A Novel Preparation Method of Two Polymer Dyes with Low Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Structure Characterization of Prepared Polymer Dyes
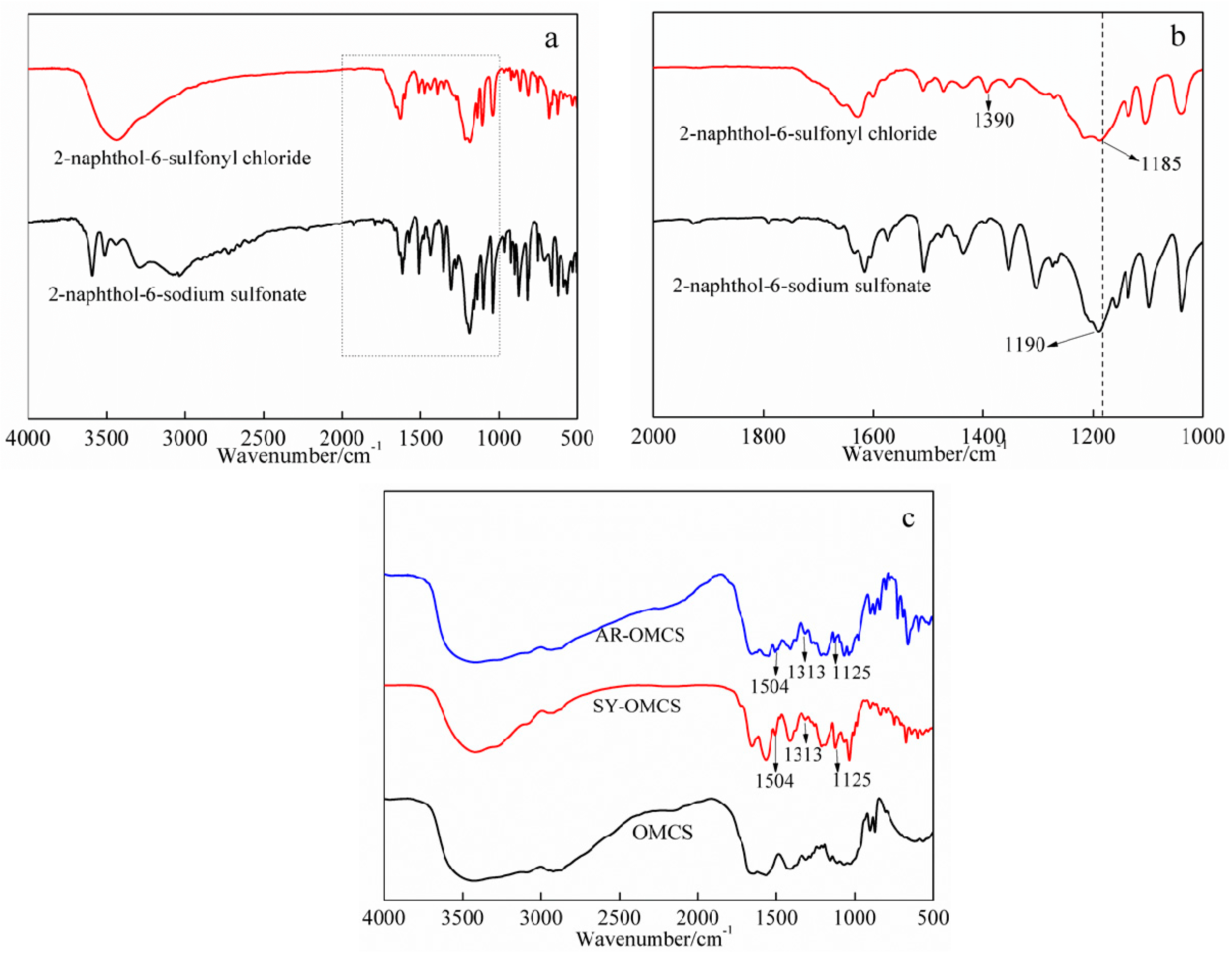
2.1.1. FT-IR Analysis
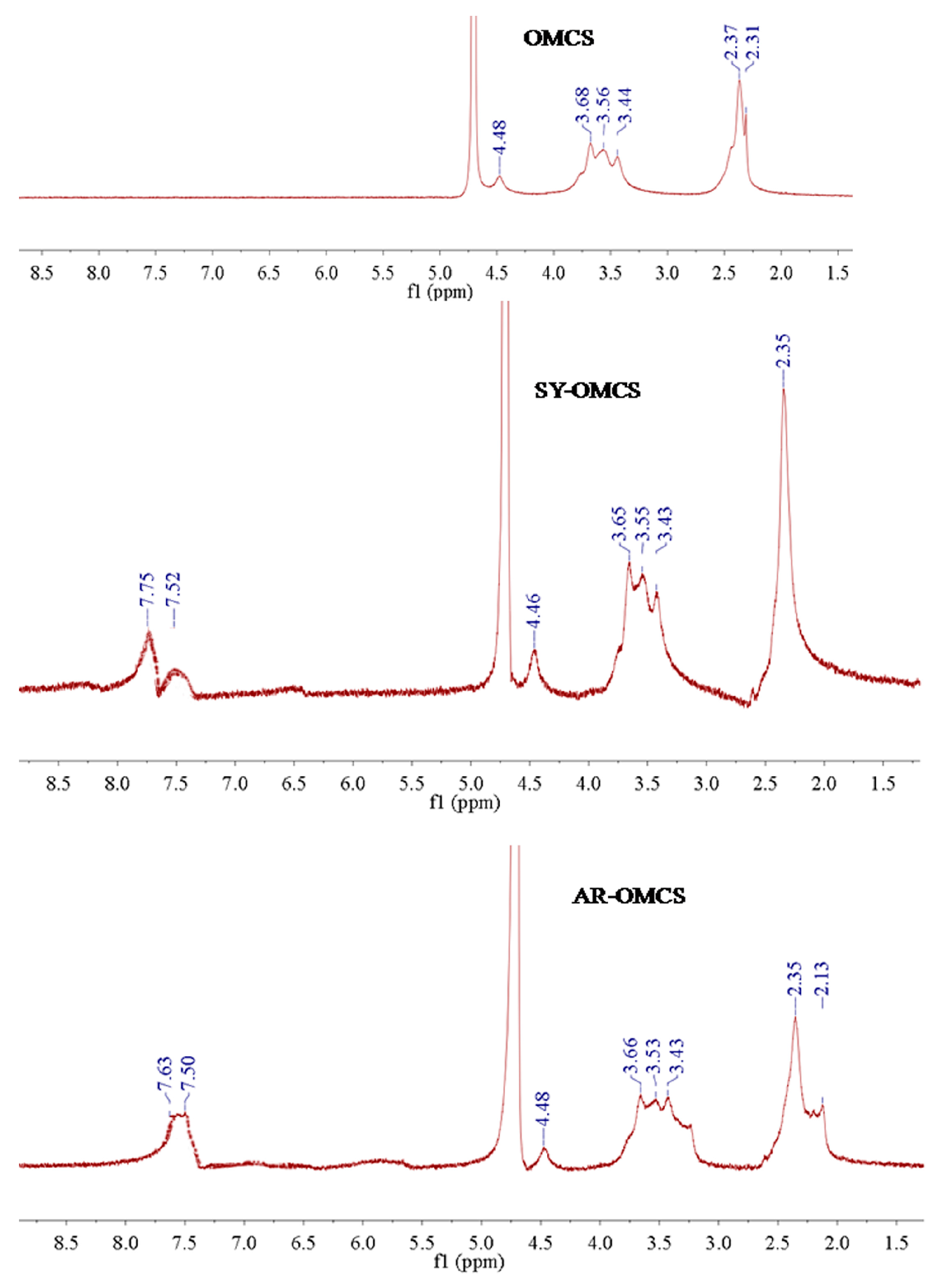
2.1.2. 1H-NMR Analysis
2.2. Grafting Degree and Water Solubility of Prepared Polymer Dyes
2.2.1. Grafting Degree Determination by Element Analysis
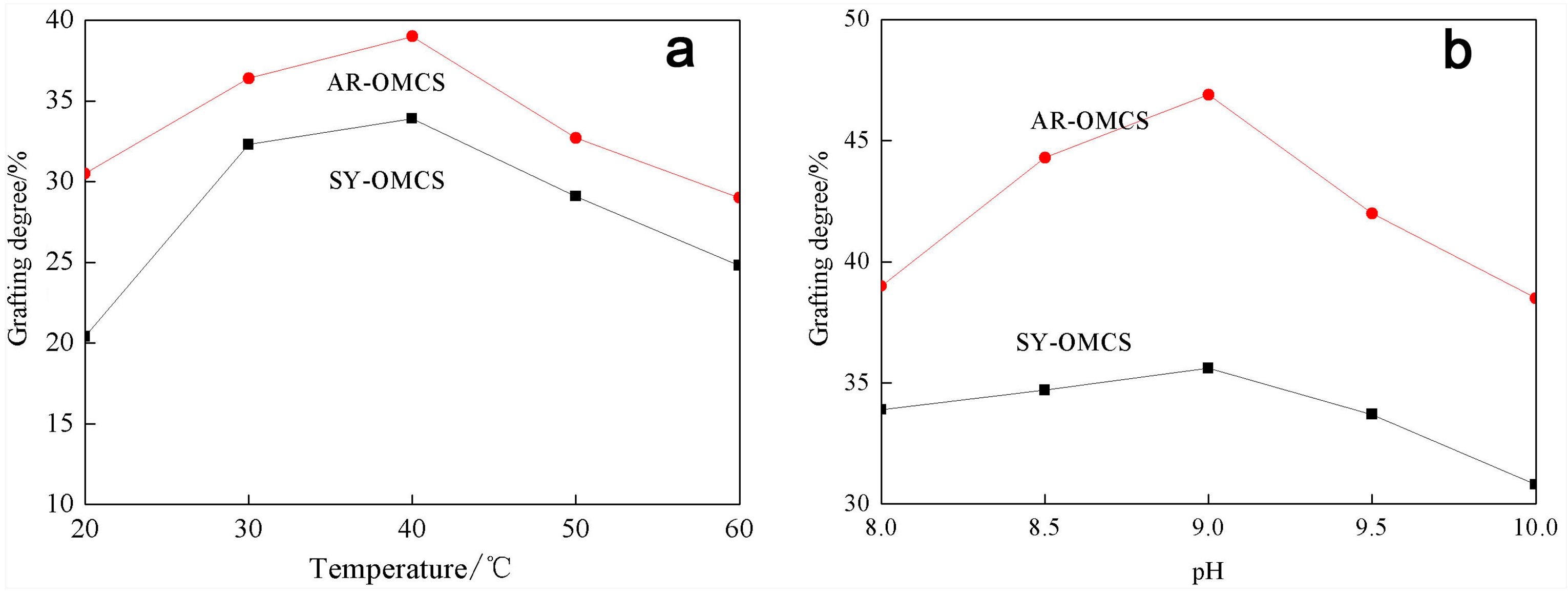
2.2.2. Effect of Amidation Reaction Conditions on the Grafting Degree of SY and AR onto OMCS
2.2.3. Water Solubility Measurement of Prepared Polymer Dyes
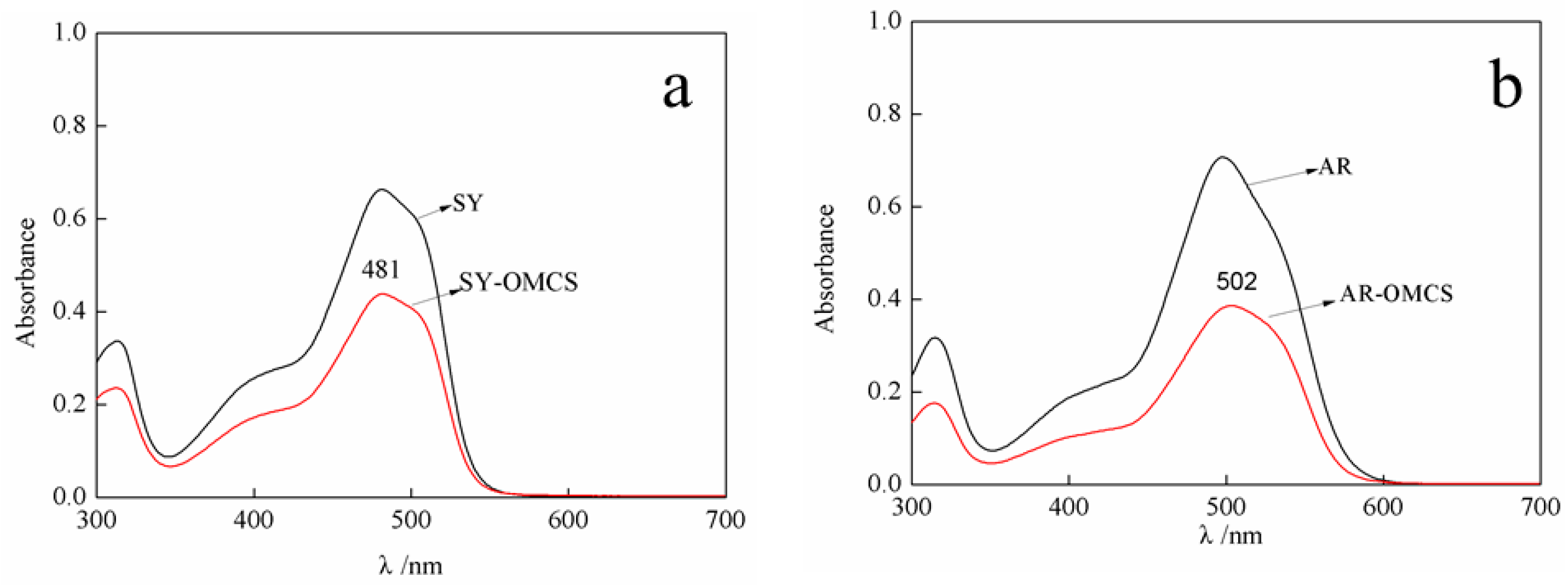
2.3. UV-Vis Spectra Analysis of Prepared Polymer Dyes
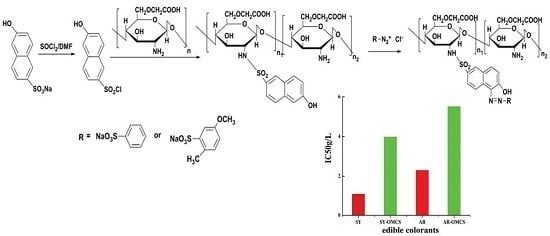
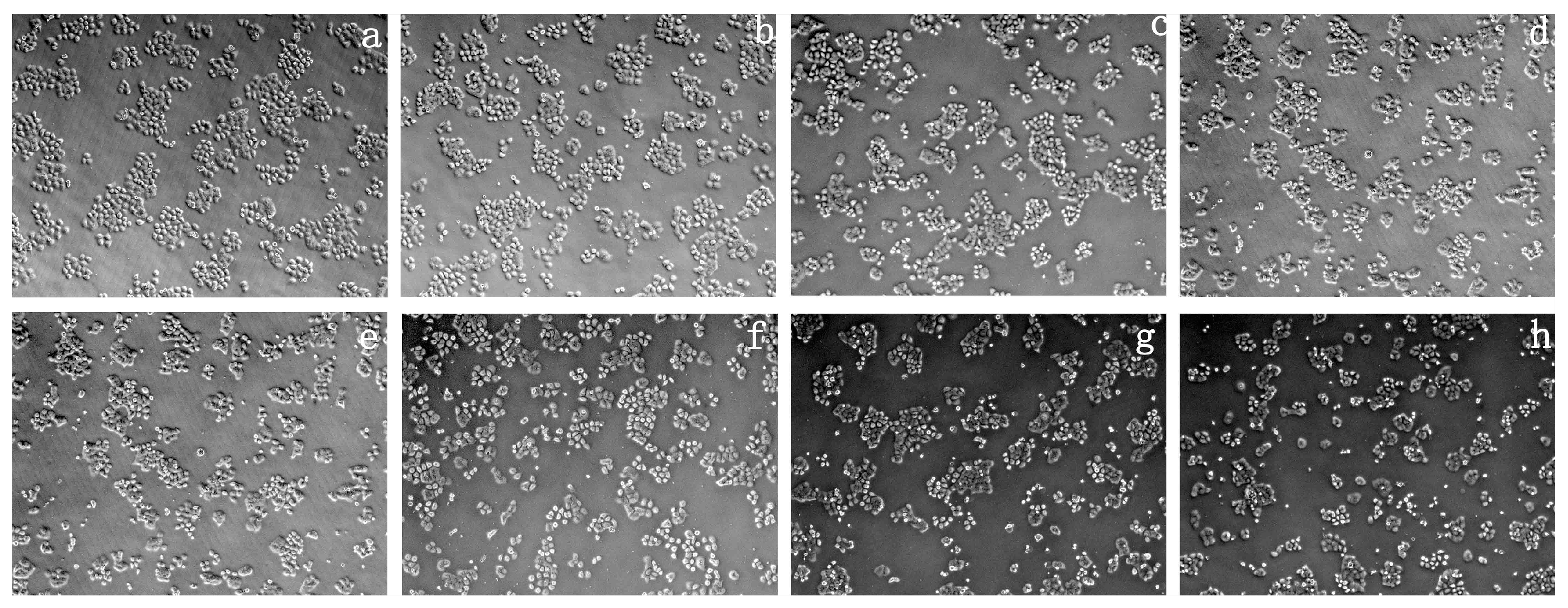
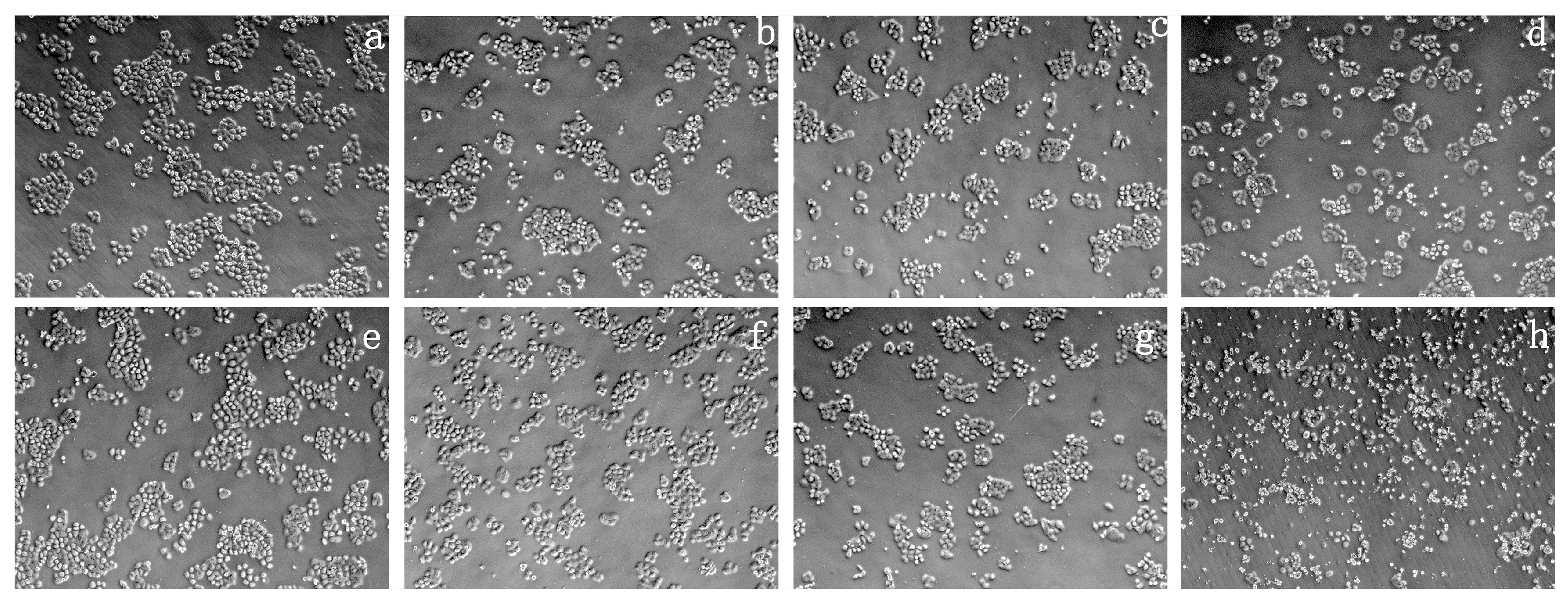
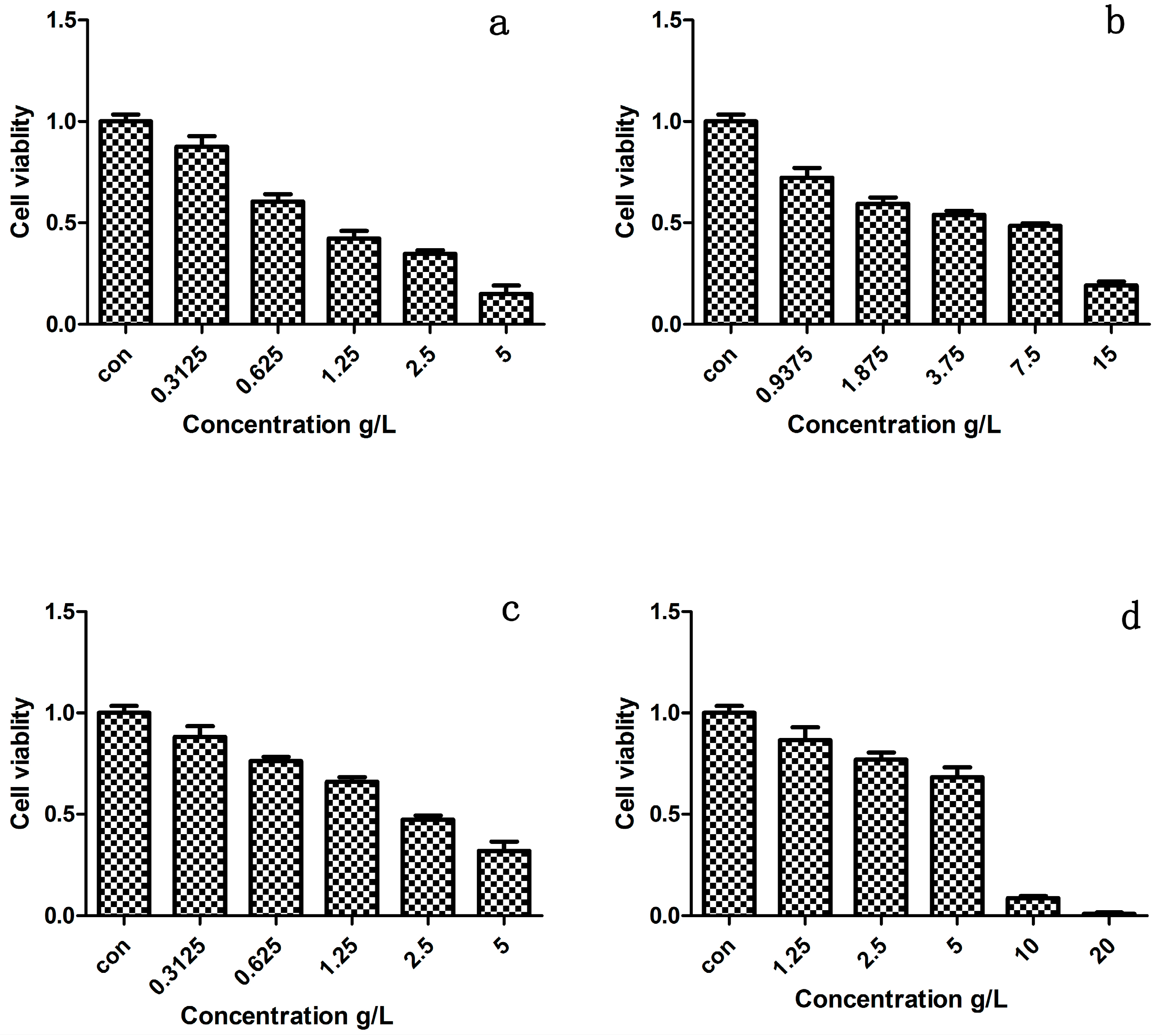
2.4. Cytotoxicity Test of Prepared Polymer Dyes
3. Materials and Methods
3.1. Material
3.2. Methods
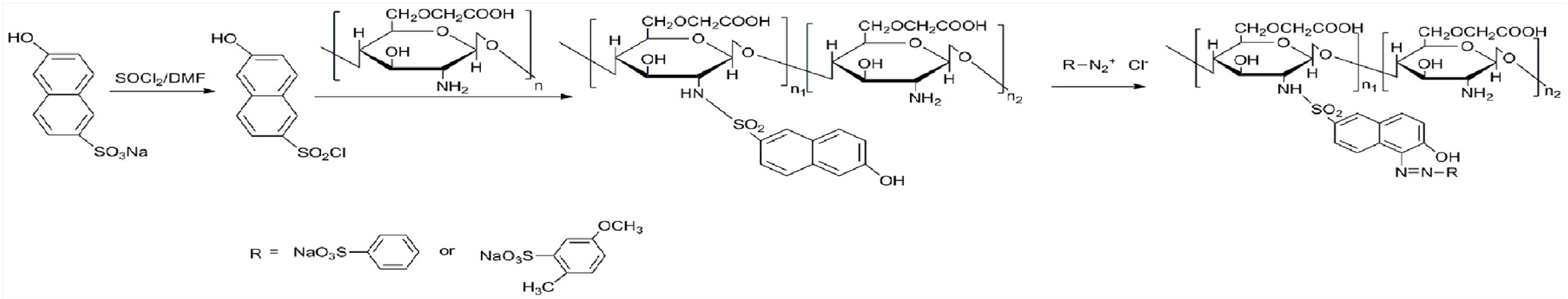
3.2.1. Preparation of Diazonium Salt
3.2.2. Preparation of Sulfonyl Chloride
3.2.3. Preparation of Polymer Dyes
3.2.4. Determination of Grafting Degree of the Azo Dyes onto OMCS
3.2.5. Water Solubility Measurement of Prepared Polymer Dyes
3.2.6. Characterization of Prepared Polymer Dyes
3.2.7. Cytotoxicity in LO2 Cell Line
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tikhomirova, T.I.; Ramazanova, G.R.; Apyari, V.V. A hybrid sorption–spectrometric method for determination of synthetic anionic dyes in foodstuffs. Food Chem. 2017, 221, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.I.D.; Guedes, M.I.F.; Vieira, Í.G.P.; Mendes, F.N.P.; Rodrigues, P.A.S.; Maia, C.S.C.; Ávila, M.M.M.; Ribeiro, L.D.M. Determination of synthetic food dyes in commercial soft drinks by TLC and ion-pair HPLC. Food Chem. 2014, 157, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Hallagan, J.B.; Allen, D.C.; Borzelleca, J.F. The safety and regulatory status of food, drug and cosmetics colour additives exempt from certification. Food Chem. Toxicol. 1995, 33, 515–528. [Google Scholar] [CrossRef]
- Amin, K.A.; Abd Elsttar, A.H. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem. Toxicol. 2010, 48, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Mehedi, N.; Mokrane, N.; Alami, O.; Ainad-Tabet, S.; Zaoui, C.; Kheroua, O.; Saidi, D. A thirteen week ad libitum administration toxicity study of Tartrazine in Swiss mice. Afr. J. Biotechnol. 2013, 12, 4519–4529. [Google Scholar]
- Ceyhan, B.M.; Gultekin, F.; Doguc, D.K.; Kulac, E. Effects of maternally exposed coloring food additives on receptor expressions related to learning and memory in rats. Food Chem. Toxicol. 2013, 56, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, A.; Tripathi, A.; Das, M. Sunset yellow FCF, a permitted food dye, alters functional responses of splenocytes at non-cytotoxic dose. Toxicol. Lett. 2013, 217, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Srivastava, N. Genotoxic effects of two commonly used food additives of boric acid and sunset yellow in root meristems of trigonella foenum-graecum. Iran. J. Environ. Health Sci. Eng. 2011, 8, 361–366. [Google Scholar]
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelatos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 2010, 48, 2934–2944. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.F.; Kawaguchi, S.; Kamaya, A.; Ohshita, M.; Kabasawa, K.; Iwama, K.; Taniguchi, K.; Tsuda, S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. 2002, 519, 103–119. [Google Scholar] [CrossRef]
- Wu, D.; Jin, Y.; Wang, J.; Wang, Q.; Li, H. Characterisation of interaction between food colourant allura red AC and human serum albumin: Multispectroscopic analyses and docking simulations. Food Chem. 2015, 170, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N. Determination of Allura red in food samples after cloud point extraction using mixed micelles. Food Chem. 2011, 126, 1465–1469. [Google Scholar] [CrossRef]
- Stevens, L.J.; Kuczek, T.; Burgess, J.R.; Stochelski, M.A.; Arnold, L.E.; Galland, L. Mechanisms of behavioral, atopic, and other reactions to artificial food colors in children. Nutr. Rev. 2013, 71, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.M.; Atta, A.H.; Arbid, M.S.; Nada, S.A.; Asaad, G.F. Immunological studies on Amaranth, Sunset Yellow and Curcumin as food colouring agents in albino rats. Food. Chem. Toxicol. 2010, 48, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Refined exposure assessment for Allura Red AC (E 129). Efsa J. 2015, 13, 4007. [Google Scholar] [CrossRef]
- Biswas, S.J.; Khuda-Bukhsh, A.R. Cytotoxic and genotoxic effects of the azo-dye p-dimethyl aminoazobenzene in mice: A time-course study. Mutat. Res. 2005, 587, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Honma, M. Evaluation of the in vivo genotoxicity of Allura Red AC (Food Red No. 40). Food. Chem. Toxicol. 2015, 84, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Renganathan, V. Non-enzymatic reduction of azo dyes by NADH. Chemosphere 2000, 40, 351–357. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, S.; Yang, J.; Liu, F. Synthesis of a novel water-soluble crosslinking polymeric dye with good dyeing properties. Dyes Pigment. 2006, 68, 69–73. [Google Scholar] [CrossRef]
- Li, B.; Shen, J.; Liang, R.; Ji, W.; Kan, C. Synthesis and characterization of covalently colored polymer latex based on new polymerizable anthraquinone dyes. Colloid Polym. Sci. 2012, 290, 1893–1900. [Google Scholar] [CrossRef]
- Bunes, L.A. Products Including Edibles Colored with Polymeric Red Colors. U.S. Patent 4,316,918, 23 February 1982. [Google Scholar]
- Sereshti, H.; Samadi, S.; Asgari, S.; Karimi, M. Preparation and application of magnetic graphene oxide coated with a modified chitosan pH-sensitive hydrogel: An efficient biocompatible adsorbent for catechin. Rsc Adv. 2015, 5, 9396–9404. [Google Scholar] [CrossRef]
- Sahu, S.K.; Maiti, S.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Hydrophobically modified carboxymethyl chitosan nanoparticles targeted delivery of paclitaxel. J. Drug Target. 2011, 19, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Chen, X.G.; Wang, Z.; Liu, W.S.; Park, H.J. The effect of carboxymethyl-chitosan on proliferation and collagen secretion of normal and keloid skin fibroblasts. Biomaterials 2002, 23, 4609–4614. [Google Scholar] [CrossRef]
- Zhu, A.; Chanpark, M.B.; Dai, S.; Li, L. The aggregation behavior of O-carboxymethylchitosan in dilute aqueous solution. Colloids Surf. B Biointerfaces 2005, 43, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Han, B.; Dong, W.; Yang, Z.; Lv, Y.; Liu, W. Effects of carboxymethyl chitosan on the blood system of rats. Biochem. Biophs. Res. Commun. 2011, 408, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhang, M.; Cui, J.; Lu, J.; Li, W. Grafting of edible colorants onto O-carboxymethyl chitosan: Preparation, characterization and anti-reduction property evaluation. New J. Chem. 2016, 40, 3363–3369. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J. Converting waste expanded polystyrene into polymeric azo dyes containing the sulfonamide group. J. Mater. Cycles Waste 2014, 16, 557–565. [Google Scholar] [CrossRef]
- Villalba, M.L.; Enrique, A.V.; Higgs, J.; Castaño, R.A.; Goicoechea, S.; Taborda, F.D.; Gavernet, L.; Lick, I.D.; Marder, M.; Bruno Blanch, L.E. Novel sulfamides and sulfamates derived from amino esters: Synthetic studies and anticonvulsant activity. Eur. J. Pharmacol. 2016, 774, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Han, B.; Yang, Y.; Liu, W. Synthesis, characterization and biological safety of O-carboxymethyl chitosan used to treat Sarcoma 180 tumor. Carbohydr. Polym. 2011, 86, 231–238. [Google Scholar] [CrossRef]
| Sample | Elemental Content/% | |||
|---|---|---|---|---|
| C | N | S | H | |
| OMCS | 41.2 | 6.5 | - | 5.3 |
| SY-OMCS | 38.0 | 4.9 | 4.1 | 4.8 |
| AR-OMCS | 36.9 | 5.0 | 5.1 | 5.1 |
| Sample | SY-OMCS | SY-OMCS 1 | AR-OMCS | AR-OMCS 1 |
|---|---|---|---|---|
| Solubility | 9.2 | 5.7 | 7.4 | 4.2 |
| Compounds | SY | SY-OMCS | AR | AR-OMCS |
|---|---|---|---|---|
| IC50 (g/L) | 1.105 | 3.981 | 2.298 | 5.513 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, D.; Zhang, M.; Cui, J.; Li, W.; Zhu, G. A Novel Preparation Method of Two Polymer Dyes with Low Cytotoxicity. Materials 2017, 10, 219. https://doi.org/10.3390/ma10030219
Lv D, Zhang M, Cui J, Li W, Zhu G. A Novel Preparation Method of Two Polymer Dyes with Low Cytotoxicity. Materials. 2017; 10(3):219. https://doi.org/10.3390/ma10030219
Chicago/Turabian StyleLv, Dongjun, Mingjie Zhang, Jin Cui, Weixue Li, and Guohua Zhu. 2017. "A Novel Preparation Method of Two Polymer Dyes with Low Cytotoxicity" Materials 10, no. 3: 219. https://doi.org/10.3390/ma10030219
APA StyleLv, D., Zhang, M., Cui, J., Li, W., & Zhu, G. (2017). A Novel Preparation Method of Two Polymer Dyes with Low Cytotoxicity. Materials, 10(3), 219. https://doi.org/10.3390/ma10030219