A Photoluminescence Study of the Changes Induced in the Zinc White Pigment by Formation of Zinc Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Methods
2.2.1. FTIR Spectroscopy
2.2.2. TRPL Spectroscopy
- Fast recombination emission was detected using a gate width of 5 ns and recording the emission decay kinetic for the first 50 ns following excitation. The spectrometer slit was set to 50 μm, giving rise to a spectral bandwidth of 2 nm.
- Long-lived emission at the microsecond timescale was detected using a gate width of 1 μs and recording the decay kinetic for 100 μs following excitation. The fainter microsecond emission was detected by increasing the spectrometer slit to 200 μm, equivalent to a spectral bandwidth of 4 nm.
3. Results
3.1. FTIR Spectroscopy
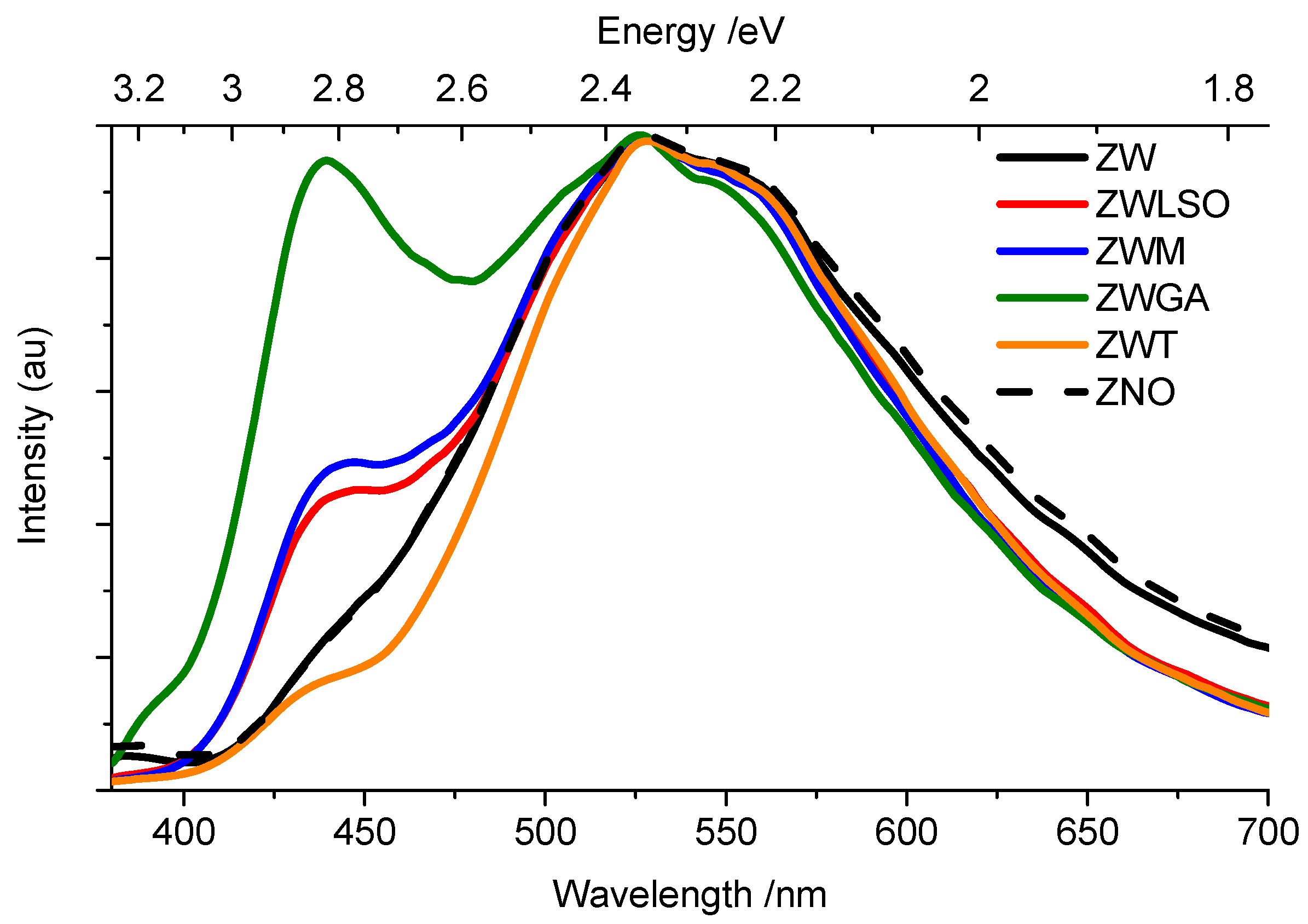
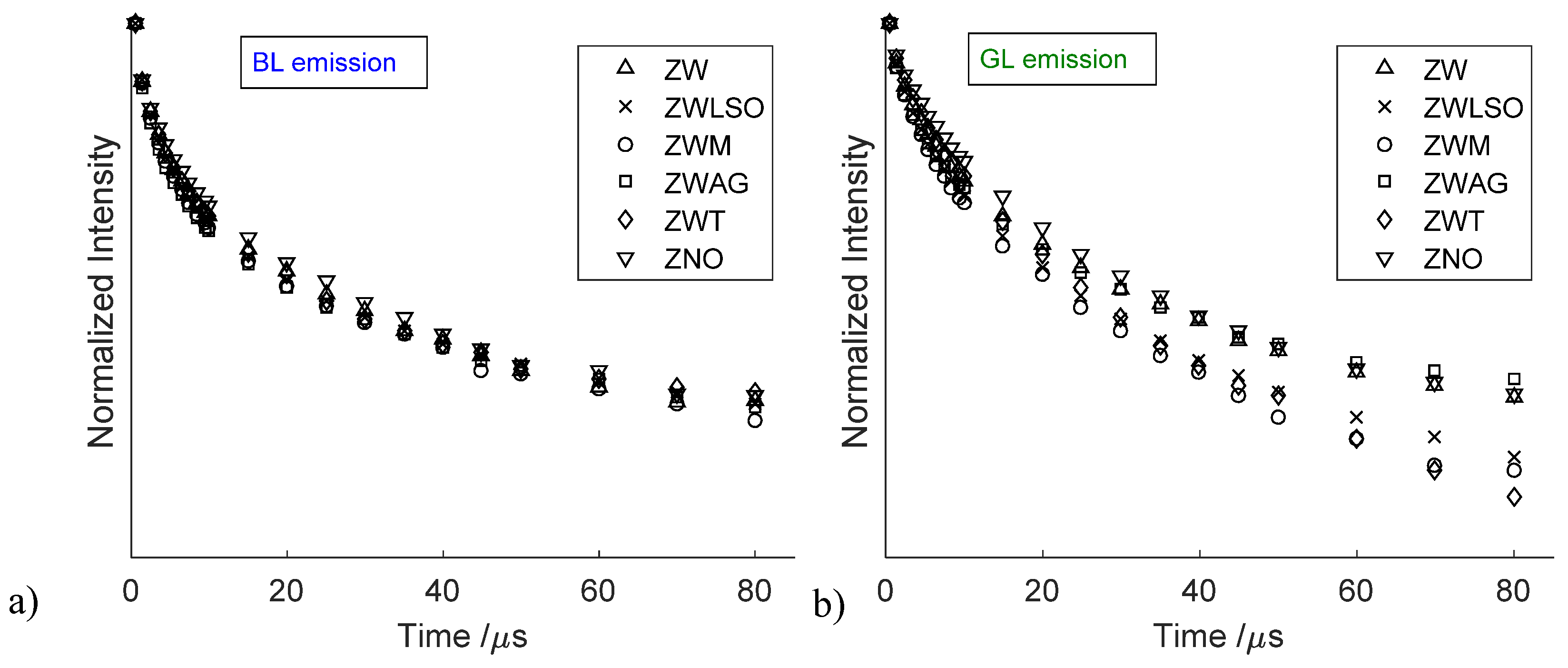
3.2. Time-Resolved Photoluminescence Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pelant, I.; Valenta, J. Luminescence Spectroscopy of Semiconductors; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Chen, Q.; Zhou, H.; Song, T.; Luo, S.; Hong, Z.; Duan, H.; Dou, L.; Liu, Y.; Yang, Y. Controllable Self-Induced Passivation of Hybrid Lead Iodide Perovskites toward High Performance Solar Cells. Nano Lett. 2014, 14, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, Y.; Kawakami, Y.; Fujita, S.; Fujita, S. Recombination dynamics of localized excitons in In0.20Ga0.80N-In0.05Ga0.95 multiple quantum wells. Phys. Rev. B 1997, 55, R1938–R1941. [Google Scholar]
- Knowles, K.E.; McArthur, E.A.; Weiss, E.A. A Multi-Timescale Map of Radiative and Non-radiative Decay Pathways for Excitons in CdSe Quantum Dots. ACS Nano 2011, 5, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Gorgis, A.; Flissikowski, T.; Brandt, O.; Chèze, C.; Geelhaar, L.; Riechert, H.; Grahn, H.T. Time-resolved photoluminescence spectroscopy of individual GaN nanowires. Phys. Rev. B 2012, 86, 041302(R). [Google Scholar] [CrossRef]
- Osmond, G. Zinc white: A review of zinc oxide pigment properties and implications for stability in oil-based paintings. AICCM Bull. 2012, 33, 20–29. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T. Pigment Compendium: A Dictionary of Historical Pigments; Routledge: London, UK, 2007. [Google Scholar]
- Jacobsen, A.E. Zinc soaps in paints. Ind. Eng. Ind. 1941, 33, 1254–1256. [Google Scholar] [CrossRef]
- Van Loon, A.; Noble, P.; Boon, J. White Hazes and Surface Crusts in Rembrandt’s Homer and Related Paintings. In Proceedings of the 16th triennial conference ICOM-CC 2011, Lisbon, Portugal, 2011. [Google Scholar]
- Keune, K.; Mass, J.; Mehta, A.; Church, J.; Meirer, F. Analytical imaging studies of the migration of degraded orpiment, realgar, and emerald green pigments in historic paintings and related conservation issues. Herit. Sci. 2016, 4. [Google Scholar] [CrossRef]
- Robinet, L.; Corbeil, M.-C. The Characterization of Metal Soaps. Stud. Conserv. 2003, 48, 23–40. [Google Scholar]
- Brambilla, L.; Riedo, C.; Baraldi, C.; Nevin, A.; Gamberini, M.C.; D’Andrea, C.; Chiantore, O.; Goidanich, S.; Toniolo, L. Characterization of fresh and aged natural ingredients used in historical ointments by molecular spectroscopic techniques: IR, Raman and fluorescence. Anal. Bioanal. Chem. 2011, 401. [Google Scholar] [CrossRef] [PubMed]
- Clementi, C.; Rosi, F.; Romani, A.; Vivani, R.; Brunetti, B.G.; Miliani, C. Photoluminescence properties of zinc oxide in paints: a study of the effect of self-absorption and passivation. Appl. Spectrosc. 2012, 66, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.J.; Keune, K.; van Loon, A.; Corkery, R.W.; Iedema, P.D. Ionomer-like structure in mature oil paint binding media. RSC Adv. 2016, 6, 93363–93369. [Google Scholar] [CrossRef]
- Kryven, I.; Duivenvoorden, J.; Hermans, J.; Iedema, P.D. Random Graph Approach to Multifunctional Molecular Networks. Macromol. Theory Simul. 2016, 25, 431–431. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; van Loon, A.; Iedema, P.D. The crystallization of metal soaps and fatty acids in oil paint model systems. Phys. Chem. Chem. Phys. 2016, 28, 10896–10905. [Google Scholar] [CrossRef] [PubMed]
- Rodnyi, P.A.; Khodyuk, I.V. Optical and luminescence properties of zinc oxide (Review). Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef]
- Umit, O.; Daniel, H.; Hadis, M. ZnO devices and applications: A review of current status and future prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar]
- Wang, Z.G.; Zu, X.T.; Zhu, S.; Wang, L.M. Green luminescence originates from surface defects in ZnO nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 35, 199–202. [Google Scholar] [CrossRef]
- Fabbri, F.; Villani, M.; Catellani, A.; Calzolari, A.; Cicero, G.; Calestani, D.; Calestani, G.; Zappettini, A.; Dierre, B.; Sekiguchi, T.; et al. Zn vacancy induced green luminescence on non-polar surfaces in ZnO nanostructures. Sci. Rep. (Nat. Publ. Group) 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Artesani, A.; Bellei, S.; Capogrosso, V.; Cesaratto, A.; Mosca, S.; Nevin, A.; Valentini, G.; Comelli, D. Photoluminescence properties of zinc white: An insight into its emission mechanisms through the study of historical artist materials. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Comelli, D.; Nevin, A.; Brambilla, A.; Osticioli, I.; Valentini, G.; Toniolo, L.; Fratelli, M.; Cubeddu, R. On the Discovery of an Unusual Luminescent Pigment in Van Gogh’s Painting “Les bretonnes et le pardon de pont Aven”. Appl. Phys. A 2012, 106, 25–34. [Google Scholar] [CrossRef]
- Seoane, E. Further crystalline constituents of gum mastic. J. Chem. Soc. (Resumed) 1956, 4158–4160. [Google Scholar] [CrossRef]
- Gunn, M.; Chottard, G.; Rivière, E.; Girerd, J.-J.; Chottard, J.-C. Chemical Reactions between Copper Pigments and Oleoresinous Media. Stud. Conserv. 2002, 47, 12–23. [Google Scholar] [CrossRef]
- Poli, T.; Piccirillo, A.; Zoccali, A.; Conti, C.; Nervo, M.; Chiantore, O. The role of zinc white pigment on the degradation of shellac resin in artworks. Polym. Degrad. Stab. 2014, 102, 138–144. [Google Scholar]
- Doménech-Carbó, M.T.; Kuckova, S.; de la Cruz-Cañizares, J.; Osete-Cortina, L. Study of the influencing effect of pigments on the photoageing of terpenoid resins used as pictorial media. J. Chromatogr. A 2006, 1121, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Bonaduce, I.; Brecoulaki, H.; Colombini, M.P.; Lluveras, A.; Restivo, V.; Ribechini, E. Gas chromatographic–mass spectrometric characterisation of plant gums in samples from painted works of art. J. Chromatogr. A 2007, 1175, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.J.; Keune, K.; van Loon, A.; Iedema, P.D. An infrared spectroscopic study of the nature of zinc carboxylates in oil paintings. J. Anal. At. Spectrom. 2015, 30, 1600–1608. [Google Scholar] [CrossRef]
- Mazzeo, R.; Prati, S.; Quaranta, M.; Joseph, E.; Kendix, E.; Galeotti, M. Attenuated total reflection micro FTIR characterisation of pigment–binder interaction in reconstructed paint films. Anal. Bioanal. Chem. 2008, 392, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Osmond, G.; Boon, J.J.; Puskar, L.; Drennan, J. Metal Stearate Distributions in Modern Artists' Oil Paints: Surface and Cross-Sectional Investigation of Reference Paint Films Using Conventional and Synchrotron Infrared Microspectroscopy. Appl. Spectrosc. 2012, 66, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Zhang, J. A simple preparation technique of ZnO thin film with high crystallinity and UV luminescence intensity. J. Phys. Chem. Solids 2008, 69, 531–534. [Google Scholar] [CrossRef]
- Thoury, M.; Echard, J.-P.; Réfrégiers, M.; Berrie, B.; Nevin, A.; Jamme, F.; Bertrand, L. Synchrotron UV−Visible Multispectral Luminescence Microimaging of Historical Samples. Anal. Chem. 2011, 83, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Réfrégiers, M.; Berrie, B.; Échard, J.P.; Thoury, M.A. A multiscalar photoluminescence approach to discriminate among semiconducting historical zinc white pigments. Analyst 2013, 138, 4463–4469. [Google Scholar] [CrossRef] [PubMed]
- Anžlovar, A.; Marinšekb, M.; Orela, Z.C.; Žigona, M. Basic zinc carbonate as a precursor in the solvothermal synthesis of nano-zinc oxide. Mater. Des. 2015, 86, 347–353. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Yang, S.; Xu, X.; Cai, W. Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Mishra, S.K.; Srivastava, R.K.; Prakash, S.G.; Yadav, R.S.; Panday, A.C. Photoluminescence and photoconductive characteristics of hydrothermally synthesized ZnO nanoparticles. Opto-Electron. Rev. 2010, 18, 467–473. [Google Scholar] [CrossRef]
- Chen, H.; Gu, S.; Tang, K.; Zhu, S.; Zhu, Z.; Ye, J.; Zhang, R.; Zheng, Y. Origins of green band emission in high-temperature annealed N-doped ZnO. J. Lumin. 2011, 131, 1189–1192. [Google Scholar] [CrossRef]

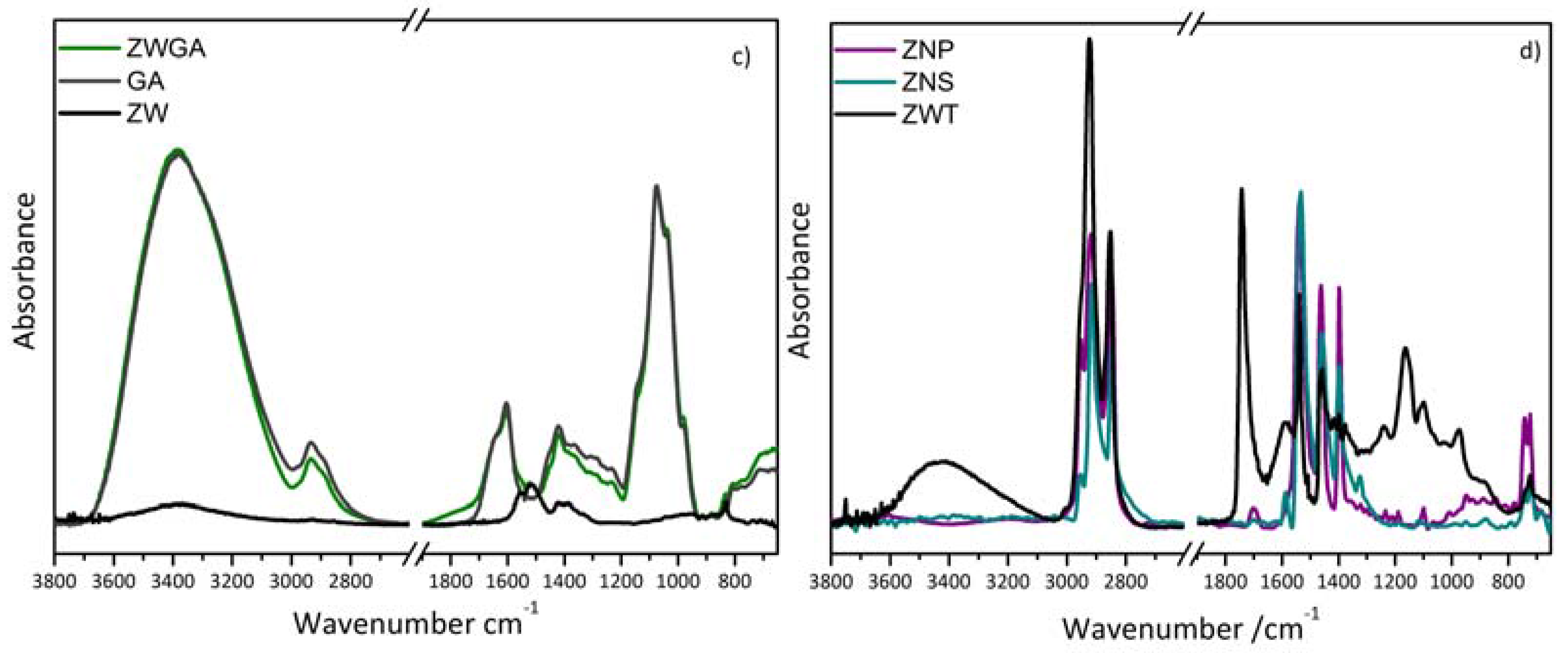
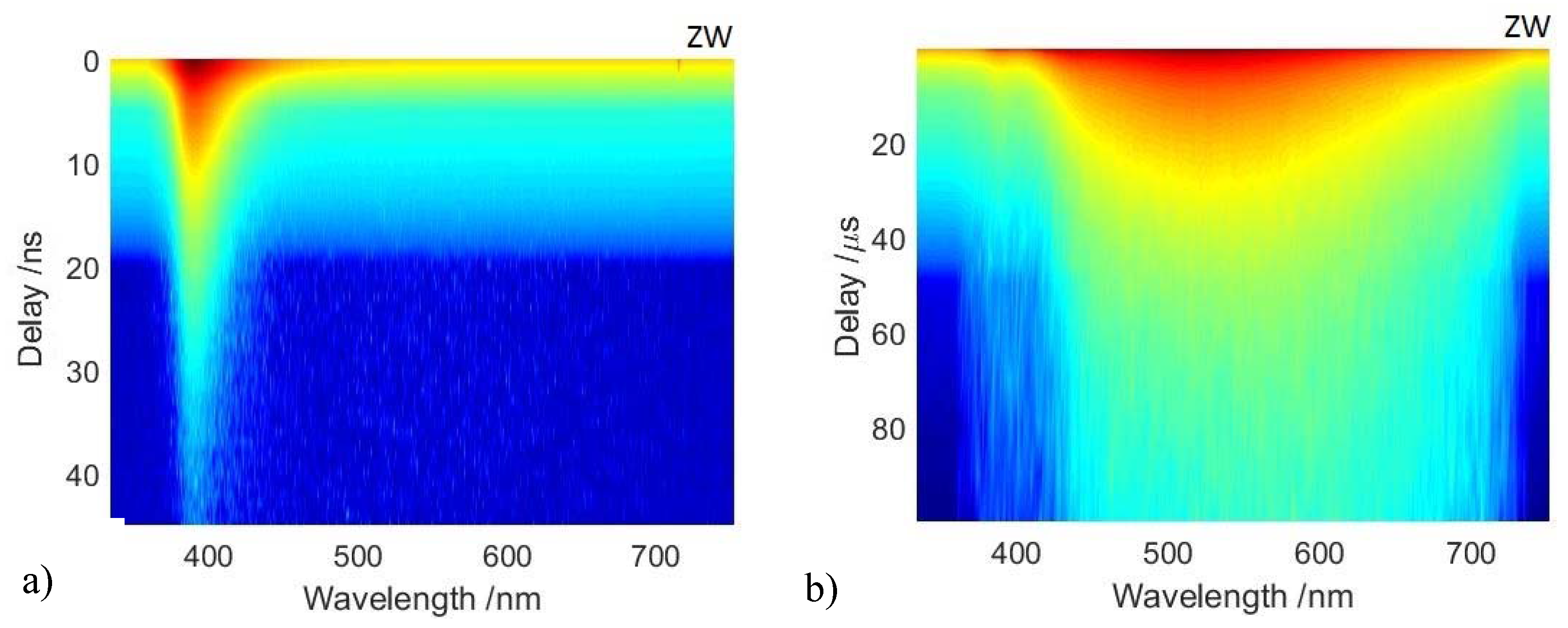
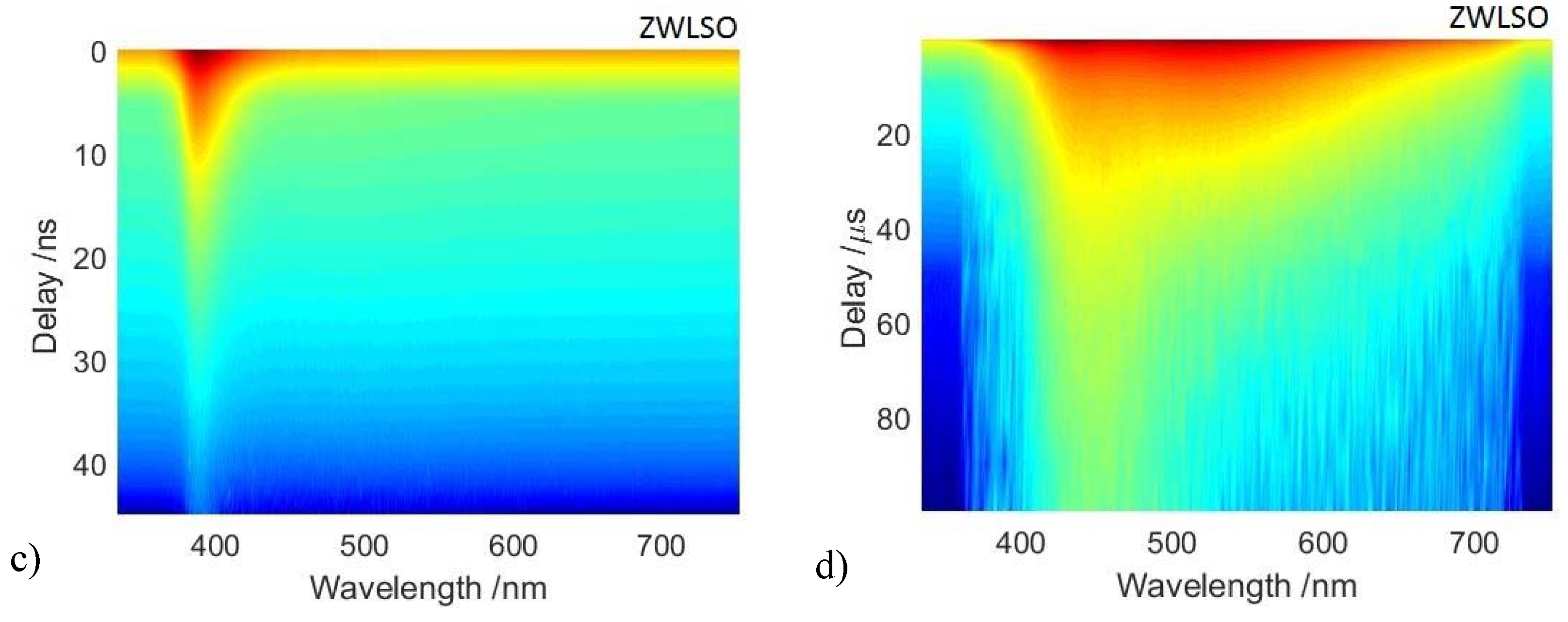

| Paint Mock Ups | Reference Samples |
|---|---|
| Zinc white + linseed oil (ZWLSO) | Zinc oxide (analytical grade) (ZNO) |
| Zinc white + mastic (ZWM) | Zinc white powder (ZW) |
| Zinc white + gum arabic (ZWGA) | Linseed oil (LSO) |
| Zinc oxide + linseed oil (ZNOLSO) | Mastic (M) |
| Zinc oxide + mastic (ZNOM) | Gum arabic (GA) |
| Zinc oxide + gum arabic (ZNOGA) | Zinc stereate (ZNS) |
| Commerical zinc white oil paint (ZWT) | Zinc palmitate (ZNP) |
| Sample | Band/cm−1 | Assignment | Attribution | Reference |
|---|---|---|---|---|
| Zinc white (ZW) | 3390 | ν O-H | Hydrated zinc carbonate zinc carbonate | [13] |
| 1550 | CO3(ν3) | |||
| 1420 | CO3 | |||
| 1510 | CO3 | |||
| 1380 | CO3 | |||
| 830 | CO3 (ν2) | |||
| Zinc white in linseed oil (ZWLSO) | 3400 | ν O-H | Linseed oil | [11,12] |
| 2920 | νs CH2 | |||
| 2850 | νasCH2 | |||
| 1740 | ν C=O ester | |||
| 1460 | δ CH2 | |||
| 1170 | ν C-O-C ester | |||
| 1598 | sh COO− | Carboxylate formation | [11,12,16] | |
| 1550 | νas COO− | |||
| 1410 | νs COO− | |||
| Zinc white in mastic (ZWM) | 1587 | ν COO− | Carboxylate complex | [23,29] |
| Zinc white in gum arabic (ZWGA) | 3370 | ν O-H | Gum arabic | [11,12] |
| 2930 | νs CH2 | |||
| 1600 | νs COO− | Organic acids in gum arabic | ||
| 1420 | δ CH2 | Gum arabic | ||
| 1070 | νas C-O-C ether (ring) | |||
| Zinc white commercial paint (ZWT) | 1585 | ν COO− | Amorphous zinc carboxylates | [16] |
| Zinc palmitate (ZNP) | 1700 | ν C=O | Unreacted palmitic acid | [28] |
| 1540 | νas COO− | Carboxylate formation | [11,12] | |
| 1400 | νs COO− | |||
| Zinc staerate (ZNS) | 1700 | ν C=O | Unreacted staeric acid | [29] |
| 1530 | νas COO− | Carboxylate formation | [11,12] | |
| 1400 | νs COO− |
| Samples | (BL/BE)sample/(BL/BE)ZW | (GL/BE)sample/(GL/BE)ZW |
|---|---|---|
| ZNO | 1.2 | 1.1 |
| ZW | 1.0 | 1.0 |
| ZWLSO | 8.3 | 4.0 |
| ZWM | 4.8 | 2.1 |
| ZWGA | 10.3 | 2.3 |
| ZWT | 2.9 | 3.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artesani, A.; Gherardi, F.; Nevin, A.; Valentini, G.; Comelli, D. A Photoluminescence Study of the Changes Induced in the Zinc White Pigment by Formation of Zinc Complexes. Materials 2017, 10, 340. https://doi.org/10.3390/ma10040340
Artesani A, Gherardi F, Nevin A, Valentini G, Comelli D. A Photoluminescence Study of the Changes Induced in the Zinc White Pigment by Formation of Zinc Complexes. Materials. 2017; 10(4):340. https://doi.org/10.3390/ma10040340
Chicago/Turabian StyleArtesani, Alessia, Francesca Gherardi, Austin Nevin, Gianluca Valentini, and Daniela Comelli. 2017. "A Photoluminescence Study of the Changes Induced in the Zinc White Pigment by Formation of Zinc Complexes" Materials 10, no. 4: 340. https://doi.org/10.3390/ma10040340
APA StyleArtesani, A., Gherardi, F., Nevin, A., Valentini, G., & Comelli, D. (2017). A Photoluminescence Study of the Changes Induced in the Zinc White Pigment by Formation of Zinc Complexes. Materials, 10(4), 340. https://doi.org/10.3390/ma10040340








