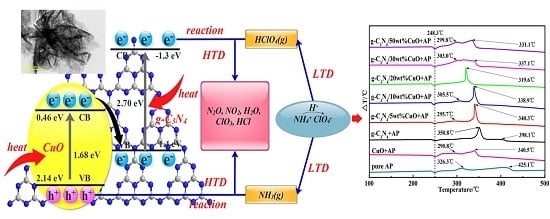
Direct Growth of CuO Nanorods on Graphitic Carbon Nitride with Synergistic Effect on Thermal Decomposition of Ammonium Perchlorate
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of g-g-C3N4/CuO Nanocomposite
2.3. Characterization
2.4. Catalytic Activity Measurement
3. Result and Discussion
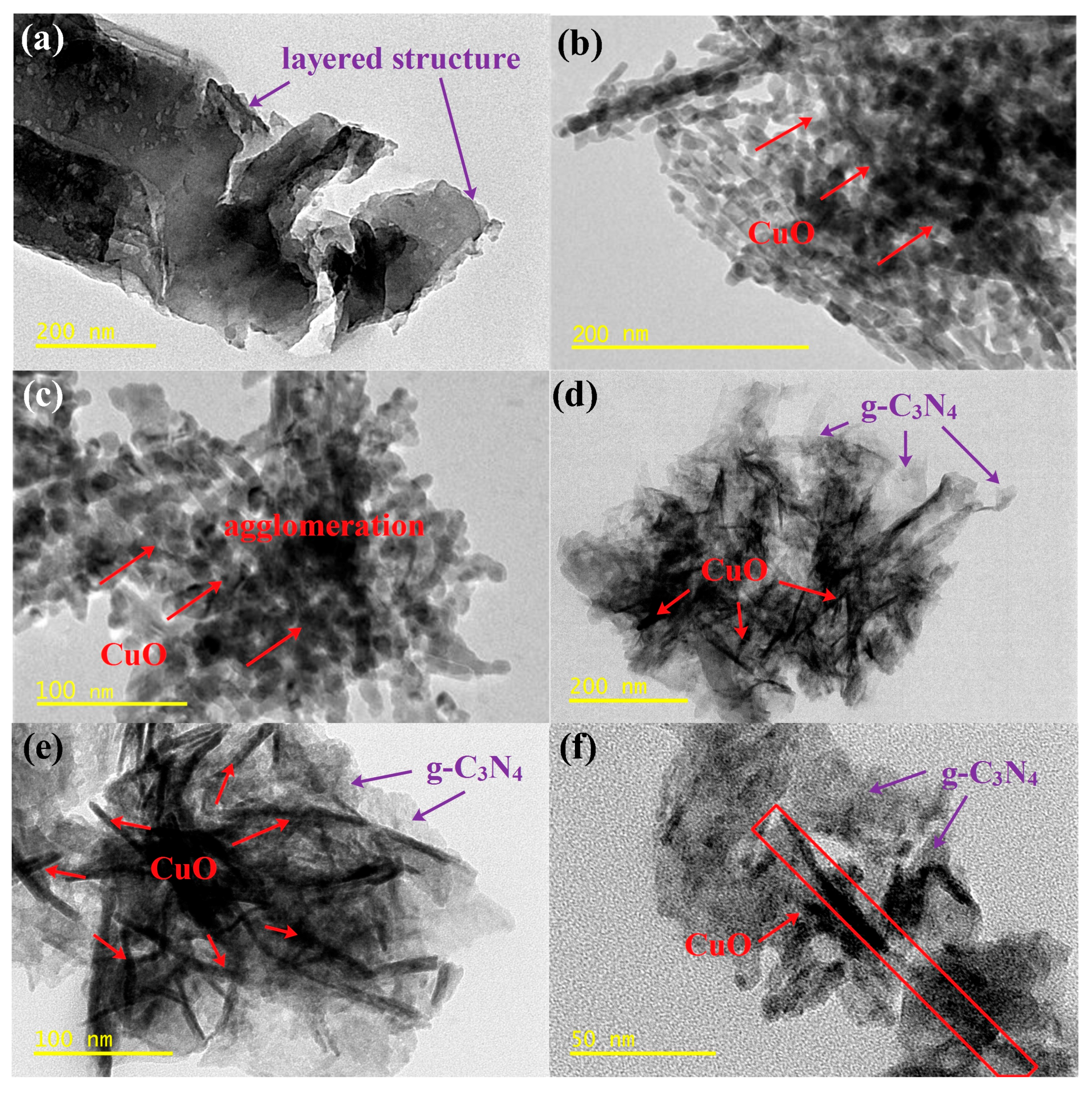
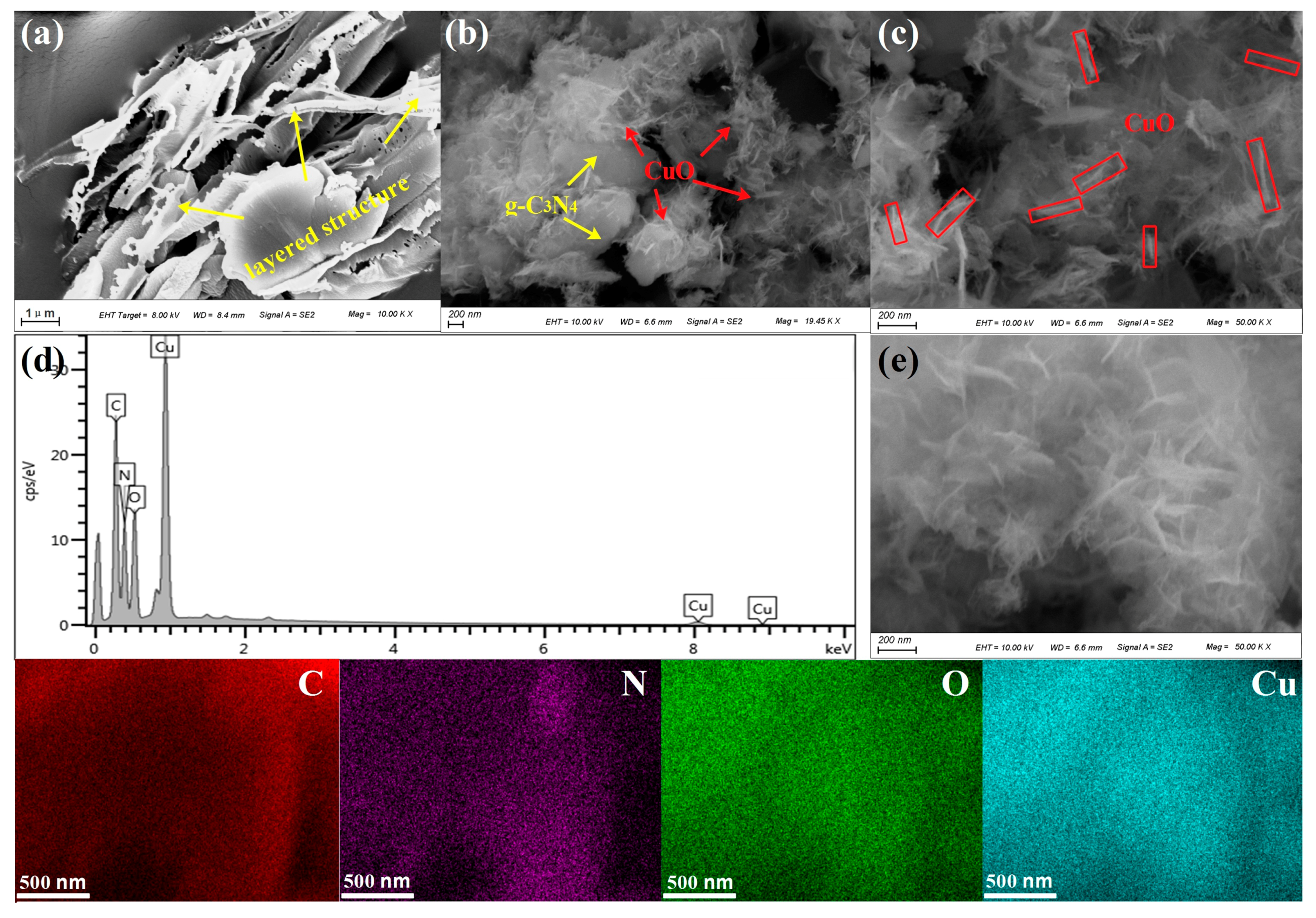
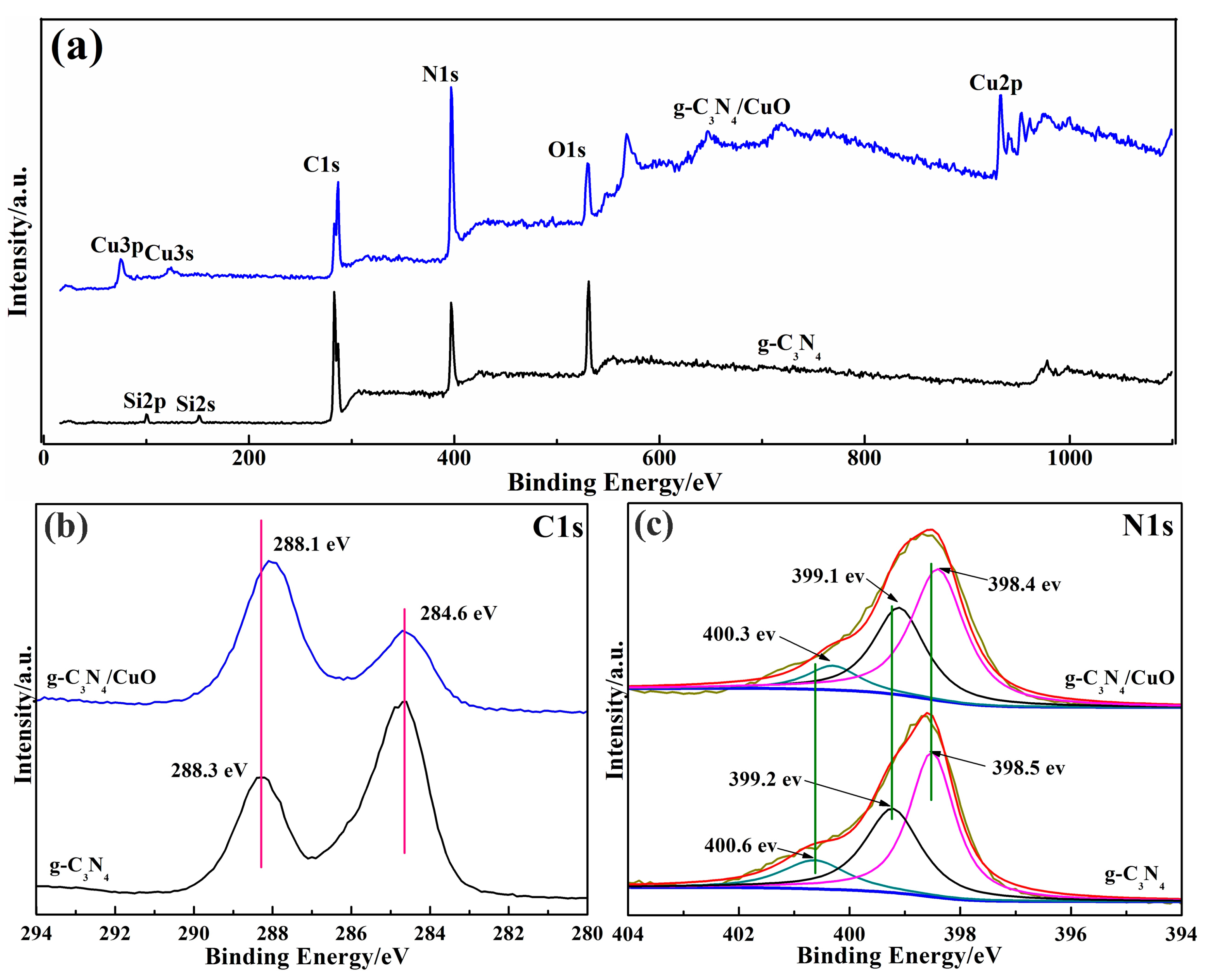
3.1. Morphology and Textural Property
3.2. Catalytic Activity of Nanocomposite Materials for Ammonium Perchlorate (AP) Decomposition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.W.H.; Mesch, M.B.; Duppel, V.; Blum, V.; Senker, J.; Lotsch, B.V. Low-molecular-weight carbon nitrides for solar hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.Q.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo) catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Yun, H.N.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.M.; Han, J.Y.; Zhou, T.H.; Xu, R. Recent progress in g-C3N4 based low cost photocatalytic system: Activity enhancement and emerging applications. Catal. Sci. Technol. 2015, 5, 5048–5061. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Sun, Y.J.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2014, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Dante, R.C.; Martín-Ramos, P.; Navas-Gracia, L.M.; Sánchez-Arévalo, F.M.; Martín-Gil, J. Polymeric carbon nitride nanosheets. J. Macromol. Sci. B 2013, 52, 623–631. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Correa-Guimaraes, A.; Martín-Gil, J. Synthesis of graphitic carbon nitride by reaction of melamine and uric acid. Mater. Chem. Phys. 2011, 130, 1094–1102. [Google Scholar] [CrossRef]
- Kong, H.J.; Won, D.H.; Kim, J.; Woo, S.I. Sulfur-doped g-C3N4/BiVO4 composite photocatalyst for water oxidation under visible light. Chem. Mater. 2016, 28, 1318–1324. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Miao, J.W.; Wang, Z.X.; Su, D.M.; Liu, S.; Cai, W.Z.; Zhang, L.P.; Hao, S.; Liu, B. Sulfur-Mediated self-templating synthesis of tapered C-PAN/g-C3N4 composite nanotubes toward efficient photocatalytic H2 evolution. ACS Energy Lett. 2016, 1, 969–975. [Google Scholar] [CrossRef]
- Zada, A.; Humayun, M.; Raziq, F.; Zhang, X.L.; Qu, Y.; Bai, L.L.; Qin, C.L.; Jing, L.Q.; Fu, H.G. Exceptional visible-light-driven cocatalyst-free photocatalytic activity of g-C3N4 by well designed nanocomposites with plasmonic Au and SnO2. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.G.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.; Park, T.J. Novel visible light active graphitic C3N4–TiO2 composite photocatalyst: Synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B Environ. 2013, 142, 718–728. [Google Scholar] [CrossRef]
- She, X.J.; Xu, H.; Wang, H.F.; Xia, J.X.; Song, Y.H.; Yan, J.; Xu, Y.G.; Zhang, Q.; Du, D.L.; Li, H.M. Controllable synthesis of CeO2/g-C3N4 composites and their applications in the environment. Dalton Trans. 2015, 44, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Huang, H.W.; Liu, C.Y.; Dong, F.; Zhang, T.R.; Du, X.; Yu, S.X.; Zhang, Y.H. In situ co-pyrolysis fabrication of CeO2/g-C3N4 n–n type heterojunction for synchronously promoting photo-induced oxidation and reduction properties. J. Mater. Chem. A 2015, 3, 17120–17129. [Google Scholar] [CrossRef]
- Li, M.L.; Zhang, L.X.; Fan, X.Q.; Zhou, Y.J.; Wu, M.Y.; Shi, J.L. Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A 2015, 3, 5189–5196. [Google Scholar] [CrossRef]
- Tian, Y.L.; Chang, B.B.; Lu, J.L.; Fu, J.; Xi, F.N.; Dong, X.P. Hydrothermal synthesis of graphitic carbon nitride–Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. ACS Appl. Mater. Interfaces 2013, 5, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lu, J.; Wang, F.Q.; Wei, W.H.; Chang, Y.; Dong, S.J. Preparation, characterization and photocatalytic performance of g-C3N4/Bi2WO6 composites for methyl orange degradation. Ceram. Int. 2014, 40, 9077–9086. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, L.; Yuan, Q.; He, J.; Luo, S.L.; Au, C.T.; Yin, S.F. Controlled synthesis of graphitic carbon nitride/beta bismuth oxide composite and its high visible-light photocatalytic activity. Carbon 2015, 86, 217–224. [Google Scholar] [CrossRef]
- Sierra, M.; Borges, E.; Esparza, P.; Méndez-Ramos, J.; Martín-Gil, J.; Martín-Ramos, P. Photocatalytic activities of coke carbon/g-C3N4 and Bi metal/Bi mixed oxides/g-C3N4 nanohybrids for the degradation of pollutants in waste water. Sci. Technol. Adv. Mater. 2016, 17, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.E.; Lopes, O.F.; Neto, A.B.S.; Ribeiro, C. Enhanced Cr(VI) photoreduction in aqueous solution using Nb2O5/CuO heterostructures under UV and visible irradiation. Chem. Eng. J. 2017, 312, 220–227. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, U.; Aravindan, V.; Srinivasan, M.; Ogale, S. Synthesis of CuO nanostructures from Cu-based metal organic framework (MOF-199) for application as anode for Li-ion batteries. Nano Energy 2013, 2, 1158–1163. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhang, K.L.; Xu, D.G.; Yang, G.C.; Huang, H.; Nie, F.D.; Liu, C.M.; Yang, S.H. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Liu, H.; Liu, P.F.; Yang, Z.H.; Feng, X.; Huo, F.W.; Lu, X.H. A template-free method for stable CuO hollow microspheres fabricated from a metal organic framework (HKUST-1). Nanoscale 2015, 7, 9411–9415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Zeng, G.Y.; Nie, F.D.; Xu, X.M.; Chen, S.; Han, Q.F.; Wang, X. Decorating graphene oxide with CuO nanoparticles in a water-isopropanol system. Nanoscale 2010, 2, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Zabilskiy, M.; Djinović, P.; Erjavec, B.; Dražić, G.; Pintar, A. Small CuO clusters on CeO2 nanospheres as active species for catalytic N2O decomposition. Appl. Catal. B Environ. 2015, 163, 113–122. [Google Scholar] [CrossRef]
- Wang, J.P.; Xu, H.; Qian, X.F.; Dong, Y.Y.; Gao, J.K.; Qian, G.D.; Yao, J.M. Direct synthesis of porous nanorod-type graphitic carbon nitride/CuO composite from Cu–melamine supramolecular framework towards enhanced photocatalytic performance. Chem. Asian J. 2015, 10, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Chandru, R.A.; Patra, S.; Oommen, C.; Munichandraiah, N.; Raghunandan, B.N. Exceptional activity of mesoporous β-MnO2 in the catalytic thermal sensitization of ammonium perchlorate. J. Mater. Chem. 2012, 22, 6536–6538. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, W.; Wang, Y.J.; Shen, P.; Li, F.S.; Li, P.Y.; Zhao, F.Q.; Gao, H.X. Hydrothermal preparation of Fe2O3/graphene nanocomposite and its enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2014, 303, 354–359. [Google Scholar] [CrossRef]
- Tan, L.H.; Xu, J.H.; Zhang, X.J.; Hang, Z.S.; Jia, Y.Q.; Wang, S.B. Synthesis of g-C3N4/CeO2 nanocomposites with improved catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Yu, J.G.; Ran, J.R. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364–1371. [Google Scholar] [CrossRef]
- Dong, M.; Wu, J.; Gao, M.C.; Xin, Y.J.; Ma, T.J.; Sun, Y.Y. Fabrication of Z-scheme g-C3N4/RGO/Bi2WO6 photocatalyst with enhanced visible-light photocatalytic activity. Chem. Eng. J. 2016, 290, 136–146. [Google Scholar]
- Chetia, T.R.; Ansari, M.S.; Qureshi, M. Graphitic carbon nitride as a photovoltaic booster in quantum dot sensitized solar cells: A synergistic approach for enhanced charge separation and injection. J. Mater. Chem. A 2016, 4, 5528–5541. [Google Scholar] [CrossRef]
- Auxilia, F.M.; Ishihara, S.; Mandal, S.; Tanabe, T.; Saravanan, G.; Ramesh, G.V.; Umezawa, N.; Hara, T.; Xu, Y.; Hishita, S. Catalysis: Low-temperature remediation of NO catalyzed by interleaved CuO nanoplates. Adv. Mater. 2014, 26, 4481–4485. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.C.; Huang, H.; Xiao, S.H.; Deng, J.W.; Zhou, G.; Li, Q.H.; Wang, T.H. 3D hierarchical CuO mesocrystals from ionic liquid precursors: Towards better electrochemical performance for Li-ion batteries. J. Mater. Chem. A 2016, 4, 8402–8411. [Google Scholar] [CrossRef]
- Hong, Y.Z.; Li, C.C.; Zhang, G.Y.; Meng, Y.D.; Yin, B.X.; Zhao, Y.; Shi, W.D. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.L.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.R.; Chen, Z.H.; Zhang, Z.G.; Fang, X.M. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Gao, H.L.; Yan, S.C.; Wang, J.J.; Zou, Z.G. Ion coordination significantly enhances the photocatalytic activity of graphitic-phase carbon nitride. Dalton Trans. 2014, 43, 8178–8183. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.W.; Fan, H.Q.; Zhu, Z.Y.; Kong, L.B.; Ma, L.T. “Dyed” graphitic carbon nitride with greatly extended visible-light-responsive range for hydrogen evolution. J. Catal. 2016, 339, 93–101. [Google Scholar] [CrossRef]
- Zhu, T.T.; Song, Y.H.; Ji, H.Y.; Xu, Y.G.; Song, Y.X.; Xia, J.X.; Yin, S.; Li, Y.P.; Xu, H.; Zhang, Q. Synthesis of g-C3N4/Ag3VO4 composites with enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2015, 44, 96–105. [Google Scholar] [CrossRef]
- He, Y.M.; Wang, Y.; Zhang, L.H.; Teng, B.T.; Fan, M.H. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Wu, W.T.; Zhang, J.Q.; Fan, W.Y.; Li, Z.T.; Wang, L.Z.; Li, X.M.; Wang, Y.; Wang, R.Q.; Zheng, J.T.; Wu, M.B. Remedying defects in carbon ntride to improve both photooxidation and H2 generation efficiencies. ACS Catal. 2016, 6, 3365–3371. [Google Scholar] [CrossRef]
- Huang, Z.A.; Sun, Q.; Lv, K.L.; Zhang, Z.H.; Mei, L.; Bing, L. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs. (101) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Meng, C.G. Facile fabrication of Fe3O4 and Co3O4 microspheres and their influence on the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2016, 674, 259–265. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, P.; Xu, H.B.; Sun, R.D.; Qing, P.H.; Zhang, Y. Thermal decomposition of ammonium perchlorate in the presence of Al(OH)3·Cr(OH)3 nanoparticles. J. Hazard. Mater. 2014, 268, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Fertassi, M.A.; Alali, K.T.; Liu, Q.; Li, R.Z.; Liu, P.G.; Liu, J.Y.; Liu, L.H.; Wang, J. Catalytic effect of CuO nanoplates, a graphene (G)/CuO nanocomposite and an Al/G/CuO composite on the thermal decomposition of ammonium perchlorate. RSC Adv. 2016, 6, 74155–74161. [Google Scholar] [CrossRef]
- Shi, W.L.; Guo, F.; Chen, J.B.; Che, G.B.; Lin, X. Hydrothermal synthesis of InVO4/Graphitic carbon nitride heterojunctions and excellent visible-light-driven photocatalytic performance for rhodamine B. J. Alloys Compd. 2014, 612, 143–148. [Google Scholar] [CrossRef]
- Hu, B.; Cai, F.P.; Chen, T.J.; Fan, M.S.; Song, C.J.; Yan, X.; Shi, W.D. Hydrothermal synthesis g-C3N4/Nano-InVO4 nanocomposites and enhanced photocatalytic activity for hydrogen production under visible light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 18247–18256. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Tian, S.Q.; Zhou, Z.X.; Wen, Y.W.; Pang, A.M.; Zhang, Y.G.; Zeng, D.W.; Li, H.T.; Shan, B.; Xie, C.S. ZnO micro/nanocrystals with tunable exposed (0001) facets for enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. J. Phys. Chem. C 2014, 118, 11833–11841. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Thermal decomposition of ammonium perchlorate. Thermochim. Acta 2006, 443, 1–36. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, L.P.; Li, G.S. Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2008, 464, 532–536. [Google Scholar] [CrossRef]
- Su, F.Z.; Antonietti, M.; Wang, X.C. mpg-C3N4 as a solid base catalyst for Knoevenagel condensations and transesterification reactions. Catal. Sci. Technol. 2012, 43, 1005–1009. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Peng, R.F. Graphitic carbon nitride (g-C3N4) as a metal-free catalyst for thermal decomposition of ammonium perchlorate. RSC Adv. 2015, 5, 24507–24512. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Peng, R.F. One-step synthesis of SnO2 nanoparticles-loaded graphitic carbon nitride and their application in thermal decomposition of ammonium perchlorate. New J. Chem. 2015, 39, 8703–8707. [Google Scholar] [CrossRef]


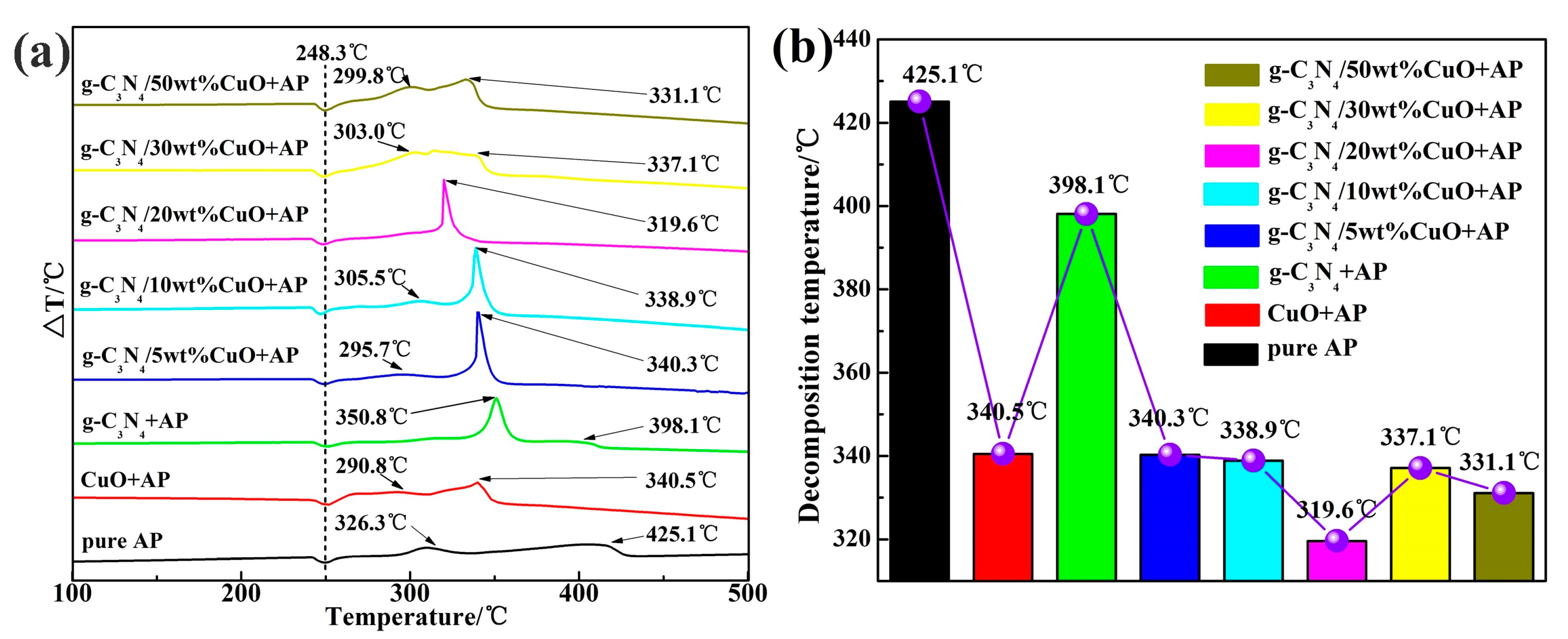
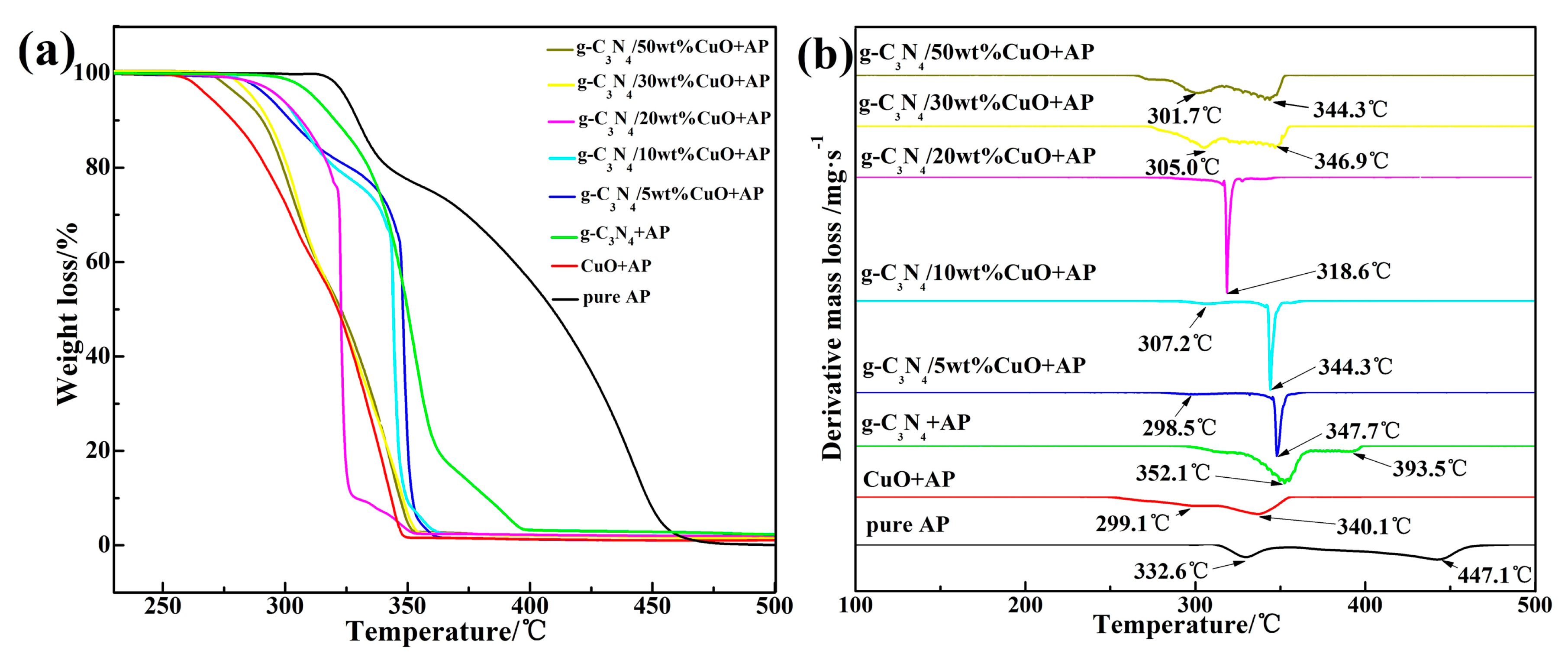
| Sample | High Decomposition Temperature (°C) | High Weight-Loss Decomposition Temperature (°C) |
|---|---|---|
| Pure AP | 425.1 | 447.1 |
| CuO + AP | 340.5 | 340.1 |
| g-C3N4 + AP | 398.1 | 393.5 |
| g-C3N4/5 wt % CuO + AP | 340.3 | 347.7 |
| g-C3N4/10 wt % CuO + AP | 338.9 | 344.3 |
| g-C3N4/20 wt % CuO + AP | 319.6 | 318.6 |
| g-C3N4/30 wt % CuO + AP | 337.1 | 346.3 |
| g-C3N4/50 wt % CuO + AP | 331.1 | 344.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.; Xu, J.; Li, S.; Li, D.; Dai, Y.; Kou, B.; Chen, Y. Direct Growth of CuO Nanorods on Graphitic Carbon Nitride with Synergistic Effect on Thermal Decomposition of Ammonium Perchlorate. Materials 2017, 10, 484. https://doi.org/10.3390/ma10050484
Tan L, Xu J, Li S, Li D, Dai Y, Kou B, Chen Y. Direct Growth of CuO Nanorods on Graphitic Carbon Nitride with Synergistic Effect on Thermal Decomposition of Ammonium Perchlorate. Materials. 2017; 10(5):484. https://doi.org/10.3390/ma10050484
Chicago/Turabian StyleTan, Linghua, Jianhua Xu, Shiying Li, Dongnan Li, Yuming Dai, Bo Kou, and Yu Chen. 2017. "Direct Growth of CuO Nanorods on Graphitic Carbon Nitride with Synergistic Effect on Thermal Decomposition of Ammonium Perchlorate" Materials 10, no. 5: 484. https://doi.org/10.3390/ma10050484
APA StyleTan, L., Xu, J., Li, S., Li, D., Dai, Y., Kou, B., & Chen, Y. (2017). Direct Growth of CuO Nanorods on Graphitic Carbon Nitride with Synergistic Effect on Thermal Decomposition of Ammonium Perchlorate. Materials, 10(5), 484. https://doi.org/10.3390/ma10050484