Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success?
Abstract
:1. Concrete and Its Problems
1.1. The Global Use of Concrete
1.2. Ecological Disadvantages Accompanying Concrete
1.3. Durability (and Sustainability) of Concrete
2. Superabsorbent Polymers
2.1. Natural versus Synthetic SAPs
2.2. Stimuli-Responsive Superabsorbent Polymers
3. Solving Crack Issues in Concrete
3.1. Tackling the Problem of Crack Formation: Autogenous Shrinkage
3.2. Influences on the Microstructural Properties and Moisture Transport Processes
3.3. Strength Decrease or Increase Due to Superabsorbent Polymers?
3.4. A Way to Increase the Freeze/Thaw Resistance
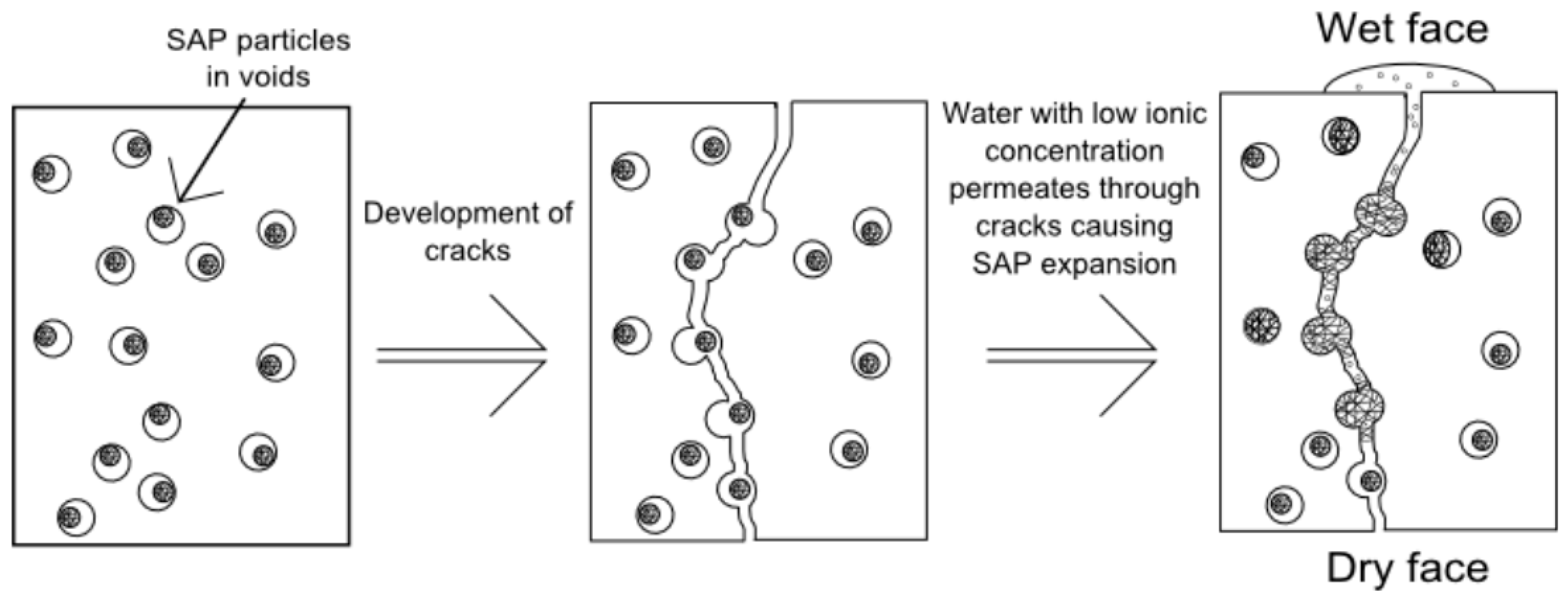
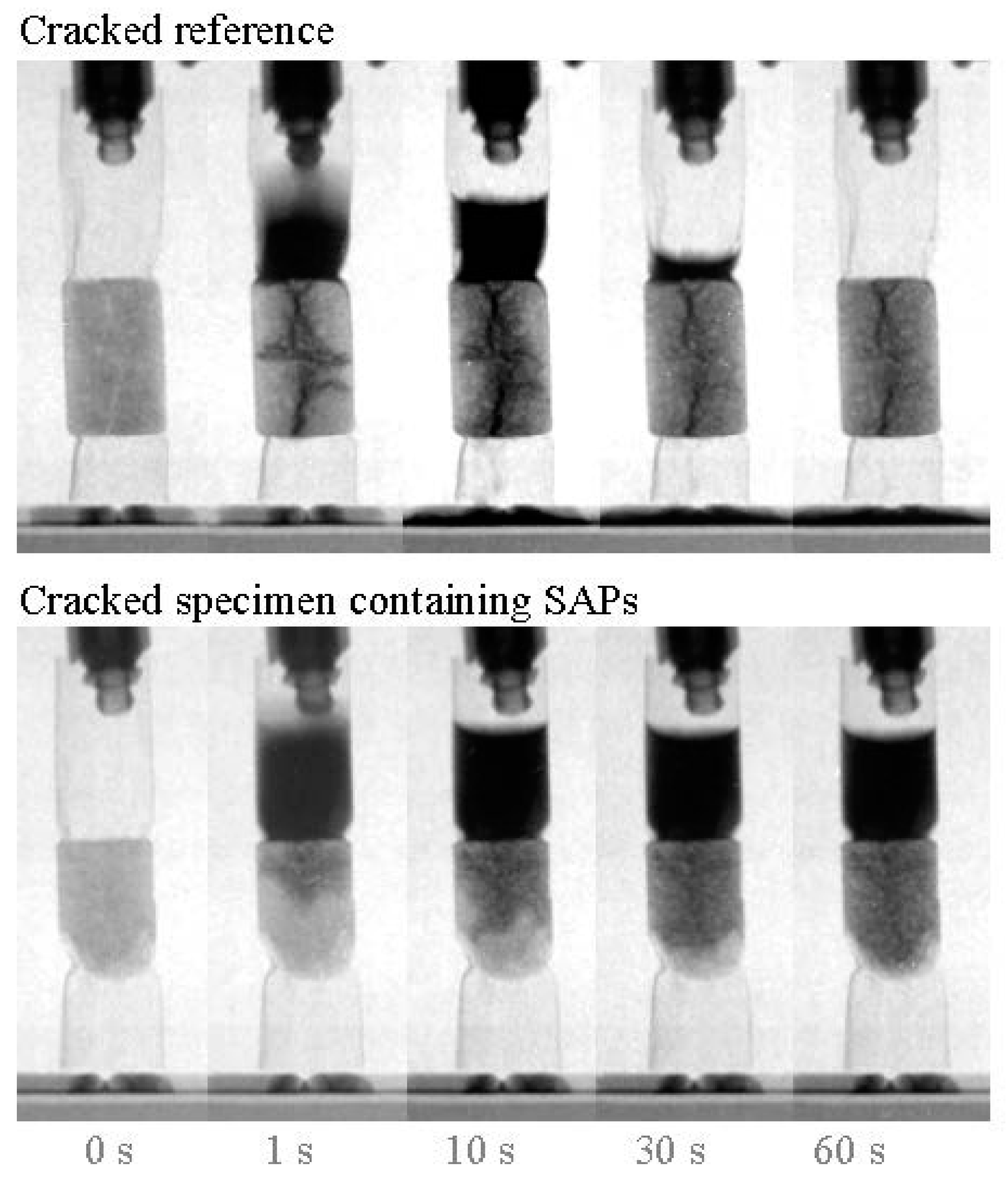
3.5. Sealing Cracks and Regaining the Water-Tightness of Structures
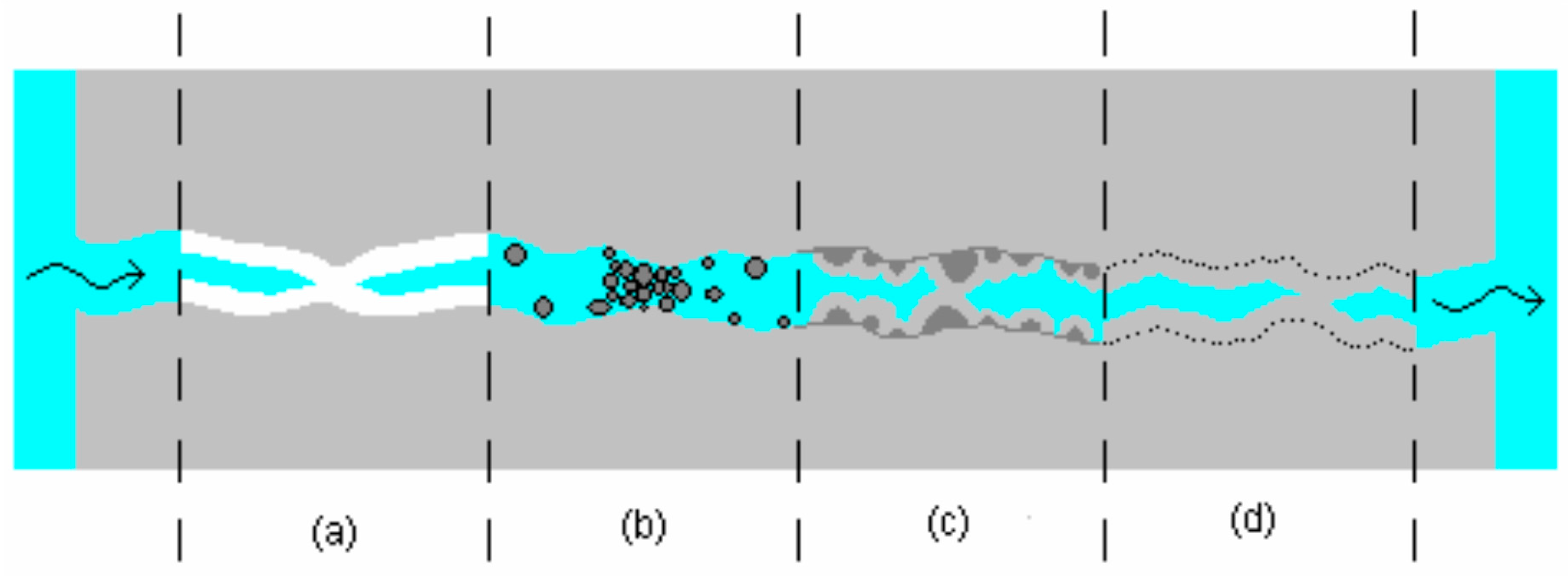
3.6. Repairing Formed Cracks by Promoting Autogenous Healing
4. Challenges to Overcome When Using SAPs in Cementitious Materials
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acrylic acid |
| AM | Acrylamide |
| AMPS | 2-acrylamido-2-methylpropane sulfonic acid |
| CaCO3 | Calcium carbonate |
| CaO | Calcium oxide—Free lime |
| Ca(OH)2 | Calcium hydroxide—Portlandite |
| CO2 | Carbon dioxide |
| C-S-H | Calcium-Silicate-Hydrate(s) |
| DMAEMA | Dimethylaminoethyl methacrylate |
| DMAPMA | Dimethylaminopropyl methacrylamide |
| LWA | Light-weight aggregate |
| SAP(s) | Superabsorbent polymer(s) |
| m% | Mass percent of cement weight |
| MAA | Methacrylic acid |
| MBA | N,N′-methylenebisacrylamide |
| MgO | Magnesium oxide—Periclase |
| W/C | Water-to-Cement ratio |
References
- World Business Council for Sustainable Development (WBCSD). The Cement Sustainability Initiative—Recycling Concrete; World Business Council for Sustainable Developments: Geneva, Switzerland, 2016. [Google Scholar]
- CEMBUREAU—The European Cement Association. Key Facts and Figures. Available online: http://www.cembureau.be/about-cement/key-facts-figures (accessed on 31 March 2016).
- Van Breugel, K. Is there a market for self-healing cement-based materials. In Proceedings of the First International Conference on Self-Healing Materials 2007, Noordwijk aan Zee, The Netherlands, 18–20 April 2007.
- Mahasenan, N.; Smith, S.; Humphreys, K.; Kaya, Y. The cement industry and global climate change: Current and potential future cement industry CO2 emissions. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Kyoto, Japan, 1–4 October 2002; pp. 995–1000.
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Asadi, M.; Jafari, H.; Moradi, A.; Asadollahfardi, R. Experimental and statistical studies of using wash water from ready-mix concrete trucks and a batching plant in the production of fresh concrete. Constr. Build. Mater. 2015, 98, 305–314. [Google Scholar] [CrossRef]
- Aggoune, S.; Imache, R.; Khadraoui, A.; Mezghiche, M. Evaluation of e-government information systems agility in the perspective of sustainability. In Electronic Government and the Information Systems Perspective; Springer: New York, NY, USA, 2011; pp. 315–329. [Google Scholar]
- Mihashi, H.; Nishiwaki, T. Development of engineered self-healing and self-repairing concrete-state-of-the-art report. J. Adv. Concr. Technol. 2012, 10, 170–184. [Google Scholar] [CrossRef]
- Ferrara, L.; Krelani, V.; Carsana, M. A “fracture testing” based approach to assess crack healing of concrete with and without crystalline admixtures. Constr. Build. Mater. 2014, 68, 535–551. [Google Scholar] [CrossRef]
- Wu, M.; Johannesson, B.; Geiker, M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 2012, 28, 571–583. [Google Scholar] [CrossRef]
- Stewart, M.G.; Rosowsky, D.V. Time-dependent reliability of deteriorating reinforced concrete bridge decks. Struct. Saf. 1998, 20, 91–109. [Google Scholar] [CrossRef]
- Arιoglu, N.; Girgin, Z.C.; Arιoglu, E. Evaluation of ratio between splitting tensile strength and compressive strength for concretes up to 120 MPa and its application in strength criterion. ACI Mater. J. 2006, 103, 18–24. [Google Scholar]
- Van Tittelboom, K.; de Belie, N.; de Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Sellevold, E.J.; Bjøntegaard, Ø. Coefficient of thermal expansion of cement paste and concrete: Mechanisms of moisture interaction. Mater. Struct. 2006, 39, 809–815. [Google Scholar] [CrossRef]
- Wang, K.; Jansen, D.C.; Shah, S.P.; Karr, A.F. Permeability study of cracked concrete. Cem. Concr. Res. 1997, 27, 381–393. [Google Scholar] [CrossRef]
- Bentz, D.P.; Jensen, O.M. Mitigation strategies for autogenous shrinkage cracking. Cem. Concr. Compos. 2004, 26, 677–685. [Google Scholar] [CrossRef]
- Snoeck, D.; Jensen, O.M.; de Belie, N. The influence of superabsorbent polymers on the autogenous shrinkage properties of cement pastes with supplementary cementitious materials. Cem. Concr. Res. 2015, 74, 59–67. [Google Scholar] [CrossRef]
- Weber, S.; Reinhardt, H.W. A new generation of high performance concrete: Concrete with autogenous curing. Adv. Cem. Based Mater. 1997, 6, 59–68. [Google Scholar] [CrossRef]
- Shen, D.; Jiang, J.; Shen, J.; Yao, P.; Jiang, G. Influence of curing temperature on autogenous shrinkage and cracking resistance of high-performance concrete at an early age. Constr. Build. Mater. 2016, 103, 67–76. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Tazawa, E.-I.; Miyazawa, S. Influence of cement and admixture on autogenous shrinkage of cement paste. Cem. Concr. Res. 1995, 25, 281–287. [Google Scholar] [CrossRef]
- Reynolds, D.; Browning, J.; Darwin, D. Lightweight Aggregates as an Internal Curing Agent for Low-Cracking High-Performance Concrete; Technical Report; The University of Kansas Center for Research, Inc.: Lawrence, KS, USA, 2009. [Google Scholar]
- Ribeiro, M.S.S. Expansive cement blend for use in shrinkage-compensating mortars. Mater. Struct. 1998, 31, 400–404. [Google Scholar] [CrossRef]
- Koerner, R.M.; Koerner, G.R. Geotextiles used as flexible forms. Geotext. Geomembr. 1996, 14, 301–311. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Kim, D.; Park, K. Swelling and mechanical properties of superporous hydrogels of poly(acrylamide-co-acrylic acid)/polyethylenimine interpenetrating polymer networks. Polymer 2004, 45, 189–196. [Google Scholar] [CrossRef]
- Abd Alla, S.G.; Sen, M.; El-Naggar, A.W.M. Swelling and mechanical properties of superabsorbent hydrogels based on Tara gum/acrylic acid synthesized by gamma radiation. Carbohydr. Polym. 2012, 89, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, L.; Kong, Y.; Zhang, E.; Liu, Y. Synthesis and properties of N-maleyl chitosan-cross-linked poly(acrylic acid-co-acrylamide) superabsorbents. J. Polym. Environ. 2011, 19, 926–934. [Google Scholar] [CrossRef]
- Buchholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; John Wiley & Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Mignon, A.; Snoeck, D.; Schaubroeck, D.; Luickx, N.; Dubruel, P.; van Vlierberghe, S.; de Belie, N. pH-responsive superabsorbent polymers: A pathway to self-healing of mortar. React. Funct. Polym. 2015, 93, 68–76. [Google Scholar] [CrossRef]
- Mignon, A.; Graulus, G.-J.; Snoeck, D.; Martins, J.; de Belie, N.; Dubruel, P.; van Vlierberghe, S. pH-sensitive superabsorbent polymers: A potential candidate material for self-healing concrete. J. Mater. Sci. 2014, 50, 970–979. [Google Scholar] [CrossRef]
- Snoeck, D.; van Tittelboom, K.; Steuperaert, S.; Dubruel, P.; de Belie, N. Self-healing cementitious materials by the combination of microfibres and superabsorbent polymers. J. Intell. Mater. Syst. Struct. 2014, 25, 13–24. [Google Scholar] [CrossRef]
- Snoeck, D.; Dubruel, P.; de Belie, N. How to seal and heal cracks in cementitious materials by using superabsorbent polymers. In Proceedings of the Conference on Application of Superabsorbent Polymers and Other New Admixtures in Concrete Construction, Dresden, Germany, 14–17 September 2014; pp. 375–384.
- Yeong, C.J.L.; Lim, Y.Y.; Goy, Y. Evaluation of Superabsorbent Polymers in Diapers, 2014. SP LABRARY Website. Available online: http://dspace.lib.sp.edu.sg:80/xmlui/handle/get/5907 (accessed on 4 January 2017).
- Buchholz, F.L. Superabsorbent Polymers: An Idea Whose Time Has Come. J. Chem. Educ. 1996, 73, 512–515. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Silva, D.; Fernandes, A.C.; Nunes, T.G.; Colaço, R.; Serro, A.P. The effect of albumin and cholesterol on the biotribological behavior of hydrogels for contact lenses. Acta Biomater. 2015, 26, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.S.; Seo, Y.G.; Lee, B.-J.; Park, Y.J.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Jin, S.G.; Choi, H.-G. Novel sodium fusidate-loaded film-forming hydrogel with easy application and excellent wound healing. Int. J. Pharm. 2015, 495, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Hosseinzadeh, H. Synthesis of starch—Poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel with salt and pH-responsiveness properties as a drug delivery system. J. Bioact. Compat. Polym. 2008, 23, 381–404. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S. Synthesis and evaluation of pH and thermosensitive pectin-based superabsorbent hydrogel for oral drug delivery systems. Starch Stärke 2009, 61, 161–172. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Peppas, N.A. Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules 1999, 32, 6646–6651. [Google Scholar] [CrossRef]
- Cheung, S.-W.; Cho, P.; Chan, B.; Choy, C.; Ng, V. A comparative study of biweekly disposable contact lenses: Silicone hydrogel versus hydrogel. Clin. Exp. Optom. 2007, 90, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Novais, J.M.; Martins-Dias, S. Detoxification of olive mill wastewater using superabsorbent polymers. Environ. Technol. 2004, 25, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J. Cables Such as Optical Fiber Cables including Superabsorbent Polymeric Materials which Are Temperature and Salt Tolerant. U.S. Patent US5163115 A, 10 November 1992. [Google Scholar]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Vundavalli, R.; Vundavalli, S.; Nakka, M.; Rao, D.S. Biodegradable nano-hydrogels in agricultural farming-alternative source for water resources. Proc. Mater. Sci. 2015, 10, 548–554. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of cellulose-based superabsorbent hydrogels as water reservoir in agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Woodhouse, J.; Johnson, M.S. Effect of superabsorbent polymers on survival and growth of crop seedlings. Agric. Water Manag. 1991, 20, 63–70. [Google Scholar] [CrossRef]
- Mo, C.; Shu-Quan, Z.; Hua-Min, L.; Zhan-Bin, H.; Shu-Qin, L. Synthesis of poly(acrylic acid)/sodium humate superabsorbent composite for agricultural use. J. Appl. Polym. Sci. 2006, 102, 5137–5143. [Google Scholar] [CrossRef]
- Viktor, M.; Hans-Wolf, R. Application of Superabsorbent Polymers (SAP) in Concrete Construction; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Craeye, B.; Geirnaert, M.; Schutter, G.D. Super absorbing polymers as an internal curing agent for mitigation of early-age cracking of high-performance concrete bridge decks. Constr. Build. Mater. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Snoeck, D.; van Vlierberghe, S.; Verstraete, W.; de Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Dudziak, L.; Schulze, J. Internal Curing by Super Absorbent Polymers (SAP)—Effects on Material Properties of Self-Compacting Fibre-Reinforced High Performance Concrete; Jensen, O.M., Lura, P., Kovler, K., Eds.; RILEM Publications SARL: Reykjavik, Iceland, 2006; pp. 87–96. [Google Scholar]
- Mignon, A.; Snoeck, D.; D’Halluin, K.; Balcaen, L.; Vanhaecke, F.; Dubruel, P.; van Vlierberghe, S.; de Belie, N. Alginate biopolymers: Counteracting the impact of superabsorbent polymers on mortar strength. Constr. Build. Mater. 2016, 110, 169–174. [Google Scholar] [CrossRef]
- Snoeck, D.; de Belie, N. From straw in bricks to modern use of microfibres in cementitious composites for improved autogenous healing—A review. Constr. Build. Mater. 2015, 95, 774–787. [Google Scholar] [CrossRef]
- Snoeck, D.; Steuperaert, S.; van Tittelboom, K.; Dubruel, P.; de Belie, N. Visualization of water penetration in cementitious materials with superabsorbent polymers by means of neutron radiography. Cem. Concr. Res. 2012, 42, 1113–1121. [Google Scholar] [CrossRef]
- Sterile Hydrogel Dressings for Moist Wound Management. Available online: http://www.kikgel.com.pl/neoheal/ (accessed on 17 March 2014).
- Ferreira, P.; Coelho, J.F.J.; Almeida, J.F.; Gil, M.H. Photocrosslinkable polymers for biomedical applications. In Biomedical Engineering—Frontiers and Challenges; Fazel-Rezai, P.R., Ed.; INTECH: Vienna, Austria, 2011. [Google Scholar]
- Cotthem, W.V. TerraCottem: Water Saving Technique Allows Plants to Grow Anywhere (Ecofriend/Popsci). Available online: https://desertification.wordpress.com/2011/01/07/terracottem-water-saving-technique -allows-plants-to-grow-everywhere-ecofriend-popsci/ (accessed on 17 March 2014).
- Mohammad, J.; Zohuriaan-Mehr, K.K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Mahdavinia, G.R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M.J. Modified chitosan 4. Superabsorbent hydrogels from poly(acrylic acid-co-acrylamide) grafted chitosan with salt- and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite superabsorbent composites. Carbohydr. Polym. 2007, 68, 367–374. [Google Scholar] [CrossRef]
- Hua, S.; Wang, A. Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Rinaudo, M. Biomaterials based on a natural polysaccharide: Alginate. TIP 2014, 17, 92–96. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, K.J.; Ko, S.W. Preparation and properties of alginate superabsorbent filament fibers crosslinked with glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804. [Google Scholar] [CrossRef]
- Dutkiewicz, J.K. Superabsorbent materials from shellfish waste—A review. J. Biomed. Mater. Res. 2002, 63, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Farhadpour, B.; Seidi, F. Synthesis and investigation of swelling behavior of new agar based superabsorbent hydrogel as a candidate for agrochemical delivery. J. Polym. Res. 2009, 16, 655–665. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, L.; Liu, P.S.; Zhang, J.; Zhou, N.L.; Lu, S.; Wei, S.H.; Shen, J. The preparation and properties of dextrin-graft-acrylic acid/montmorillonite superabsorbent nanocomposite. Polym. Compos. 2009, 30, 976–981. [Google Scholar] [CrossRef]
- Nnadi, F.; Brave, C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J. Soil Sci. Environ. Manag. 2011, 2, 206–211. [Google Scholar]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Q.; Embree, H.D.; Balgley, B.M.; Smith, P.J.; Payne, G.F. Utilizing renewable resources to create functional polymers: Chitosan-based associative thickener. Environ. Sci. Technol. 2002, 36, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Mignon, A.; Devisscher, D.; Graulus, G.-J.; Stubbe, B.; Martins, J.; Dubruel, P.; de Belie, N.; van Vlierberghe, S. Combinatory approach of methacrylated alginate and acid monomers for concrete applications. Carbohydr. Polym. 2017, 155, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mignon, A.; Snoeck, D.; Wiktor, V.; Boon, N.; de Belie, N. Application of modified-alginate encapsulated carbonate producing bacteria in concrete: A promising strategy for crack self-healing. Front. Microbiol. 2015, 6, 1088. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, D. Self-Healing and Microstructure of Cementitious Materials with Microfibres and Superabsorbent Polymers; Ghent University: Ghent, Belgium, 2015. [Google Scholar]
- Farzanian, K.; Pimenta Teixeira, K.; Perdigão Rocha, I.; de Sa Carneiro, L.; Ghahremaninezhad, A. The mechanical strength, degree of hydration, and electrical resistivity of cement pastes modified with superabsorbent polymers. Constr. Build. Mater. 2016, 109, 156–165. [Google Scholar] [CrossRef]
- Colby, R.H.; Rubinstein, M. Polymer Physics; Oxford University: New York, NY, USA, 2003; pp. 274–281. [Google Scholar]
- Zhang, B.; Cui, Y.; Yin, G.; Li, X.; Liao, L.; Cai, X. Synthesis and swelling properties of protein-poly(acrylic acid-co-acrylamide) superabsorbent composite. Polym. Compos. 2011, 32, 683–691. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2013. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Phunchareon, P. Influence of reaction parameters on water absorption of neutralized poly(acrylic acid-co-acrylamide) synthesized by inverse suspension polymerization. J. Appl. Polym. Sci. 1999, 72, 1349–1366. [Google Scholar] [CrossRef]
- Katono, H.; Maruyama, A.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Thermo-responsive swelling and drug release switching of interpenetrating polymer networks composed of poly(acrylamide-co-butyl methacrylate) and poly(acrylic acid). J. Control. Release 1991, 16, 215–227. [Google Scholar] [CrossRef]
- Horkay, F.; Tasaki, I.; Basser, P.J. Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 2000, 1, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Siriwatwechakul, W.; Siramanont, J.; Vichit-Vadakan, W. Behavior of superabsorbent polymers in calcium-and sodium-rich solutions. J. Mater. Civ. Eng. 2012, 24, 976–980. [Google Scholar] [CrossRef]
- Zhu, Q.; Barney, C.W.; Erk, K.A. Effect of ionic crosslinking on the swelling and mechanical response of model superabsorbent polymer hydrogels for internally cured concrete. Mater. Struct. 2015, 48, 2261–2276. [Google Scholar] [CrossRef]
- Don, T.-M.; Huang, M.-L.; Chiu, A.-C.; Kuo, K.-H.; Chiu, W.-Y.; Chiu, L.-H. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater. Chem. Phys. 2008, 107, 266–273. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M.; Mahdavinia, R.G.; Hosseinzadeh, H. Synthesis and super-swelling behavior of a novel protein-based superabsorbent hydrogel. Polym. Bull. 2006, 57, 813–824. [Google Scholar] [CrossRef]
- Dimonie, M.V.; Boghina, C.M.; Marinescu, N.N.; Marinescu, M.M.; Cincu, C.I.; Oprescu, C.G. Inverse suspension polymerization of acrylamide. Eur. Polym. J. 1982, 18, 639–645. [Google Scholar] [CrossRef]
- Pang, X.; Cheng, G.; Li, R.; Lu, S.; Zhang, Y. Bovine serum albumin-imprinted polyacrylamide gel beads prepared via inverse-phase seed suspension polymerization. Anal. Chim. Acta 2005, 550, 13–17. [Google Scholar] [CrossRef]
- Zhou, X.; Weng, L.; Chen, Q.; Zhang, J.; Shen, D.; Li, Z.; Shao, M.; Xu, J. Investigation of pH sensitivity of poly(acrylic acid-co-acrylamide) hydrogel. Polym. Int. 2003, 52, 1153–1157. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Phillips, G.O.; Al-Assaf, S. Hydrogels: Methods of Preparation, Characterisation and Applications; INTECH: Rijeka, Croatia, 2011. [Google Scholar]
- Kim, J.J.; Park, K. Smart hydrogels for bioseparation. Bioseparation 1998, 7, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Yong Qiu, K.P. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar]
- Best, J.P.; Neubauer, M.P.; Javed, S.; Dam, H.H.; Fery, A.; Caruso, F. Mechanics of pH-responsive hydrogel capsules. Langmuir 2013, 29, 9814–9823. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.R.; Peppas, N.A. Release behavior of bioactive agents from pH-sensitive hydrogels. J. Biomater. Sci. Polym. Ed. 1993, 4, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.F.S.; Cussler, E.L. Temperature sensitive gels as extraction solvents. Chem. Eng. Sci 1987, 42, 97–103. [Google Scholar] [CrossRef]
- Bromberg, L.E.; Ron, E.S. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug Deliv. Rev. 1998, 31, 197–221. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Molecular separation by thermosensitive hydrogel membranes. J. Membr. Sci. 1991, 64, 283–294. [Google Scholar] [CrossRef]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion–contraction of photoresponsive artificial muscle regulated by host–guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Tanaka, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Lee, K.K.; Cussler, E.L.; Marchetti, M.; McHugh, M.A. Pressure-dependent phase transitions in hydrogels. Chem. Eng. Sci 1990, 45, 766–767. [Google Scholar] [CrossRef]
- Mahkam, M. Novel pH-sensitive hydrogels for colon-specific drug delivery. Drug Deliv. 2010, 17, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Rana, V.; Rai, P.; Tiwary, A.K.; Singh, R.S.; Kennedy, J.F.; Knill, C.J. Modified gums: Approaches and applications in drug delivery. Carbohydr. Polym. 2011, 83, 1031–1047. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Murthy, P.S.; Sreeramulu, J.; Raju, K.M. Swelling behavior of semi-interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and poly(acrylamide-co-sodium methacrylate). J. Appl. Polym. Sci. 2005, 98, 302–314. [Google Scholar] [CrossRef]
- Bentz, D.P.; Weiss, W.J. Internal Curing: A 2010 State-of-the-Art Review; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011; p. 82.
- Mechtcherine, V.; Gorges, M.; Schröfl, C.; Assmann, A.; Brameshuber, W.; Bettencourt Ribeiro, V.; Cusson, D.; Custódio, J.; Fonseca da Silva, E.; Ichimiya, K.; et al. Effect of internal curing by using superabsorbent polymers (SAP) on autogenous shrinkage and other properties of a high-performance fine-grained concrete: Results of a RILEM round-robin test, TC 225-SAP. Mater. Struct. 2014, 47, 541–562. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Dudziak, L.; Hempel, S. Mitigating early age shrinkage of ultra-high performance concrete by using super absorbent polymers (SAP). In Proceedings of the Conference on Creep, Shrinkage and Durability Mechanics of Concrete and Concrete Structures, Ise-Shima, Japan, 30 September–2 October 2008; Sato, R., Maekawa, K., Tanabe, T., Sakata, K., Nakamura, H., Mihashi, H., Eds.; Taylor & Francis: Ise-Shima, Japan, 2009; pp. 847–853. [Google Scholar]
- Craeye, B.; De Schutter, S. Experimental evaluation of mitigation of autogenous shrinkage by means of a vertical dilatometer for concrete. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, Lyngby, Denmark, 20–23 August 2006; Jensen, O.M., Lura, P., Kovler, K., Eds.; RILEM Publications SARL: Lyngby, Denmark, 2006; pp. 21–30. [Google Scholar]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Igarashi, S.; Watanabe, A. Experimental study on prevention of autogenous deformation by internal curing using super-absorbent polymer particles. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, Lyngby, Denmark, 20–23 August 2006; Jensen, O.M., Lura, P., Kovler, K., Eds.; RILEM Publications SARL: Lyngby, Denmark, 2006; pp. 77–86. [Google Scholar]
- Assmann, A. Physical Properties of Concrete Modified with Superabsorbent Polymers; Stuttgart University: Stuttgart, Germany, 2013. [Google Scholar]
- Van Tittelboom, K.; de Belie, N. Self-healing in cementitious materials—A review. Materials 2013, 6, 2182–2217. [Google Scholar] [CrossRef]
- Schröfl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- Ferraris, C.F. Measurement of the rheological properties of high performance concrete: State of the art report. J. Res. Natl. Inst. Stand. Technol. 1999, 104, 461. [Google Scholar] [CrossRef]
- Snoeck, D.; de Belie, N. Effect of superabsorbent polymers, superplasticizer and additional water on the setting of cementitious materials. Int. J. 3R’s 2015, 5, 721–729. [Google Scholar]
- Secrieru, E.; Mechtcherine, V.; Schröfl, C.; Borin, D. Rheological characterisation and prediction of pumpability of strain-hardening cement-based-composites (SHCC) with and without addition of superabsorbent polymers (SAP) at various temperatures. Constr. Build. Mater. 2016, 112, 581–594. [Google Scholar] [CrossRef]
- Toledo Filho, R.D.; Silva, E.F.; Lopes, A.N.M.; Mechtcherine, V.; Dudziak, L. Effect of superabsorbent polymers on the workability of concrete and mortar. In Application of Super Absorbent Polymers (SAP) in Concrete Construction; Springer: New York, NY, USA, 2012; pp. 39–50. [Google Scholar]
- Paiva, H.; Esteves, L.P.; Cachim, P.B.; Ferreira, V.M. Rheology and hardened properties of single-coat render mortars with different types of water retaining agents. Constr. Build. Mater. 2009, 23, 1141–1146. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Secrieru, E.; Schröfl, C. Effect of superabsorbent polymers (SAPs) on rheological properties of fresh cement-based mortars—Development of yield stress and plastic viscosity over time. Cem. Concr. Res. 2015, 67, 52–65. [Google Scholar] [CrossRef]
- Powers, T.C.; Brownyard, T.L. Studies of the Physical Properties of Hardened Portland Cement Paste; Portland Cement Association, Research Laboratories: Cornell, NY, USA, 1948; p. 892. [Google Scholar]
- Wyrzykowski, M.; Lura, P. In Reduction of autogenous shrinkage in OPC and BFSC pastes with internal curing. In Proceedings of the XIII International Conference on Durability of Building Materials and Components, São Paulo, Brazil, 2–5 September 2014; pp. 1010–1017.
- Almeida, F.D.C.R.; Klemm, A.J. Effect of superabsorbent polymers (SAP) on fresh state mortars with ground granulated blast-furnace slag (GGBS). In Proceedings of the 5th International Conference on Durability of Concrete Structures, Shenzhen, China, 30 June 2016; pp. 1–7.
- Lura, P.; Ye, G.; Cnudde, V.; Jacobs, P. Preliminary results about 3D distribution of superabsorbent polymers in mortars. In Proceedings of the International Conference on Microstructure Related Durability of Cementitious Composites, Nanjing, China, 13–15 October 2008; pp. 1341–1348.
- Mönnig, S. Water saturated super-absorbent polymers used in high strength concrete. Otto-Graf-J. 2005, 16, 193–202. [Google Scholar]
- Reinhardt, H.W.; Assmann, A. Enhanced durability of concrete by superabsorbent polymers. In Proceedings of the International Symposium Brittle Matrix Composites 9, Warsaw, Poland, 25–28 October 2009; Brandt, A.M., Olek, J., Marshal, I.H., Eds.; Woodhead Publishing: Warsaw, Poland, 2009; pp. 291–300. [Google Scholar]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; van Vlierberghe, S.; Dubruel, P.; Lodewyckx, P.; de Belie, N. The influence of different drying techniques on the water sorption properties of cement-based materials. Cem. Concr. Res. 2014, 64, 54–62. [Google Scholar] [CrossRef]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; van Vlierberghe, S.; Dubruel, P.; Lodewyckx, P.; de Belie, N. The effects of superabsorbent polymers on the microstructure of cementitious materials studied by means of sorption experiments. Cem. Concr. Res. 2015, 77, 26–35. [Google Scholar] [CrossRef]
- Lura, P.; Durand, F.; Loukili, A.; Kovler, K.; Jensen, O.M. Compressive strength of cement pastes and mortars with superabsorbent polymers. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, Lyngby, Denmark, 20–23 August 2006; Jensen, O.M., Lura, P., Kovler, K., Eds.; RILEM Publications SARL: Lyngby, Denmark, 2006; pp. 117–125. [Google Scholar]
- Klemm, A.J.; Sikora, K.S. The effect of superabsorbent polymers (SAP) on microstructure and mechanical properties of fly ash cementitious mortars. Constr. Build. Mater. 2013, 49, 134–143. [Google Scholar] [CrossRef]
- Snoeck, D.; Schaubroeck, D.; Dubruel, P.; de Belie, N. Effect of high amounts of superabsorbent polymers and additional water on the workability, microstructure and strength of mortars with a water-to-cement ratio of 0.50. Constr. Build. Mater. 2014, 72, 148–157. [Google Scholar] [CrossRef]
- Dudziak, L.; Mechtcherine, V. Enhancing early-age resistance to cracking in high-strength cement based materials by means of internal curing using super absorbent polymers. In Proceedings of the International RILEM Conference on Material Science, Aachen, Germany, 6–8 September 2010; Brameshuber, W., Ed.; RILEM Publications SARL: Aachen, Germany, 2010; pp. 129–139. [Google Scholar]
- Lura, P.; Jensen, O.M.; Igarashi, S. Experimental observation of internal curing of concrete. Mater. Struct. 2007, 40, 211–220. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jespersen, M.H.S.; Jensen, O.M. Mechanical properties of concrete with SAP part I: Development of compressive strength. In Proceedings of the International RILEM Conference on Use of Superabsorbent Polymers and Other New Additives in Concrete, Lyngby, Denmark, 15–18 August 2010; Jensen, O.M., Hasholt, M.T., Laustsen, S., Eds.; RILEM Publications SARL: Lyngby, Denmark, 2010; pp. 117–126. [Google Scholar]
- Laustsen, S.; Hasholt, M.T.; Jensen, O.M. Void structure of concrete with superabsorbent polymers and its relation to frost resistance of concrete. Mater. Struct. 2015, 48, 357–368. [Google Scholar] [CrossRef]
- Mönnig, S.; Lura, P. Superabsorbent polymers—An additive to increase the freeze-thaw resistance of high strength concrete. In Advances in Construction Materials 2007; Springer: New York, NY, USA, 2007; pp. 351–358. [Google Scholar]
- Jones, W.A.; Weiss, W.J. Freeze thaw durability of internally cured concrete made using superabsorbent polymers. In Proceedings of the 4th International Conference on the Durability of Concrete Structure, West Lafayette, IN, USA, 24–26 July 2014.
- Mechtcherine, V.; Schröfl, C.; Wyrzykowski, M.; Gorges, M.; Cusson, D.; Margeson, J.; de Belie, N.; Snoeck, D.; Ichimiya, K.; Igarashi, S.-I.; et al. Effect of superabsorbent polymers (SAP) on the freeze-thaw resistance of concrete: Results of a RILEM interlaboratory test. Mater. Struct. 2017, 50, 1–19. [Google Scholar] [CrossRef]
- Lura, P.; Ye, G.; Cnudde, V.; Jacobs, P. Preliminary results about 3D distribution of superabsorbent polymers in mortars. In International Conference on Microstructure Related Durability of Cementitious Composites; Sun, W., van Breugel, K., Miao, C., Ye, G., Chen, H., Eds.; RILEM Publications SARL: Reykjavik, Iceland, 2008; p. 8. [Google Scholar]
- Snoeck, D.; van Tittelboom, K.; de Belie, N.; Steuperaert, S.; Dubruel, P. The use of superabsorbent polymers as a crack sealing and crack healing mechanism in cementitious materials. In Proceedings of the 3rd International Conference on Concrete Repair, Rehabilitation and Retrofitting, Cape Town, South Afrcia, 3–5 September 2012; pp. 152–157.
- Van Tittelboom, K.; Wang, J.; Araújo, M.; Snoeck, D.; Gruyaert, E.; Debbaut, B.; Derluyn, H.; Cnudde, V.; Tsangouri, E.; van Hemelrijck, D. Comparison of different approaches for self-healing concrete in a large-scale lab test. Constr. Build. Mater. 2016, 107, 125–137. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Potential of superabsorbent polymer for self-sealing cracks in concrete. Adv. Appl. Ceram. 2010, 109, 296–302. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Self-sealing cement-based materials using superabsorbent polymers. In Proceedings of the International RILEM Conference on Use of Superabsorbent Polymers and Other New Additives in Concrete, Lyngby, Denmark, 15–18 August 2010; Jensen, O.M., Hasholt, M.T., Laustsen, S., Eds.; RILEM Publications SARL: Lyngby, Denmark, 2010; pp. 171–178. [Google Scholar]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef]
- Snoeck, D.; Dubruel, P.; De Belie, N. Superabsorbent polymers to prevent water movement in cementitious materials. Int. J. 3R’s 2012, 3, 432–440. [Google Scholar]
- Song, X.F.; Wei, J.F.; He, T.S. A method to repair concrete leakage through cracks by synthesizing super-absorbent resin in situ. Constr. Build. Mater. 2009, 23, 386–391. [Google Scholar] [CrossRef]
- Cailleux, E.; Pollet, V. Investigations on the development of self-healing properties in protective coatings for concrete and repair mortars. In Proceedings of the 2nd International Conference on Self Healing Materials, Chicago, IL, USA, 28 June–1 July 2009.
- Joseph, C.; Jefferson, A.D.; Cantoni, M.B. Issues relating to the autonomic healing of cementitious materials. In Proceedings of the 1st International Conference on Self-Healing Materials, Noordwijk, The Netherlands, 13 April 2007.
- Sanjay, P.; Kshitij, C.S.; Yusuke, S.; Toshihiro, O.; Ryosuke, K.; Yoshikazu, A. Feasibility of externally activated self-repairing concrete with epoxy injection network and Cu-Al-Mn superelastic alloy reinforcing bars. Smart Mater. Struct. 2014, 23, 105027. [Google Scholar]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Ryu, J.S. An experimental study on the repair of concrete crack by electrochemical technique. Mater. Struct. 2001, 34, 433–437. [Google Scholar] [CrossRef]
- Allen, R.T.L.; Edwards, S.C.; Shaw, D.N. Repair of Concrete Structures; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Broomfield, J.P. Corrosion of Steel in Concrete: Understanding, Investigation and Repair; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. Mater. J. 1999, 96, 448–454. [Google Scholar]
- Neville, A. Autogenous healing—A concrete miracle? Concr. Int. 2002, 24, 76–82. [Google Scholar]
- Hearn, N. Self-sealing, autogenous healing and continued hydration: What is the difference? Mater. Struct. 1998, 31, 563–567. [Google Scholar] [CrossRef]
- Yang, Y.; Lepech, M.D.; Yang, E.-H.; Li, V.C. Autogenous healing of engineered cementitious composites under wet–dry cycles. Cem. Concr. Res. 2009, 39, 382–390. [Google Scholar] [CrossRef]
- Ter Heide, N. Crack Healing in Hydrating Concrete; Delft University of Technology: Delft, The Netherlands, 2005. [Google Scholar]
- Joseph, C.; Gardner, D.; Jefferson, T.; Isaacs, B.; Lark, B. Self-healing cementitious materials: A review of recent work. Constr. Mater. 2011, 164, 29–41. [Google Scholar] [CrossRef]
- Talaiekhozan, A.; Keyvanfar, A.; Shafaghat, A.; Andalib, R.; Abd Majid, M.Z.; Fulazzaky, M.A.; Zin, R.M.; Lee, C.T.; Hussin, M.W.; Hamzah, N.; et al. A review of self-healing concrete research development. J. Environ. Treat. Tech. 2014, 2, 1–11. [Google Scholar]
- Yang, Y.; Yang, E.-H.; Li, V.C. Autogenous healing of engineered cementitious composites at early age. Cem. Concr. Res. 2011, 41, 176–183. [Google Scholar] [CrossRef]
- Li, V.C. Engineered cementitious composites (ECC)—Material, structural, and durability performance. In Concrete Construction Engineering Handbook; Nawy, E., Ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 78. [Google Scholar]
- Li, V.C.; Wang, S.; Wu, C. Tensile strain-hardening behavior of polyvinyl alcohol engineered cementitious composites (PVA-ECC). ACI Mater. J. 1997, 98, 483–492. [Google Scholar]
- Snoeck, D.; de Belie, N. Repeated autogenous healing in strain-hardening cementitious composites by using superabsorbent polymers. J. Mater. Civ. Eng. 2015, 28. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; de Belie, N. Autogenous healing of cracks in cementitious materials with varying mix compositions. In Proceedings of the 2nd International conference on Self-Healing Materials (ICSHM 2009), Chicago, IL, USA, 28 June–1 July 2009.
- Van Tittelboom, K. Self-Healing Concrete through Incorporation of Encapsulated Bacteria-or Polymer-Based Healing Agents. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2012. [Google Scholar]
- Jacobsen, S.; Marchand, J.; Hornain, H. SEM observations of the microstructure of frost deteriorated and self-healed concrete. Cem. Concr. Res. 1995, 25, 55–62. [Google Scholar] [CrossRef]
- Snoeck, D.; de Belie, N. Mechanical and self-healing properties of cementitious composites reinforced with flax and cottonised flax, and compared with polyvinyl alcohol fibres. Biosyst. Eng. 2012, 111, 325–335. [Google Scholar] [CrossRef]
- Snoeck, D.; Smetryns, P.-A.; de Belie, N. Improved multiple cracking and autogenous healing in cementitious materials by means of chemically-treated natural fibres. Biosyst. Eng. 2015, 139, 87–99. [Google Scholar] [CrossRef]
- Yang, E.-H. Designing Added Functions in Engineered Cementitious Composites; University of Michigan: Ann Arbor, MI, USA, 2008. [Google Scholar]
- Reinhardt, H.W.; Jooss, M. Permeability and self-healing of cracked concrete as a function of temperature and crack width. Cem. Concr. Res. 2003, 33, 981–985. [Google Scholar] [CrossRef]
- Aldea, C.; Wong, W.; Popovics, J.S.; Shah, S.P. Extent of healing of cracked normal strength concrete. Mater. Civ. Eng. 2000, 12, 92–96. [Google Scholar] [CrossRef]
- Clear, C.A. The Effects of Autogenous Healing upon the Leakage of Water through Cracks in Concrete; Cement and Concrete Association: Buckinghamshire, UK, 1985; p. 31. [Google Scholar]
- Li, V.C. On engineered cementitious composites (ECC). J. Adv. Concr. Technol. 2003, 1, 215–230. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Yamashiro, Y.; Saito, T. Self-healing ability of fly ash–cement systems. Cem. Concr. Compos. 2009, 31, 195–203. [Google Scholar] [CrossRef]
- Qian, S.Z.; Zhou, J.; Schlangen, E. Influence of curing condition and precracking time on the self-healing behavior of engineered cementitious composites. Cem. Concr. Compos. 2010, 32, 686–693. [Google Scholar] [CrossRef]
- Qian, S.Z.; Zhou, J.; de Rooij, M.R.; Schlangen, E.; Ye, G.; van Breugel, K. Self-healing behavior of strain hardening cementitious composites incorporating local waste materials. Cem. Concr. Compos. 2009, 31, 613–621. [Google Scholar] [CrossRef]
- Kan, L.; Shi, H. Investigation of self-healing behavior of engineered cementitious composites (ECC) materials. Constr. Build. Mater. 2012, 29, 348–356. [Google Scholar] [CrossRef]
- Lauer, K.R.; Slate, F.O. Autogenous healing of cement paste. ACI Mater. J. 1956, 52, 1083–1097. [Google Scholar]
- Janssen, D. Water Encapsulation to Initiate Self-Healing in Cementitious Materials. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2011. [Google Scholar]
- Akcay, B.; Tasdemir, M.A. Effects of lightweight aggregates on autogenous deformation in concrete. In Measuring, Monitoring and Modeling Concrete Properties; Springer: New York, NY, USA, 2006; pp. 163–170. [Google Scholar]
- Bentz, D.P.; Snyder, K.A. Protected paste volume in concrete: Extension to internal curing using saturated lightweight fine aggregate. Cem. Concr. Res. 1999, 29, 1863–1867. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Yang, Y. Incorporation of SAP particles as controlling pre-existing flaws to improve the performance of ECC. Constr. Build. Mater. 2011, 28, 139–145. [Google Scholar] [CrossRef]
- Kim, J.S.; Schlangen, E. Super absorbent polymers to simulate self healing in ECC. In 2nd International Symposium on Service Life Design for Infrastructures; van Breugel, K., Ye, G., Yuan, Y., Eds.; RILEM Publications SARL: Delft, The Netherlands, 2010; pp. 849–858. [Google Scholar]
- Snoeck, D.; Dewanckele, J.; Cnudde, V.; de Belie, N. X-ray computed microtomography to study autogenous healing of cementitious materials promoted by superabsorbent polymers. Cem. Concr. Compos. 2016, 65, 83–93. [Google Scholar] [CrossRef]
- Fan, S.; Li, M. X-ray computed microtomography of three-dimensional microcracks and self-healing in engineered cementitious composites. Smart Mater. Struct. 2015, 24, 015021. [Google Scholar] [CrossRef]
- Kong, X.-M.; Zhang, Z.-L.; Lu, Z.-C. Effect of pre-soaked superabsorbent polymer on shrinkage of high-strength concrete. Mater. Struct. 2015, 48, 2741–2758. [Google Scholar] [CrossRef]
- AzariJafari, H.; Kazemian, A.; Rahimi, M.; Yahia, A. Effects of pre-soaked super absorbent polymers on fresh and hardened properties of self-consolidating lightweight concrete. Constr. Build. Mater. 2016, 113, 215–220. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jensen, O.M.; Kovler, K.; Zhutovsky, S. Can superabsorbent polymers mitigate autogenous shrinkage of internally cured concrete without compromising the strength? Constr. Build. Mater. 2012, 31, 226–230. [Google Scholar] [CrossRef]
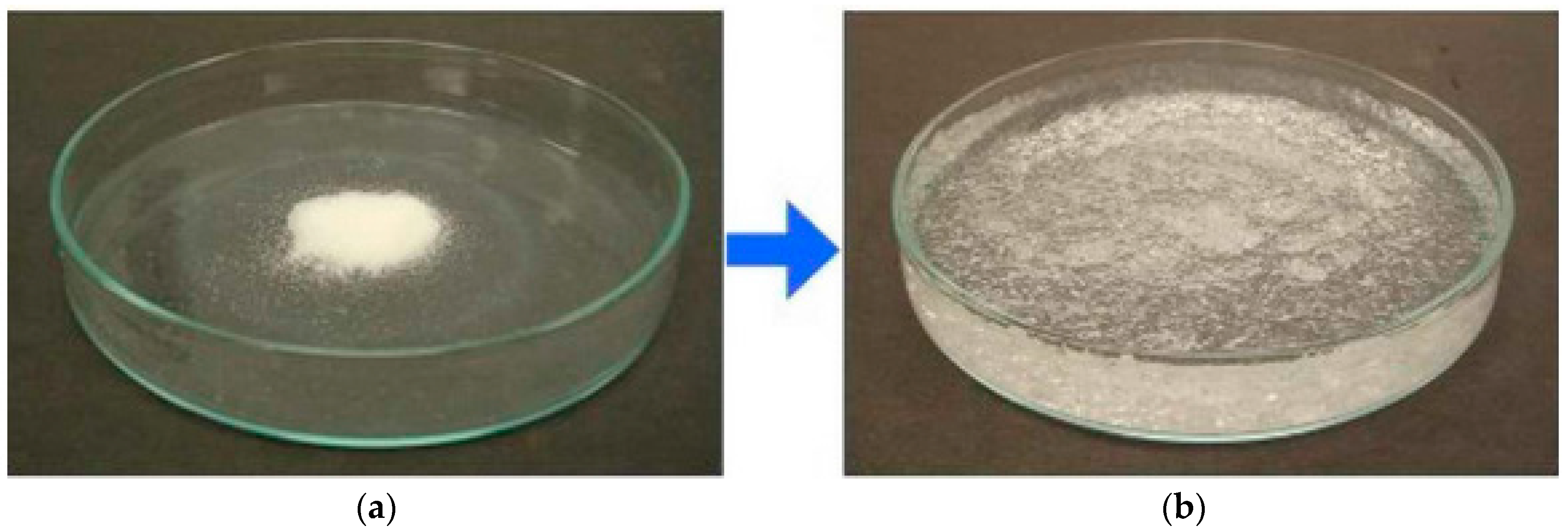
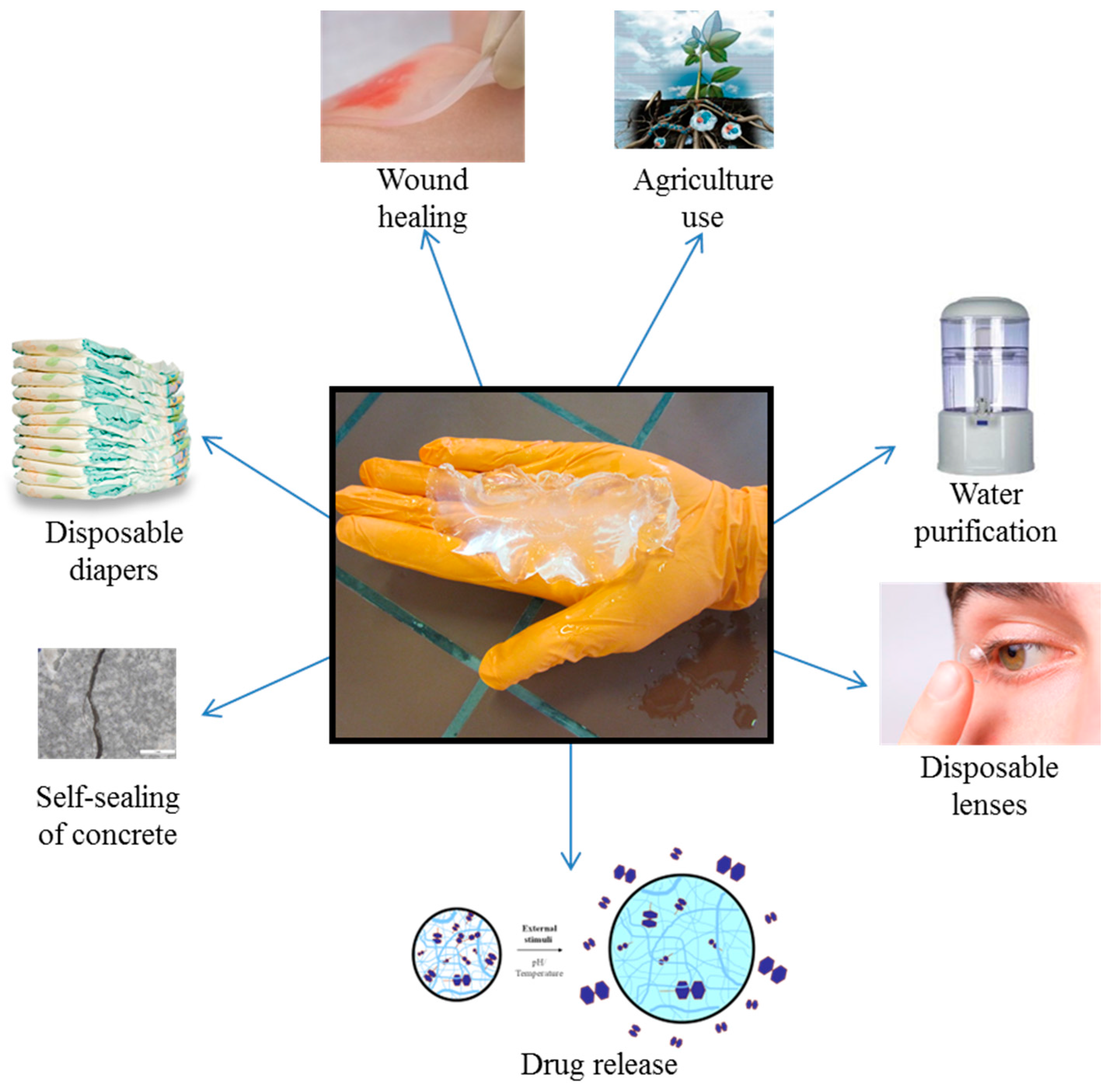
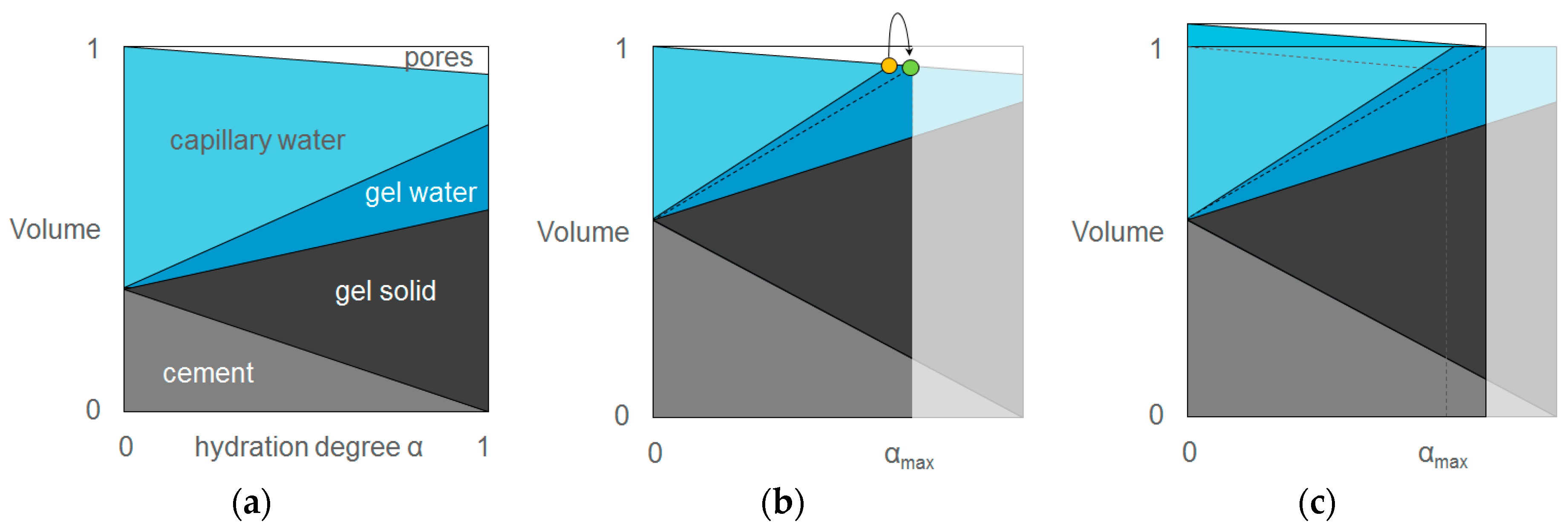
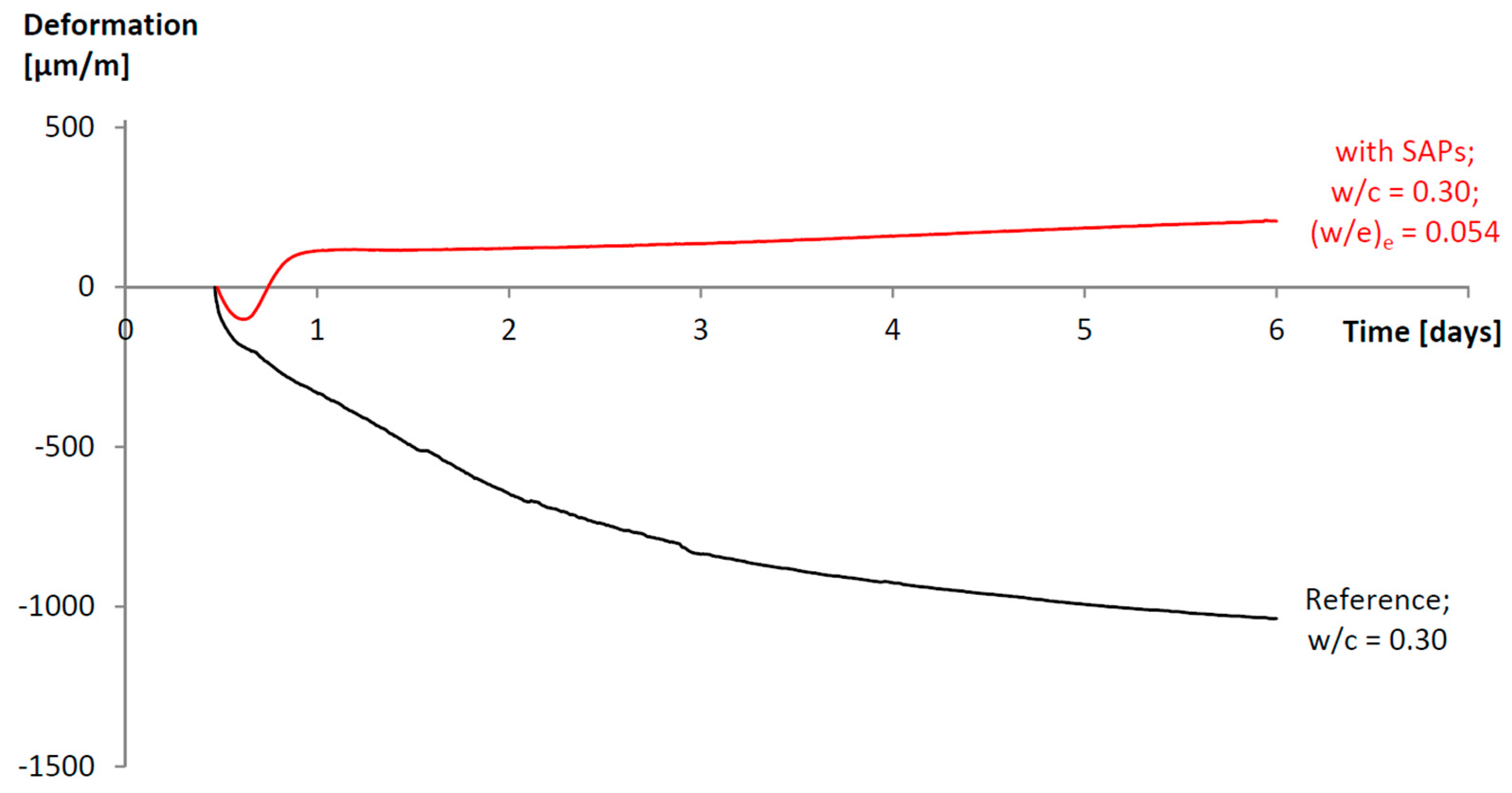

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignon, A.; Snoeck, D.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success? Materials 2017, 10, 237. https://doi.org/10.3390/ma10030237
Mignon A, Snoeck D, Dubruel P, Van Vlierberghe S, De Belie N. Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success? Materials. 2017; 10(3):237. https://doi.org/10.3390/ma10030237
Chicago/Turabian StyleMignon, Arn, Didier Snoeck, Peter Dubruel, Sandra Van Vlierberghe, and Nele De Belie. 2017. "Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success?" Materials 10, no. 3: 237. https://doi.org/10.3390/ma10030237
APA StyleMignon, A., Snoeck, D., Dubruel, P., Van Vlierberghe, S., & De Belie, N. (2017). Crack Mitigation in Concrete: Superabsorbent Polymers as Key to Success? Materials, 10(3), 237. https://doi.org/10.3390/ma10030237








