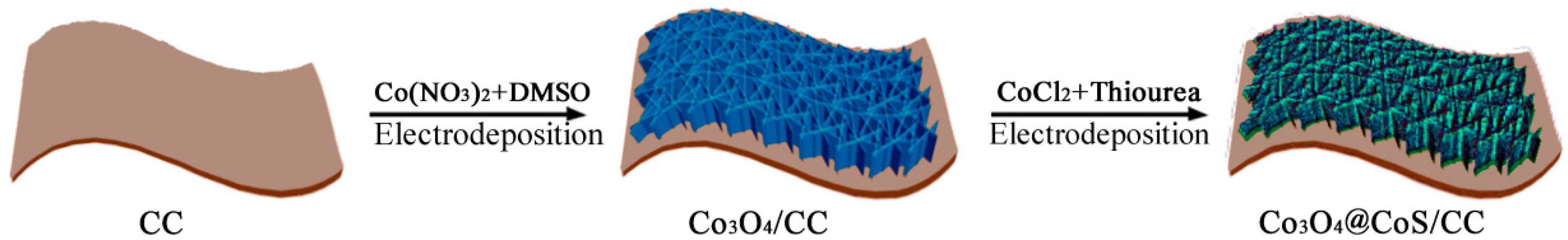
2.1. Synthesis of Co3O4/CC and CoS/CC Composites
2.2. Synthesis of Co3O4@CoS/CC Composites
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
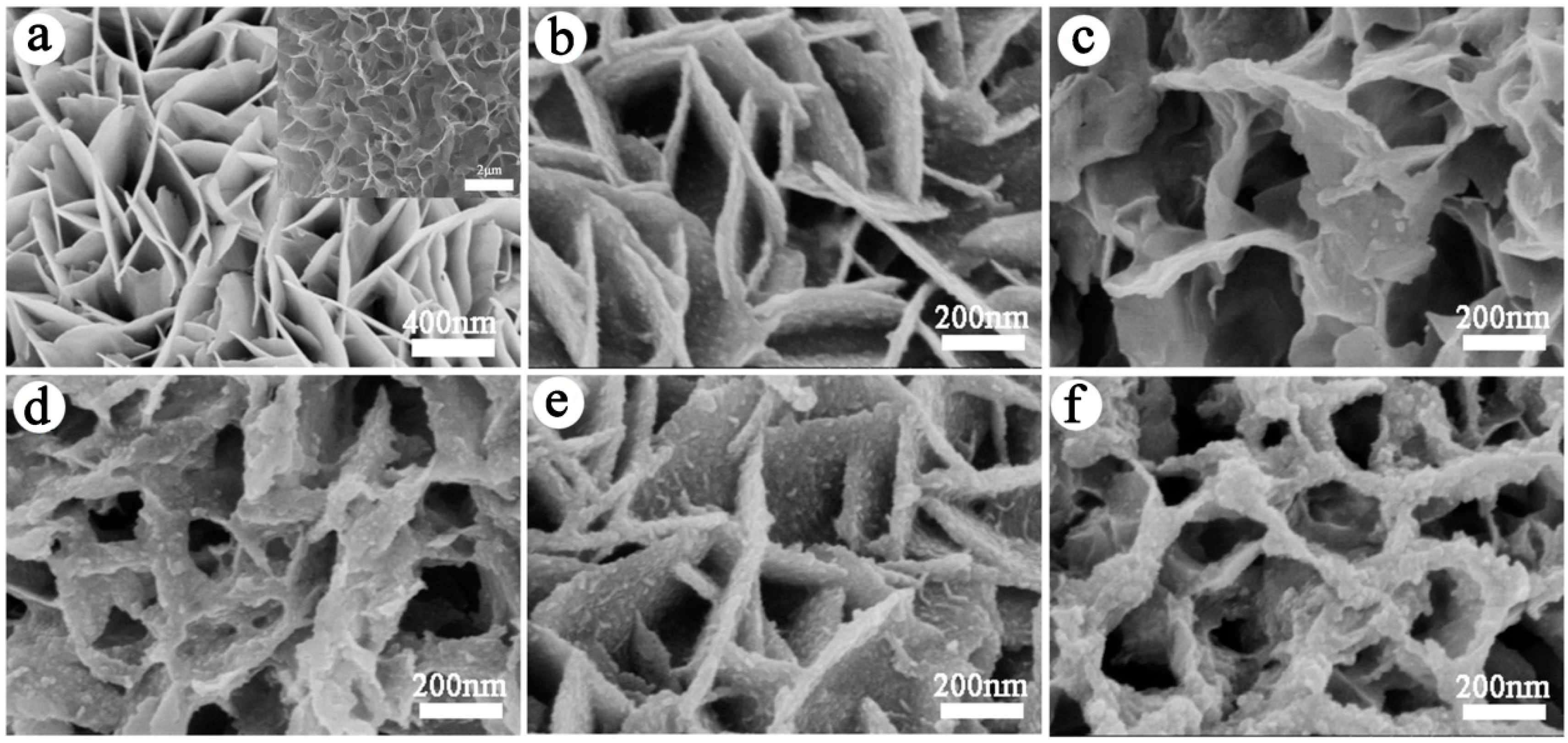
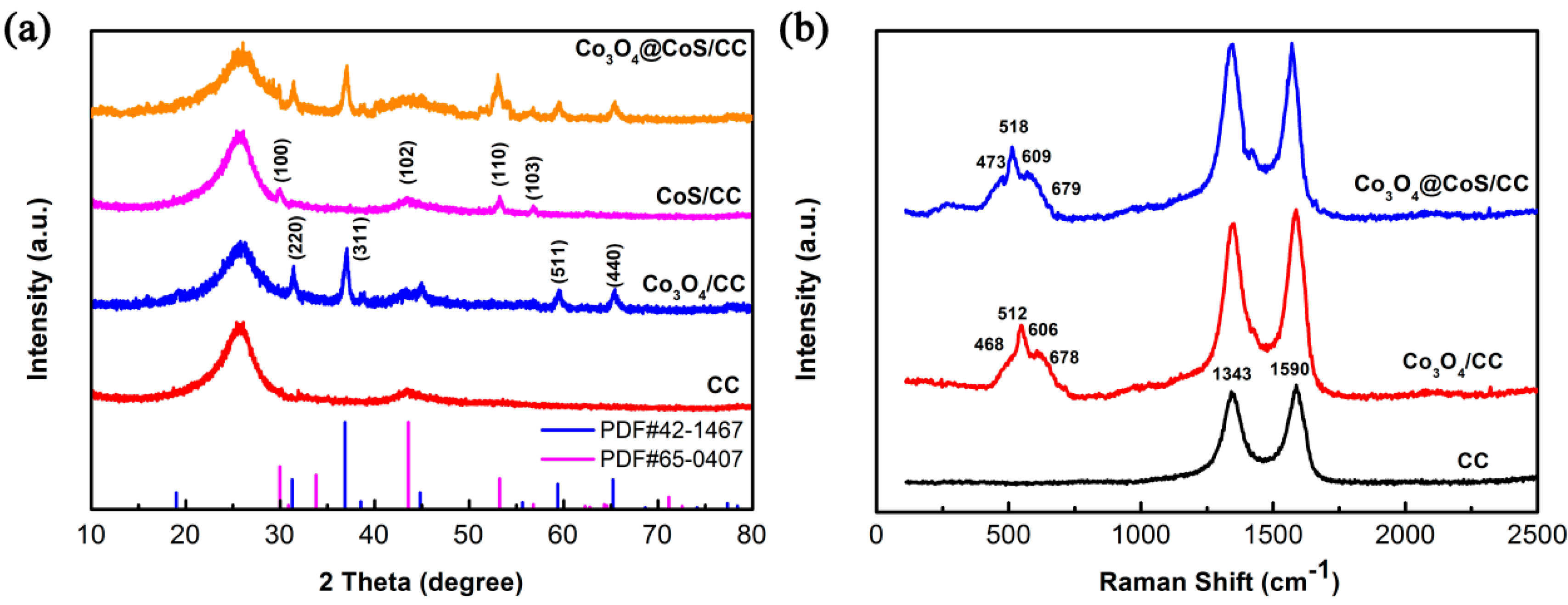
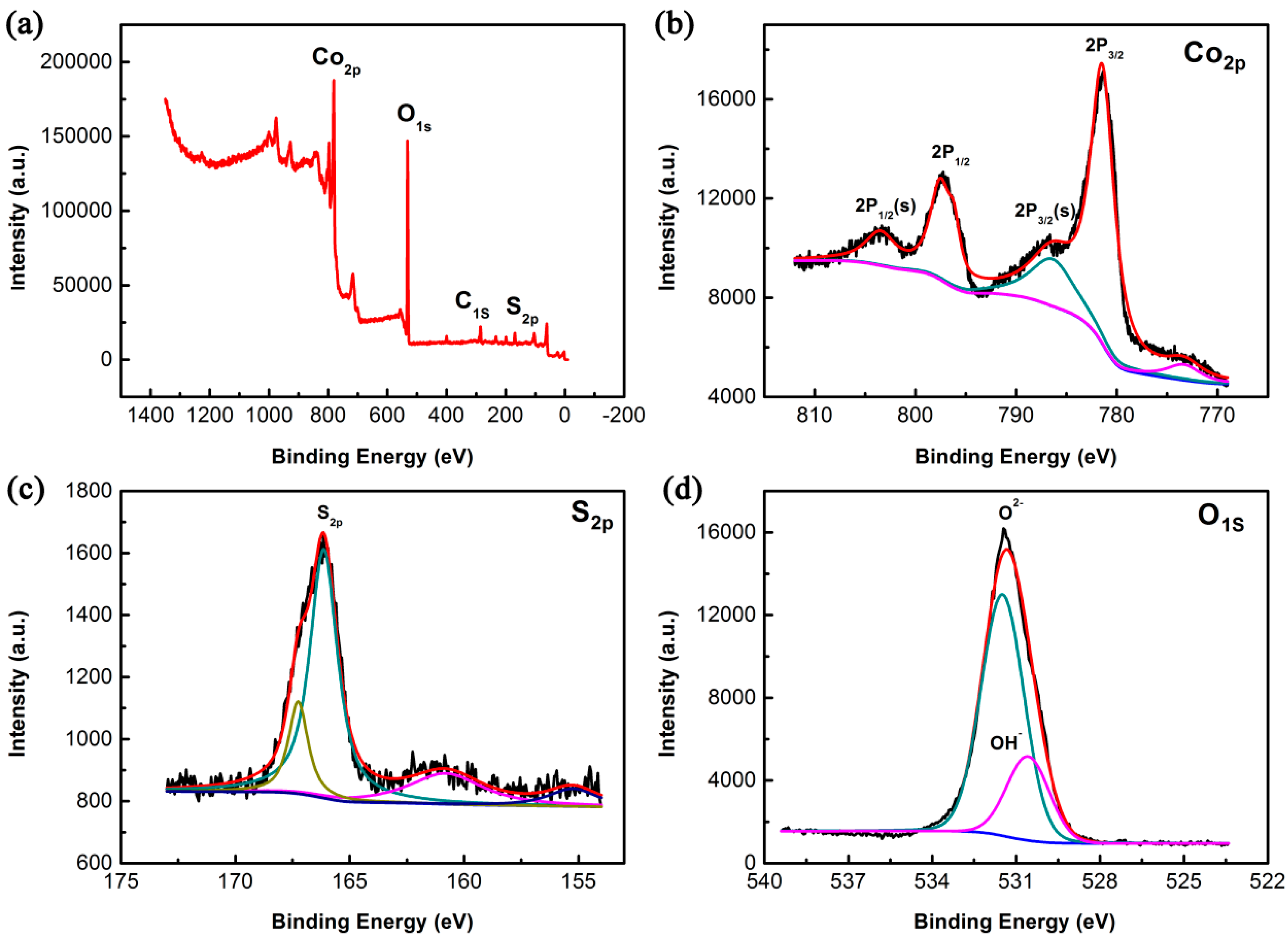
3.1. Morphology and Structural Characterization
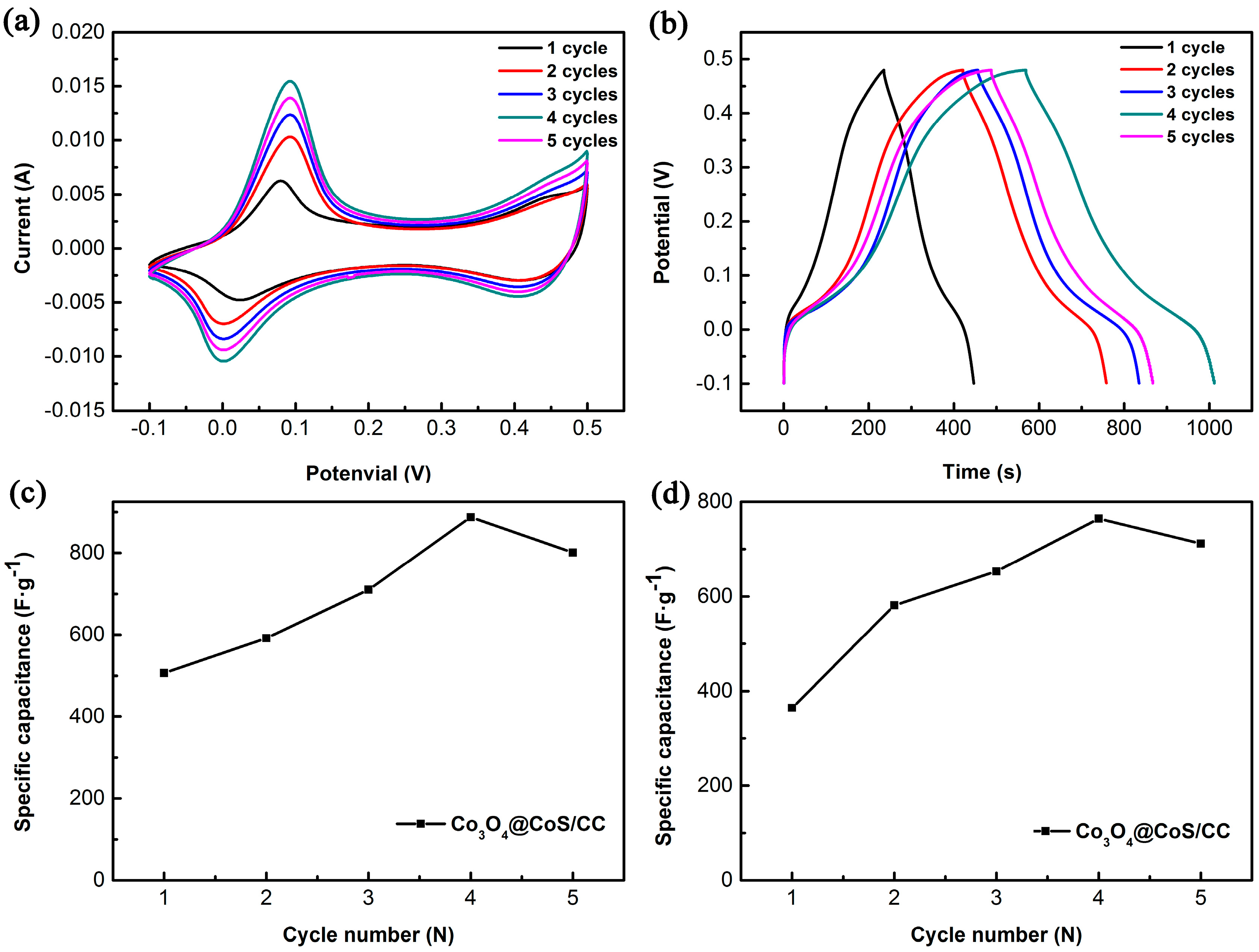
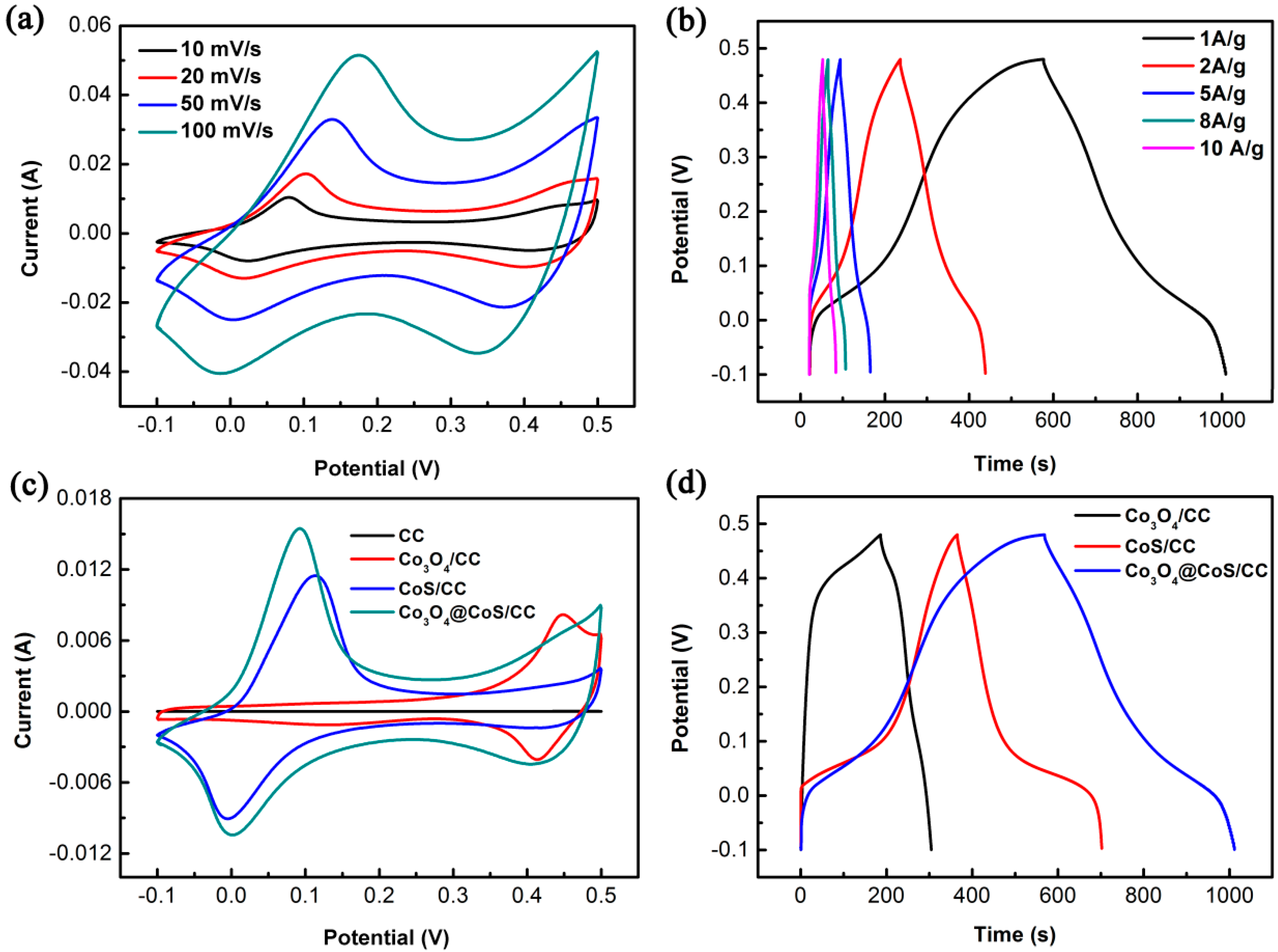
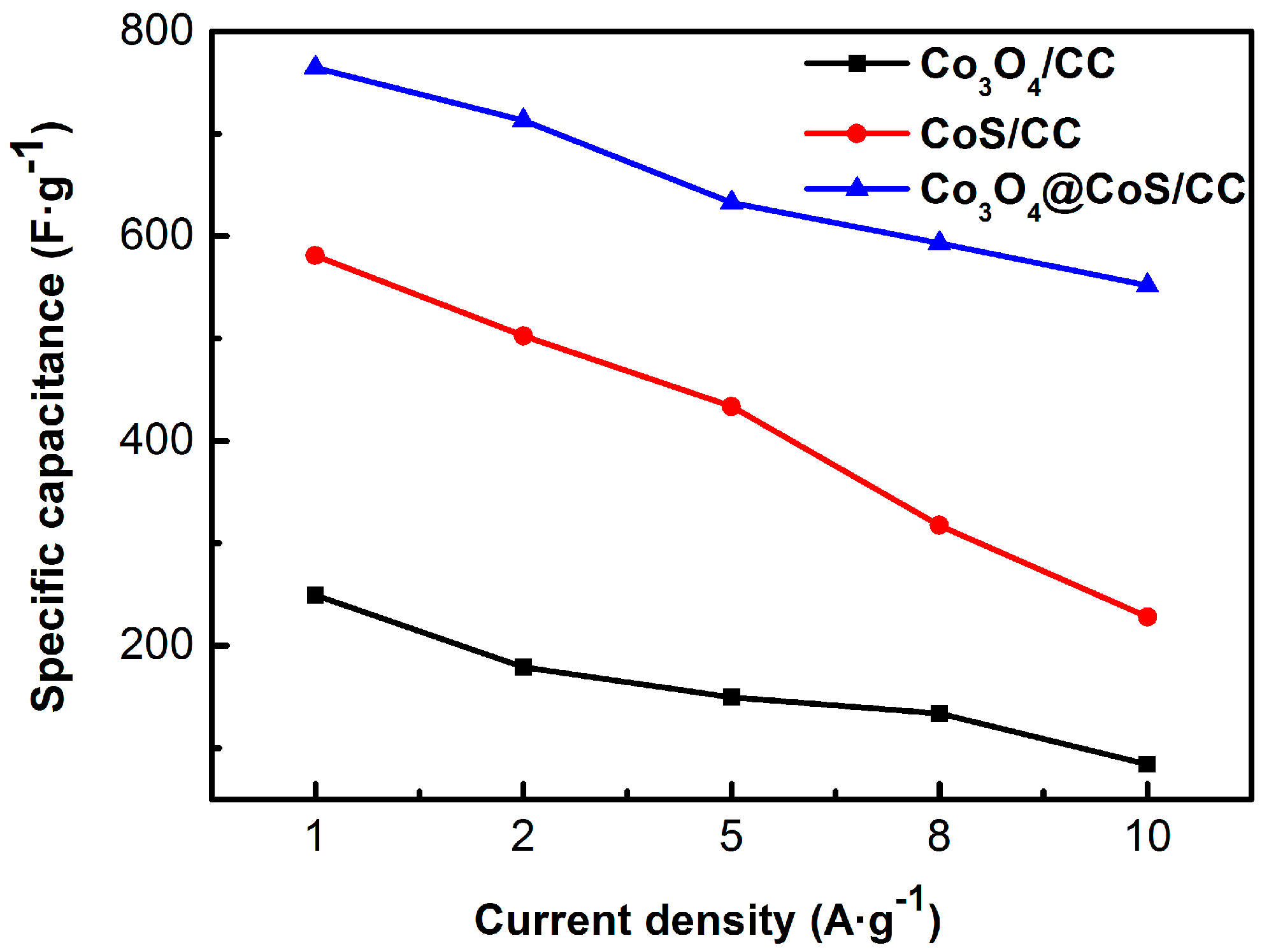
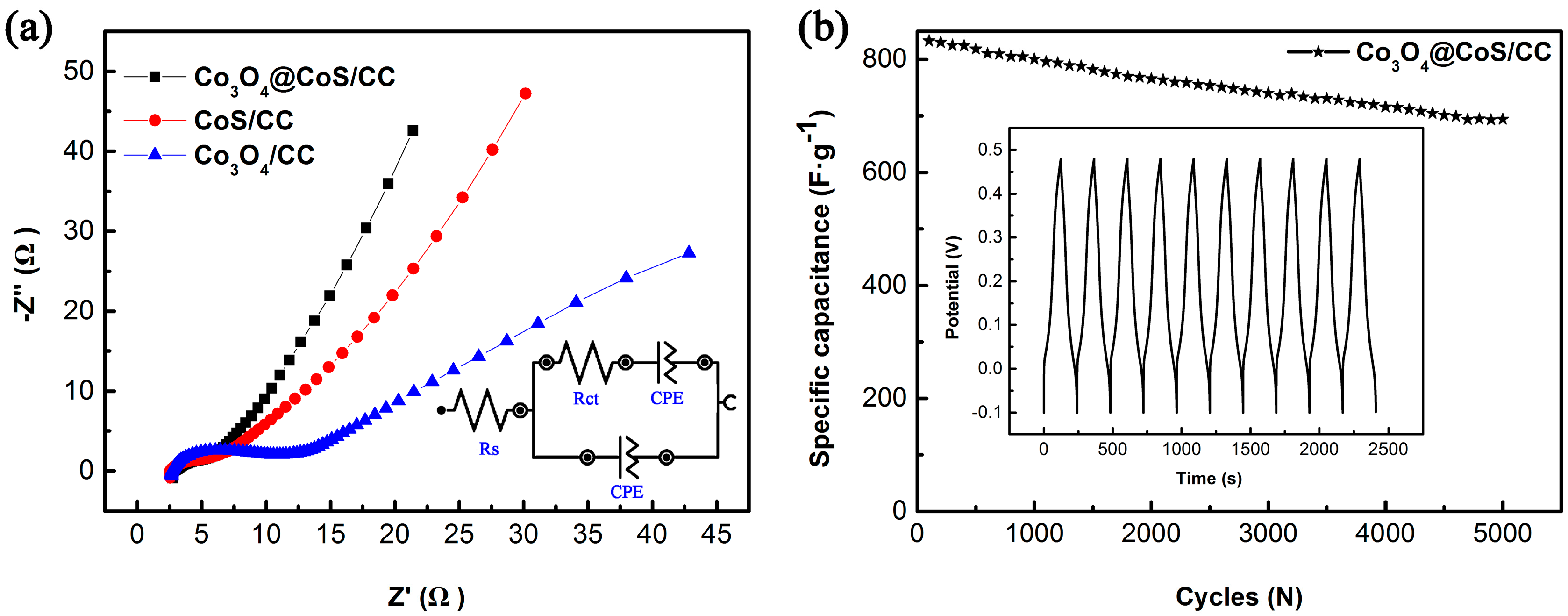
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, 28–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Y. Recent progress in supercapacitors: From materials design to system construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gu, Z.; Zheng, X.; Zhang, X. Three-dimensional Co3O4@NiO hierarchical nanowire arrays for solid-state symmetric supercapacitor with enhanced electrochemical performances. Chem. Eng. J. 2016, 304, 223–231. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2011, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Li, Z.; Zhang, R.; Ning, F.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical conducting polymer@clay core-shell arrays for flexible all-solid-state supercapacitor devices. Small 2015, 11, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Tu, J.P.; Mai, Y.J.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011, 21, 9319–9325. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultra layered Co3O4 for high-performance supercapacitor applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, Y.Q.; Mai, Y.J.; Wang, X.L.; Gu, C.D.; Zhao, X.B. Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv. 2012, 2, 1835–1841. [Google Scholar] [CrossRef]
- Silva, R.; Pereira, G.M.; Voiry, D.; Chhowalla, M.; Asefa, T. Co3O4 nanoparticles/cellulose nanowhiskers derived amorphous carbon nanoneedles: Sustainable materials for supercapacitors and oxygen reduction electrocatalysis. RSC Adv. 2015, 5, 49385–49391. [Google Scholar] [CrossRef]
- Sun, G.; Ma, L.; Ran, J.; Shen, X.; Tong, H. Incorporation of homogeneous Co3O4 into a nitrogen-doped carbon aerogel: Via a facile in situ synthesis method: Implications for high performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 9542–9554. [Google Scholar] [CrossRef]
- Ke, Q.; Tang, C.; Yang, Z.C.; Zheng, M.; Mao, L.; Liu, H.; Wang, J. 3D Nanostructure of carbon nanotubes decorated Co3O4 nanowire arrays for high performance supercapacitor electrode. Electrochim. Acta 2015, 163, 9–15. [Google Scholar] [CrossRef]
- Raj, R.P.; Ragupathy, P.; Mohan, S. Remarkable capacitive behavior of a Co3O4-polyindole composite as electrode material for supercapacitor applications. J. Mater. Chem. A 2015, 3, 24338–24348. [Google Scholar] [CrossRef]
- Lin, H.; Huang, Q.; Wang, J.; Jiang, J.; Liu, F.; Chen, Y.; Wang, C.; Lu, D.; Han, S. self-assembled graphene/polyaniline/Co3O4 ternary hybrid aerogels for supercapacitors. Electrochim. Acta 2016, 191, 444–451. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.; Li, Z.; Yang, S. Hierarchical Co3O4@Au-decorated PPy core/shell nanowire arrays: An efficient integration of active materials for energy storage. J. Mater. Chem. A 2015, 3, 2535–2540. [Google Scholar] [CrossRef]
- Kong, D.; Luo, J.; Wang, Y.; Ren, W.; Yu, T.; Luo, Y.; Yang, Y.; Cheng, C. Three-dimensional Co3O4@MnO2 hierarchical nanoneedle arrays: Morphology control and electrochemical energy etorage. Adv. Funct. Mater. 2014, 24, 3815–3826. [Google Scholar] [CrossRef]
- Tang, C.H.; Yin, X.; Gong, H. Superior performance asymmetric supercapacitors based on a directly grown commercial mass 3D Co3O4@Ni(OH)2 core-shell electrode. ACS Appl. Mater. Interfaces 2013, 5, 10574–10582. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Wang, A.L.; Li, G.R.; Wang, J.W.; Ou, Y.N.; Tong, Y.X. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J. Mater. Chem. 2012, 22, 5656–5665. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, T.; Yu, X.; Zhang, H.; Duan, H.; Lu, B. Nanoforest of hierarchical Co3O4@NiCo2O4 nanowire arrays for high-performance supercapacitors. Nano Energy 2013, 2, 586–594. [Google Scholar] [CrossRef]
- Li, S.; Wen, J.; Chen, T.; Xiong, L.; Wang, J.; Fang, G. In situ synthesis of 3D CoS nanoflake/Ni(OH)2 nanosheet nanocomposite structure as a candidate supercapacitor electrode. Nanotechnology 2016, 27, 1–9. [Google Scholar]
- Luo, F.; Li, J.; Yuan, H.; Xiao, D. Rapid synthesis of three-dimensional flower-like cobalt sulfide hierarchitectures by microwave assisted heating method for high-performance supercapacitors. Electrochim. Acta 2014, 123, 183–189. [Google Scholar] [CrossRef]
- Ray, R.S.; Sarma, B.; Jurovitzki, A.L.; Misra, M. Fabrication and characterization of titania nanotube/cobalt sulfide supercapacitor electrode in various electrolytes. Chem. Eng. J. 2015, 260, 671–683. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.; Zhang, S.; Mao, C.; Song, J.; Niu, H.; Jin, B.; Tian, Y. Controlled synthesis of nickel sulfide/graphene oxide nanocomposite for high-performance supercapacitor. Appl. Surf. Sci. 2013, 282, 704–708. [Google Scholar] [CrossRef]
- Wu, J.; Ouyang, C.; Dou, S.; Wang, S. Hybrid NiS/CoO mesoporous nanosheet arrays on Ni foam for high-rate supercapacitors. Nanotechnology 2015, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Sourous 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Shen, L.; Song, X.; Chen, H.; Lou, X.W. General formation of MS (M = Ni, Cu, Mn) box-in-box hollow structures with enhanced pseudocapacitive properties. Adv. Funct. Mater. 2014, 24, 7440–7446. [Google Scholar] [CrossRef]
- Wang, H.Y.; Xiao, F.X.; Yu, L.; Liu, B.; Lou, X.W. Hierarchical α-MnO2 nanowires@Ni1−xMnxOy nanoflakes core-shell nanostructures for supercapacitors. Small 2014, 10, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible energy-storage devices: Design consideration and recent progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, K.; Zu, S.Z.; Han, B.H.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kong, D.; Zhang, J.; Wang, Y.; Chen, T.; Cheng, C.; Yang, H.Y. 3D hierarchical Co3O4@Co3S4 nanoarrays as cathode materials for asymmetric pseudocapacitors. J. Mater. Chem. A 2016, 4, 3287–3296. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, T.J.; Gong, H. Remarkable improvement in supercapacitor performance by sulfur introduction during a one-step synthesis of nickel hydroxide. Phys. Chem. Chem. Phys. 2017, 19, 10462–10469. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, S.; Ding, Y.; Zhu, X.; Wang, Z.L.; Liu, M. Hierarchical network architectures of carbon fiber paper supported cobalt oxide nanonet for high-capacity pseudocapacitors. Nano Lett. 2012, 12, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Wang, J.; Huang, Z. N3S2@CoS core-shell nano-triangular pyramid arrays on Ni foam for high-performance supercapacitors. Phys. Chem. Chem. Phys. 2015, 17, 16434–16442. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, G.; Du, X.; Jagadale, A.D.; Cao, J.; Hao, X.; Ma, X.; Abudula, A. Homogeneous nanosheet Co3O4 film prepared by novel unipolar pulse electro-deposition method for electrochemical water splitting. RSC Adv. 2015, 5, 76026–76031. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Pavlinek, V.; Cheng, Q.; Saha, P.; Li, C. MnO2 nanoflake/polyaniline nanorod hybrid nanostructures on graphene paper for high-performance flexible supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 17165–17171. [Google Scholar] [CrossRef]
- Kim, C.; Yang, K.; Kojima, M.; Yoshida, K.; Kim, Y.J.; Kim, Y.A.; Endo, M. Fabrication of electrospinning-derived carbon nanofiber webs for the anode material of lithium-ion secondary batteries. Adv. Funct. Mater. 2006, 16, 2393–2397. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, 199–201. [Google Scholar] [CrossRef]
- Xu, J.; Fu, G.; Tang, Y.; Zhao, T.S. Non-precious Co3O4 nano-rod electrocatalyst for oxygen reduction reaction in anion-exchange membrane fuel cells. Energy Environ. Sci. 2012, 5, 5333–5339. [Google Scholar] [CrossRef]
- Sun, H.; Qin, D.; Huang, S.; Guo, X.; Li, D.; Luo, Y.; Meng, Q. Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique. Energy Environ. Sci. 2011, 4, 2630–2637. [Google Scholar] [CrossRef]
- Mate, V.R.; Shirai, M.; Rode, C.V. Heterogeneous Co3O4, catalyst for selective oxidation of aqueous veratryl alcohol using molecular oxygen. Catal. Commun. 2013, 33, 66–69. [Google Scholar] [CrossRef]
- Tao, F.; Zhao, Y.Q.; Zhang, G.Q.; Li, H.L. Electrochemical characterization on cobalt sulfide for electrochemical supercapacitors. Electrochem. Commun. 2007, 9, 1282–1287. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shih, Z.Y.; Yang, Z.; Chang, H.T. Carbon nanotubes/cobalt sulfide composites as potential high-rate and high-efficiency supercapacitors. J. Power Sourous 2012, 215, 43–47. [Google Scholar] [CrossRef]
- Huang, C.W.; Teng, H. Influence of carbon nanotube grafting on the impedance behavior of activated carbon capacitors. J. Electrochem. Soc. 2008, 155, 739–744. [Google Scholar] [CrossRef]
- Li, W.; Li, G.; Sun, J.; Zou, R.; Xu, K.; Sun, Y.; Chen, Z.; Yang, J.; Hu, J. Hierarchical heterostructures of MnO2 nanosheets or nanorods grown on Au-coated Co3O4 porous nanowalls for high-performance pseudocapacitance. Nanoscale 2013, 5, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; Zhang, T.; He, Y.; Jia, C.; Saha, P.; Cheng, Q. Co3O4@CoS Core-Shell Nanosheets on Carbon Cloth for High Performance Supercapacitor Electrodes. Materials 2017, 10, 608. https://doi.org/10.3390/ma10060608
Ning J, Zhang T, He Y, Jia C, Saha P, Cheng Q. Co3O4@CoS Core-Shell Nanosheets on Carbon Cloth for High Performance Supercapacitor Electrodes. Materials. 2017; 10(6):608. https://doi.org/10.3390/ma10060608
Chicago/Turabian StyleNing, Jinfeng, Tianyu Zhang, Ying He, Congpu Jia, Petr Saha, and Qilin Cheng. 2017. "Co3O4@CoS Core-Shell Nanosheets on Carbon Cloth for High Performance Supercapacitor Electrodes" Materials 10, no. 6: 608. https://doi.org/10.3390/ma10060608
APA StyleNing, J., Zhang, T., He, Y., Jia, C., Saha, P., & Cheng, Q. (2017). Co3O4@CoS Core-Shell Nanosheets on Carbon Cloth for High Performance Supercapacitor Electrodes. Materials, 10(6), 608. https://doi.org/10.3390/ma10060608




