Influence of Curing Mode on the Surface Energy and Sorption/Solubility of Dental Self-Adhesive Resin Cements
Abstract
:1. Introduction
2. Results
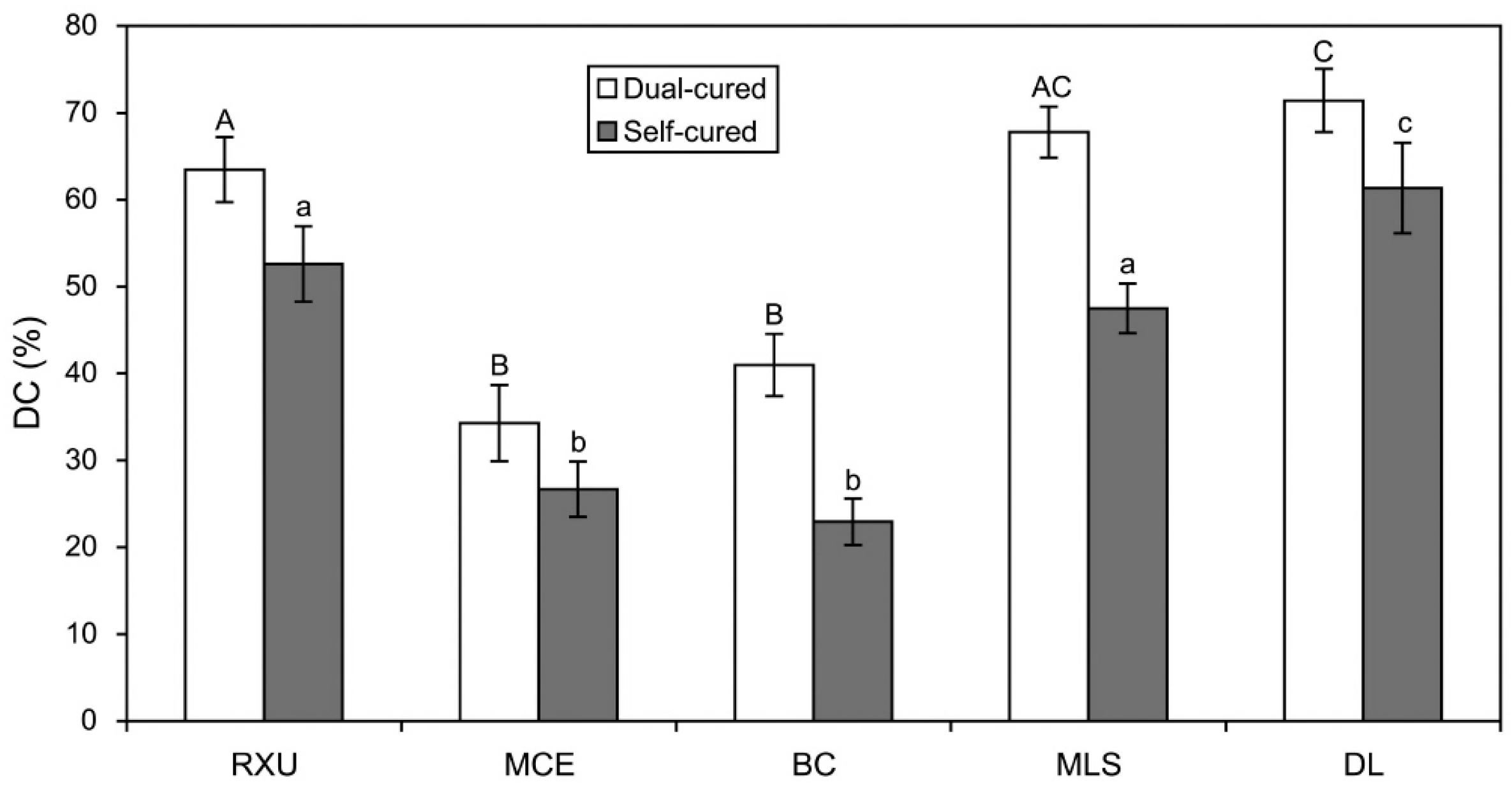
2.1. Degree of Conversions (DC)
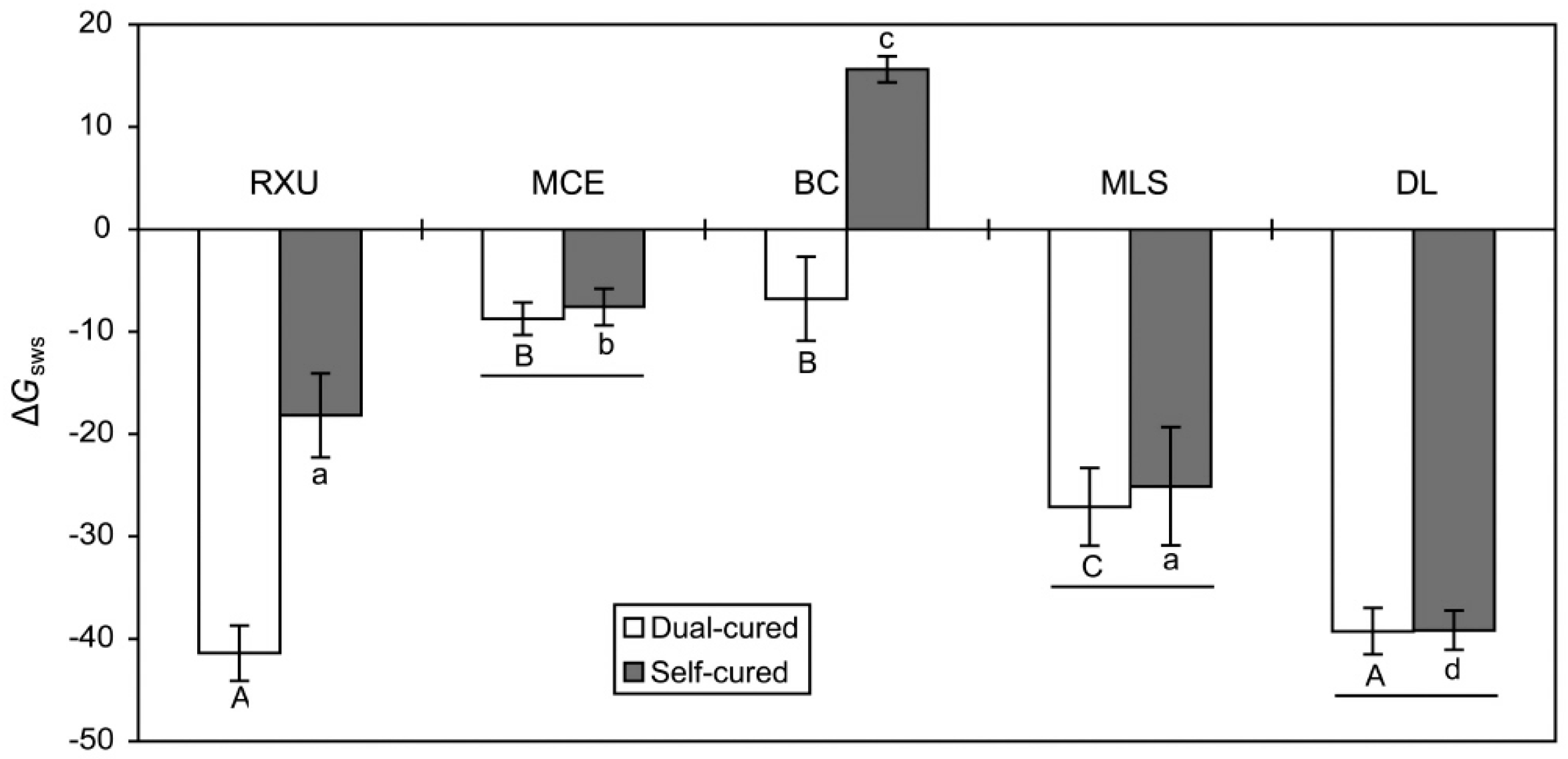
2.2. Surface Energy Parameters
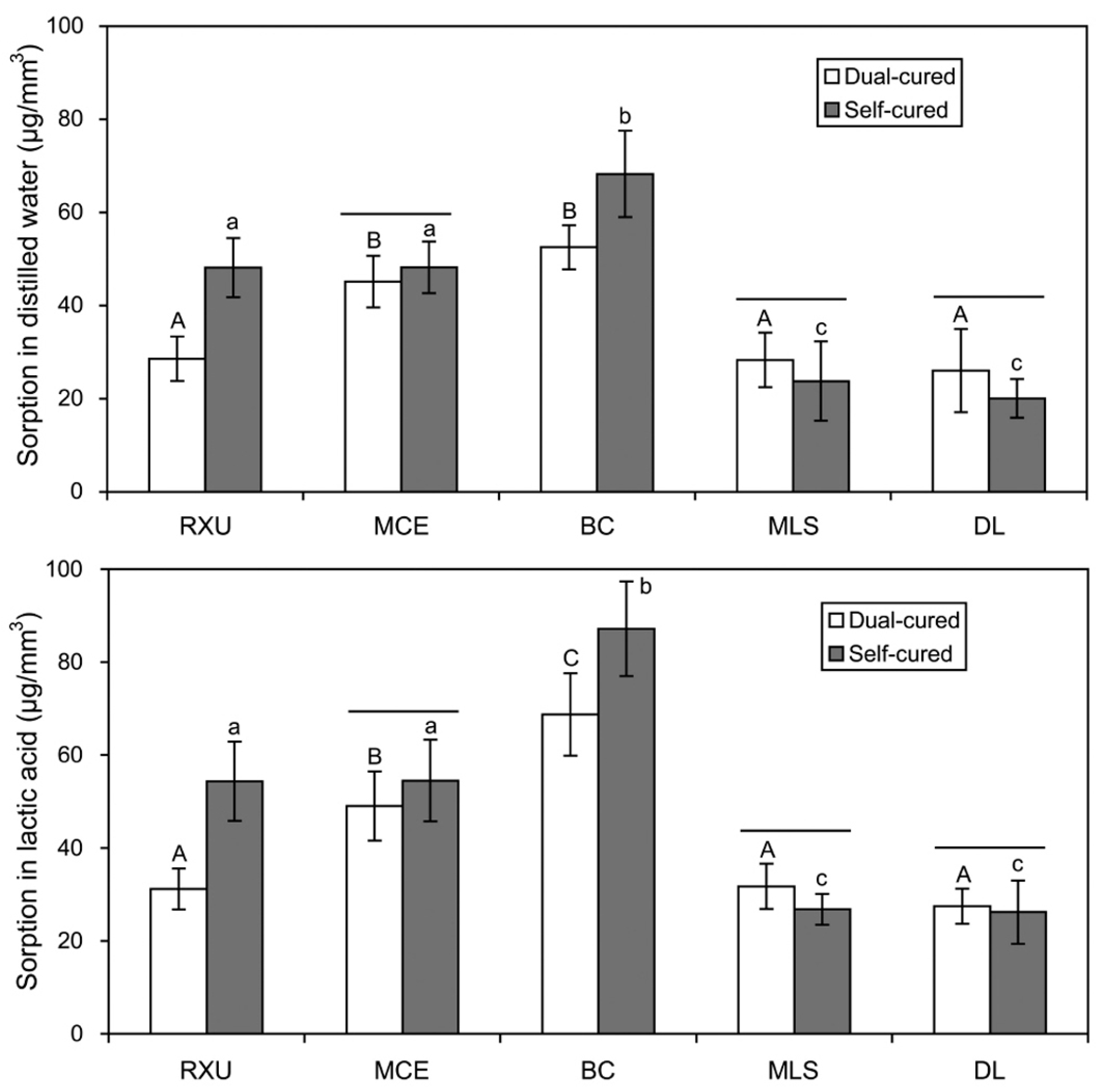
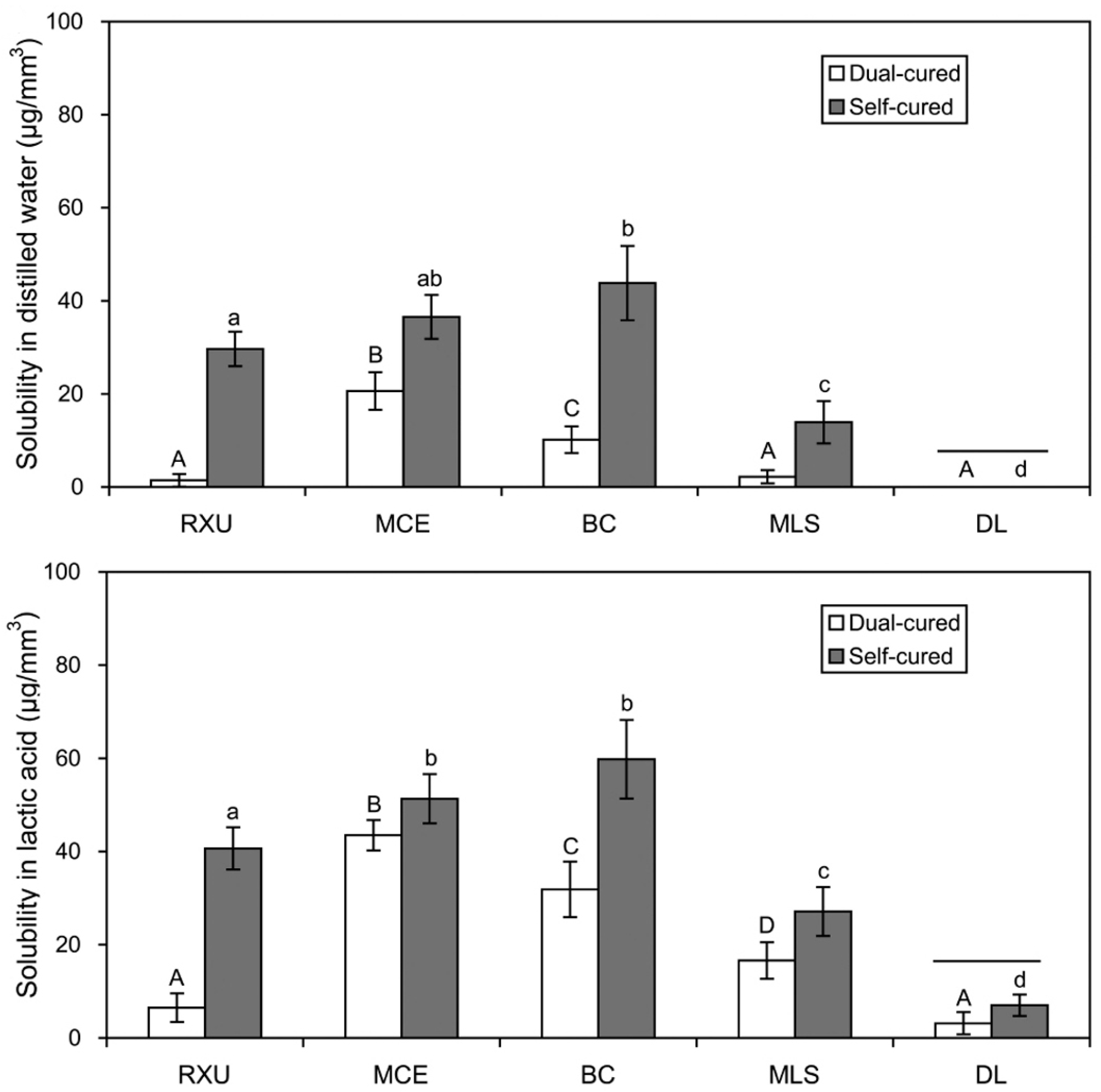
2.3. Sorption and Solubility
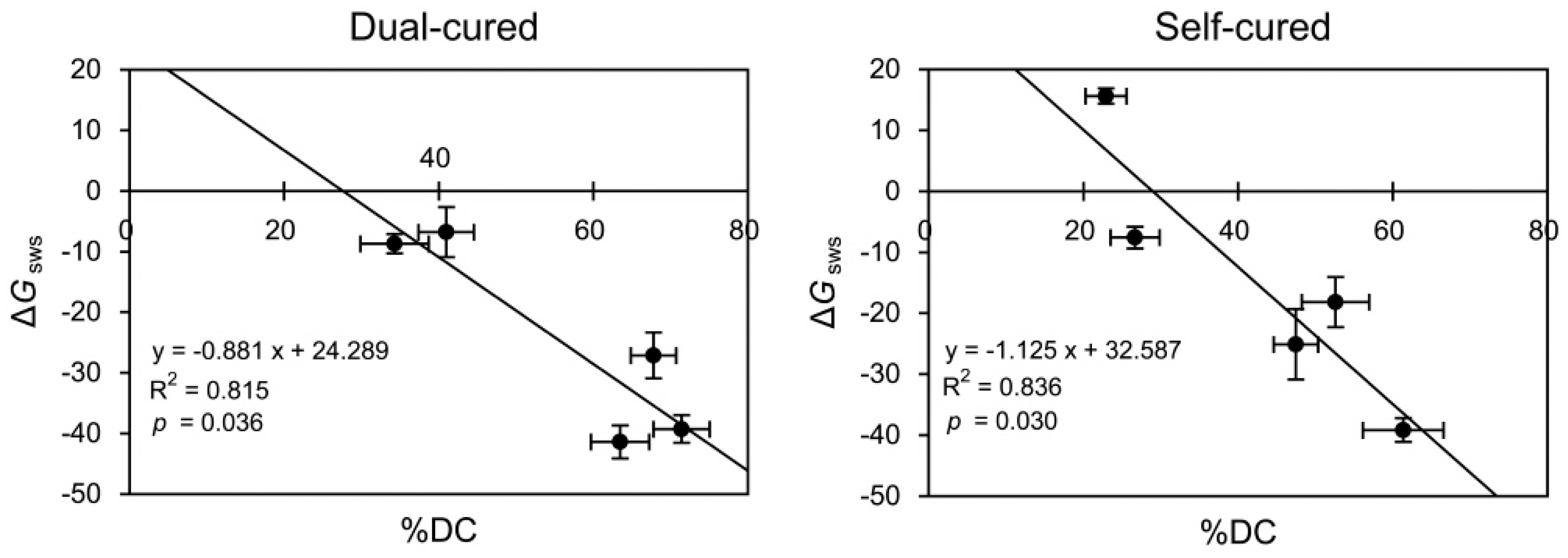
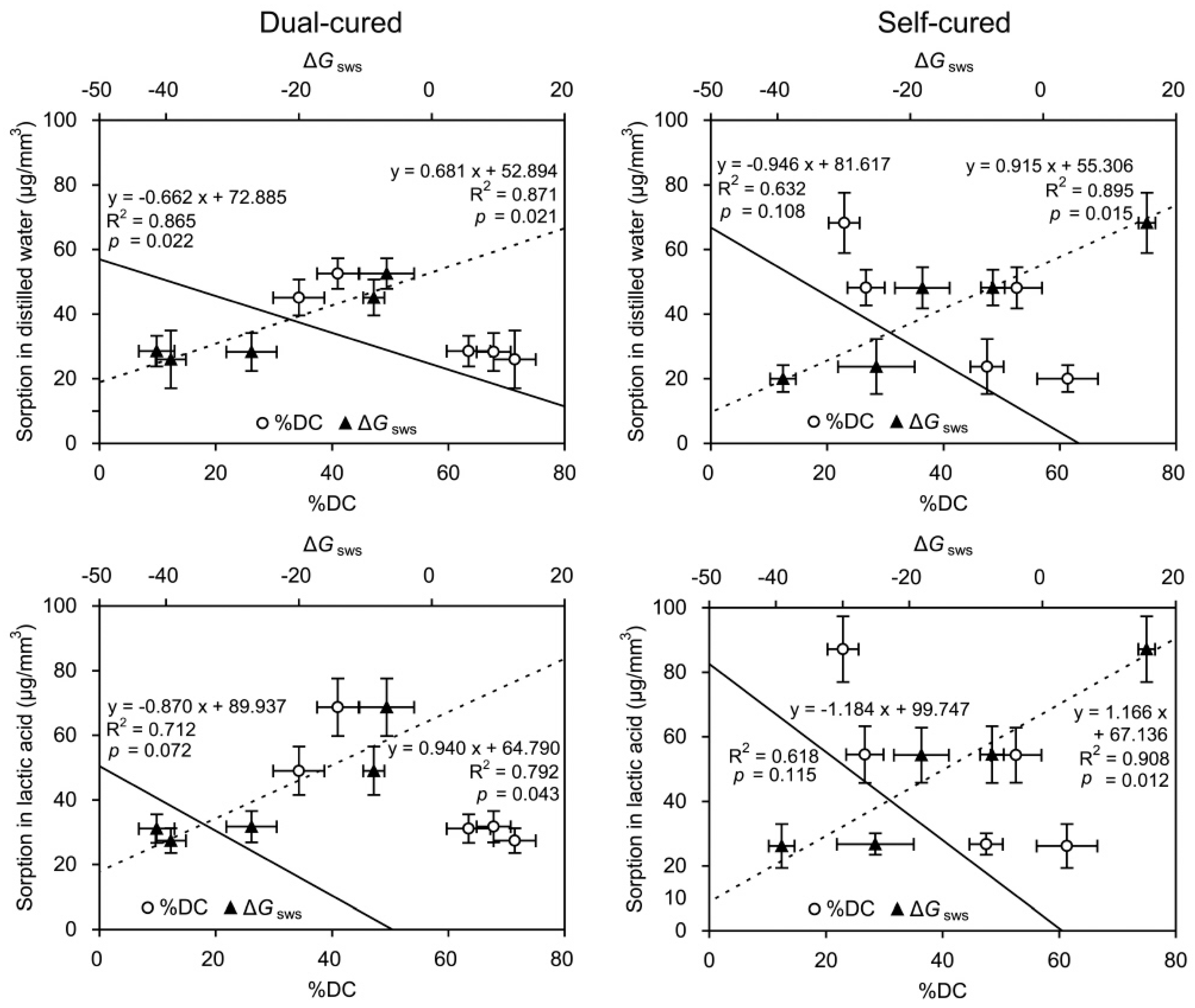
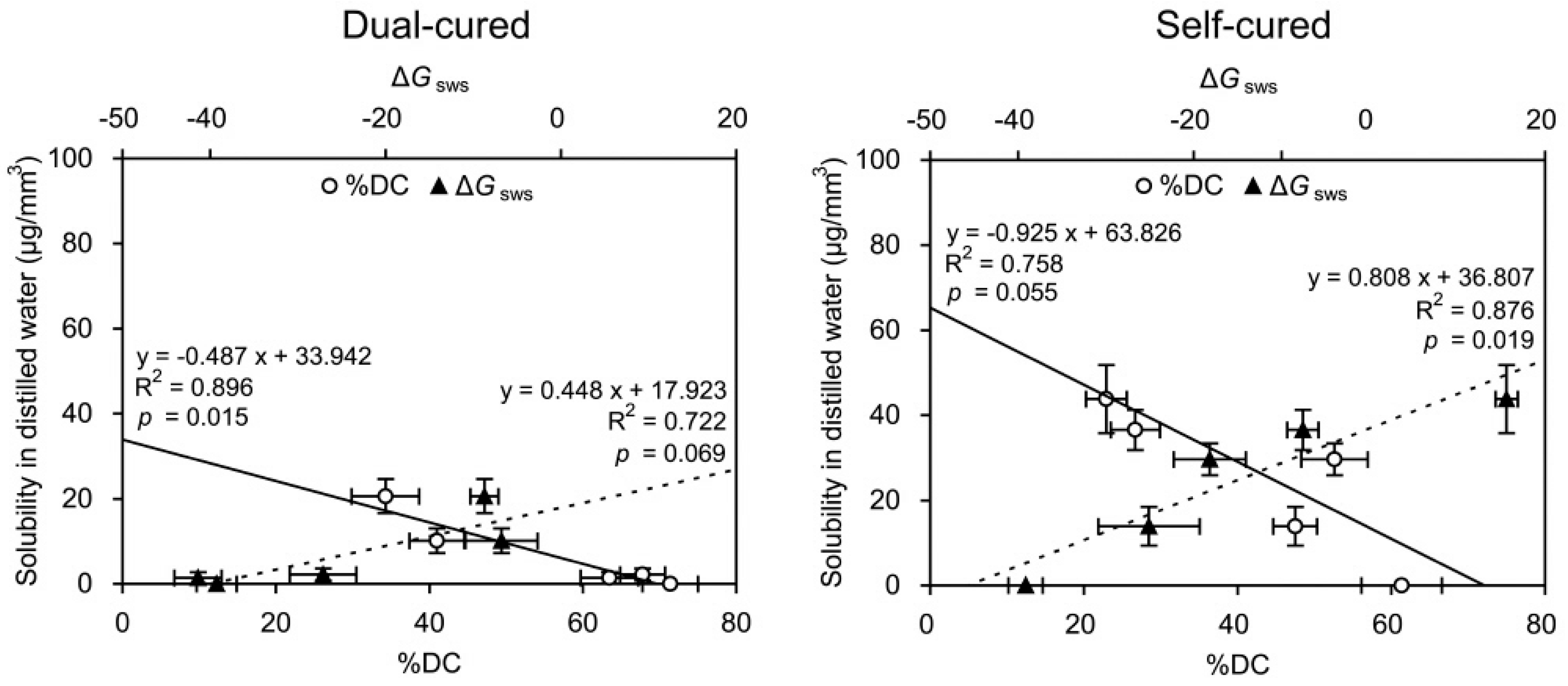
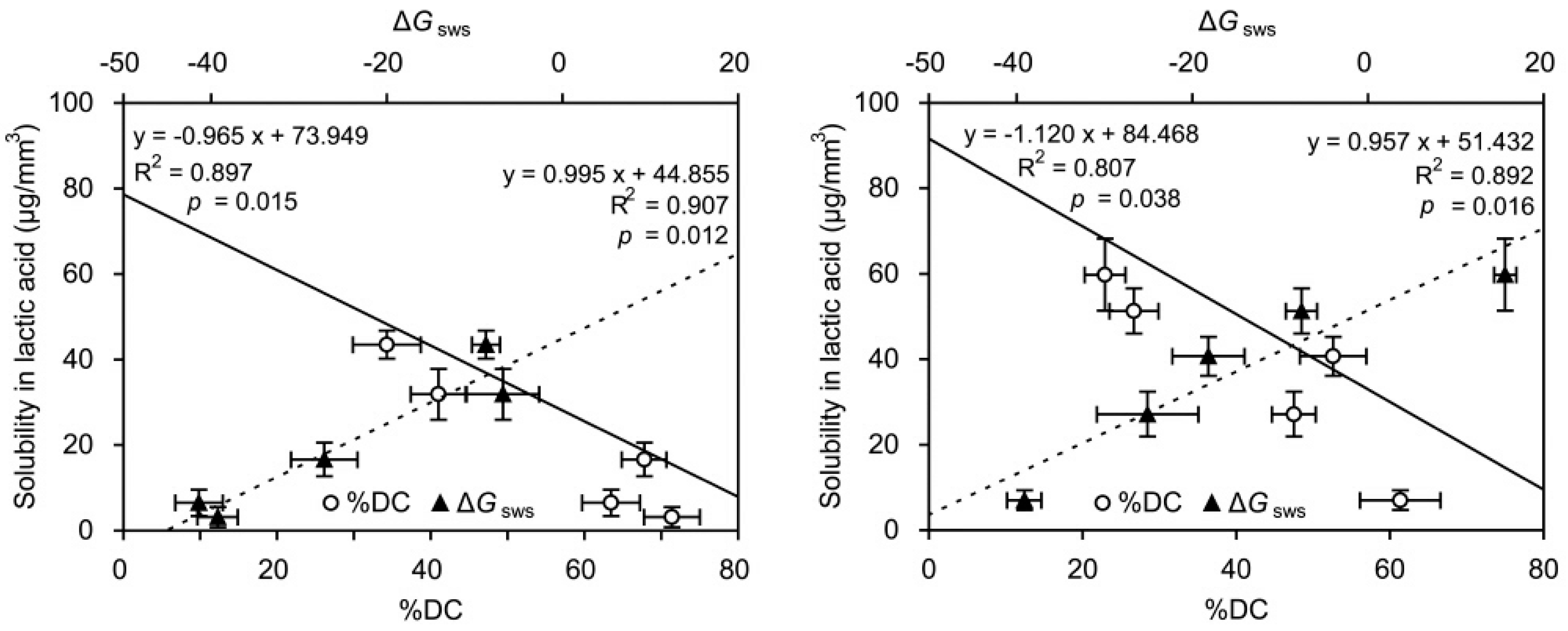
2.4. Linear Regressions
3. Discussion
4. Materials and Methods
4.1. Degree of Conversions (DC)
4.2. Surface Energy Parameters
4.3. Sorption and Solubility
4.4. Statistical Analysis
5. Conclusions
- For all the resin cements tested, the dual-cure mode consistently produced significantly higher %DC values than the self-cure mode.
- Dual-curing of the two SARCs (RXU and BC) resulted in greater surface hydrophobicity as compared with self-curing, whereas curing mode had no significant effect in the other two SARCS (MCE and MLS) or in the conventional resin cement (DL).
- Overall, the SARCs exhibited greater sorption/solubility values than the conventional resin cement (DL), especially when self-cured. The effect of lactic acid on the materials was generally more deleterious than that of distilled water.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marghalani, H.Y. Sorption and solubility characteristics of self-adhesive resin cements. Dent. Mater. 2012, 28, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.R.; Boscato, N.; Jardim, P.S.; Schneider, L.F. Dual and self-curing potential of self-adhesive resin cements as thin films. Oper. Dent. 2011, 36, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Vrochari, A.D.; Eliades, G.; Hellwig, E.; Wrbas, K.T. Curing efficiency of four self-etching, self-adhesive resin cements. Dent. Mater. 2009, 25, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Chun, J.N.; Kwon, P.C.; Kim, K.H.; Kwon, T.Y. Polymerization kinetics of dual-curing adhesive systems when used solely or in conjunction with chemically-cured resin cement. J. Adhes. Dent. 2013, 15, 453–459. [Google Scholar] [PubMed]
- Moosavi, H.; Hariri, I.; Sadr, A.; Thitthaweerat, S.; Tagami, J. Effects of curing mode and moisture on nanoindentation mechanical properties and bonding of a self-adhesive resin cement to pulp chamber floor. Dent. Mater. 2013, 29, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.R.; André, C.B.; Ambrosano, G.M.B.; Giannini, M. The effect of light exposure on water sorption and solubility of self-adhesive resin cements. Int. Sch. Res. Notices 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Son, J.S.; Kim, K.H.; Kwon, T.Y. Influence of surface energy parameters of dental self-adhesive resin cements on bond strength to dentin. J. Adhes. Sci. Technol. 2013, 27, 1778–1789. [Google Scholar] [CrossRef]
- Petropoulou, A.; Vrochari, A.D.; Hellwig, E.; Stampf, S.; Polydorou, O. Water sorption and water solubility of self-etching and self-adhesive resin cements. J. Prosthet. Dent. 2015, 114, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Filho, L.E.; Burger, L.A.; Kenshima, S.; Bauer, J.R.; Medeiros, I.S.; Muench, A. Effect of light-activation methods and water storage on the flexural strength of two composite resins and a compomer. Braz. Oral Res. 2006, 20, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Fowler, B.O.; Dickens, S.H.; Richards, N.D. Novel dental resins from trialkoxysilanes and dental monomers by in situ formation of oligomeric silyl ethers and silsesquioxanes. Polym. Prep. 2002, 43, 633–634. [Google Scholar]
- Kim, Y.K.; Min, B.K.; Son, J.S.; Kim, K.H.; Kwon, T.Y. Influence of different drying methods on microtensile bond strength of self-adhesive resin cements to dentin. Acta Odontol. Scand. 2014, 72, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Shear bond strengths of various luting cements to zirconia ceramic: Surface chemical aspects. J. Dent. 2011, 39, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van Pelt, A.W.J.; de Boer, P.; de Jong, H.P.; Arends, J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984, 9, 319–331. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Resin composites: Strength of the bond to dentin versus surface energy parameters. Dent. Mater. 2005, 21, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J.; Giese, R.F. The hydrophilicity and hydrophobicity of clay minerals. Clays Clay Miner. 1995, 43, 474–477. [Google Scholar] [CrossRef]
- Ma, K.X.; Chung, T.S. Surface tension investigations of thermotropic liquid crystalline polymers. In Thermotropic Liquid Crystal Polymers: Thin-Film Polymerization, Characterization, Blends, and Applications, 1st ed.; Chung, T.S., Ed.; CRC Press: Lancaster, UK, 2001; pp. 157–182. [Google Scholar]
- Bagheri, R.; Azar, M.R.; Tyas, M.J.; Burrow, M.F. The effect of aging on the fracture toughness of esthetic restorative materials. Am. J. Dent. 2010, 23, 142–146. [Google Scholar] [PubMed]
- Da Silva, E.M.; Gonçalves, L.; Guimarães, J.G.; Poskus, L.T.; Fellows, C.E. The diffusion kinetics of a nanofilled and a midifilled resin composite immersed in distilled water, artificial saliva, and lactic acid. Clin. Oral Investig. 2011, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Zamboniota, S.; Valandro, F.; Bottino, M.; Bagis, B. Microhardness of dual-polymerized resin cement around a translucent fiber post in the intraradicular environment. J. Conserv. Dent. 2011, 14, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Tyas, M.J.; Burrow, M.F. Subsurface degradation of resin-based composites. Dent. Mater. 2007, 23, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W.; Leyhausen, G.; Garcia-Godoy, F. Effect of storage media on the fluoride release and surface microhardness of four polyacid-modified composite resins (“compomers”). Dent. Mater. 1999, 15, 196–201. [Google Scholar] [CrossRef]
- Azar, M.R.; Bagheri, R.; Burrow, M.F. Effect of storage media and time on the fracture toughness of resin-based luting cements. Aust. Dent. J. 2012, 57, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Noronha-Filho, J.D.; Amaral, C.M.; Poskus, L.T.; Guimarães, J.G. Long-term degradation of resin-based cements in substances present in the oral environment: Influence of activation mode. J. Appl. Oral Sci. 2013, 21, 271–277. [Google Scholar] [PubMed]
- Mendes, L.C.; Matos, I.C.; Miranda, M.S.; Benzi, M.R. Dual-curing, self-adhesive resin cement: Influence of the polymerization modes on the degree of conversion and microhardness. Mater. Res. 2010, 13, 171–176. [Google Scholar] [CrossRef]
- Deb, S.; Sehmi, H. A comparative study of the properties of dental resin composites polymerized with plasma and halogen light. Dent. Mater. 2003, 19, 517–522. [Google Scholar] [CrossRef]
- Ramos, M.B.; Pegoraro, T.A.; Pegoraro, L.F.; Carvalho, R.M. Effects of curing protocol and storage time on the micro-hardness of resin cements used to lute fiber-reinforced resin posts. J. Appl. Oral Sci. 2012, 20, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Øysæd, H.; Ruyter, I.E. Composites for use in posterior teeth: Mechanical properties tested under dry and wet conditions. J. Biomed. Mater. Res. 1986, 20, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Dentistry-Polymer-Based Filling, Restorative and Luting Materials; ISO 4049:2009; International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- Guerra, R.M.; Duran, I.; Ortiz, P. FTIR monomer conversion analysis of UDMA-based dental resins. J. Oral Rehabil. 1996, 23, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, P.M.; Giese, R.F.; van Oss, C.J. Determination of the acid-base characteristics of clay mineral surfaces by contact angle measurements-implications for the adsorption of organic solutes from aqueous media. J. Adhes. Sci. Technol. 1990, 4, 267–275. [Google Scholar] [CrossRef]
- Takimoto, M.; Ishii, R.; Iino, M.; Shimizu, Y.; Tsujimoto, A.; Takamizawa, T.; Ando, S.; Miyazaki, M. Influence of temporary cement contamination on the surface free energy and dentine bond strength of self-adhesive cements. J. Dent. 2012, 40, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Good, R.J. Contact angle, wetting, and adhesion: A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, H.S.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014, 84, 680–686. [Google Scholar] [CrossRef] [PubMed]
| Code | Brand Name | Manufacturer | Composition * (Batch Number) | Filler Loading/Average Particle Size * |
|---|---|---|---|---|
| RXU | RelyX U200 | 3M ESPE, Seefeld, Germany | Base: Mono-, di-, and tri-glycerol esters of phosphoric acid dimethacrylate, TEGDMA, glass, silica, sodium persulfate, tert-butyl peroxy-3,5,5-trimethylhexanoate; Catalyst: substituted dimethacrylate, 1,12-dodecane dimethacrylate, glass, silica, calcium hydroxide, calcium salt of 1-benzyl-5-phenyl-barbic-acid, sodium p-toluenesulfinate (574751) | 43 vol %/12.5 μm |
| MCE | Maxcem Elite | Kerr Corp., Orange, CA, USA | GPDM, TEGDMA, fillers, activators, stabilizers, HEMA, cumene hydroperoxide, titanium dioxide, pigments (5427018) | 69 wt % |
| BC | BisCem | Bisco Inc., Schaumberg, IL, USA | Di-HEMA phosphate, Tetra-EGDMA, glass (1500001067) | Base: 36 vol % (60 wt %); Catalyst: 40 vol % (62 wt %)/Base: 1.0 μm; Catalyst 3.5 μm |
| MLS | Multilink Speed | Ivoclar Vivadent, Schaan, Liechtenstein | Base: UDMA, TEGDMA; Catalyst: UDMA, TEGDMA, methacrylated phosphoric acid ester, PEGDMA, benzoyl peroxide (U18982) | Base: 75.0 wt %; Catalyst: 47.4 wt % |
| DL | Duo-Link | Bisco Inc., Schaumberg, IL, USA | Base: Bis-GMA, TEGDMA, UDMA, glass filler; Catalyst: Bis-GMA, TEGDMA, glass filler (1500003655) | 38 vol % (60 wt %)/<1.0 μm |
| Resin Cement | Curing Mode | γs | γsLW | γs+ | γs− | γsAB |
|---|---|---|---|---|---|---|
| RXU | Dual-cured | 44.08 (0.33)A | 43.58 (0.38)A | 0.01 (0.002)A | 11.20 (1.01)A | 0.50 (0.09)A |
| Self-cured | 56.86 (0.84)a | 49.01 (0.45)a | 0.73 (0.06)a | 21.35 (2.47)ab | 7.85 (0.50)a | |
| MCE | Dual-cured | 37.90 (1.52)B | 35.90 (0.97)B | 0.05 (0.03)B | 22.86 (0.94)B | 1.99 (0.75)BC |
| Self-cured | 52.03 (0.74)b | 39.46 (0.93)b | 1.66 (0.35)b | 23.96 (0.94)a | 12.57 (1.49)b | |
| BC | Dual-cured | 42.98 (1.03)A | 40.91 (1.08)C | 0.04 (0.02)B | 25.12 (2.30)B | 2.08 (0.43)BC |
| Self-cured | 52.13 (2.06)b | 33.75 (1.38)c | 2.14 (0.61)b | 39.98 (1.65)c | 18.38 (2.97)c | |
| MLS | Dual-cured | 43.57 (0.61)A | 41.78 (0.36)C | 0.05 (0.01)B | 15.87 (1.54)C | 1.79 (0.27)B |
| Self-cured | 48.22 (1.14)c | 43.84 (0.83)d | 0.29 (0.02)c | 16.75 (2.83)b | 4.39 (0.37)d | |
| DL | Dual-cured | 46.27 (1.11)C | 43.50 (1.14)A | 0.17 (0.04)C | 11.12 (1.01)A | 2.77 (0.28)C |
| Self-cured | 46.24 (1.86)c | 43.78 (0.74)d | 0.16 (0.14)c | 11.32 (0.84)d | 2.46 (1.15)e |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Bagheri, R.; Kim, Y.K.; Son, J.S.; Kwon, T.-Y. Influence of Curing Mode on the Surface Energy and Sorption/Solubility of Dental Self-Adhesive Resin Cements. Materials 2017, 10, 129. https://doi.org/10.3390/ma10020129
Kim H-J, Bagheri R, Kim YK, Son JS, Kwon T-Y. Influence of Curing Mode on the Surface Energy and Sorption/Solubility of Dental Self-Adhesive Resin Cements. Materials. 2017; 10(2):129. https://doi.org/10.3390/ma10020129
Chicago/Turabian StyleKim, Hyun-Jin, Rafat Bagheri, Young Kyung Kim, Jun Sik Son, and Tae-Yub Kwon. 2017. "Influence of Curing Mode on the Surface Energy and Sorption/Solubility of Dental Self-Adhesive Resin Cements" Materials 10, no. 2: 129. https://doi.org/10.3390/ma10020129
APA StyleKim, H.-J., Bagheri, R., Kim, Y. K., Son, J. S., & Kwon, T.-Y. (2017). Influence of Curing Mode on the Surface Energy and Sorption/Solubility of Dental Self-Adhesive Resin Cements. Materials, 10(2), 129. https://doi.org/10.3390/ma10020129






