Lighting the Way to See Inside Two-Photon Absorption Materials: Structure–Property Relationship and Biological Imaging
Abstract
:1. Introduction
2. Measurement Methods
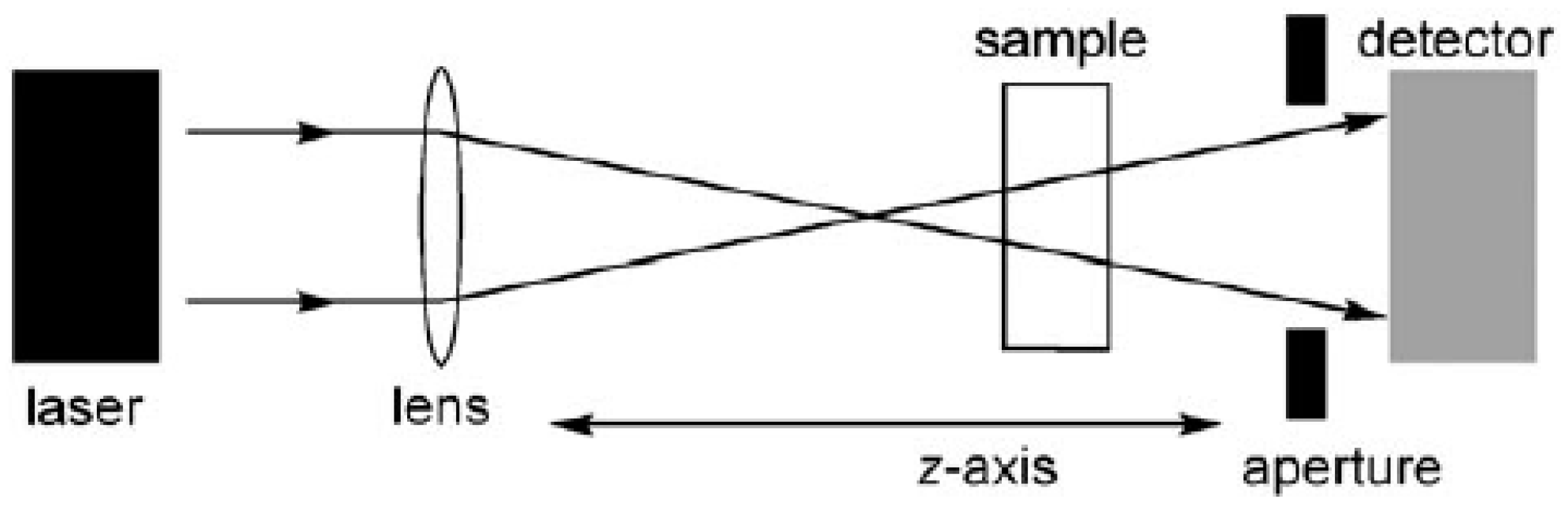
2.1. The Z-Scan Technique
- (1)
- Light can be lost due to self-defocusing (if the aperture of the detector is too narrow or too far from the sample) or because of nonlinear scattering; this results in extra contributions to the apparent nonlinear absorption.
- (2)
- A built up of excited-state populations (by either one-photon or two-photon absorption) can lead to nonlinear transmission through excited-state absorption (ESA). The contribution from ESA can be reduced by the use of wavelengths where there is negligible 1PA, very short laser pulses (<1 ps), and low repetition rates; a repetition rate of less than 1 kHz may be needed to allow excited triplet states to fully decay between pulses.
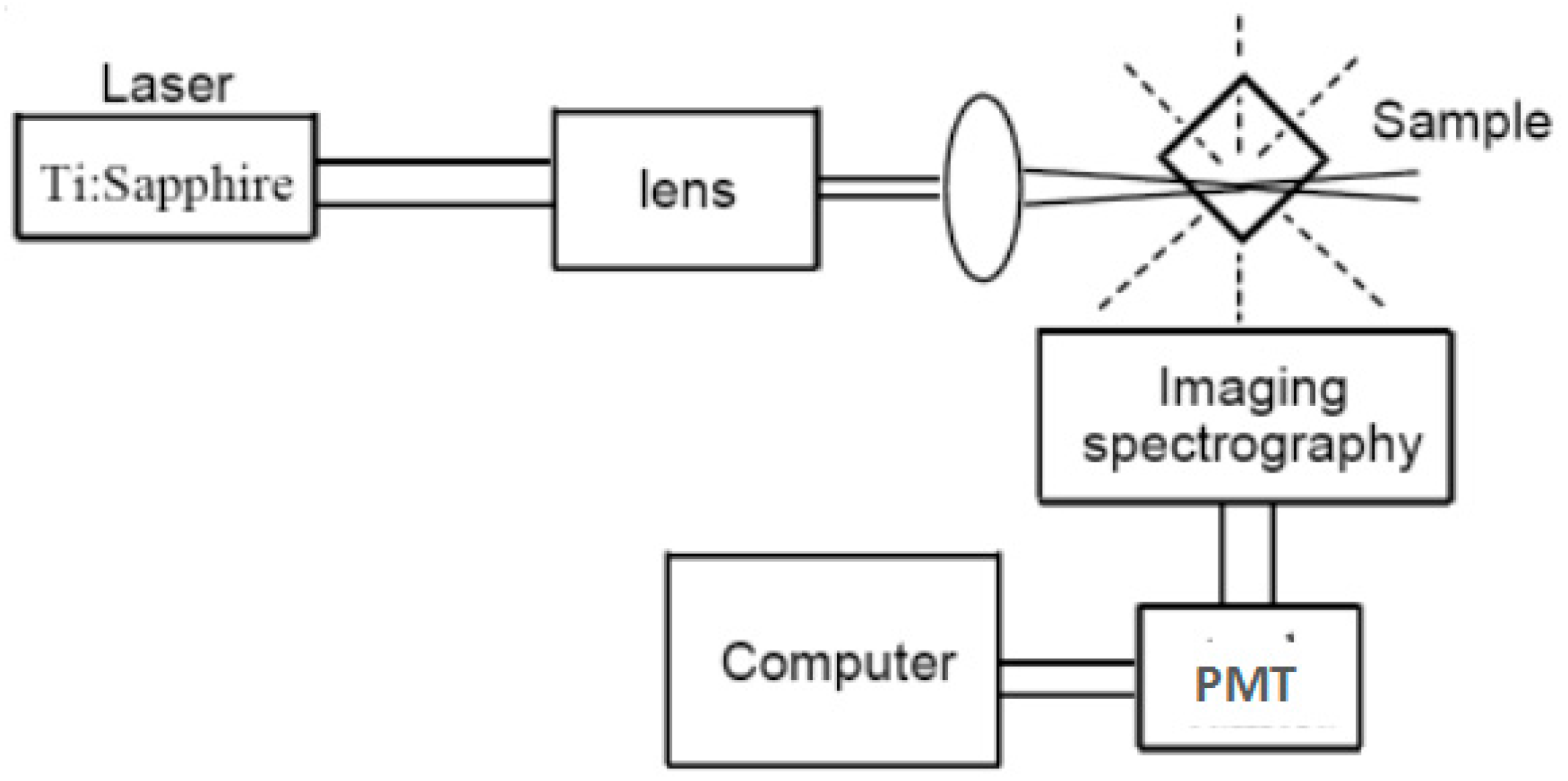
2.2. Two-Photon Excited Fluorescence Method (2PEF)
3. Design Strategies, Structure-Property Relationships, and Biological Applications
3.1. Organic 2PA Fluorophores
3.1.1. Pyridinium Derivatives
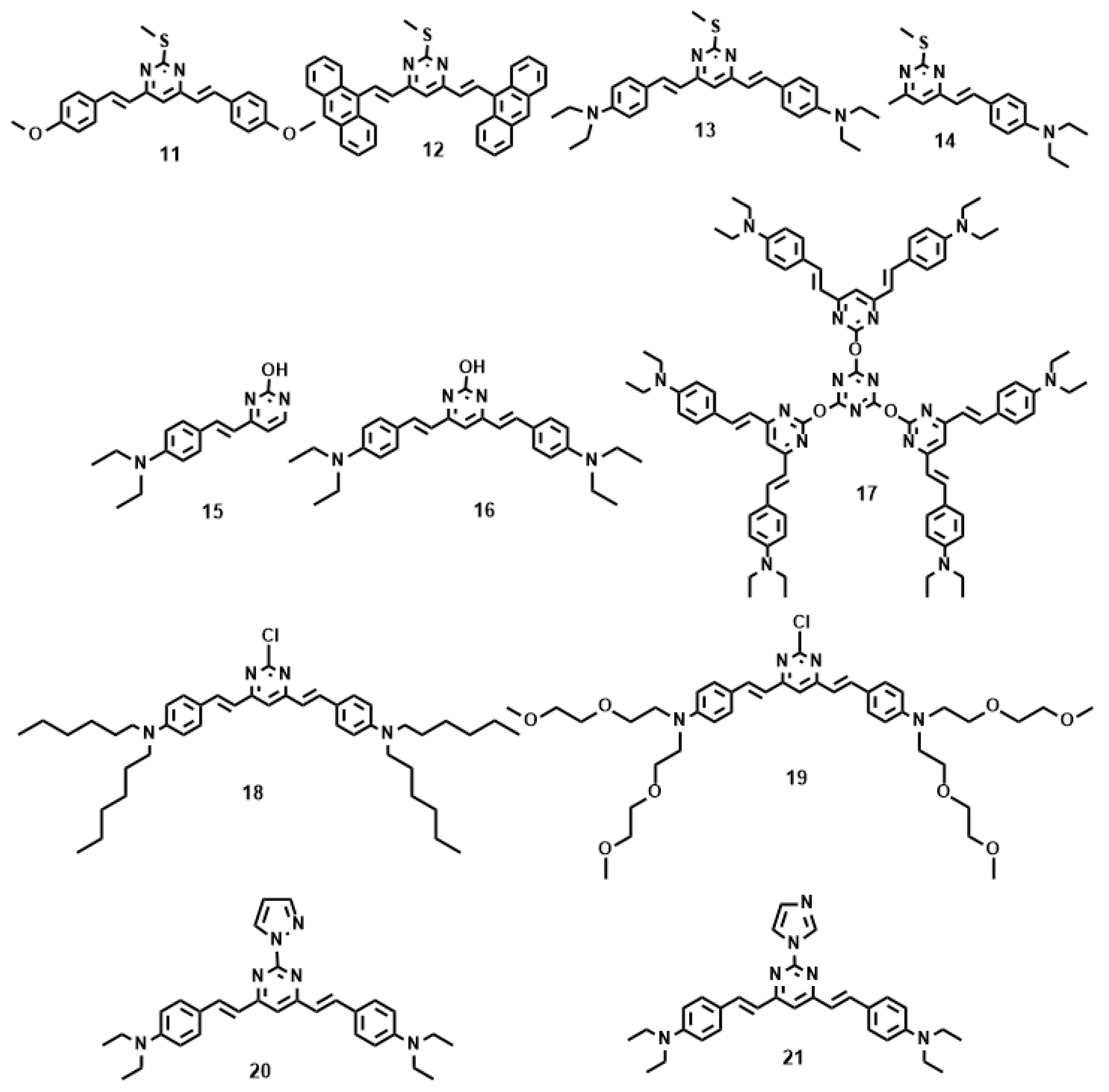
3.1.2. Pyrimidine Derivatives
3.1.3. Triphenylamine Derivatives
3.2. Organic-Inorganic Nanohybrids
3.3. Metal Complexes
3.3.1. Lanthanide-Diketonates
3.3.2. Transition Metal Complexes
Precious Metal Complexes
Transition Metal Complexes Based on Terpyridine Ligands
Transition Metal Complexes Based on Bis-β-Diketonate
3.3.3. Clusters
4. Conclusions and Outlook
- (1)
- For sensing, diagnostic or therapeutic applications, it is very important to realize the rapid and efficient cellular uptake of 2PA materials by optimizing their overall charge, size, hydrophobicity, and conjugated moiety.
- (2)
- Although a series of 2PA materials have been developed to stain different cellular organisms, their compartmentalization staining is usually not specific. Therefore, selective compartmentalization staining is still a challenge.
- (3)
- Compared with rapid developments in the synthesis of 2PA materials as staining dyes, discussions on the internalization mechanisms of phosphorescent complexes are still rare.
- (4)
- Compared with two-photon rare earth complexes, the long lifetime of phosphorescence can allow the possibility of two-photon time-resolved emission imaging. As a result, we have the emissive signals of the two-photon rare earth complexes using two-photon time-resolved emission imaging microscopy. However, the examples of two-photon lifetime-based imaging are very few. This will be another important research direction.
- (5)
- Compared with the rapid development of two-photon bio-imaging based on 2PA materials, only a few complexes have been used in multi-model bio-imaging, such as, Magnetic Resonance Imaging (MRI) combined with two-photon fluorescence microscopy (TPFM). Therefore, realizing multi-model imaging will be an important research topic in this field.
Acknowledgments
Conflicts of Interest
References
- Goppert-Mayer, M. Two-quantum processes. Ann. Phys. 1931, 9, 273–294. [Google Scholar]
- Maiman, T. NPG library. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Kaiser, W.; Garrett, C.G.B. Two-photon excitation in CaF2:Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- So, B.K.; Lee, K.S.; Lee, S.M.; Lee, M.K.; Lim, T.K. Synthesis and linear/nonlinear optical properties of new polyamides with DANS chromophore and silphenylene groups. Opt. Mater. 2003, 21, 87–92. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-molecule two-photon probes for bio-imaging applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Lee, K.T.; Suh, Y.D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.F.; Guo, L.; Li, Y.H.; Shuang, S.M.; Dong, C.; Wong, M.S. Highly selective two-photon fluorescent for ratiometric sensing and imaging cysteine in mitochondria. Anal. Chem. 2016, 88, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, F.; Huang, H.; Li, L.; Zhu, J.J. Nanomaterial-based activatable imaging probes: From design to biological applications. Chem. Soc. Rev. 2015, 44, 7855–7880. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cai, Y.B.; Jing, J.; Zhang, J.L. Unravelling the correlation between metal induced aggregation and cellular uptake/subcellular localization of Znsalen: An overlooked rule for design of luminescent metal probes. Chem. Sci. 2015, 6, 2389–2397. [Google Scholar] [CrossRef]
- Ma, D.L.; He, H.Z.; Leung, K.H.; Chan, D.S.H.; Leung, C.H. Bioactive Luminescent Transition-Metal Complexes for Biomedical Applications. Angew. Chem. Int. Ed. 2013, 52, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Li, L.; Yao, S.Q. Two-Photon Small Molecule Enzymatic Probes. Acc. Chem. Res. 2016, 49, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, L.; Agrawalla, B.K.; Park, S.J.; Zhu, H.; Sivaraman, B.; Chang, Y.T. Development of targetable two-photon fluorescent probes to image hypochlorous acid in mitochondria and lysosome in live cell and inflamed mouse model. J. Am. Chem. Soc. 2015, 137, 5930–5938. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, S.; Zhang, Z.; Gutmann, M.; Lühmann, T.; Edkins, R.M.; Clermont, G.; Blanchard-Desce, M. Water-soluble triarylborane chromophores for one-and two-photon excited fluorescence imaging of mitochondria in cells. Chem. Eur. J. 2016, 22, 14701–14706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFratta, C.N.; Fourkas, J.T.; Baldacchini, T.; Farrer, R.A. Mehrphotonen-Mikrofabrikation. Angew. Chem. Int. Ed. 2007, 119, 6352–6374. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Fourkas, J.T.; Baldacchini, T.; Farrer, R.A. Multiphoton fabrication. Angew. Chem. Int. Ed. 2007, 46, 6238–6258. [Google Scholar] [CrossRef] [PubMed]
- Teran, N.B.; He, G.S.; Baev, A.; Shi, Y.; Swihart, M.T.; Prasad, P.N.; Reynolds, J.R. Twisted Thiophene-Based Chromophores with Enhanced Intramolecular Charge Transfer for Cooperative Amplification of Third-Order Optical Nonlinearity. J. Am. Chem. Soc. 2016, 138, 6975–6984. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cui, Y.; Xu, H.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Confinement of pyridinium hemicyanine dye within an anionic metal-organic framework for two-photon-pumped lasing. Nat. Commun. 2013, 4, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cui, Y.; Wu, C.D.; Yang, Y.; Chen, B.; Qian, G. Two-photon responsive metal-organic framework. J. Am. Chem. Soc. 2015, 137, 4026–4029. [Google Scholar] [CrossRef] [PubMed]
- Huang, B. Native Point Defects in CaS: Focus on Intrinsic Defects and Rare Earth Ion Dopant Levels for Up-converted Persistent Luminescence. Inorg. Chem. 2015, 54, 11423–11440. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Chung, S.J.; Kim, K.S.; Wang, X.; He, G.S.; Swiatkiewicz, J.; Prasad, P.N. Organics and polymers with high two-photon activities and their applications. Adv. Polym. Sci. 2003, 161, 157–193. [Google Scholar]
- Lu, K.; He, C.; Lin, W. A chlorin-based nanoscale metal-organic framework for photodynamic therapy of colon cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Shang, W.; Liang, X.; Liang, X.; Chen, Q.; Chi, C.; Tian, J. Cancer Diagnosis and Imaging-guided Photothermal Therapy Using a Dual-modality Nanoparticle. ACS Appl. Mater. Interfaces 2016, 8, 29232–29241. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Fang, Q.; Wang, D.; Xue, G.; Yu, W.T.; Shao, Z.S.; Jiang, M.H. Trivalent boron as acceptor in D-π-A chromophores: Synthesis, structure and fluorescence following single-and two-photon excitation. Chem. Commun. 2002, 2900–2901. [Google Scholar] [CrossRef]
- Collings, J.C.; Poon, S.Y.; Droumaguet, C.L.; Charlot, M.; Katan, C.; Pålsson, L.-O.; Beeby, A.; Mosely, J.A.; Kaiser, H.M.; Kaufmann, D.; et al. The Synthesis and one-and two-photon optical properties of dipolar, quadrupolar and octupolar donor–acceptor molecules containing dimesitylboryl groups. Chem. Eur. J. 2009, 15, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlot, M.; Porrès, L.; Christopher, D.; Entwistle, C.C.; Beeby, A.; Marder, T.B.; Blanchard-Desce, M. Investigation of two-photon absorption behavior in symmetrical acceptor–π–acceptor derivatives with dimesitylboryl end-groups. Evidence of new engineering routes for TPA/transparency trade-off optimization. Phys. Chem. Chem. Phys. 2005, 7, 600–606. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Fang, Q.; Wang, D.; Cao, D.X.; Xue, G.; Yu, W.T.; Lei, H. Trivalent boron as an acceptor in donor-π-acceptor-type compounds for single-and two-photon excited fluorescence. Chem. Eur. J. 2003, 9, 5074–5084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Fang, Q.; Cao, D.X.; Wang, D.; Xu, G.B. Triaryl boron-based A-π-A vs triaryl nitrogen-based D-π-D quadrupolar compounds for single-and two-photon excited fluorescence. Org. Lett. 2004, 6, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, M.; Li, F.; Fang, Q.; Chen, Z.; Yi, T.; Huang, C. Highly selective two-photon chemosensors for fluoride derived from organic boranes. Org. Lett. 2005, 7, 5481–5484. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Fang, Q.; Yuan, M.; Liu, Z.; Shen, Y.; Chen, H. Switching high two-photon efficiency: From 3,8,13-substituted triindole derivatives to their 2,7,12-Isomers. Org. Lett. 2010, 12, 5192–5195. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Marshall, A.S.; Chi, H.S.; Yin, X.; Perry, J.W.; Jäkle, F. Luminescent quadrupolar borazine oligomers: Synthesis, photophysics, and two-photon absorption properties. Chem. Eur. J. 2015, 21, 18237–18247. [Google Scholar] [CrossRef] [PubMed]
- Makarov, N.S.; Mukhopadhyay, S.; Yesudas, K.; Brédas, J.L.; Perry, J.W.; Pron, A.; Mullen, K. Impact of electronic coupling, symmetry, and planarization on one-and two-photon properties of triarylamines with one, two, or three diarylboryl acceptors. J. Phys. Chem. A. 2012, 116, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Edkins, R.M.; Sewell, L.J.; Beeby, A.; Batsanov, A.S.; Fucke, K.; Boucekkine, A. Experimental and theoretical studies of quadrupolar oligothiophene-cored chromophores containing dimesitylboryl moieties as π-accepting end-groups: Syntheses, structures, fluorescence, and one-and two-photon absorption. Chem. Eur. J. 2014, 20, 13618–13635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Entwistle, C.D.; Collings, J.C.; Steffen, A.; Pålsson, L.O.; Beeby, A.; Albesa-Jové, D.; Poon, S.Y. Syntheses, structures, two-photon absorption cross-sections and computed second hyperpolarisabilities of quadrupolar A-π-A systems containing E-dimesitylborylethenyl acceptors. J. Mater. Chem. 2009, 19, 7532–7544. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zeng, S.; Zhang, B.; Swihart, M.T.; Yong, K.T.; Prasad, P.N. New Generation Cadmium-Free Quantum Dots for Biophotonics and Nanomedicine. Chem. Rev. 2016, 116, 12234–12327. [Google Scholar] [CrossRef] [PubMed]
- Carrone, G.; Etchenique, R. Chemical two-photon fluorescence. Anal. Chem. 2015, 87, 4363–4369. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.A.; Kazarian, S.G. Attenuated total reflection Fourier-transform infrared (ATR-FTIR) imaging of tissues and live cells. Chem. Soc. Rev. 2016, 45, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Goodson, T.G., III. Optical excitations in organic dendrimers investigated by time-resolved and nonlinear optical spectroscopy. Acc. Chem. Res. 2005, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Kamada, K.; Matsunaga, K.; Yoshino, A.; Ohta, K. Two-photon-absorption-induced accumulated thermal effect on femtosecond Z-scan experiments studied with time-resolved thermal-lens spectrometry and its simulation. J. Opt. Soc. Am. B 2003, 20, 529–537. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Drobizhev, M.; Stepanenko, Y.; Dzenis, Y.; Karotki, A.; Rebane, A.; Taylor, P.N.; Anderson, H.L. Extremely strong near-IR two-photon absorption in conjugated porphyrin dimers: Quantitative description with three-essential-states model. J. Phys. Chem. B 2005, 109, 7223–7236. [Google Scholar] [CrossRef] [PubMed]
- Makarov, N.S.; Drobizhev, M.; Rebane, A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 2008, 16, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Wurth, C.; Pauli, J.; Lochmann, C.; Spieles, M.; Resch-Genger, U. Integrating sphere setup for the traceable measurement of absolute photoluminescence quantum yields in the near infrared. Anal. Chem. 2012, 84, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Rumi, M.; Ehrlich, J.E.; Heikal, A.A.; Perry, J.W.; Barlow, S.; Hu, Z.; Mc Cord-Maughon, D.; Parker, T.C.; Röckel, H.; Thayu-manavan, S.; et al. Structure-Property Relationships for Two-Photon Absorbing Chromophores: Bis-Donor Diphenylpolyene and Bis(styryl)benzene Derivatives. J. Am. Chem. Soc. 2000, 122, 9500–9510. [Google Scholar] [CrossRef]
- Signorini, R.; Ferrante, C.; Pedron, D.; Zerbetto, M.; Cecchetto, E.; Slaviero, M.; Fortunati, I.; Collini, E.; Bozio, R.; Abbotto, A.; et al. Effective Two-Photon Absorption Cross Section of Heteroaromatic Quadrupolar Dyes: Dependence on Measurement Technique and Laser Pulse Characteristics. J. Phys. Chem. A 2008, 112, 4224–4234. [Google Scholar] [CrossRef] [PubMed]
- Padilha, L.A.; Webster, S.; Hu, H.; Przhonska, O.V.; Hagan, D.J.; Van Stryland, E.W.; Bondar, M.V.; Davydenko, I.G.; Slominsky, Y.L.; Kachkovski, A.D. Excited state absorption and decay kinetics of near IR polymethine dyes. Chem. Phys. 2008, 352, 97–105. [Google Scholar] [CrossRef]
- Li, D.D.; Tian, X.H.; Wang, A.D.; Guan, L.J.; Zheng, J.; Li, F.; Li, S.L.; Zhou, H.P.; Wua, J.Y.; Tian, Y.P. Nucleic acid-selective light-up fluorescent biosensors for ratiometric two-photon imaging of the viscosity of live cells and tissues. Chem. Sci. 2016, 7, 2257–2263. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Han, Z.; He, W.; Guo, Z. Photoluminescence imaging of Zn2+ in living systems. Chem. Soc. Rev. 2015, 44, 4517–4546. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.Y.; Zhang, X.J.; Tian, Y.P.; Zhou, H.P.; Li, L.; Wu, J.Y.; Zhang, S.Y.; Yang, J.X.; Jin, B.K.; Tao, X.T.; et al. Design, crystal structures and enhanced frequency-upconverted lasing efficiencies of a new series of dyes from hybrid of inorganic polymers and organic chromophores. J. Mater. Chem. 2009, 19, 9163–9169. [Google Scholar] [CrossRef]
- Zhou, S.S.; Zhang, Q.; Tian, X.H.; Hu, G.J.; Hao, F.Y.; Wu, J.Y.; Tian, Y.P. Synthesis, crystal structure, optical properties, DNA-binding and cell imaging of an organic chromophore. Dyes Pigments 2011, 92, 689–695. [Google Scholar] [CrossRef]
- Liu, Z.D.; Hao, F.Y.; Xu, H.J.; Wang, H.; Wu, J.Y.; Tian, Y.P. A-π-D-π-A pyridinium salts: Synthesis, crystal structures, two-photon absorption properties and application to biological imaging. CrystEngComm 2015, 17, 5562–5568. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, Q.; Sun, X.S.; Shao, N.Q.; Li, R.; Li, S.L.; Zhou, H.P.; Wu, J.Y.; Tian, Y.P. Hydrosoluble two-photon absorbing materials: A series of sulfonated organic inner salts in biological imaging application. Dyes Pigments 2014, 102, 79–87. [Google Scholar] [CrossRef]
- Li, D.D.; Yu, D.H.; Zhang, Q.; Li, S.L.; Zhou, H.P.; Wu, J.Y.; Tian, Y.P. Synthesis, crystal structure and third-order nonlinear optical properties in the near-IR range of a novel stilbazolium dye substituted with flexible polyether chains. Dyes Pigments 2013, 97, 278–285. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, L.; Xu, H.; Hu, Z.J.; Brommesson, C.; Wu, J.Y.; Sun, Z.Q.; Tian, Y.P.; Uvdal, K. Design, synthesis, linear and nonlinear photophysical properties of novel pyrimidine-based imidazole derivatives. New J. Chem. 2016, 40, 3456–3463. [Google Scholar] [CrossRef]
- Huang, Z.L.; Lei, H.; Li, N.; Qiu, Z.R.; Wang, H.Z.; Guo, J.D.; Luo, Y.; Zhong, Z.P.; Liu, X.F.; Zhou, Z.H. Novel heterocycle-based organic molecules with two-photon induced blue fluorescent emission. J. Mater. Chem. 2003, 13, 708–711. [Google Scholar] [CrossRef]
- Wei, P.; Bi, X.D.; Wu, Z.; Xu, Z. Synthesis of Triphenylamine-Cored Dendritic Two-Photon Absorbing Chromophores. Org. Lett. 2005, 7, 3199–3202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, Y.P.; Yang, J.X.; Sun, P.P.; Wu, J.Y.; Zhou, H.P.; Zhang, S.Y.; Jin, B.K.; Xing, X.J.; Wang, C.K.; et al. Facile Synthesis and Systematic Investigations of a Series of Novel Bent-Shaped Two-Photon Absorption Chromophores Based on Pyrimidine. Chem. Asian J. 2009, 4, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Zhang, J.; Li, L.; Zhang, Q.; Li, S.L.; Zhang, S.Y.; Wu, J.Y.; Tian, Y.P. Synthesis, two-photon absorption properties and bio-imaging applications of mono-, di- and hexa-branched pyrimidine derivatives. Dyes Pigments 2014, 102, 263–272. [Google Scholar] [CrossRef]
- Belfield, K.D.; Bondar, M.V.; Morales, A.R.; Frazer, A.; Mikhailov, I.A.; Przhonska, O.V. Photophysical properties and ultrafast excited-state dynamics of a new twophoton absorbing thiopyranyl probe. J. Phys. Chem. C 2013, 117, 11941–11952. [Google Scholar]
- Xie, Y.P.; Zhang, X.F.; Zhang, Y.D.; Zhou, F.; Qi, J.; Qu, J.L. Fusing three perylenebisimide branches and a truxene core into a star-shaped chromophore with strong two-photon excited fluorescence and high photostability. Chem. Commun. 2012, 48, 4338–4340. [Google Scholar] [CrossRef] [PubMed]
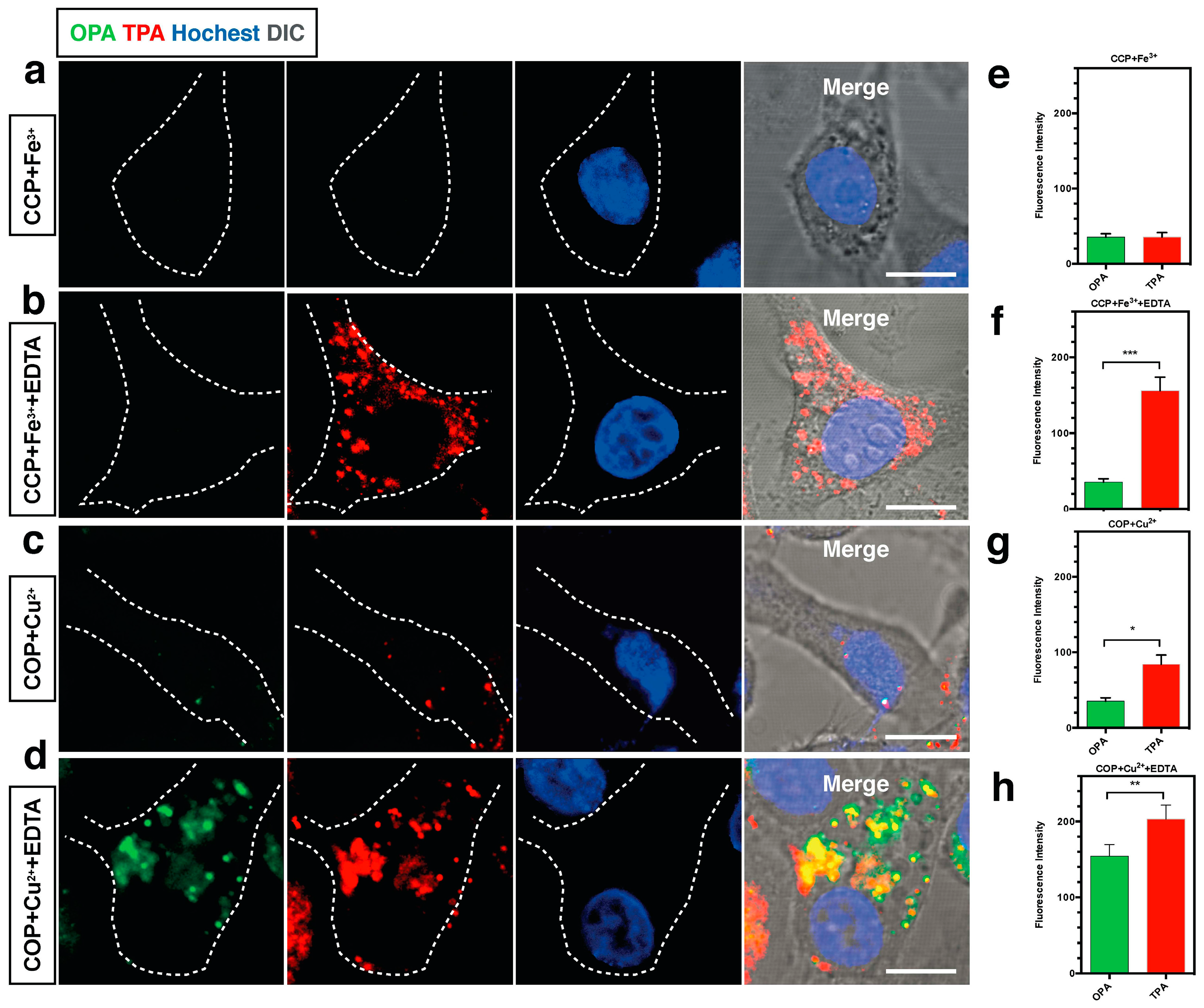
- Zhang, Q.; Tian, X.H.; Wang, H.; Hu, Z.J.; Wu, J.Y.; Zhou, H.P.; Zhang, S.Y.; Yang, J.X.; Sun, Z.Q.; Tian, Y.P.; et al. NIR-region two-photon fluorescent probes for Fe3+/Cu2+ ions based on pyrimidine derivatives with different flexible chain. Sens. Actuators B 2016, 222, 574–578. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q.; Li, D.D.; Zhang, J.; Shi, P.F.; Li, S.L.; Wu, J.Y.; Tian, Y.P. Synthesis, crystal structures, two-photon absorption and biological imaging application of two novel bent-shaped pyrimidine derivatives. Dyes Pigments 2013, 99, 20–28. [Google Scholar] [CrossRef]
- Chung, S.J.; Kim, K.S.; Lin, T.C.; He, G.S.; Swiatkiewicz, J.; Prasad, P.N. Cooperative Enhancement of Two-Photon Absorption in Multi-branched Structures. J. Phys. Chem. B 1999, 103, 10741–10745. [Google Scholar] [CrossRef]
- Paek, S.H.; Cho, N.; Cho, S.; Lee, J.K.; Ko, J.J. Planar Star-Shaped Organic Semiconductor with Fused Triphenylamine Core for Solution-Processed Small-Molecule Organic Solar Cells and Field-Effect Transistors. Org. Lett. 2012, 14, 6326–6329. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Xu, W.; Cai, F.; Chen, P.; Peng, B.; Chen, J.; Li, Z. New Triphenylamine-Based Organic Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2007, 111, 4465–4472. [Google Scholar] [CrossRef]
- Zhu, M.R.; Zou, J.H.; He, X.; Yang, C.L.; Wu, H.B.; Zhong, C.; Qin, J.G.; Cao, Y. Triphenylamine Dendronized Iridium(III) Complexes: Robust Synthesis, Highly Efficient Nondoped Orange Electrophosphorescence and the Structure–Property Relationship. Chem. Mater. 2012, 24, 174–180. [Google Scholar] [CrossRef]
- Andrade, C.D.; Yanez, C.O.; Qaddoura, M.A.; Wang, X.; Arnett, C.L.; Coombs, S.A.; Yu, J.; Bassiouni, R.; Bondar, M.V.; Belfield, K.D. Two-Photon Fluorescence Lysosomal Bio-imaging with a Micelle-Encapsulated Fluorescent Probe. J. Fluoresc. 2011, 21, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Jing, J.; Cai, Y.B.; Tang, J.; Chen, J.J.; Zhang, J.L. Construction of an orthogonal ZnSalen/Salophen library as a colour palette for one- and two-photon live cell imaging. Chem. Sci. 2014, 5, 2318–2327. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Ding, H.J.; Hu, G.J.; Du, Y.J.; Wang, C.K.; Wu, J.Y.; Li, S.L.; Zhou, H.P.; Yang, J.X.; et al. Synthesis, crystal structures and two-photon absorption properties of a series of terpyridine-based chromophores. Dyes Pigments 2012, 95, 149–160. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.Z.; Tian, X.H.; Li, F.; Xu, W.T.; Zhang, Y.J.; Wang, C.K.; Zhang, J.; Zhou, H.P.; Wu, J.Y.; et al. Synthesis, crystal structures of a series of novel 2,2′:6′,2″-terpyridine derivatives: The influences of substituents on their photophysical properties and intracellular acid organelle targeting. Dyes Pigments 2016, 128, 149–157. [Google Scholar] [CrossRef]
- Li, S.L.; Shen, W.B.; Hu, G.J.; Zhang, Q.; Liu, B.; Liu, J.; Ding, H.J.; Wu, J.Y.; Zhou, H.P.; Yang, J.X.; et al. Crystal structure, nonlinear optical and photophysical properties of a novel chromophore constructed with terpyridine, triphenylamine and ethyl cyanocaetate functional moieties. Mater. Chem. Physi. 2013, 140, 200–207. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Kong, M.; Zhang, Q.; Zhang, Z.W.; Zhou, H.P.; Zhang, S.Y.; Li, S.L.; Wu, J.Y.; Tian, Y.P. A series of triphenylamine-based two-photon absorbing materials with AIE property for biological Imaging. J. Mater. Chem. B 2014, 2, 5430–5440. [Google Scholar] [CrossRef]
- Gan, X.P.; Wang, Y.; Ge, X.P.; Li, W.; Zhang, X.Z.; Zhu, W.J.; Zhou, H.P.; Wu, J.Y.; Tian, Y.P. Triphenylamine isophorone derivatives with two photon absorption:Photo-physical property, DFT study and bio-imaging. Dyes Pigments 2015, 120, 65–73. [Google Scholar] [CrossRef]
- Macak, P.; Luo, Y.; Norman, P.; Agren, H. Electronic and vibronic contributions to two-photon absorption of molecules with multi-branched structures. J. Chem. Phys. 2000, 113, 7055–7061. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, X.H.; Hu, Z.J.; Brommesson, C.; Wu, J.Y.; Zhou, H.P.; Yang, J.X.; Sun, Z.Q.; Tian, Y.P.; Uvdal, K. Nonlinear optical response and two-photon biological applications of a new family of imidazole-pyrimidine derivatives. Dyes Pigments 2016, 126, 286–295. [Google Scholar] [CrossRef]
- Yu, Z.P.; Zheng, Z.; Yang, M.D.; Wang, L.K.; Tian, Y.P.; Wu, J.Y.; Zhou, H.P.; Xu, H.M.; Wu, Z.Q. Photon-induced intramolecular charge transfer with the influence of D/A group and mode: Optical physical properties and bio-imaging. J. Mater. Chem. C 2013, 1, 7026–7033. [Google Scholar] [CrossRef]
- Tian, Y.P.; Li, L.; Zhang, J.Z.; Yang, J.X.; Zhou, H.P.; Wu, J.Y.; Sun, P.P.; Tao, L.M.; Gao, Y.H.; Wang, C.K.; et al. Investigations and facile synthesis of a series of novel multi-functional two-photon absorption materials. J. Mater. Chem. 2007, 17, 3646–3654. [Google Scholar] [CrossRef]
- Murphy, S.; Huang, L.; Kamat, P.V. Charge-Transfer Complexation and Excited-State Interactions in Porphyrin-Silver Nanoparticle Hybrid Structures. J. Phys. Chem. C 2011, 115, 22761–22769. [Google Scholar] [CrossRef]
- Kometani, N.; Tsubonishi, M.; Fujita, T.; Asami, K.; Yonezawa, Y. Preparation and Optical Absorption Spectra of Dye-Coated Au, Ag, and Au/Ag Colloidal Nanoparticles in Aqueous Solutions and in Alternate Assemblies. Langmuir 2001, 17, 578–580. [Google Scholar] [CrossRef]
- Nabika, H.; Takase, M.; Nagasawa, F.; Murakoshi, K. Toward Plasmon-Induced Photoexcitation of Molecules. J. Phys. Chem. Lett. 2010, 1, 2470–2487. [Google Scholar] [CrossRef]
- Lee, J.; Hernandez, P.; Lee, J.W.; Govorov, A.O.; Kotov, N.A. Exciton–plasmon interactions in molecular spring assemblies of nanowires and wavelength-based protein detection. Nat. Mater. 2007, 6, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Wu, J.Y.; Zhao, Q.; Zheng, L.X.; Zhou, H.P.; Zhang, S.Y.; Yang, J.X.; Tian, Y.P. Solvent-resolved fluorescent Ag nanocrystals capped with a novel terpyridine-based dye. New J. Chem. 2009, 33, 607–611. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.H.; Kim, H.; Laquai, F.; Lee, J.K.; Yoo, S.; Sohn, B.H. Switching off FRET in the Hybrid Assemblies of Diblock Copolymer Micelles, Quantum Dots, and Dyes by Plasmonic Nanoparticles. ACS Nano 2012, 6, 5051–5059. [Google Scholar] [CrossRef] [PubMed]
- Cohanoschi, I.; Hernandez, F.E. Surface Plasmon Enhancement of Two- and Three-Photon Absorption of Hoechst 33 258 Dye in Activated Gold Colloid Solution. J. Phys. Chem. B 2005, 109, 14506–14512. [Google Scholar] [CrossRef] [PubMed]
- Stellacci, F.; Bauer, C.A.; Meyer-Friedrichsen, T.; Wenseleers, W.; Marder, S.R.; Perry, J.W. Ultrabright Supramolecular Beacons Based on the Self-Assembly of Two-Photon Chromophores on Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Wenseleers, W.; Stellacci, F.; Meyer-Friedrichsen, T.; Mangel, T.; Bauer, C.A.; Pond, S.J.K.; Marder, S.R.; Perry, J.W. Five Orders-of-Magnitude Enhancement of Two-Photon Absorption for Dyes on Silver Nanoparticle Fractal Clusters. J. Phys. Chem. B 2002, 106, 6853–6863. [Google Scholar] [CrossRef]
- Lal, M.; Levy, L.; Kim, K.S.; He, G.S.; Wang, X.; Min, Y.H.; Pakatchi, S.; Prasad, P.N. Silica Nanobubbles Containing an Organic Dye in a Multilayered Organic/Inorganic Heterostructure with Enhanced Luminescence. Chem. Mater. 2000, 12, 2632–2639. [Google Scholar] [CrossRef]
- Kim, S.; Pudavar, H.E.; Prasad, P.N. Dye-concentrated organically modified silica nanoparticles as a ratiometric fluorescent pH probe by one- and two-photon excitation. Chem. Commun. 2006, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zheng, Q.D.; He, G.S.; Bharali, D.J.; Pudavar, H.E.; Baev, A.; Prasad, P.N. Aggregation-Enhanced Fluorescence and Two-Photon Absorption in Nanoaggregates of a 9,10-Bis[4′-(4″-aminostyryl)styryl]anthracene Derivative. Adv. Funct. Mater. 2006, 16, 2317–2323. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Xu, G.X.; Prasad, P.N.; Swihart, M.T. Biocompatible Luminescent Silicon Quantum Dots for Imaging of Cancer Cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Tian, Y.P.; Jin, F.; Wu, J.Y.; Xie, Y.; Tao, X.T.; Jiang, M.H. Self-Assembly of An Organic Chromophore with Cd−S Nanoclusters: Supramolecular Structures and Enhanced Emissions. Cryst. Growth Des. 2005, 5, 565–570. [Google Scholar] [CrossRef]
- Gao, Y.H.; Wu, J.Y.; Li, Y.M.; Sun, P.P.; Zhou, H.P.; Yang, J.X.; Zhang, S.Y.; Jin, B.K.; Tian, Y.P. A Sulfur-Terminal Zn(II) Complex and Its Two-Photon Microscopy Biological Imaging Application. J. Am. Chem. Soc. 2009, 131, 5208–5231. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Kageyama, H. Charge Carrier Transporting Molecular Materials and Their Applications in Devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, N.N.; Wang, W.D.; Kong, L.; Wu, J.Y.; Li, S.L.; Tian, Y.P. NIR laser induced TPA enhancement of Zn(II)-terpyridine capped gold nanoparticles for targeting mitochondria. Dyes Pigments 2016, 133, 86–92. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y.P.; Yang, J.X.; Sun, P.P.; Kong, L.; Wu, J.Y.; Zhou, H.P.; Zhang, S.Y.; Jin, B.K.; Tao, X.T.; et al. Two-photon absorption enhancement induced by aggregation with accurate photophysical data: Spontaneous accumulation of dye in silica nanoparticles. Chem. Commun. 2010, 46, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Picot, A.; D’Aléo, A.; Baldeck, P.L.; Grichine, A.; Duperray, A.; Andraud, C.; Maury, O. Long-Lived Two-Photon Excited Luminescence of Water-Soluble Europium Complex: Applications in Biological Imaging Using Two-Photon Scanning Microscopy. J. Am. Chem. Soc. 2008, 130, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G. Conversion of Ag Nanowires to AgCl Nanowires Decorated with Au Nanoparticles and Their Photocatalytic Activity. J. Phys. Chem. 2010, 114, 2127–2133. [Google Scholar] [CrossRef]
- Chen, D.L.; Yoo, S.H.; Huang, Q.S.; Ali, G.; Cho, S.O. Sonochemical Synthesis of Ag/AgCl Nanocubes and Their Efficient Visible-Light-Driven Photocatalytic Performance. Chem. Eur. J. 2012, 18, 5192–5200. [Google Scholar] [CrossRef] [PubMed]
- Tagar, Z.A.; Memon, S.N.; Agheem, M.H.; Junejo, Y.; Hassan, S.S.; Kalwar, N.H.; Khattak, M.I. Selective, simple and economical lead sensor based on ibuprofen derived silver nanoparticles. Sens. Actuators B 2011, 157, 430–437. [Google Scholar] [CrossRef]
- Yan, J.; Han, X.J.; He, J.J.; Kang, L.L.; Zhang, B.; Du, Y.C.; Zhao, H.T.; Dong, C.K.; Wang, H.L.; Xu, P. Highly Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Platforms Based on Silver Nanostructures Fabricated on Polyaniline Membrane Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Bell, S.E.J. Label-Free Detection of Nanomolar Unmodified Single- and Double-Stranded DNA by Using Surface-Enhanced Raman Spectroscopy on Ag and Au Colloids. Chem. Eur. J. 2012, 18, 5394–5400. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.H.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z. Biomedical Applications of Shape-Controlled Plasmonic Nanostructures: A Case Study of Hollow Gold Nanospheres for Photothermal Ablation Therapy of Cancer. Chem. Lett. 2010, 1, 686–695. [Google Scholar] [CrossRef]
- Parab, H.J.; Chen, H.M.; Lai, T.; Huang, J.H.; Chen, P.H.; Liu, R.S.; Hsiao, M.; Chen, C.; Tsai, D.; Hwu, Y. Biosensing, Cytotoxicity, and Cellular Uptake Studies of Surface-Modified Gold Nanorods. J. Phys. Chem. C 2009, 113, 7574–7578. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Parent, K.L.; Buttry, D.A. Electrochemical Solid-State Phase Transformations of Silver Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Zhang, J.; Huang, L.; He, D.; Ma, L.; Ouyang, J.; Jiang, F. Novel Application of Ag Nanoclusters in Fluorescent Imaging of Human Serum Proteins after Native Polyacrylamide Gel Electrophoresis (PAGE). Chem. Eur. J. 2012, 18, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Cho, B.R. Two-Photon Probes for Intracellular Free Metal Ions, Acidic Vesicles, and Lipid Rafts in Live Tissues. Acc. Chem. Res. 2009, 42, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zhu, A.W.; Ding, C.Q.; Zhao, X.M.; Li, B.; Tian, Y. Carbon Dot-Based Inorganic–Organic Nanosystem for Two-Photon Imaging and Biosensing of pH Variation in Living Cells and Tissues. Adv. Mater. 2012, 24, 5844–5848. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yang, J.X.; Li, S.L.; Zhang, Q.; Xue, Z.M.; Zhou, H.P.; Wu, J.Y.; Jin, B.K.; Tian, Y.P. A Self-Assembled Nanohybrid Composed of Fluorophore-Phenylamine Nanorods and Ag Nanocrystals: Energy Transfer, Wavelength Shift of Fluorescence and TPEF Applications for Live-Cell Imaging. Chem. Eur. J. 2013, 19, 16625–16633. [Google Scholar] [CrossRef] [PubMed]
- De Sa, G.F.; Malta, O.L.; Donega, C.D.; Simas, A.M.; Longo, R.L.; Santa-Cruz, P.A.; da Silva, E.F. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 2000, 196, 165–195. [Google Scholar]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Filipescu, N.; Sager, W.F.; Serafin, F.A. Substituent Effects on Intramolecular Energy Transfer. II. Fluorescence Spectra of Europium and Terbium β-Diketone Chelates. J. Phys. Chem. 1964, 68, 3324–3346. [Google Scholar] [CrossRef]
- Rendell, D. Fluorescence and Phosphorescence Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Hu, Z.J.; Tian, X.H.; Zhao, X.H.; Wang, P.; Zhang, Q.; Sun, P.P.; Wu, J.Y.; Yang, J.X.; Tian, Y.P. Efficient two-photon-sensitized luminescence of a novel europium(III) β-diketonate complex and application in biological imaging. Chem. Commun. 2011, 47, 12467–12469. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, G.; Maliwal, B.P.; Grycaynski, I.; Dattelbaum, J.; Lakowicz, J.R.; Fluoresc, J. Multiphoton Ligand-Enhanced Excitation of Lanthanides. J. Fluoresc. 2001, 11, 101–107. [Google Scholar] [CrossRef]
- Zhou, S.S.; Xue, X.; Wang, J.F.; Dong, Y.; Jiang, B.; Wei, D.; Wan, M.L.; Jia, Y. Synthesis, optical properties and biological imaging of the rare earth complexes with curcumin and pyridine. J. Mater. Chem. 2012, 22, 22774–22780. [Google Scholar] [CrossRef]
- Wang, J.S.; Jin, F.Z.; Ma, H.C.; Li, X.B.; Liu, M.Y.; Kan, J.K.; Chen, G.J.; Dong, Y.B. Au@Cu(II)-MOF: Highly Efficient Bifunctional Heterogeneous Catalyst for Successive Oxidation-Condensation Reactions. Inorg. Chem. 2016, 55, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Fleetham, T.B.; Huang, L.; Klimes, K.; Brooks, J.; Li, J. Tetradentate Pt(II) Complexes with 6-Membered Chelate Rings: A New Route for Stable and Efficient Blue Organic Light Emitting Diodes. Chem. Mater. 2016, 28, 3276–3282. [Google Scholar] [CrossRef]
- Garah, M.E.; Sinn, S.; Diana, A.; Santana-Bonilla, A.; Gutierrez, R.; De Cola, L.; Cuniberti, G.; Ciesielskia, A.; Samorì, P. Discrete polygonal supramolecular architectures of isocytosine-based Pt(II) complexes at the solution/graphite interface. Chem. Commun. 2016, 52, 11163–11166. [Google Scholar] [CrossRef] [PubMed]
- Romanova, J.; Prabhath, M.R.R.; Jarowski, P. Relationship between Metallophilic Interactions and Luminescent Properties in Pt(II) Complexes: TD-DFT Guide for the Molecular Design of Light-Responsive Materials. J. Phys. Chem. C 2016, 120, 2002–2012. [Google Scholar] [CrossRef]
- Zou, H.H.; Wang, L.; Long, Z.X.; Qin, Q.P.; Song, Z.K.; Xie, T.; Zhang, S.H.; Liu, Y.C.; Lin, B.; Chen, Z.F. Preparation of 4-([2,2′:6′,2″-terpyridin]-4′-yl)-N,N-diethylaniline NiII and PtII complexes and exploration of their in vitro cytotoxic activities. Eur. J. Med. Chem. 2016, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wawrznek, R.; Muhieddine, K.; Ullah, M.; Koszo, P.B.; Shaw, P.E.; Grosjean, A.; Maasoumi, F.; Stoltzfus, D.M.; Clegg, J.K.; Burn, P.L.; et al. Orange-Red-Light-Emitting Field-Effect Transistors Based on Phosphorescent Pt(II) Complexes with Area Emission. Adv. Opt. Mater. 2016, 4, 1867–1874. [Google Scholar] [CrossRef]
- Kantoury, M.; Moghadam, M.E.; Tarlani, A.A.; Divsalar, A. Structure Effect of Some New Anticancer Pt(II) Complexes of Amino Acid Derivatives with Small Branched or Linear Hydrocarbon Chains on Their DNA Interaction. Chem. Biol. Drug Des. 2016, 88, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Yu, Z.Q.; Wong, W.Y. Multifunctional polymetallaynes: Properties, functions and applications. Chem. Soc. Rev. 2016, 45, 5264–5295. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, U.; Pellegrin, Y.; Devocelle, M.; Forster, R.J.; Signac, W.; Morand, N.; Keyes, T.E. Ruthenium polypyridyl peptide conjugates: Membrane permeable probes for cellular imaging. Chem. Commun. 2008, 5307–5309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, A.E.; Chambron, J.C.; Sauvage, J.P.; Turro, N.J.; Barton, J.K. Ru(bpy)2(dppz)2+. J. Am. Chem. Soc. 1990, 112, 4960–4962. [Google Scholar] [CrossRef]
- Jiménez-Hernández, M.E.; Orellana, G.; Montero, F.; Portolés, M.T. A Ruthenium Probe for Cell Viability Measurement Using Flow Cytometry, Confocal Microscopy and Time-resolved Luminescence. Photochem. Photobiol. 2000, 72, 28–34. [Google Scholar] [CrossRef]
- Xiao, L.F.; Wang, H.; Zhang, Q.; Zhu, Y.Z.; Luo, J.S.; Liang, Y.K.; Zhang, S.Y.; Zhou, H.P.; Tian, Y.P.; Wu, J.Y. Novel ruthenium(II) polypyridyl complexes containing carbazole with flexible substituents: Crystal structure, nonlinear optical properties and DNA-binding interaction. Dyes Pigments 2015, 113, 165–173. [Google Scholar] [CrossRef]
- Puckett, C.A.; Barton, J.K. Fluorescein Redirects a Ruthenium–Octaarginine Conjugate to the Nucleus. J. Am. Chem. Soc. 2009, 131, 8738–8739. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.R.; Garica-Lara, J.; Foster, S.J.; Smythe, C.; Battaglia, G.; Thomas, J.A. A ruthenium(II) polypyridyl complex for direct imaging of DNA structure in living cells. Nat. Chem. 2009, 1, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, X.H.; Guan, L.J.; Zhang, Q.; Zhang, S.Y.; Zhou, H.P.; Wu, J.Y.; Tian, Y.P. Targeting mitochondrial DNA with a two-photon active Ru(II) phenanthroline derivative. J. Mater. Chem. B 2016, 4, 2895–2902. [Google Scholar] [CrossRef]
- Liu, J.P.; Chen, Y.; Li, G.Y.; Zhang, P.Y.; Jin, C.Z.; Zeng, L.L.; Ji, L.N.; Chao, H. Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, C.H.; Li, F.Y. Phosphorescent heavy-metal complexes for bio-imaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.F.; Coe, B.J.; Sánchez, S.; Wang, D.Q.; Tian, Y.P.; Nyk, M.; Samoc, M. Uniting Ruthenium(II) and platimum(II) polypyridine centers in heteropolymetallic complexes giving strong two-photon absorption. Inorg. Chem. 2015, 54, 11450–11456. [Google Scholar] [CrossRef] [PubMed]
- Kaes, C.; Katz, A.; Hosseini, M.W. Bipyridine: The Most Widely Used Ligand. A Review of Molecules Comprising at Least Two 2,2′-Bipyridine Units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef] [PubMed]
- Hofmeier, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine-metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Zhang, Q.; Wang, P.; Wu, J.Y.; Kan, Y.H.; Tian, Y.P.; Zhou, H.P.; Yang, J.X.; Tao, X.T.; Jiang, M.H. Studies of the isomerization and photophysical properties of a novel 2,2′:6′,2″-terpyridine-based ligand and its complexes. Dalton Trans. 2011, 40, 8170–8178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.X.; Zheng, Z.; Zhou, H.P.; Ke, W.Z.; Wang, J.Q.; Yu, Z.P.; Jin, F.; Yang, J.X.; Wu, J.Y.; Tian, Y.P. A new 2,2′:6′,2″-terpyridine-based ligand and its complexes: Structures, photophysical properties and DFT calculations to evaluate the halogen effect on the TPA. Cryst. Eng. Comm. 2012, 14, 5613–5621. [Google Scholar] [CrossRef]
- Shi, P.F.; Jiang, Q.; Zhao, X.S.; Zhang, Q.; Tian, Y.P. Study of the one-photon and two-photon properties of two water-soluble terpyridines and their zinc complexes. Dalton Trans. 2015, 44, 8041–8048. [Google Scholar] [CrossRef] [PubMed]
- Albert, I.D.; Marks, T.J.; Ratner, M.A. Remarkable NLO Response and Infrared Absorption in Simple Twisted Molecular π-Chromophores. J. Am. Chem. Soc. 1998, 120, 11174–11181. [Google Scholar] [CrossRef]
- Righetto, S.; Rondena, S.; Locatelli, D.; Roberto, D.; Tessore, F.; Ugo, R.; Quici, S.; Roma, S.; Korystov, D.; Srdanov, V.I. An investigation on the two-photon absorption activity of various terpyridines and related homoleptic and heteroleptic cationic Zn(II) complexes. J. Mater. Chem. 2006, 16, 1439–1444. [Google Scholar] [CrossRef]
- Ruiz Delgado, M.C.; Casado, J.; Hernandez, V.; López Navarrete, J.T.; Orduna, J.; Villacampa, B.; Alicante, R.; Raimundo, J.M.; Blanchard, P.; Roncali, J.J. Electronic, Optical, and Vibrational Properties of Bridged Dithienylethylene-Based NLO Chromophores. J. Phys. Chem. C 2008, 112, 3109–3120. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.; Locatelli, D.; Roberto, D.; Tessore, F.; Ugo, R.; Cavazzini, M.; Quici, S.; De Angelis, F.; Fantacci, S.; Ledoux-Rak, I.; et al. New [(D-terpyridine)-Ru-(D or A-terpyridine)][4-EtPhCO2]2 complexes (D = electron donor group; A = electron acceptor group) as active second-order non linear optical chromophores. Dalton Trans. 2012, 41, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Schwich, T.; Cifuentes, M.P.; Gugger, P.A.; Samoc, M.; Humphrey, M.G. Electronic, Molecular Weight, Molecular Volume, and Financial Cost-Scaling and Comparison of Two-Photon Absorption Efficiency in Disparate Molecules (Organometallic Complexes for Nonlinear Optics. 48.)—A Response to “Comment on ‘Organometallic Complexes for Nonlinear Optics. 45. Dispersion of the Third-Order Nonlinear Optical Properties of Triphenylamine-Cored Alkynylruthenium Dendrimers’. Increasing the Nonlinear Response by Two Orders of Magnitude”. Adv. Mater. 2011, 23, 1433–1435. [Google Scholar]
- Roberts, R.L.; Schwich, T.; Corkery, T.C.; Cifuentes, M.P.; Green, K.A.; Farmer, J.D.; Low, P.J.; Marder, T.B.; Samoc, M.; Humphrey, M.G. Organometallic Complexes for Nonlinear Optics. 45. Dispersion of the Third-Order Nonlinear Optical Properties of Triphenylamine-Cored Alkynylruthenium Dendrimers. Adv. Mater. 2009, 21, 2318–2322. [Google Scholar] [CrossRef]
- Tan, J.Y.; Li, R.; Li, D.D.; Zhang, Q.; Li, S.L.; Zhou, H.P.; Yang, J.X.; Wu, J.Y.; Tian, Y.P. Thiophene-based terpyridine and its zinc halide complexes: Third-order nonlinear optical properties in the near-infrared region. Dalton Trans. 2015, 44, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Schlutter, F.; Wild, A.; Winter, A.; Hager, M.D.; Baumgaertel, A.; Friebe, C.; Schubert, U.S. Synthesis and Characterization of New Self-Assembled Metallo-Polymers Containing Electron-Withdrawing and Electron-Donating Bis(terpyridine) Zinc(II) Moieties. Macromolecules 2010, 43, 2759–2771. [Google Scholar] [CrossRef]
- Schmittel, M.; Kalsani, V.; Kishore, R.S.; Cölfen, H.; Bats, J.W. Dynamic and Fluorescent Nanoscale Phenanthroline/Terpyridine Zinc(II) Ladders. Self-Recognition in Unlike Ligand/Like Metal Coordination Scenarios. J. Am. Chem. Soc. 2005, 127, 11544–11545. [Google Scholar] [CrossRef] [PubMed]
- He, G.S.; Tan, L.S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tian, X.H.; Hu, Z.J.; Brommesson, C.; Wu, J.Y.; Zhou, H.P.; Li, S.L.; Yang, J.X.; Sun, Z.Q.; Tian, Y.P.; et al. A series of Zn(II) terpyridine complexes with enhanced two-photon-excited fluorescence in vitro and in vivo Bio-imaging. J. Mater. Chem. B 2015, 3, 7213–7221. [Google Scholar] [CrossRef]
- Rendón, N.; Bourdolle, A.; Baldeck, P.L.; Bozec, H.L.; Andraud, C.; Brasselet, S.; Copéret, C.; Maury, O. Bright Luminescent Silica Nanoparticles for Two-Photon Microscopy Imaging via Controlled Formation of 4,4′-Diethylaminostyryl-2,2′-bipyridine Zn(II) Surface Complexes. Chem. Mater. 2011, 23, 3228–3236. [Google Scholar] [CrossRef]
- Barzoukas, M.; Runser, C.; Fort, A.; Blanchard-Desce, M. A two-state description of (hyper) polarizabilities of push-pull molecules based on a two-form model. Chem. Phys. Lett. 1996, 257, 531–537. [Google Scholar] [CrossRef]
- Tian, X.H.; Zhang, Q.; Zhang, M.Z.; Uvdal, K.; Wang, Q.; Chen, J.Y.; Du, W.; Huang, B.; Wu, J.Y.; Tian, Y.P. Probe for simultaneous membrane and nucleus labeling in living cells and in vivo bio-imaging using a two-photon absorption water-soluble Zn(II) terpyridine complex with a reduced π-conjugation system. Chem. Sci. 2017. [Google Scholar] [CrossRef]
- Li, D.M.; Tian, X.H.; Hu, G.J.; Zhang, Q.; Wang, P.; Sun, P.P.; Zhou, H.P.; Meng, X.M.; Yang, J.X.; Wu, J.Y.; et al. Synthesis, Crystal Structures, Photophysical Properties, and Bio-imaging of Living Cells of Bis-β-Diketonate Phenothiazine Ligands and Its Cyclic Dinuclear Complexes. Inorg. Chem. 2011, 50, 7997–8006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, Y.; Zhang, J.F.; Humphrey, M.G. Modulation of Third-Order Nonlinear Optical Properties by Backbone Modification of Polymeric Pillared-Layer Heterometallic Clusters. Adv. Mater. 2008, 20, 1870–1875. [Google Scholar] [CrossRef]
- Ford, P.C.; Cariati, E.; Bourassa, J. Photoluminescence Properties of Multinuclear Copper(I) Compounds. Chem. Rev. 1999, 99, 3625–3647. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Tian, X.H.; Zhang, Q.; Sun, P.P.; Wu, J.Y.; Zhou, H.P.; Jin, B.K.; Yang, J.X.; Zhang, S.Y.; Wang, C.K.; et al. Assembly, Two-Photon Absorption and Bio-imaging of Living Cells of A Cuprous Cluster. Chem. Mater. 2012, 24, 954–961. [Google Scholar] [CrossRef]
- Katan, C.; Charlot, M.; Mongin, O.; Droumaguet, C.; Jouikov, V.; Terenziani, F.; Badaeva, E.; Tretiak, S.; Blanchard-Desce, M.J. Simultaneous Control of Emission Localization and Two-Photon Absorption Efficiency in Dissymmetrical Chromophores. Phys. Chem. B 2010, 114, 3152–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Zipfel, W.; Shear, J.B.; Williams, R.M.; Webb, W.W. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Picot, A.; Malvolti, F.; Le Guennic, B.; Baldeck, P.L.; Williams, J.A.G.; Andraud, C.; Maury, O. Two-Photon Antenna Effect Induced in Octupolar Europium Complexes. Inorg. Chem. 2007, 46, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Chen, J.J.; Zhao, P.; Lv, H.B.; Yu, Y.; Xu, P.Y.; Zhang, J.L. Luminescent zinc salen complexes as single and two-photon fluorescence subcellular imaging probes. Chem. Commun. 2011, 47, 2435–2437. [Google Scholar] [CrossRef] [PubMed]
- Leydet, Y.; Bassani, D.M.; Jonusauskas, G.; McClenaghan, N.D. Equilibration between Three Different Excited States in a Bichromophoric Copper(I) Polypyridine Complex. J. Am. Chem. Soc. 2007, 129, 8688–8689. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Casini, A. A golden future in medicinal inorganic chemistry: The promise of anticancer gold organometallic compounds. Dalton Trans. 2014, 43, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Yu, J.C.; Cui, Y.J.; Rao, X.T.; Yang, Y.; Qian, G.D. Assembly and tunable luminescence of lanthanide-organic frameworks constructed from 4-(3,5-dicarboxyphenyl)pyridine-2,6-dicarboxylate ligand. J. Alloys Compd. 2013, 551, 616–620. [Google Scholar] [CrossRef]
- Wang, W.H.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Hydroxy-substituted pyridine-like N-heterocycles: Versatile ligands in organometallic catalysis. New J. Chem. 2013, 37, 1860–1866. [Google Scholar] [CrossRef]
- Denard, C.A.; Huang, H.; Bartlett, M.J.; Lu, L.; Tan, Y.C.; Zhao, H.M.; Hartwig, J.F. Cooperative Tandem Catalysis by an Organometallic Complex and a Metalloenzyme. Angew. Chem. 2014, 126, 475–479. [Google Scholar] [CrossRef]
- Shaheen, F.; Ali, S.; Meetsma, A. Structural properties and antibacterial potency of new supramolecular organotin(IV) dithiocarboxylates. Polyhedron 2012, 31, 697–703. [Google Scholar] [CrossRef]
- Wobser, S.D.; Stephenson, C.J.; Delferro, M.; Marks, T.J. Carbostannolysis Mediated by Bis(pentamethylcyclopentadienyl)lanthanide Catalysts. Utility in Accessing Organotin Synthons. Organometallics 2012, 32, 1317–1327. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, S.; Shah, N.A.; Muhammad, N.; Tahir, M.N.; Khalid, N.; Khan, M.R. Catalytic, biological and DNA binding studies of organotin(IV) carboxylates of 3-(2-fluorophenyl)-2-methylacrylic acid: Synthesis, spectroscopic characterization and X-ray structure analysis. Polyhedron 2013, 57, 127–137. [Google Scholar] [CrossRef]
- Hadjikakou, S.K.; Hadjiliadis, N. Antiproliferative and anti-tumor activity of organotin compounds. Coord. Chem. Rev. 2009, 253, 235–249. [Google Scholar] [CrossRef]
- Ma, C.L.; Zhang, S.L.; Zhang, R.F. Chiral self-assembly of triorganotin complexes: Syntheses, characterization, crystal structures and antitumor activity of organotin(IV) complexes containing (R)-(+)-methylsuccinic acid, (S)-(+)-methylglutaric acid and l-(−)-malic acid ligands. Polyhedron 2012, 31, 478–485. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Mohapatra, C.; Banerjee, R.; Mallick, A. Synthesis, Structure, and H2/CO2 Adsorption in a Three-Dimensional 4-Connected Triorganotin Coordination Polymer with a sqc Topology. Inorg. Chem. 2013, 52, 3579–3581. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Shao, K.Z.; Yan, L.S.; Mei, Z.M.; Zhu, D.S.; Xu, L. A novel macrocyclic organotin carboxylate containing a nona-nuclear long ladder. Dalton Trans. 2013, 42, 15387–15390. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, V.; Mahapatra, C. 2D-Coordination Polymer Containing Interconnected 82-Membered Organotin Macrocycles. Cryst. Growth Des. 2013, 13, 4655–4658. [Google Scholar] [CrossRef]
- Li, D.M.; Hu, R.T.; Zhou, W.; Sun, P.P.; Kan, Y.H.; Tian, Y.P.; Zhou, H.P.; Wu, J.Y.; Tao, X.T.; Jiang, M.H. Synthesis, Structures, and Photophysical Properties of Two Organostannoxanes from a Novel Acrylic Acid Derived from Phenothiazine. Eur. J. Inorg. Chem. 2009, 18, 2664–2672. [Google Scholar] [CrossRef]
- Zhao, X.S.; Liu, J.; Wang, H.; Zou, Y.; Li, S.L.; Zhang, S.Y.; Zhou, H.P.; Wu, J.Y.; Tian, Y.P. Synthesis, crystal structures and two-photon absorption properties of triphenylamine cyanoacetic acid derivative and its organooxotin complexes. Dalton Trans. 2015, 44, 701–709. [Google Scholar] [CrossRef] [PubMed]

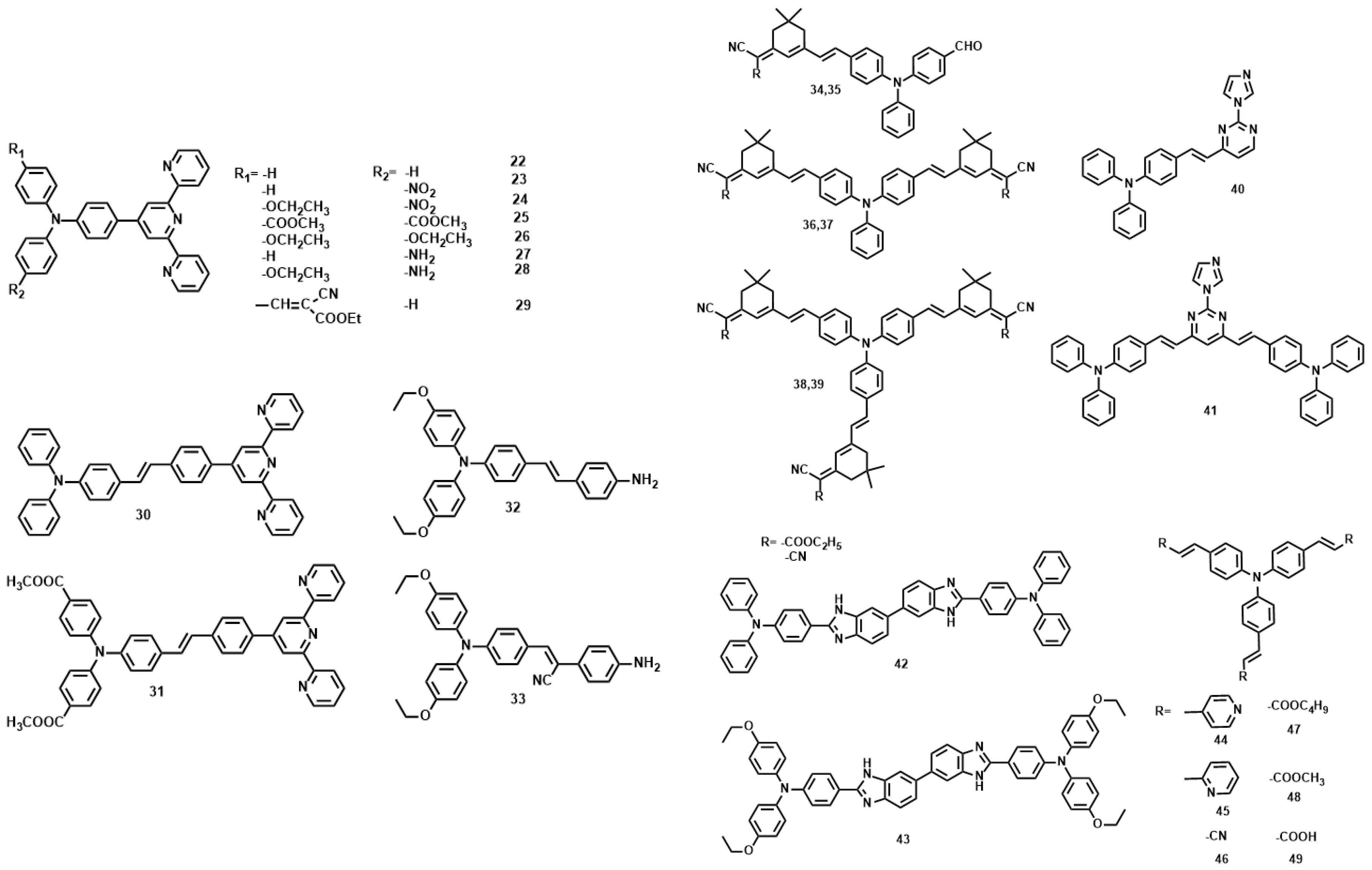
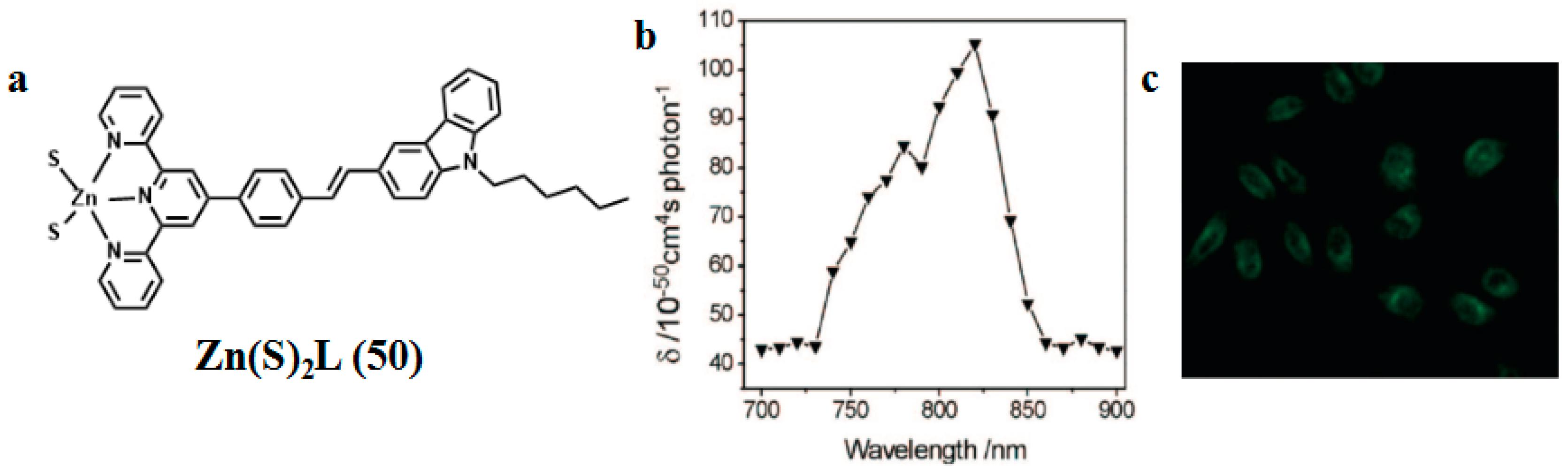
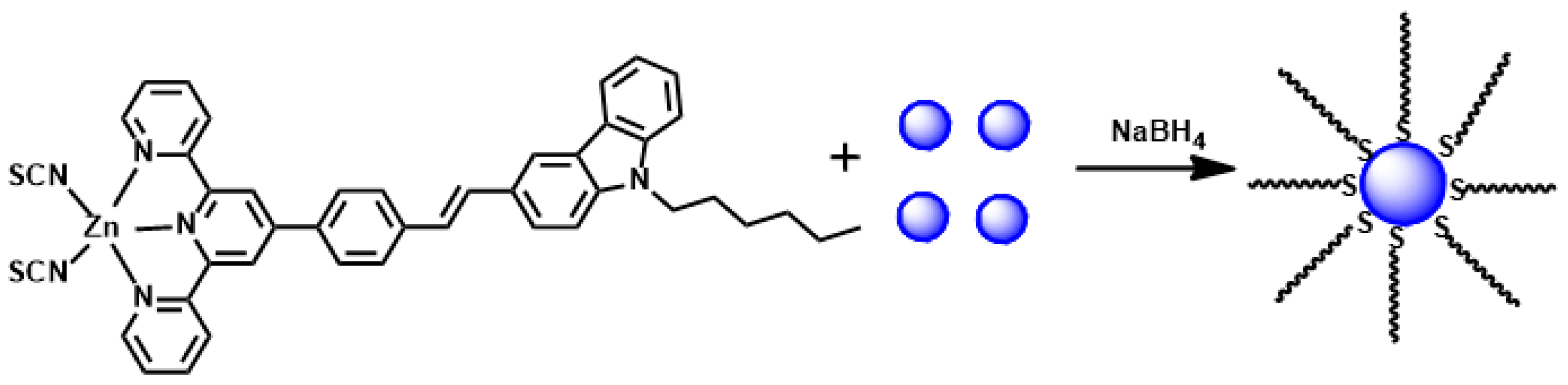

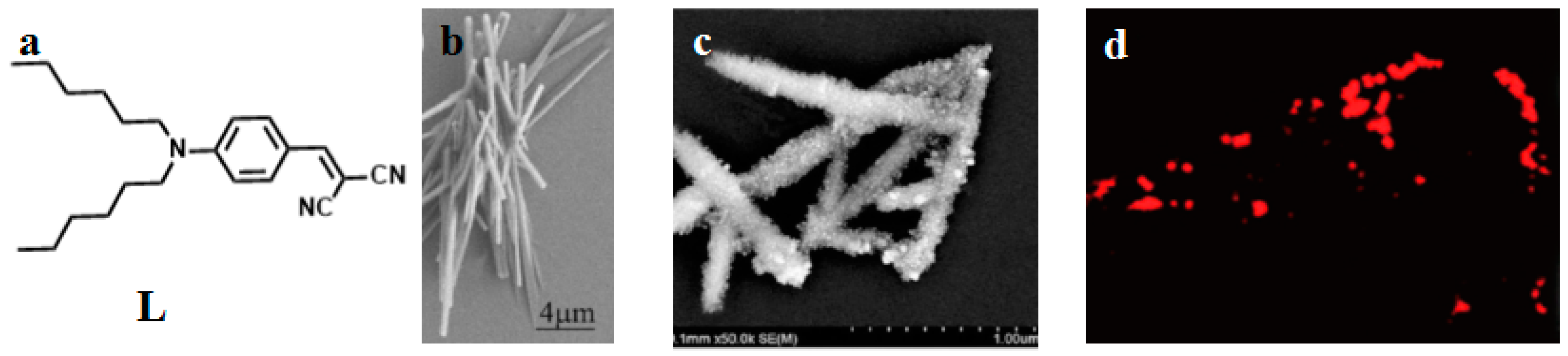
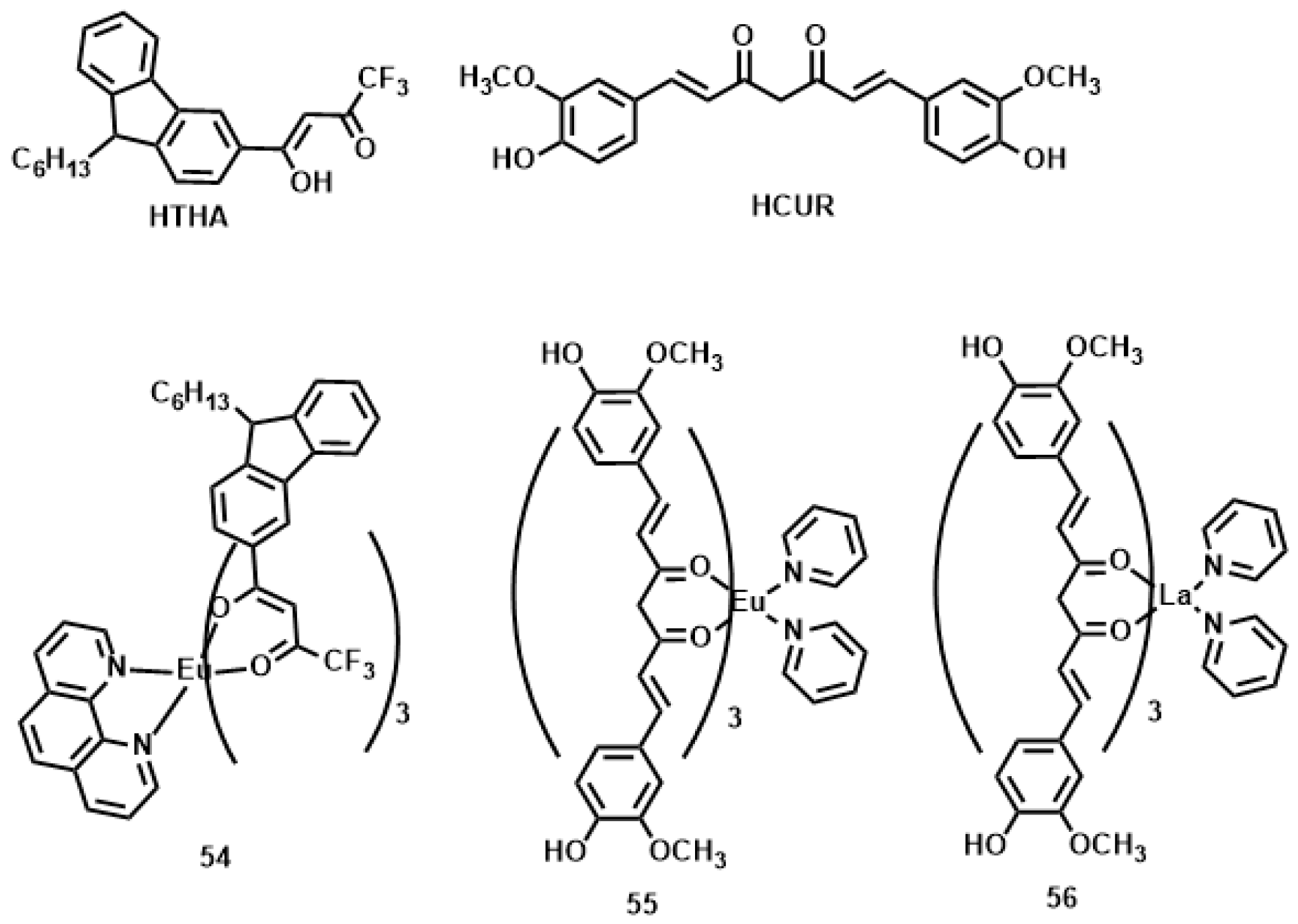
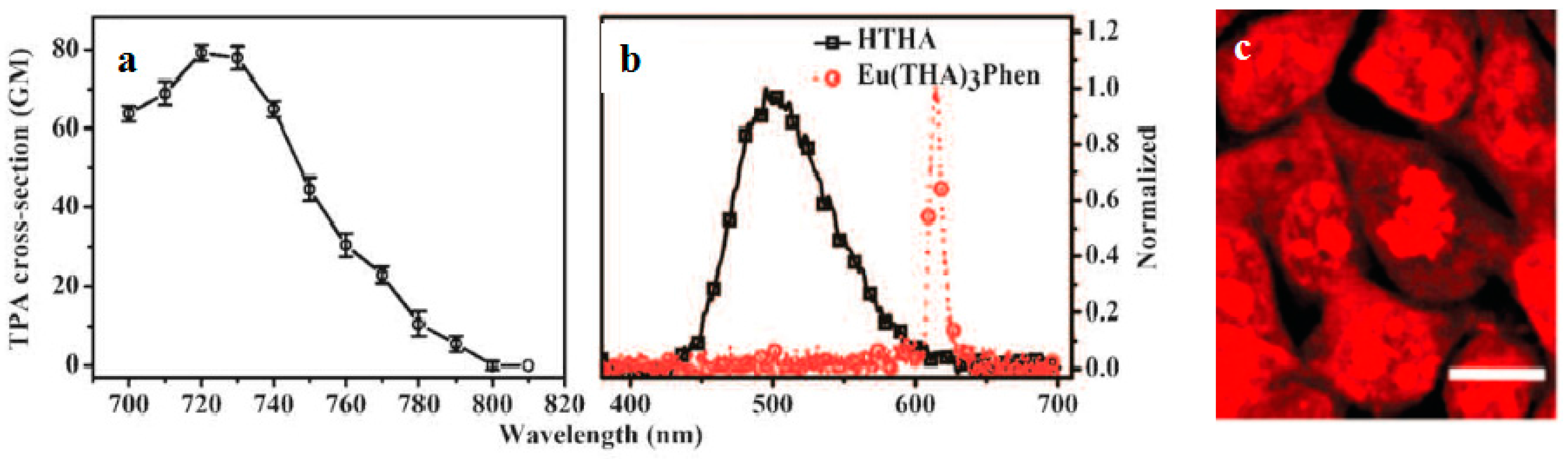
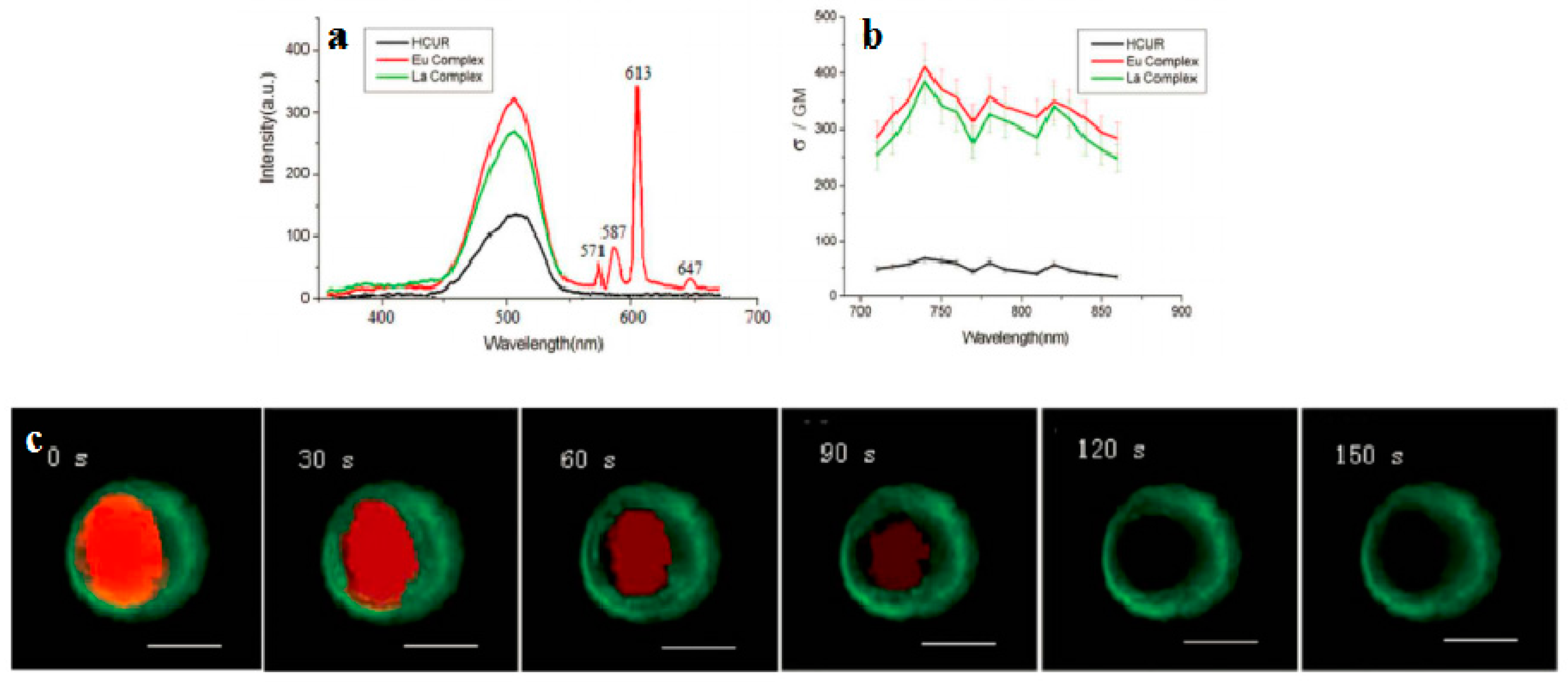
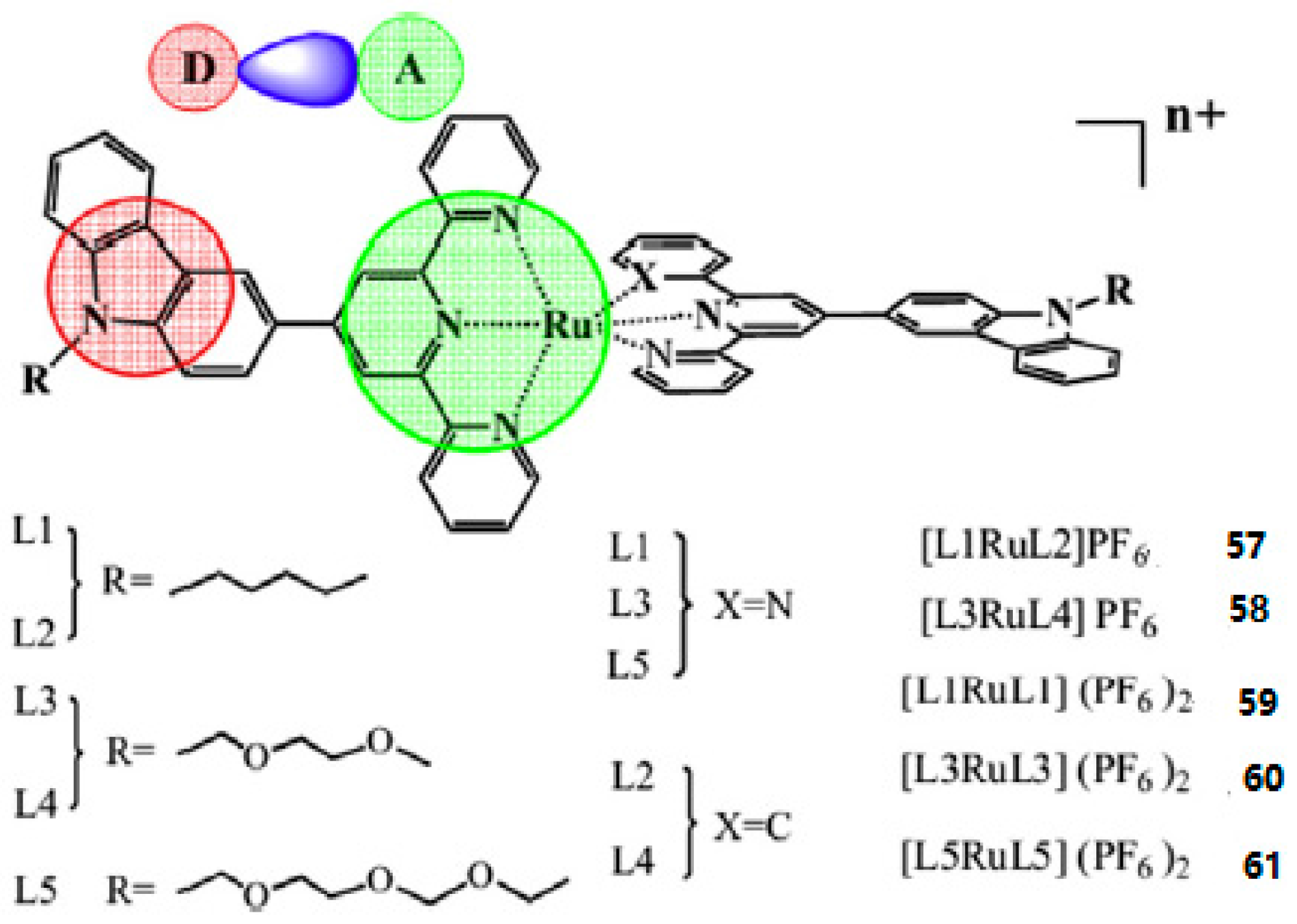
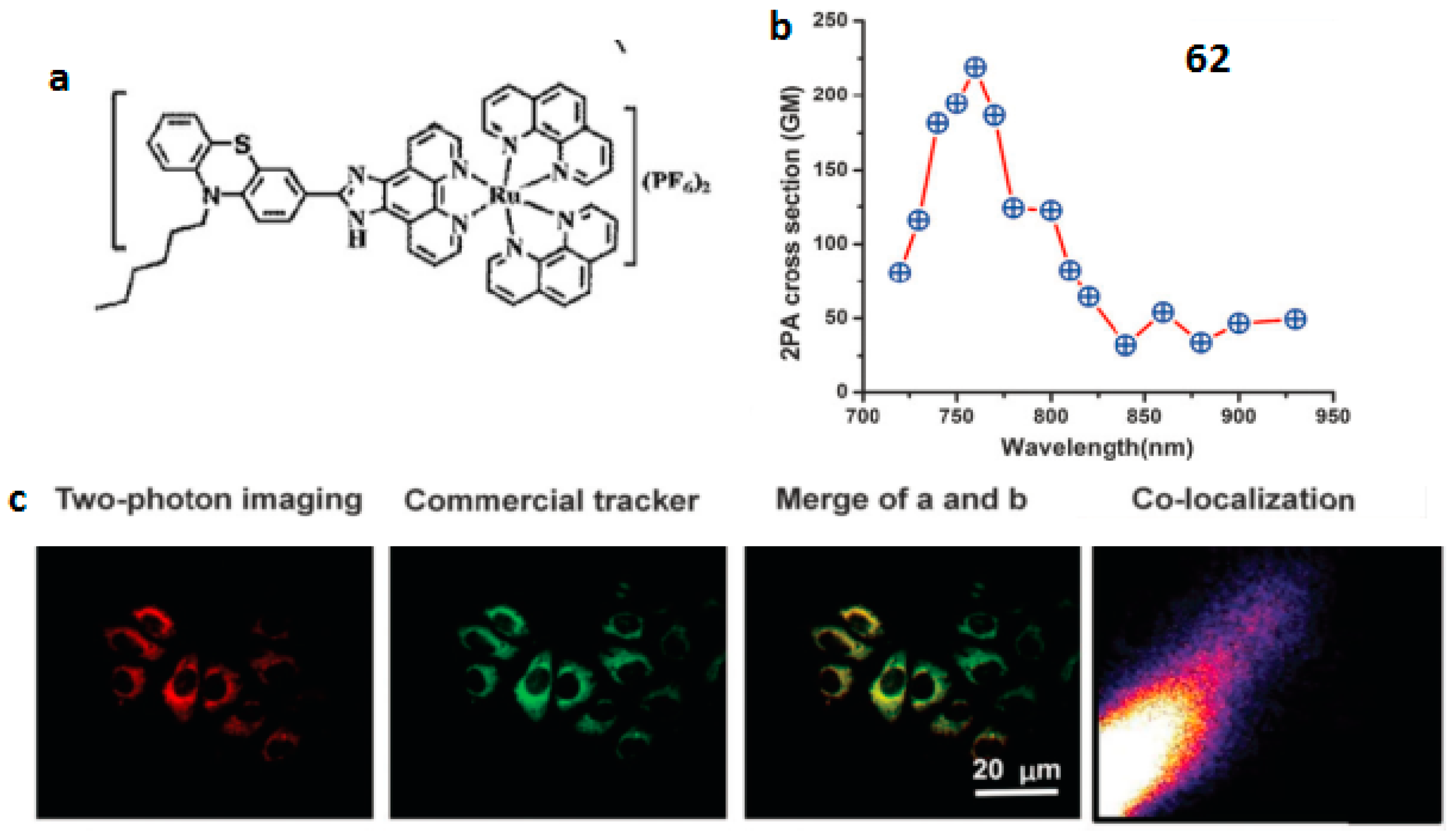
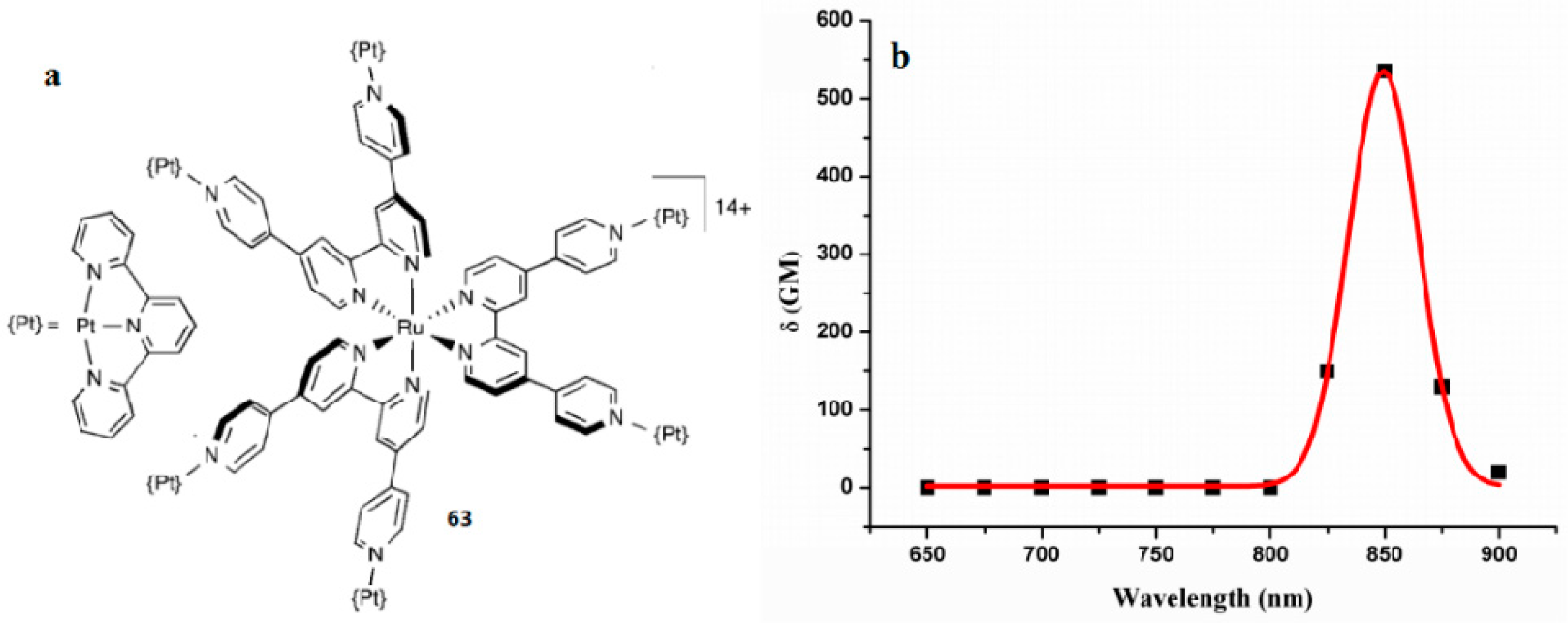
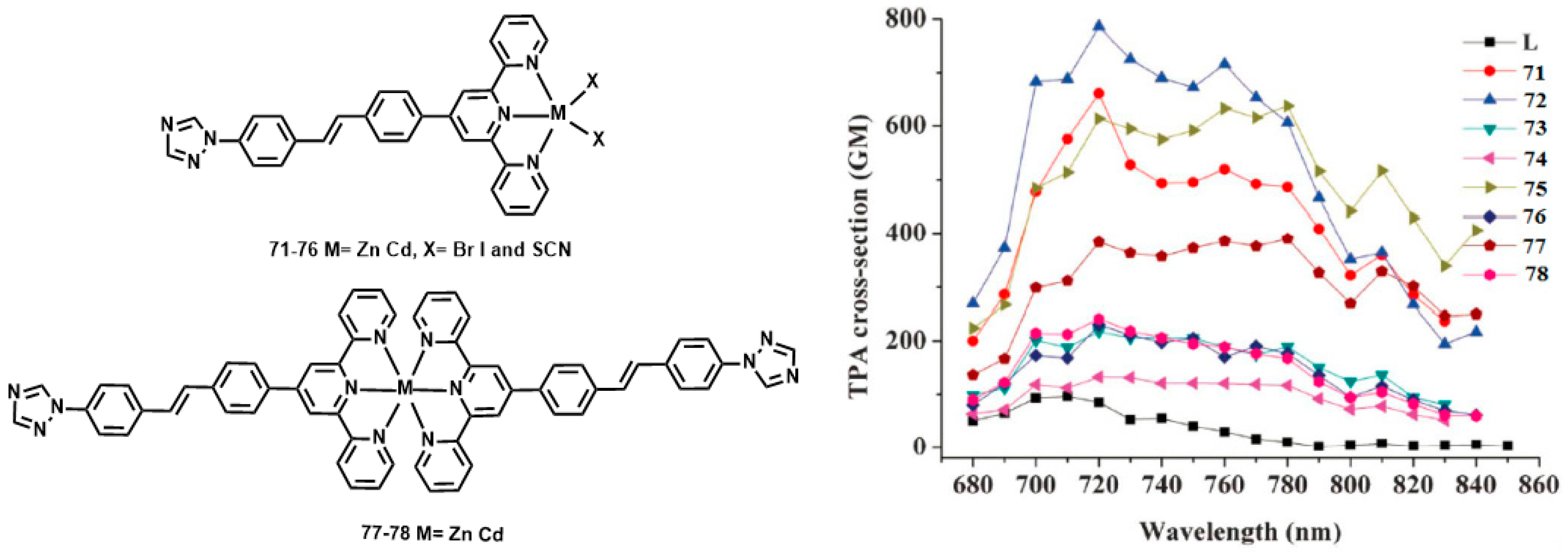
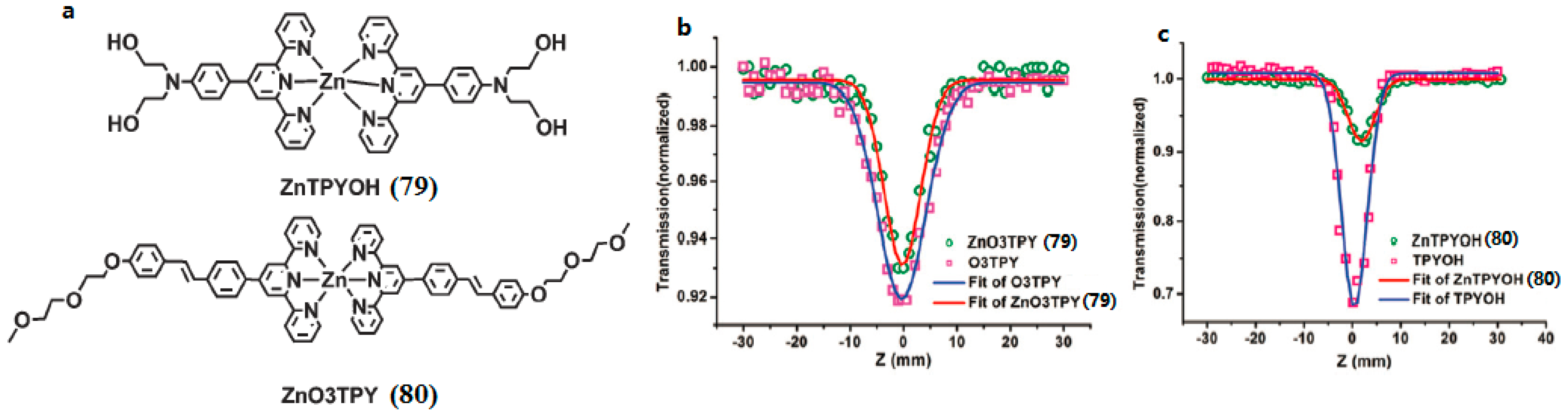
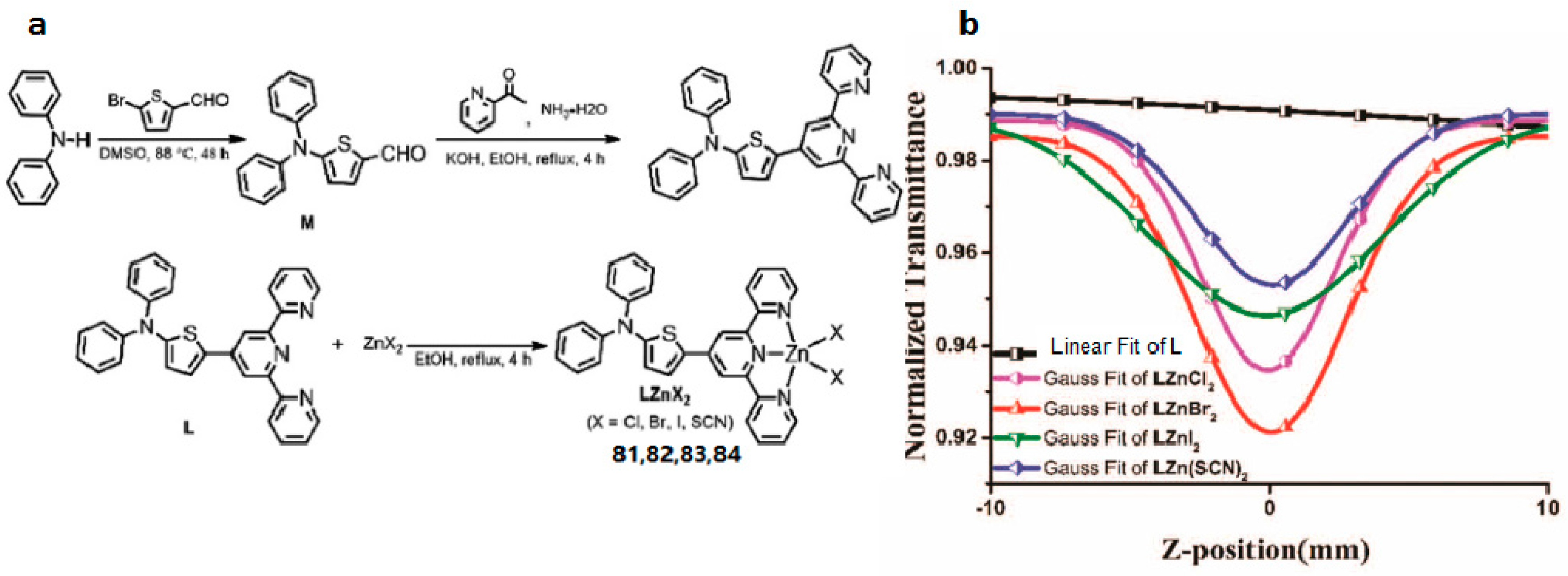
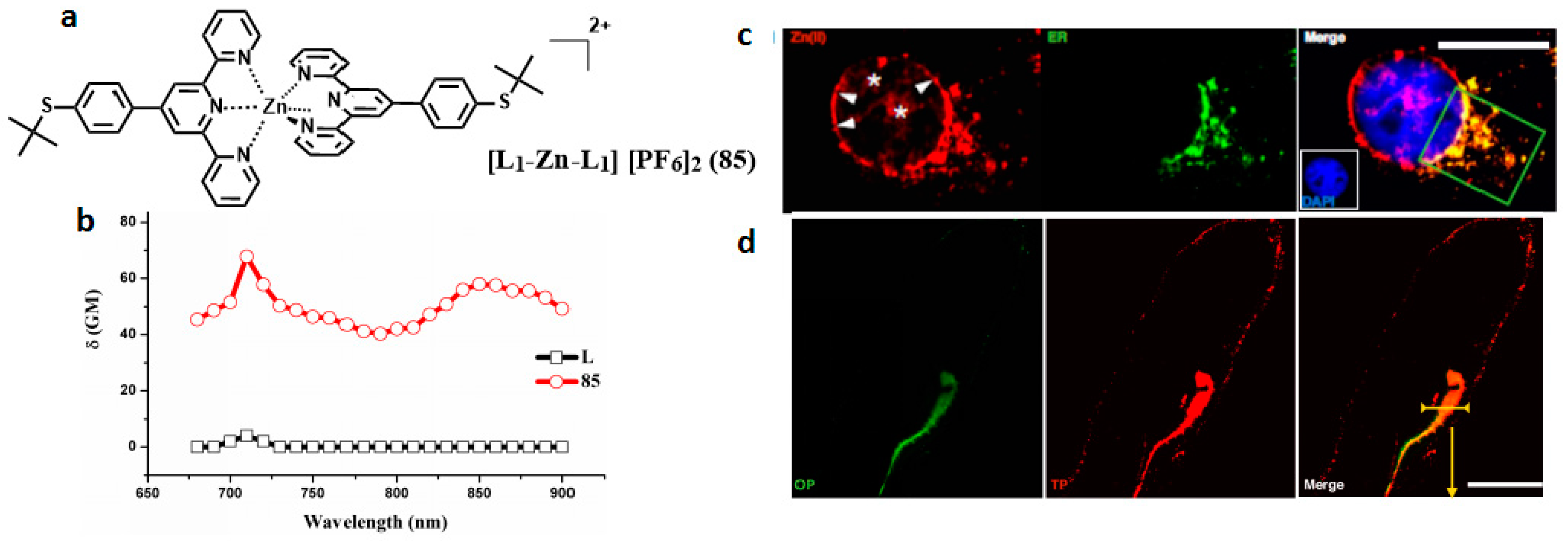
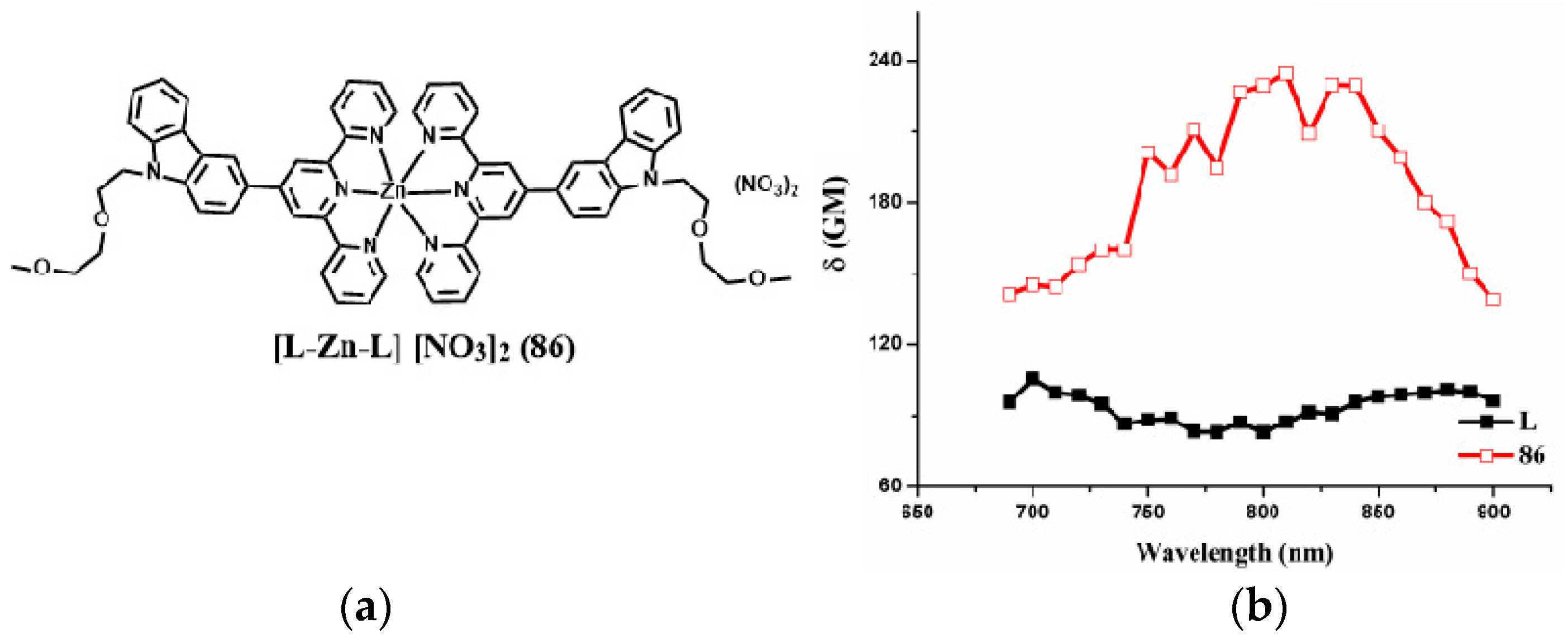

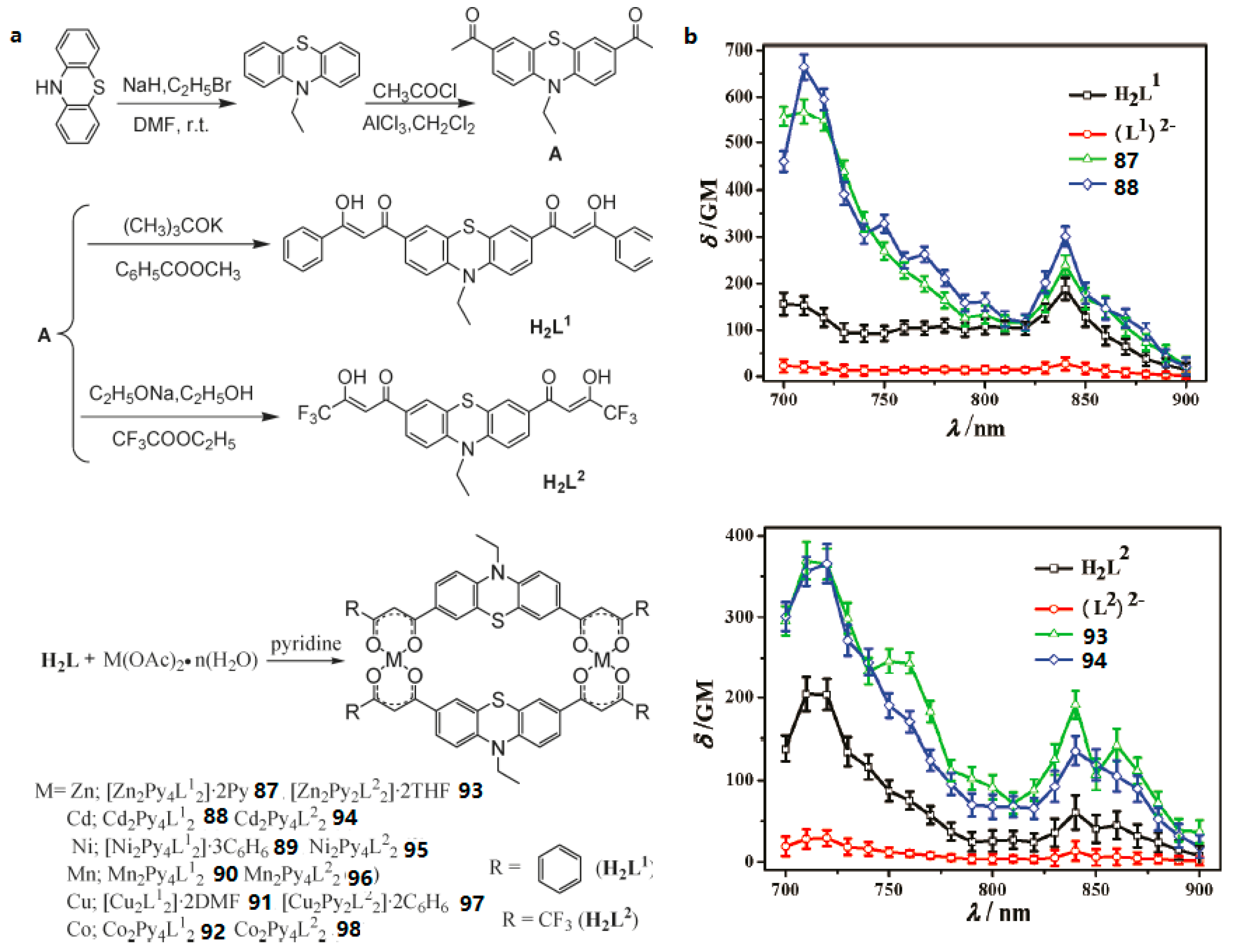
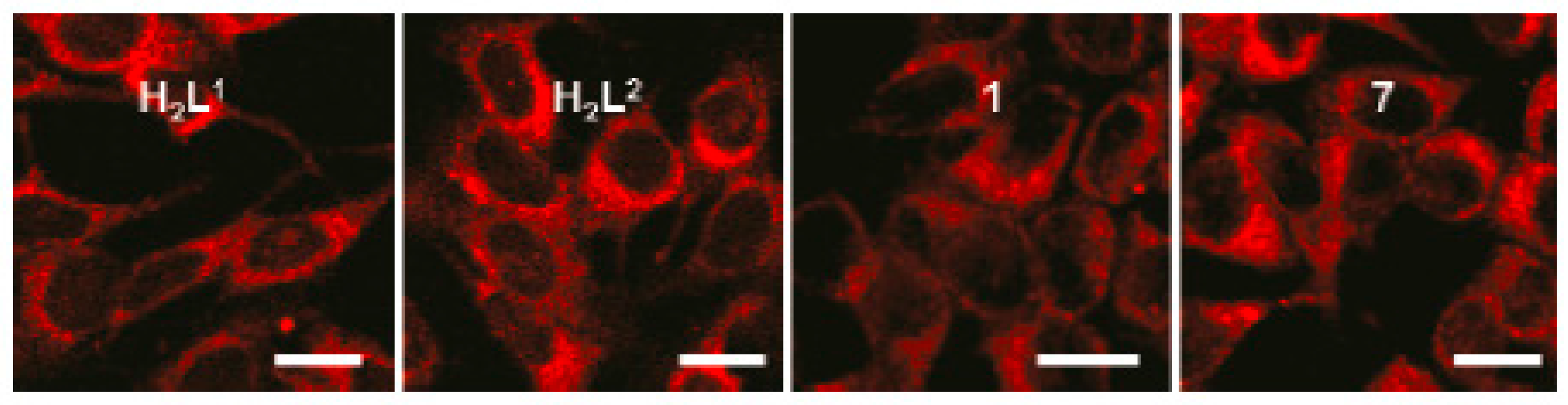
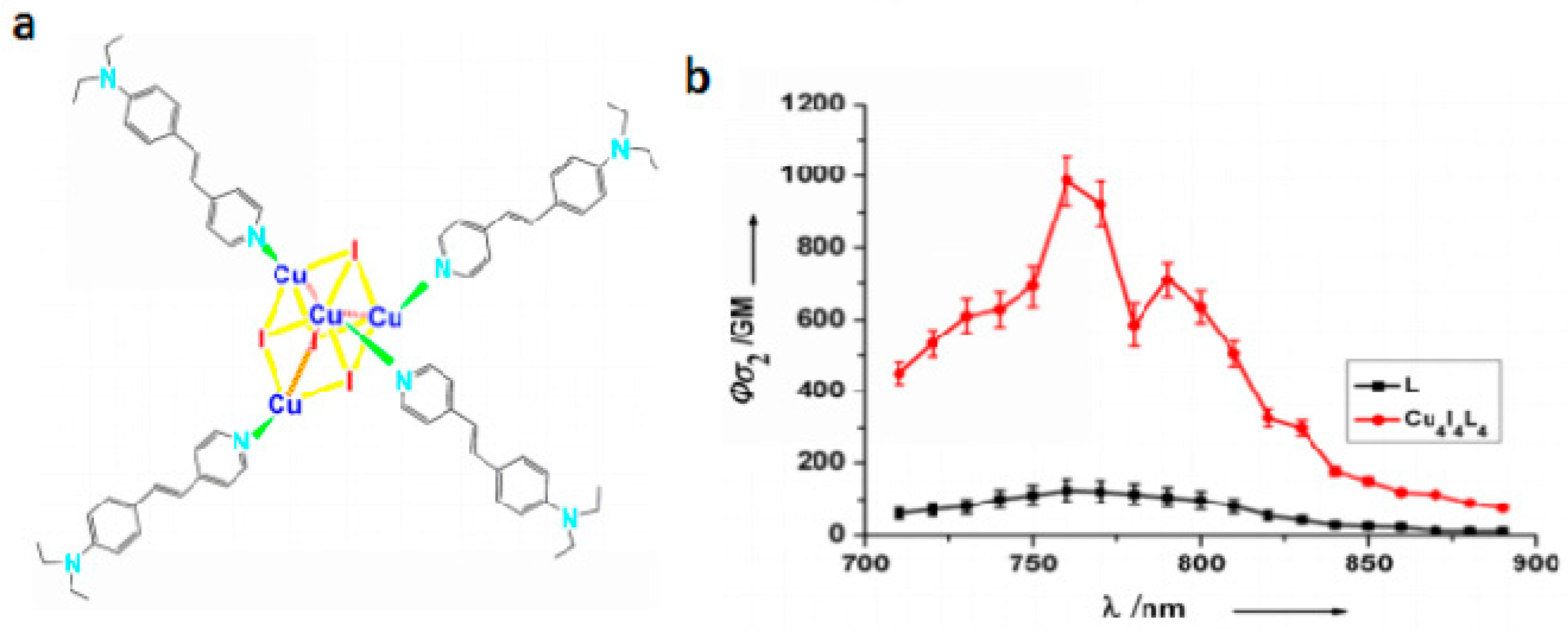

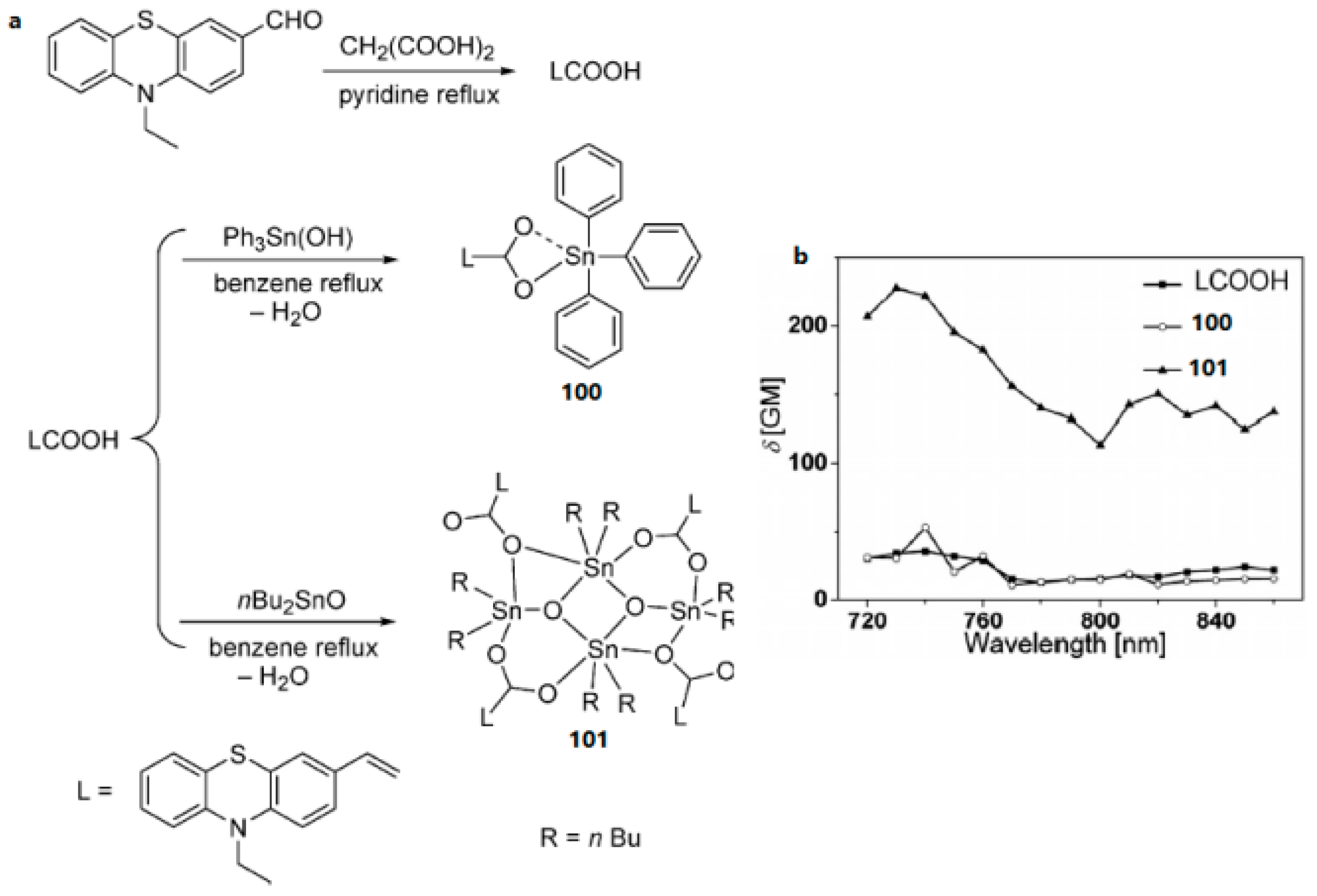

| Compounds | max a (nm) | max b (nm) | max c (nm) | max d (nm) | δ e |
|---|---|---|---|---|---|
| 1 | 471 | 603 | 621 | 1064 | 134 |
| 2 | 482 | 601 | 627 | 1064 | 122 |
| 3 | 486 | 604 | 627 | 1064 | 136 |
| 4 | 486 | 605 | 625 | 1064 | 123 |
| 5 | 426 | 521 | 586 | 760 | 14.8 |
| 6 | 340 | 585 | 615 | 750 | 309 |
| 7 | 480 | 603 | 598 | 960 | 48 |
| 8 | 485 | 606 | 598 | 960 | 57 |
| 9 | 474 | 597 | 600 | 960 | 115 |
| 10 | 472 | 595 | 620 | 960 | 517 |
| Compounds | max a (nm) | max b (nm) | max c (nm) | max d (nm) | δ e |
|---|---|---|---|---|---|
| 11 | 380 | 457 | 495 | 720 | 8 |
| 12 | 402 | 443 | 483 | 740 | 10 |
| 13 | 452 | 566 | 572 | 820 | 498 |
| 14 | 406 | 520 | 570 | 800 | 104 |
| 15 | 416 | 529 | 550 | 870 | 151 |
| 16 | 471 | 573 | 600 | 830 | 1319 |
| 17 | 460 | 569 | 590 | 800 | 1885 |
| 18 | 480 | 630 | 610 | 820 | 375 |
| 19 | 451 | 600 | 590 | 840 | 216 |
| 20 | 458 | 561 | 560 | 840 | 742 |
| 21 | 459 | 561 | 562 | 840 | 170 |
| Compounds | max a (nm) | max b (nm) | max c (nm) | max d (nm) | δ e |
|---|---|---|---|---|---|
| 22 | 286, 357 | 487 | 525 | 720 | 434 |
| 23 | 276, 327, 406 | - | - | 760 (Z-scan) | 415 (Z-scan) |
| 24 | 276, 327, 409 | - | - | 760 (Z-scan) | 462 (Z-scan) |
| 25 | 277, 352 | 446 | 495 | 720 | 179 |
| 26 | 286, 369 | 526 | 575 | 750 | 70 |
| 27 | 285, 370 | 485 | 580 | 750 | 295 |
| 28 | 285, 375 | 487 | 574 | 750 | 308 |
| 29 | 338, 429 | 494 | - | 690 (Z-scan) | 7938 (Z-scan) |
| 30 | 294, 526 | 526 | 562 | 720 | 2869 |
| 31 | 290, 360 | 505 | 520 | 840 | 1019 |
| 32 | 370 | 470 | 500 | 690 | 327 |
| 33 | 412 | 500 | 550 | 700 | 394 |
| 34 | 347, 438 | 570 | 605 | 940 | 660 |
| 35 | 349, 449 | 583 | 608 | 940 | 999 |
| 36 | 348, 484 | 591 | 613 | 860 | 1830 |
| 37 | 350, 497 | 602 | 620 | 880 | 2087 |
| 38 | 355, 484 | 596 | 623 | 860 | 5382 |
| 39 | 355, 499 | 602 | 629 | 860 | 9398 |
| 40 | 295, 410 | 548 | 566 | 890 | 121 |
| 41 | 297, 442 | 569 | 603 | 890 | 138 |
| 42 | 374 | 417 | 442 | 720 | 220 |
| 43 | 374 | 478 | 528 | 700 | 777 |
| 44 | 396 | 517 | 536 | 780 | 623 |
| 45 | 387 | 498 | 527 | 780 | 595 |
| 46 | 387 | 495 | 510 | 780 | 285 |
| 47 | 398 | 509 | 522 | 780 | 392 |
| 48 | 397 | 505 | 530 | 780 | 287 |
| 49 | 388 | 503 | 523 | 780 | 190 |
| Non-linearity Parameters | S | S-Au NPs | Au NPs |
|---|---|---|---|
| λmax a (nm) | 790 | 790 | 790 |
| β (cm·GW−1) | 0.11 | 0.14 | 0.069 |
| δ (GM) | 4595 | 5849 | 2874 |
| γ (cm2·GW−1) (×10−14) | 4.62 | 5.19 | - |
| Re(χ(3)) [esu] (×10−14) | 2.40 | 2.69 | - |
| Im(χ(3)) [esu] (×10−16) | 3.59 | 4.60 | - |
| Non-Linearity Parameters | L Nanorods | Nanohybrid 53 |
|---|---|---|
| λmax a (nm) | 800 | 840 |
| β (cm·GW−1) | 0.58 | 1.60 |
| δ (GM) | 1353 | 5731 |
| γ (cm2·GW−1) | 5.81 × 10−16 | 1.10 × 10−14 |
| Re(χ(3)) [esu] | 3.01 × 10−14 | 5.70 × 10−13 |
| Im(χ(3)) [esu] | 1.07 × 10−7 | 5.07 × 10−7 |
| Non-linearity Parameters | 57 | 58 | 59 | 60 | 61 |
|---|---|---|---|---|---|
| λmax a | 730 | 730 | 740 | 730 | 730 |
| β (cm·GW−1) | 0.086 | 0.105 | 0.032 | 0.049 | 0.083 |
| δ (GM) | 3888 | 4747 | 1427 | 2228 | 3766 |
| γ (cm2·GW−1) (×10−15) | 8.12 | 5.02 | 4.30 | 5.25 | 7.09 |
| Re(χ(3)) [esu] × 10−13 | 4.51 | 2.79 | 2.39 | 2.92 | 3.94 |
| Im(χ(3)) [esu] × 10−14 | 2.78 | 3.39 | 1.05 | 1.59 | 2.69 |
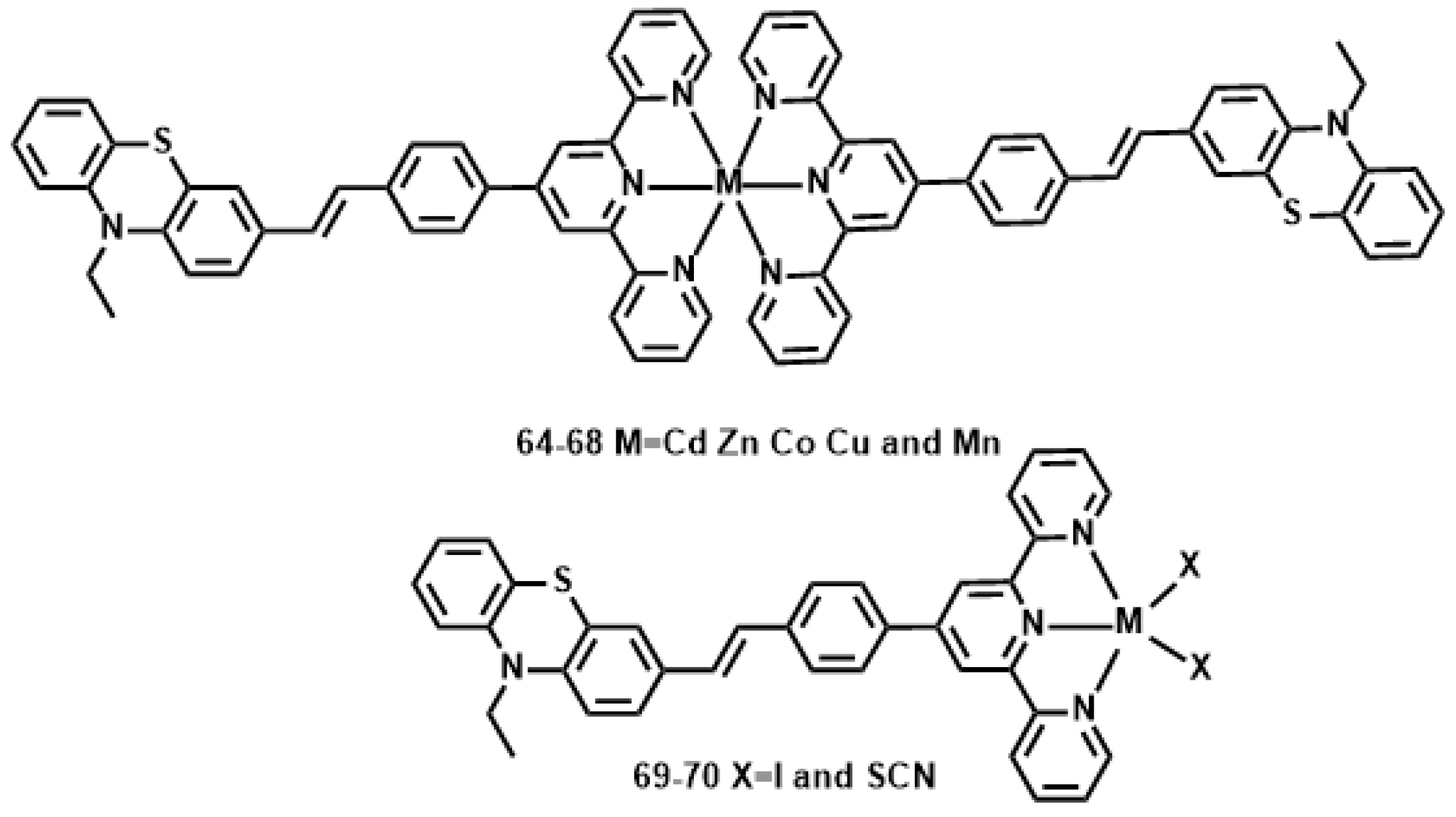
| Compound | λmax a | λmax b | β × 10−3 (cm·GW−1) | δ × 103 GM |
|---|---|---|---|---|
| L | 306,394 | 491,561 | 14 | 5.8 |
| 64 | 310,396 | 492,564 | 49 | 20 |
| 65 | 315,397 | 494,562 | 51 | 21 |
| 66 | 326,401 | 496,564 | 45 | 19 |
| 67 | 308,396 | 499,572 | 38 | 15 |
| 68 | 311,397 | 495,572 | 34 | 14 |
| 69 | 320,301 | 491,571 | 47 | 19 |
| 70 | 320,400 | 491,570 | 43 | 18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Tian, X.; Zhou, H.; Wu, J.; Tian, Y. Lighting the Way to See Inside Two-Photon Absorption Materials: Structure–Property Relationship and Biological Imaging. Materials 2017, 10, 223. https://doi.org/10.3390/ma10030223
Zhang Q, Tian X, Zhou H, Wu J, Tian Y. Lighting the Way to See Inside Two-Photon Absorption Materials: Structure–Property Relationship and Biological Imaging. Materials. 2017; 10(3):223. https://doi.org/10.3390/ma10030223
Chicago/Turabian StyleZhang, Qiong, Xiaohe Tian, Hongping Zhou, Jieying Wu, and Yupeng Tian. 2017. "Lighting the Way to See Inside Two-Photon Absorption Materials: Structure–Property Relationship and Biological Imaging" Materials 10, no. 3: 223. https://doi.org/10.3390/ma10030223
APA StyleZhang, Q., Tian, X., Zhou, H., Wu, J., & Tian, Y. (2017). Lighting the Way to See Inside Two-Photon Absorption Materials: Structure–Property Relationship and Biological Imaging. Materials, 10(3), 223. https://doi.org/10.3390/ma10030223






