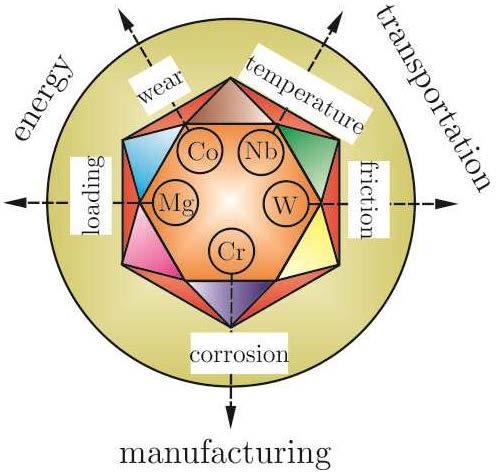
Solutions for Critical Raw Materials under Extreme Conditions: A Review
Abstract
:1. Introduction
2. State of the Art
2.1. Co and W in WC-Co Cemented Carbide Wear-Resistant Tool Materials
2.2. Co and Other CRMs in High-Temperature Ni-Based Superalloys
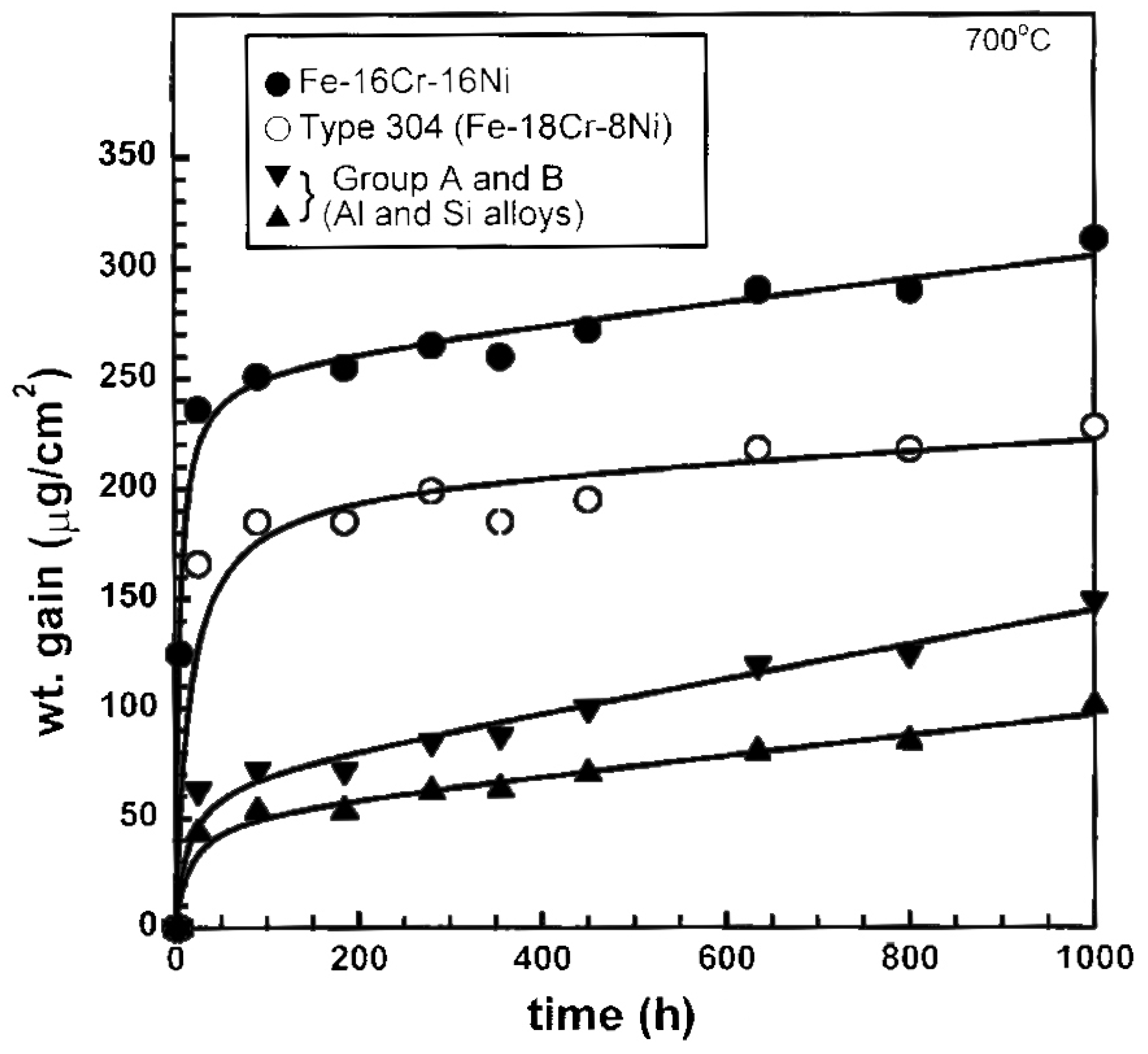
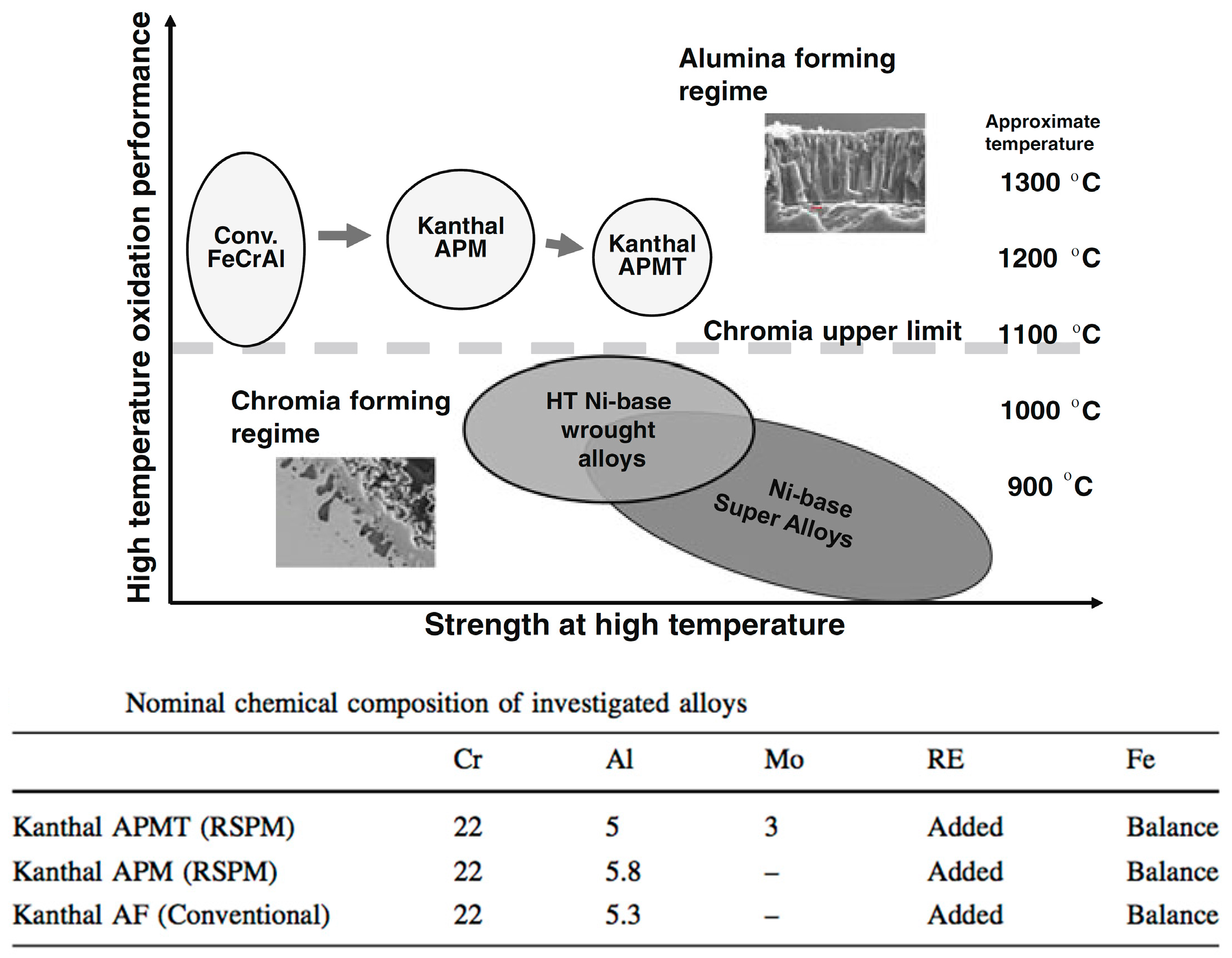
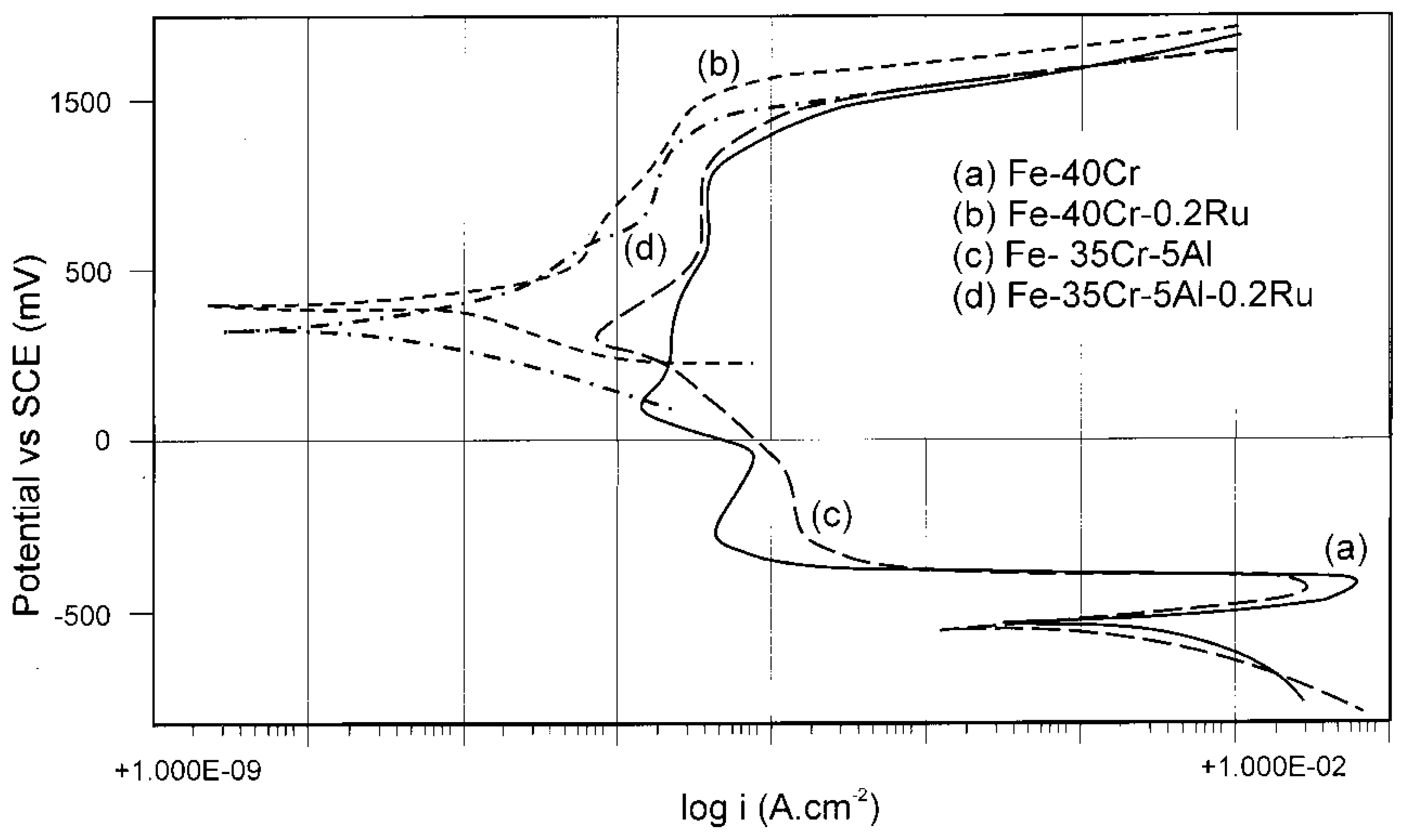
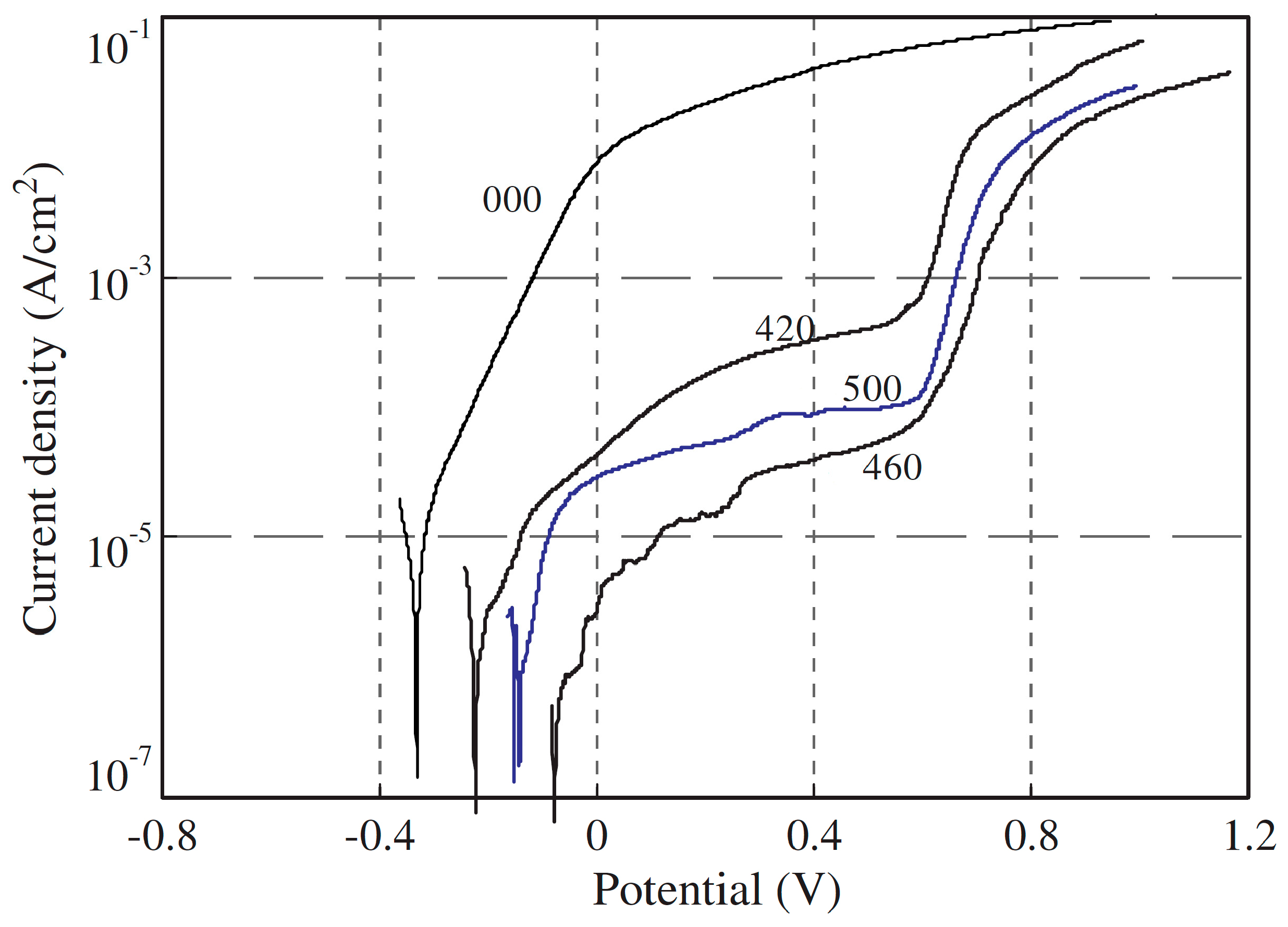
2.3. Cr in Alloys and Surface Coatings
2.4. Nb in Stainless Steel and High-Strength Low-Alloy Steels
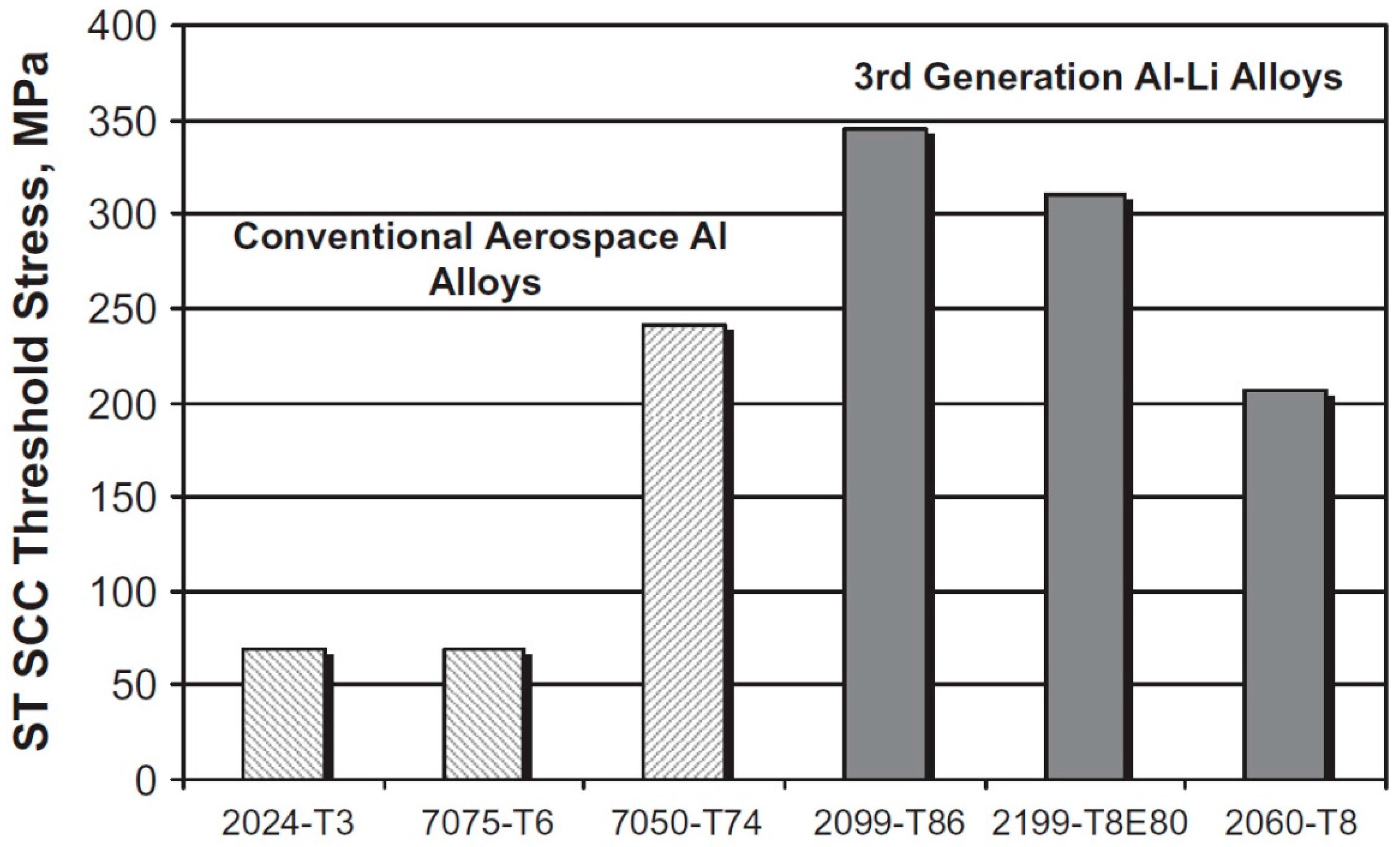
2.5. Mg in Aerospace Industry Al-Alloys
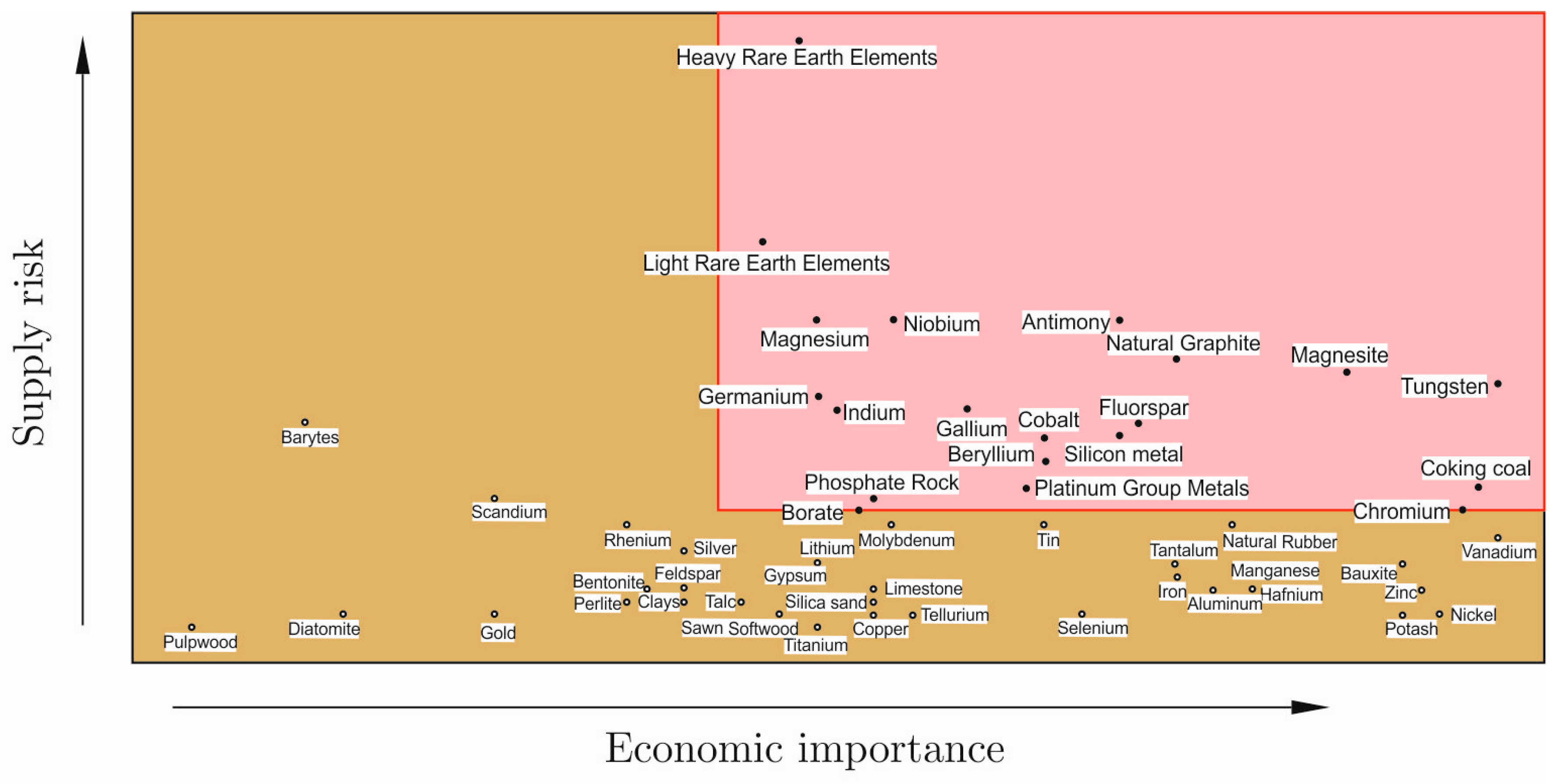
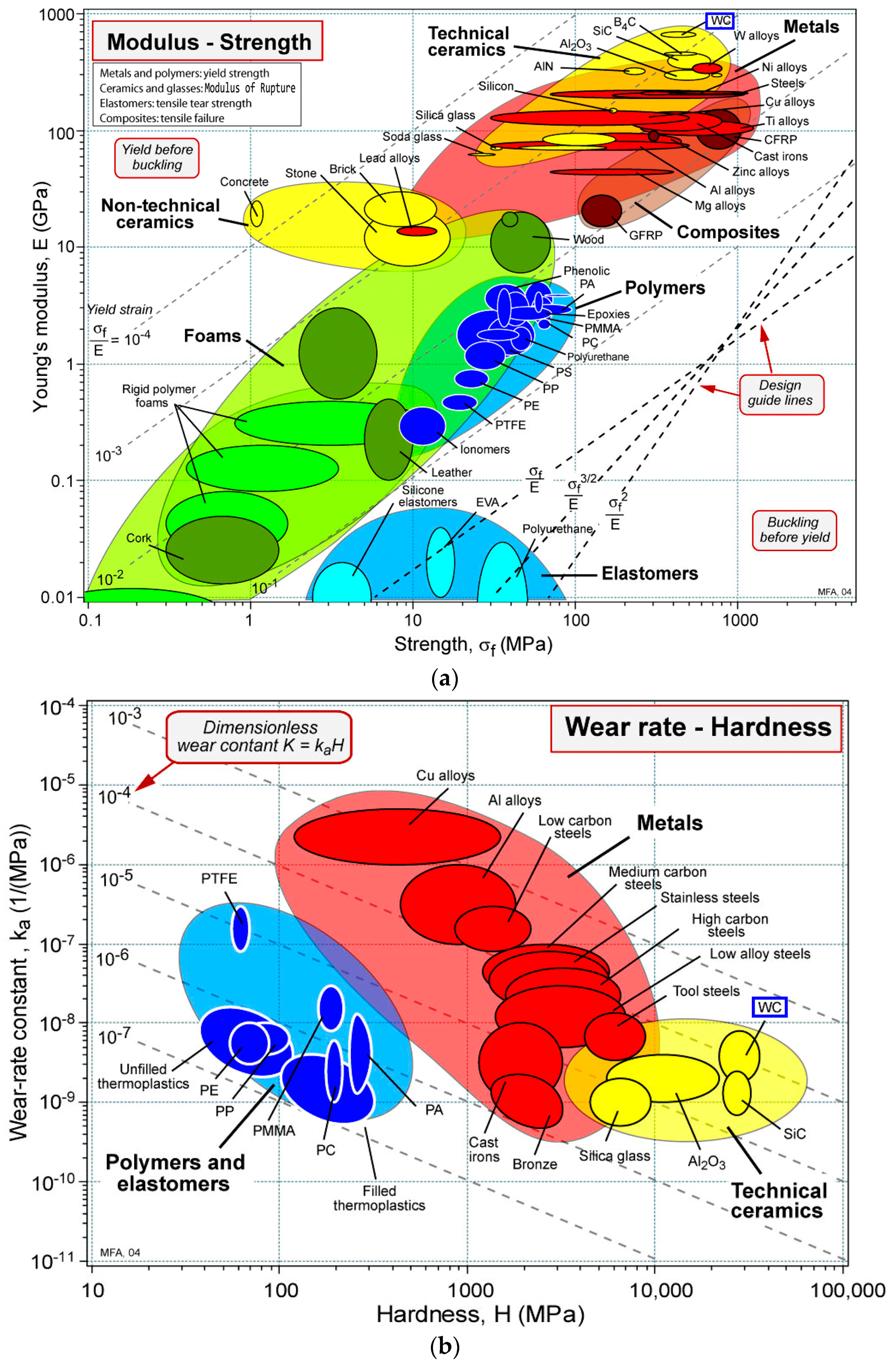
3. Possible CRM-Friendly Solutions
3.1. Alternatives to W and Co in WC-Co Cemented Carbides
3.2. Alternative to Co and Other CRMs in High-Temperature Ni-Based Superalloys
3.3. Alternative to Cr in Stainless Alloys and Surface Coatings
3.4. Alternative to Nb in Stainless Steel and HSLA Steels
3.5. Alternative to Mg in Aerospace Industry Al Alloys
4. Outlook
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Communication from the Commission to the European Parliament and the Council. The Raw Materials Initiative—Meeting Our Critical Needs for Growth and Jobs in Europe. Available online: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2008:0699:FIN:en:PDF (accessed on 10 March 2017).
- Report on Critical Raw Materials for EU, Report of the Ad-Hoc Working Group on Defining Critical Raw Materials for EU. May 2014. Available online: http://mima.geus.dk/report-on-critical-raw-materials_en.pdf (accessed on 10 March 2017).
- Strategic Implementation Plan for the European Innovation Partnership. 2013. Available online: https://ec.europa.eu/growth/tools-databases/eip-raw-materials/en/system/files/ged/20130731_SIP%20Part%20%20I%20complet%20clean.pdf (accessed on 10 March 2017).
- EIT Knowledge and Innovation Community (KIC) by the EIT Governing Board. Available online: https://eit.europa.eu/eit-community/eit-raw-materials (accessed on 10 March 2017).
- ERA-MIN Roadmap. 2013. Available online: http://www.era-min-eu.org/images/documents/public/Roadmap10.pdf (accessed on 10 March 2017).
- EU, Directorate General for Internal Policies, Policy Department A, Economic and Scientific Policy, Substitutionability of critical raw materials, Study IP/A/ITRE/ST/2011-15. October 2012. Available online: https://ec.europa.eu/growth/tools-databases/eip-raw-materials/en/system/files/ged/75%20Substitutability%20of%20CRM%20-%20DG%20Internal%20Policies.pdf (accessed on 10 March 2017).
- CRM-EXTREME COST Action. Available online: http://www.crm-extreme.eu (accessed on 10 March 2017).
- EXTREME, A European Network for Substitution of CRMs Used under Extreme Conditions. Available online: http://www.network-extreme.eu (accessed on 10 March 2017).
- Flintstone2020, Project Reference: 689279. Next Generation of Superhard Non-CRM Materials and Solutions in Tooling. Available online: http://cordis.europa.eu/project/rcn/199891_en.html (accessed on 10 March 2017).
- EQUINOX, Project Reference: 689510. A Novel Process for Manufacturing Complex Shaped Fe-Al Intermetallic Parts Resistant to Extreme Environments. Available online: http://www.equinox-project.eu/ (accessed on 10 March 2017).
- Cemented Carbide, Sandvik New Developments and Applications. Available online: http://www2.sandvik.com/sandvik/0130/HI/SE03411.nsf/7a5364adb7735b05412568c70034ea1b/651f6e334db04c46c125707600562c88/$FILE/Cemented+Carbide.pdf (accessed on 10 March 2017).
- Liu, C. Alternative Binder Phases for WC Cemented Carbides. Master’s Thesis. Engineering Materials Science KTH, School of Industrial Engineering and Management (ITM), Materials Science and Engineering, 2014. Available online: http://www.diva-portal.se/smash/get/diva2:815039/FULLTEXT01.pdf (accessed on 10 March 2017).
- Ashby, M.F. The CES EduPack Resource Booklet 2: Material and Process Selection Charts. 2009. Available online: http://www.grantadesign.com/download/pdf/teaching_resource_books/2-Materials-Charts-2010.pdf (accessed on 10 March 2017).
- Mannesson, K.; Borgh, I.; Borgenstam, A.; Ågren, J. Abnormal grain growth in cemented carbides—Experiments and simulation. Int. J. Refract. Met. Hard Mater. 2011, 29, 488–494. [Google Scholar] [CrossRef]
- General Carbide. The Designer’s Guide to Tungsten Carbide. Available online: http://www.generalcarbide.com/assets/pdf/GCDesignerGuide.pdf (accessed on 10 March 2017).
- Schubert, W.F.; Lassner, E.; Böhlke, W. CEMENTED CARBIDES—A SUCCESS STORY, Tungsten 2010, ITIA (International Tungsten Industry Association). Available online: http://www.itia.info/assets/files/Newsletter_2010_06.pdf (accessed on 10 March 2017).
- Critical Raw Materials Innovation Network (CRM_InnoNet)—Substitution of Critical Raw Materials, Critical Raw Materials Substitution Profiles. September 2013, Revised May 2015. Available online: http://www.criticalrawmaterials.eu/wp-content/uploads/D3.3-Raw-Materials-Profiles-final-submitted-document.pdf (accessed on 10 March 2017).
- Sakaki, M.; Bafghi, M.S.; Khaki, J.V.; Zhang, Q.; Sait, F. Conversion of W2C to WC phase during mechano-chemical synthesis of nano-size WC–Al2O3 powder using WO3–2Al–(1 + x)C mixtures. Int. J. Refract. Met. Hard Mater. 2013, 36, 116–121. [Google Scholar] [CrossRef]
- Cornwall, R.G. WC-Co enjoys proud history and bright future. Met. Powder Rep. 1998, 53, 32–36. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Nickel Based Superalloys. Available online: http://www.msm.cam.ac.uk/phase-trans/2003/Superalloys/superalloys.html (accessed on 10 March 2017).
- Tang, Q.; Ukai, S.; Oono, N.; Hayashi, S.; Leng, B.; Sugino, Y.; Han, W.; Okuda, T. Oxide Particle Refinement in 4.5 mass% Al Ni-Based ODS Superalloys. Mater. Trans. 2012, 53, 645–651. [Google Scholar] [CrossRef]
- Tian, C.; Han, G.; Cui, C.; Sun, X. Effects of Co content on tensile properties and deformation behaviors of Ni-based disk superalloys at different temperatures. Mater. Des. 2015, 88, 123–131. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.M.; Yoo, Y.S.; Jeong, H.W.; Jang, H. Statistical Study of the Effects of the Composition on the Oxidation Resistance of Ni-Based Superalloys. J. Nanomater. 2015, 2015, 929546. [Google Scholar] [CrossRef]
- Zenk, C.H.; Neumeier, S.; Engl, N.M.; Fries, S.G.; Dolotko, O.; Weiser, M.; Virtanen, S.; Göken, M. Intermediate Co/Ni-base model superalloys—Thermophysical properties, creep and oxidation. Scr. Mater. 2016, 112, 83–86. [Google Scholar] [CrossRef]
- Dong, J.; Bi, Z.; Wang, N.; Xie, X.; Wang, Z. Structure Control of a New-Type High-Cr Superalloy. In Superalloys 2008; Reed, R.C., Green, K.A., Caron, P., Gabb, T.P., Fahrman, M.G., Huron, E.S., Woodard, S.A., Eds.; TMS (The Minerals, Metals & Materials Society): Champion, PA, USA, 2008. [Google Scholar]
- Kawagishi, K.; Yeh, A.C.; Yokokawa, T.; Kobayashi, T.; Koizumi, Y.; Harada, H. Development of an Oxidation-Resistant High-Strength Sixth-Generation Single-Crystal Superalloy TMS-238. In Superalloy 2012; Huron, E.S., Reed, R.C., Hardy, M.C., Mills, M.J., Montero, R.E., Portella, P.D., Telesman, J., Eds.; TMS (The Minerals, Metals & Materials Society): Champion, PA, USA, 2012. [Google Scholar]
- Sato, A.; Harada, H.; Yeh, A.C.; Kawagishi, K.; Kobayashi, T.; Koizumi, Y.; Yokokawa, T.; Zhang, J.-X. A 5th Generation SC Superalloy with Balanced High Temperature Properties and Processability. In Superalloys 2008; Reed, R.C., Green, K.A., Caron, P., Gabb, T.P., Michael, G., Fahrmann, M.G., Huron, E.S., Woodard, S.A., Eds.; TMS (The Minerals, Metals & Materials Society): Champion, PA, USA, 2008. [Google Scholar]
- Matuszewski, K.; Rettig, R.; Matysiak, H.; Peng, Z.; Povstugar, I.; Choi, P.; Müller, J.; Raabe, D.; Spiecker, E.; Kurzydłowski, K.J.; et al. Effect of ruthenium on the precipitation of topologically close packed phases in Ni-based superalloys of 3rd and 4th generation. Acta Mater. 2015, 95, 274–283. [Google Scholar] [CrossRef]
- Stainless Steel—ASM Specialty Handbook; ASM International: Materials Park, OH, USA, 1994.
- Di Caprio, G. Gli Acciai Inossidabili, 4th ed.; Hoepli: Milano, Italy, 2003. [Google Scholar]
- Van Rooyen, G.T. The Potential of Chromium as an Alloying Element. In Proceedings of the 1st International Chromium Steel and Alloys Congress, Cape Town, South Africa, 8–11 March 1992; Volume 2, pp. 43–47.
- Metals Handobook, Volume 13—Corrosion, 9th ed.; ASM International: Metals Park, OH, USA, 1987.
- Cunat, P.J. Alloying Elements in Stainless Steel and Other Chromium-Containing Alloys. Euro Inox2004. Available online: http://www.bssa.org.uk/cms/File/Euro%20Inox%20Publications/Alloying%20Elements.pdf (accessed on 10 March 2017).
- Bellezze, T.; Roventi, G.; Fratesi, R. Electrochemical study on the corrosion resistance of Cr III-based conversion layers on zinc coatings. Surf. Coat. Technol. 2002, 155, 221–230. [Google Scholar] [CrossRef]
- Wynn, P.C.; Bishop, C.V. Replacing hexavalent chromium. Trans. Inst. Met. Finish. 2001, 79, B27–B30. [Google Scholar]
- Militzer, M. Thermomechanical Processed Steels. Reference Module in Materials Science and Materials Engineering. Compr. Mater. Process. 2014, 1, 191–216. [Google Scholar]
- Tamarelli, C.M. AHSS 101: The Evolving Use of Advanced High-Strength Steels for Automotive Applications; Steel Market Development Institute, Student Intern-Summer 2011, University of Michigan: Southfield, MI, USA, 2011. [Google Scholar]
- Bigot, A.; Auger, P.; Chambreland, S.; Blavette, D.; Reeves, A. Atomic Scale Imaging and Analysis of T′ Precipitates in Al-Mg-Zn Alloys. Microsc. Microanal. Microstruct. 1997, 8, 103–113. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Smallman, R.; Ngan, A. Physical Metallurgy and Advanced Materials; Elsevier Ltd.: Oxford, UK, 2007; p. 390. [Google Scholar]
- Mosbah, A.Y.; Wexler, D.; Calka, A. Abrasive wear of WC-FeAl composites. Wear 2005, 258, 1337–1341. [Google Scholar] [CrossRef]
- Yusoff, M.; Othman, R.; Hussain, Z. Mechanical alloying and sintering of nanostructured tungsten carbide-reinforced carbon composite and its characterization. Mater. Des. 2011, 32, 3293–3298. [Google Scholar] [CrossRef]
- Makarov, S.; Poletika, I.; Krylova, T. Tungsten carbide by boron replacement under electron beam surfacing. In Proceedings of the 9th International Conference “Interaction of Radiation with Solids”, Minsk, Belarus, 20–22 September 2011.
- Goljandin, D.; Sarjas, H.; Kulu, P.; Käerdi, H.; Mikli, V. Metal Matrix Hardmetal/Cermet Reinforced Composite Powder for Thermal Spray. Mater. Sci. Medžiagotyra 2012, 18, 84–89. [Google Scholar] [CrossRef]
- Zhang, S. Titanium carbonitride-based cermets processes and properties. Mater. Sci. Eng. A 1993, 163, 141–148. [Google Scholar] [CrossRef]
- Kumar, B.V.M.; Basu, B.; Vizintin, J.; Kalin, M. Tribochemistry in sliding wear of TiCN–Ni-based cermets. J. Mater. Res. 2008, 23, 1214–1227. [Google Scholar] [CrossRef]
- Ishida, T.; Moriguchi, H.; Ikegaya, A. Development of Cemented Carbide Tool of Reduced Rare Metal Usage. SEI Tech. Rev. 2011, 73, 52–56. [Google Scholar]
- Szutkowska, M.; Jaworska, L.; Cygan, S.; Karolus, M.; Kalinka, A.; Leśniewski, W. WC-Co hardmetal with addition of MAX phase from Ti-Si-C system obtained by HIP method. IAAM Scientist Awarded Lecture. In Proceedings of the International Conference on Materials Science & Technology, Delhi, India, 1–4 March 2016.
- Szutkowska, M.; Jaworska, L.; Karolus, M.; Podsiadło, M. Effect of MAX phase from Ti-Si-C system on microstructure and mechanical properties of WC-Co hardmetal sintered by SPS method. In Proceedings of the International Symposium on Green Manufacturing and Applications ISGMA, Bali, Indonesia, 21–25 July 2016.
- Dutkiewicz, J.; Szutkowska, M.; Leśniewski, W.; Wieliczko, P.; Pieczara, A.; Rogal, Ł. The effect of TiC on structure and hardness of WC-Co composites prepared using various consolidation methods. Kompozyty (Compos.) 2014, 2, 91–95. [Google Scholar]
- Tarragó, J.M.; Ferrari, C.; Reig, B.; Coureaux, D.; Schneider, L.; Llanes, L. Mechanics and mechanisms of fatigue in a WC–Ni hardmetal and a comparative study with respect to WC–Co hard metals. Int. J. Fatigue 2015, 70, 252–257. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Roa, J.J.; Valle, V.; Marshall, J.M.; Llanes, L. Fracture and fatigue behavior of WC–Co and WC–CoNi cemented carbides. Int. J. Refract. Met. Hard Mater. 2015, 49, 184–191. [Google Scholar] [CrossRef]
- Viswanadham, R.K.; Lindquist, R.G. Transformation-Toughening in Cemented Carbides: Part I. Binder Composition Control. Metall. Trans. A 1987, 18, 2163–2173. [Google Scholar] [CrossRef]
- Schubert, W.D.; Fugger, M.; Wittmann, B.; Useldinger, R. Aspects of sintering of cemented carbides with Fe-based binders. Int. J. Refract. Met. Hard Mater. 2015, 49, 110–123. [Google Scholar] [CrossRef]
- Novák, P.; Šotka, D.; Novák, M.; Michalcová, A.; Šerák, J.; Vojtěch, D. Production of NiAl–matrix composites by reactive sintering. Powder Metall. 2011, 54, 308–313. [Google Scholar] [CrossRef]
- Novák, P.; Salvetr, P.; Pecenová, Z. Intermetallics–Synthesis, Production, Properties. Manuf. Technol. 2015, 15, 1024–1028. [Google Scholar]
- Novák, P.; Kříž, J.; Průša, F.; Kubásek, J.; Marek, I.; Michalcová, A.; Voděrová, M.; Vojtěch, D. Structure and properties of Ti-Al-Si-X alloys produced by SHS method. Intermetallics 2013, 39, 11–19. [Google Scholar] [CrossRef]
- Knaislová, A.; Novák, P.; Nová, K. Using of Microscopy in optimization of the Ti-Al-Si alloys preparation by powder metallurgy. Manuf. Technol. 2016, 16, 946–949. [Google Scholar]
- Novák, P.; Vojtěch, D.; Šerák, J.; Knotek, V.; Bártová, B. Duplex surface treatment of the Nb-alloyed PM tool steel. Surf. Coat. Technol. 2006, 201, 3342–3349. [Google Scholar] [CrossRef]
- Hayama, A.O.F.; Sandim, H.R.Z.; Lins, J.F.C.; Hupalo, M.F.; Padilha, A.F. Annealing behavior of the ODS nickel-based superalloy PM 1000. Mater. Sci. Eng. A 2004, 371, 198–209. [Google Scholar] [CrossRef]
- The GEnx Commercial Aircraft Engine. The GEnx Engine Delivers Proven Performance for the Boeing 787 Dreamliner and Boeing 747-8. Available online: http://www.geaviation.com/commercial/engines/genx/ (accessed on 10 March 2017).
- Schwaighofer, E.; Clemens, H.; Mayer, S.; Lindemann, J.; Klose, J.; Smarsly, W.; Güther, V. Microstructural design and mechanical properties of a cast and heat-treated intermetallic multi-phase γ-TiAl based alloy. Intermetallics 2014, 44, 128–140. [Google Scholar] [CrossRef]
- Kratochvil, P. The history of the search and use of heat resistant Pyroferal© alloys based on FeAl. Intermetallics 2008, 16, 587–591. [Google Scholar] [CrossRef]
- Novák, P.; Zelinková, M.; Šerák, J.; Michalcová, A.; Novák, M.; Vojtěch, D. Oxidation resistance of SHS Fe-Al-Si alloys at 800 °C in air. Intermetallics 2011, 19, 1306–1312. [Google Scholar] [CrossRef]
- Meher, S.; Carroll, L.J.; Pollock, T.M.; Carroll, M.C. Solute partitioning in multi-component γ/γ′ Co–Ni-base superalloys with near-zero lattice misfit. Scr. Mater. 2016, 113, 185–189. [Google Scholar] [CrossRef]
- Frick, L. Ceramic Composites Give Super-Alloys Strong Competition. 2012. Available online: http://machinedesign.com/news/ceramic-composites-give-super-alloys-strong-competition (accessed on 10 March 2017).
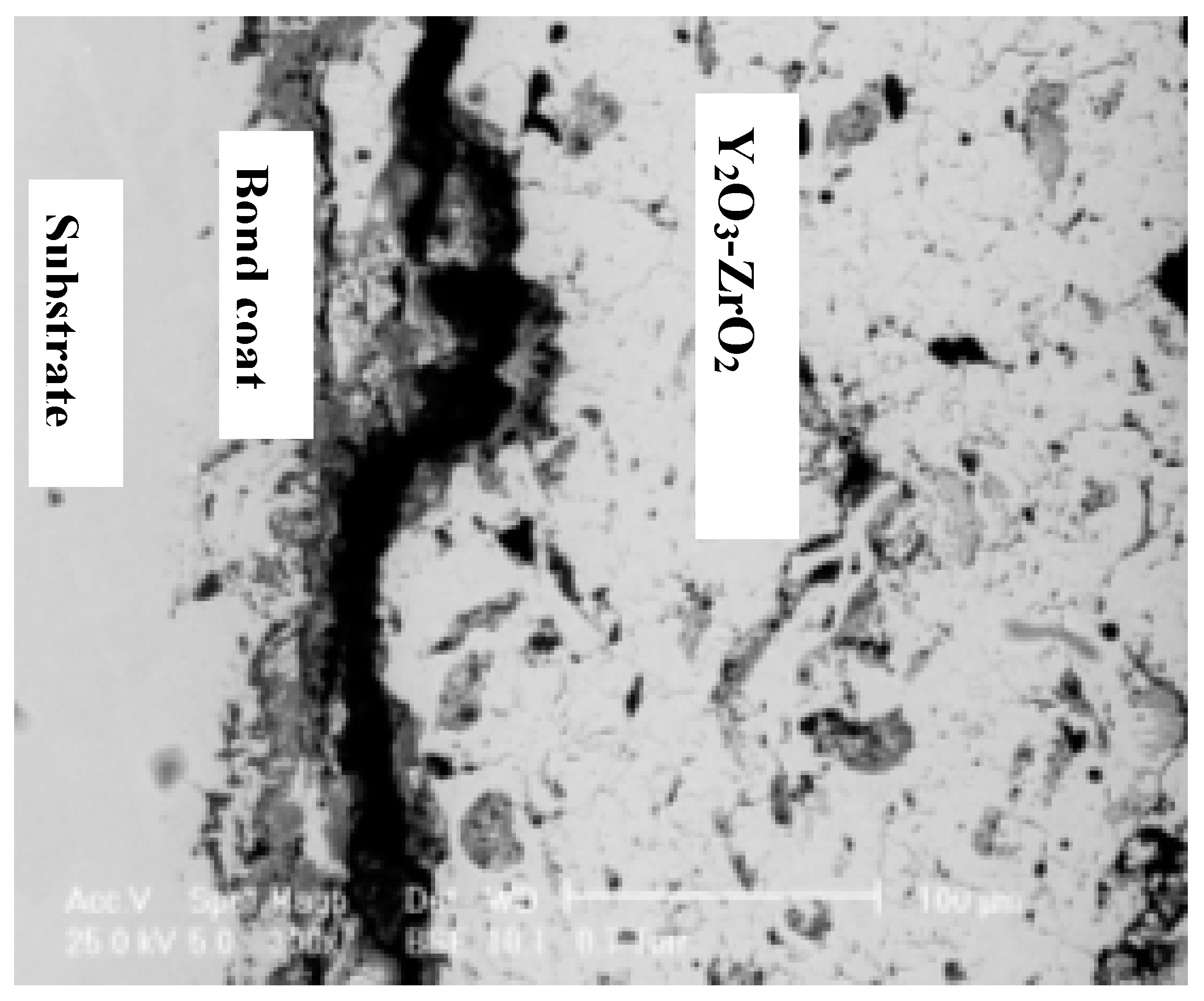
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Vassen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stover, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Ilina, S.; Ionescu, G.; Manoliu, V.; Piticescu, R.R. Nanostructured Zirconia Layers as Thermal Barrier Coatings. INCAS Bull. 2011, 3, 63–69. [Google Scholar]
- Substitution Alternatives for Strategic Materials. In Strategic Materials: Technologies to Reduce U.S. Import Vulnerability; U.S. Congress, Office of Technology Assessment, OTA-ITE-248: Washington, DC, USA, 1985; Chapter 7; pp. 263–328.
- Glenn, M.L.; Larson, D.E. Reduced-Chromium Stainless Steel Substitutes Containing Silicon and Aluminum; Report of Investigation 8918; United States Department of the Interior, Bureau of Mines: Albany, OR, USA, 1984.
- Bullard, S.J.; Larson, D.E.; Dunning, J.S. Oxidation and Corrosion Resistance of Two Fe-8Cr-16Ni-Si-Cu Alloys. Corrosion 1992, 48, 891–897. [Google Scholar] [CrossRef]
- Dunning, J.S.; Alman, D.E.; Rawers, J.C. Influence of Silicon and Aluminum Additions on the Oxidation Resistance of a Lean-Chromium Stainless Steel. Oxid. Met. 2002, 57, 409–425. [Google Scholar] [CrossRef]
- Engkvist, J.; Bexell, U.; Grehk, M.; Olsson, M. High temperature oxidation of FeCrAl-alloys—Influence of Al-concentration on oxide layer characteristics. Mater. Corros. 2009, 60, 876–881. [Google Scholar] [CrossRef]
- Wolff, I.M.; Iorio, L.E.; Rumpf, T.; Scheers, P.V.T.; Potgieter, J.H. Oxidation and corrosion behaviour of Fe–Cr and Fe–Cr–Al alloys with minor alloying additions. Mater. Sci. Eng. A 1998, 241, 264–276. [Google Scholar] [CrossRef]
- Jönsson, B.; Lu, Q.; Chandrasekaran, D.; Berglund, R.; Rave, F. Oxidation and Creep Limited Lifetime of Kanthal APMT®, a Dispersion Strengthened FeCrAlMo Alloy Designed for Strength and Oxidation Resistance at High Temperatures. Oxid. Met. 2013, 79, 29–39. [Google Scholar] [CrossRef]
- Pothen, F.; Goeschl, T.; Löschel, A.; Jaha, V. Strategic Trade Policy and Critical Raw Materials in Stainless Steel Production; Project Report; Zentrum für Europäische Wirtschaftsforschung: Mannheim, Germany, 2013. [Google Scholar]
- Cavallini, M.; Felli, F.; Fratesi, R.; Veniali, F. High temperature air oxidation behaviour of “poor man” high manganese-aluminum steels. Mater. Corros. 1982, 33, 386–390. [Google Scholar] [CrossRef]
- Casteletti, L.C.; Neto, A.L.; Totten, G.E.; Heck, S.C.; Fernandes, F.A.P. Use of Fe–31Mn–7.5Al–1.3Si–0.9C Alloy for Fabrication of Resistive Elements. J. ASTM Int. 2010, 7, 1–4. [Google Scholar]
- Bellezze, T.; Giuliani, G.; Roventi, G.; Fratesi, R.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour of austenitic and duplex stainless steels in an industrial strongly acidic solution. Mater. Corros. 2016, 67, 831–838. [Google Scholar] [CrossRef]
- Chen, W.Y.C.; Stephens, J.R. Anodic Polarization Behaviour of Austenitic Stainless Steel Alloys with Lower Chromium Content. Corrosion 1979, 35, 443–451. [Google Scholar] [CrossRef]
- Reformatskaya, I.I.; Rodionova, I.G.; Podobaev, A.N.; Ashcheulova, I.I.; Trofimova, E.V. Silicon as an Alloying Element in Ferrite Stainless Steels Containing 8–13% Cr. Prot. Met. 2006, 42, 549–554. [Google Scholar] [CrossRef]
- Wan, J.; Ran, Q.; Li, J.; Xu, Y.; Xiao, X.; Yu, H.; Jiang, L. A new resource-saving, low chromium and low nickel duplex stainless steel 15Cr–xAl–2Ni–yMn. Mater. Des. 2014, 53, 43–50. [Google Scholar] [CrossRef]
- Cavallini, M.; Felli, F.; Fratesi, R.; Veniali, F. Aqueous solution corrosion behaviour of “poor man” high manganese-aluminum steels. Mater. Corros. 1982, 33, 281–284. [Google Scholar] [CrossRef]
- Li, C.; Bell, T. Corrosion properties of plasma nitrided AISI 410 martensitic stainless steel in 3.5% NaCl and 1% HCl aqueous solutions. Corros. Sci. 2006, 48, 2036–2049. [Google Scholar] [CrossRef]
- Bellezze, T.; Roventi, G.; Quaranta, A.; Fratesi, R. Improvement of pitting corrosion resistance of AISI 444 stainless steel to make it a possible substitute for AISI 304L and 316L in hot natural waters. Mater. Corros. 2008, 59, 727–731. [Google Scholar] [CrossRef]
- Park, L.J.; Ryu, H.J.; Hong, S.H.; Kim, Y.G. Microstructure and Mechanical Behavior of Mechanically Alloyed ODS Ni-Base Superalloy for Aerospace Gas Turbine Application. Adv. Perform. Mater. 1998, 5, 279–290. [Google Scholar] [CrossRef]
- Hoornaert, T.; Hua, Z.K.; Zhang, J.H. Hard Wear-Resistance Coatings: A Review. In Advanced Tribology; Luo, L., Meng, Y., Shao, T., Zhao, Q., Eds.; Springer: Berlin, Germany, 2010; pp. 774–779. [Google Scholar]
- Subrahmanyam, J.; Srivastava, M.P.; Sivakumar, R. Characterization of plasma-sprayed WC-Co coatings. Mater. Sci. Eng. 1986, 84, 209–214. [Google Scholar] [CrossRef]
- Çalışkan, H. Effect of test parameters on the micro-abrasion behavior of PVD CrN coatings. Measurement 2014, 55, 444–451. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Espallargas, N.; Suegama, P.H.; Benedetti, A.V.; Fernández, J. High-velocity oxyfuel Cr3C2-NiCr replacing hard chromium coatings. J. Therm. Spray Technol. 2005, 14, 335–341. [Google Scholar] [CrossRef]
- Veprek, S.; Jilek, M. Superhard nanocomposite coatings. From basic science toward industrialization. Pure Appl. Chem. 2002, 74, 475–481. [Google Scholar] [CrossRef]
- Merl, D.K.; Milošev, I.; Panjan, P.; Zupanič, F. Morphology and Corrosion Properties PVD Cr-N Coatings Deposited on Aluminium Alloys. Mater. Tehnol. 2011, 45, 593–597. [Google Scholar]
- Fragiel, A.; Staia, M.H.; Muñoz-Saldaña, J.; Puchi-Cabrera, E.S.; Cortes-Escobedo, C.; Cota, L. Influence of the N2 partial pressure on the mechanical properties and tribological behavior of zirconium nitride deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2008, 202, 3653–3660. [Google Scholar] [CrossRef]
- Valerina, D.; Signore, M.A.; Tapfer, L.; Piscopiello, E.; Galietti, U.; Rizzo, A. Adhesion and wear of ZrN films sputtered on tungsten carbide substrates. Thin Solid Films 2013, 538, 42–47. [Google Scholar] [CrossRef]
- Gusmano, G.; Montesperelli, G.; Rapone, M.; Padeletti, G.; Cusmà, A.; Kaciulis, S.; Mezzi, A.; Maggio, R.D. Zirconia Primers for Corrosion Resistant Coatings. Surf. Coat. Technol. 2007, 201, 5822–5828. [Google Scholar] [CrossRef]
- Holleck, H.; Schier, V. Multilayer PVD coatings for wear protection. Surf. Coat. Technol. 1995, 76–77, 328–336. [Google Scholar] [CrossRef]
- Mahamood, R.M.; Akinlabi, E.T.; Shukla, M.; Pityana, S. Functionally Graded Material: An Overview. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012; Ao, S.I., Gelman, L., Hukins, D.W.L., Hunter, A., Korsunsky, A.M., Eds.; International Association of Engineers: London, UK, 2012; Volume III, pp. 1593–1597. Available online: http://www.iaeng.org/publication/WCE2012/WCE2012_pp1593-1597.pdf (accessed on 10 March 2017). [Google Scholar]
- Ho, S.-Y.; Kotousov, A.; Nguyen, P.; Harding, S.; Codrington, J.; Tsukamoto, H. FGM (Functionally Graded Material) Thermal Barrier Coatings for Hypersonic Structures—Design and Thermal Structural Analysis. AOARD REPORT Contract No. 064043; Department of Mechanical Engineering, University of Adelaide: Adelaide, Australia, 2007. [Google Scholar]
- Costa, J.S.; Dei Agnoli, R.; Ferreira, J.Z. Corrosion behavior of a conversion coating based on zirconium and colorants on galvanized steel by electrodeposition. Technol. Metal. Mater. Miner. 2015, 12, 167–175. [Google Scholar] [CrossRef]
- Schaffnit, P.; Stallybrass, C.; Konrad, J.; Kulgemeyer, A.; Meuser, H. Dual-scale phase field simulation of grain growth upon reheating of a microalloyed line pipe steel. Int. J. Mater. Res. 2010, 101, 549–554. [Google Scholar] [CrossRef]
- Banerjee, K.; Perez, M.; Wang, X.; Militzer, M. Nonisothermal Austenite Grain Growth Kinetics in a Microalloyed X80 Linepipe Steel. Metall. Mater. Trans. A 2010, 41, 3161–3172. [Google Scholar] [CrossRef]
- Rioja, R.R.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Metall. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Bodily, B.; Heinimann, M.; Bray, G.; Colvin, E.; Witters, J. Advanced aluminum and aluminum–lithium solutions for derivative and next generation aerospace structures. In Proceedings of the SAE 2012 Aerospace Manufacturing and Automated Fastening Conference and Exhibition, AMAF 2012, Fort Worth, TX, USA, 18–20 September 2012; Code 96078, SAE Paper No. 2012-01-1874. Volume 6.
- Moreto, J.A.; Gamboni, O.; Ruchert, C.O.F.T.; Romagnoli, F.; Moreira, M.F.; Beneduce, F.; Bose Filho, W.W. Corrosion and fatigue behavior of new Al alloys. Procedia Eng. 2011, 10, 1521–1526. [Google Scholar] [CrossRef]
- Yu, N.; Shang, J.; Cao, Y.; Ma, D.; Liu, Q. Comparative analysis of Al-Li alloy and aluminum honeycomb panel for aerospace application by structural optimization. Math. Probl. Eng. 2015, 2015, 815257. [Google Scholar] [CrossRef]
- Soltani, P.; Keikhosravy, M.; Oskouei, R.H.; Soutis, C. Studying the tensile behaviour of GLARE laminates: A finite element modelling approach. Appl. Compos. Mater. 2011, 18, 271–282. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Dou, C.; Sekulic, D.P. An effect of the rotation speed on microstructure and mechanical properties of the friction stir welded 2060-T8 Al-Li alloy. Mater. Charact. 2017, 123, 9–19. [Google Scholar] [CrossRef]
- Lertora, E.; Gambaro, C. AA8090 Al-Li alloy FSW parameters to minimize defects and increase fatigue life. Int. J. Mater. Form. 2010, 3, 1003–1006. [Google Scholar] [CrossRef]
- Cai, B.; Zheng, Z.Q.; He, D.Q.; Li, S.C.; Li, H.P. Friction stir weld of 2060 Al–Cu–Li alloy: Microstructure and mechanical properties. J. Alloys Compd. 2015, 649, 19–27. [Google Scholar] [CrossRef]
- Purdy, G.; Ågren, J.; Borgenstam, A.; Bréchet, Y.; Enomoto, M.; Furuhara, T.; Gamsjäger, E.; Gouné, M.; Hillert, M.; Hutchinson, C.; et al. ALEMI: A Ten-Year History of Discussions of Alloying-Element Interactions with Migrating Interfaces. Metall. Mater. Trans. A 2011, 42, 3703–3718. [Google Scholar] [CrossRef]
- Gouné, M.; Danoix, F.; Ågren, J.; Bréchet, Y.; Hutchinson, C.R.; Militzer, M.; Purdy, G.; van der Zwaag, S.; Zurob, H. Overview of the current issues in austenite to ferrite transformation and the role of migrating interfaces therein for low alloyed steels. Mater. Sci. Eng. R Rep. 2015, 92, 1–38. [Google Scholar] [CrossRef]
- Wang, M.-M.; Tasan, C.C.; Ponge, D.; Kostka, A.; Raabe, D. Smaller is less stable: Size effects on twinning vs. transformation of reverted austenite in TRIP-maraging steels. Acta Mater. 2014, 79, 268–281. [Google Scholar] [CrossRef]
- Wiessner, M.; Gamsjäger, E.; van der Zwaag, S.; Angerer, P. Effect of reverted austenite on tensile and impact strength in a martensitic stainless steel—An in-situ X-ray diffraction study. Mater. Sci. Eng. A 2017, 682, 117–125. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grilli, M.L.; Bellezze, T.; Gamsjäger, E.; Rinaldi, A.; Novak, P.; Balos, S.; Piticescu, R.R.; Ruello, M.L. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials 2017, 10, 285. https://doi.org/10.3390/ma10030285
Grilli ML, Bellezze T, Gamsjäger E, Rinaldi A, Novak P, Balos S, Piticescu RR, Ruello ML. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials. 2017; 10(3):285. https://doi.org/10.3390/ma10030285
Chicago/Turabian StyleGrilli, Maria Luisa, Tiziano Bellezze, Ernst Gamsjäger, Antonio Rinaldi, Pavel Novak, Sebastian Balos, Radu Robert Piticescu, and Maria Letizia Ruello. 2017. "Solutions for Critical Raw Materials under Extreme Conditions: A Review" Materials 10, no. 3: 285. https://doi.org/10.3390/ma10030285
APA StyleGrilli, M. L., Bellezze, T., Gamsjäger, E., Rinaldi, A., Novak, P., Balos, S., Piticescu, R. R., & Ruello, M. L. (2017). Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials, 10(3), 285. https://doi.org/10.3390/ma10030285