Development of AlN/Epoxy Composites with Enhanced Thermal Conductivity
Abstract
:1. Introduction
2. Results and Discussion
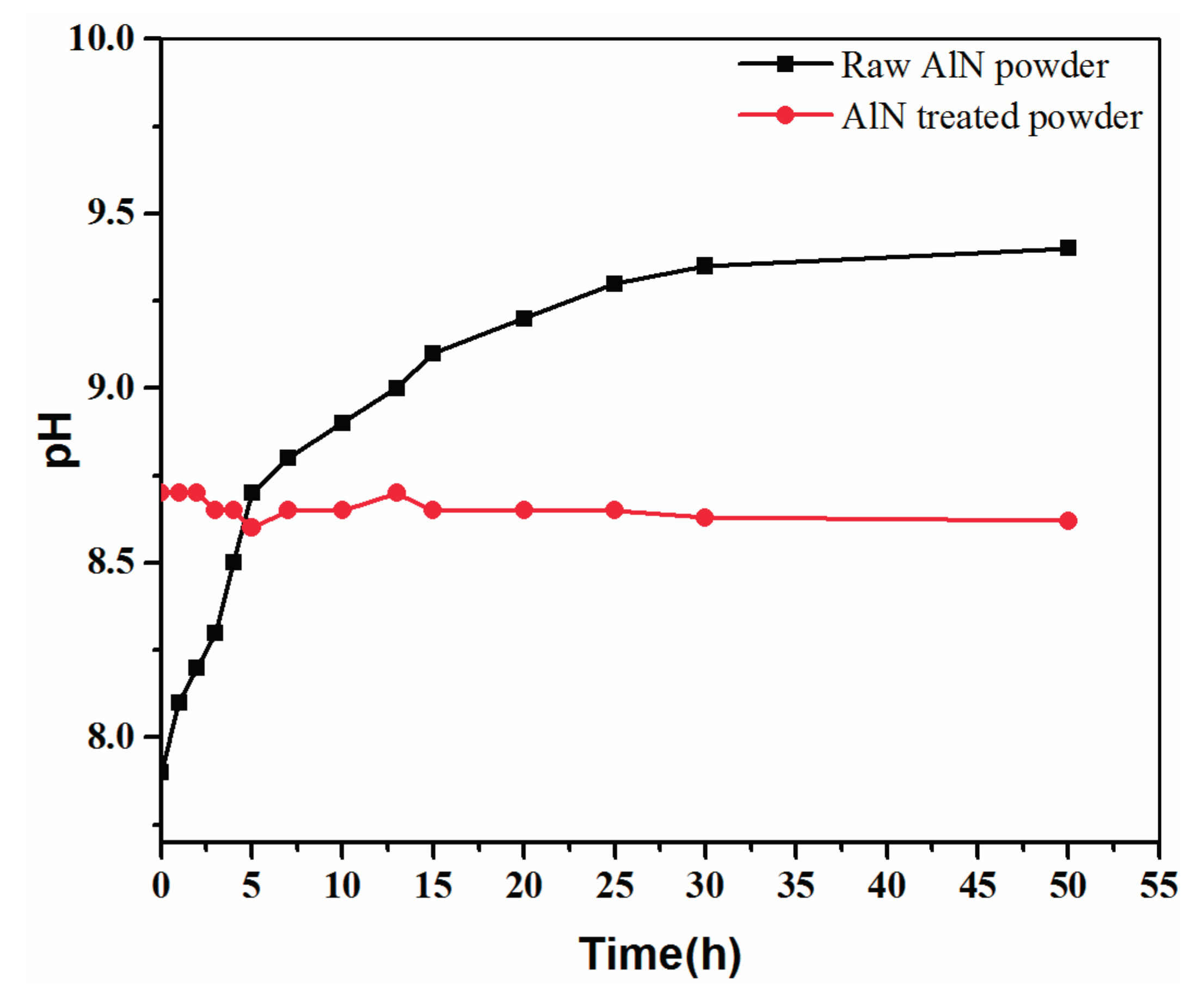
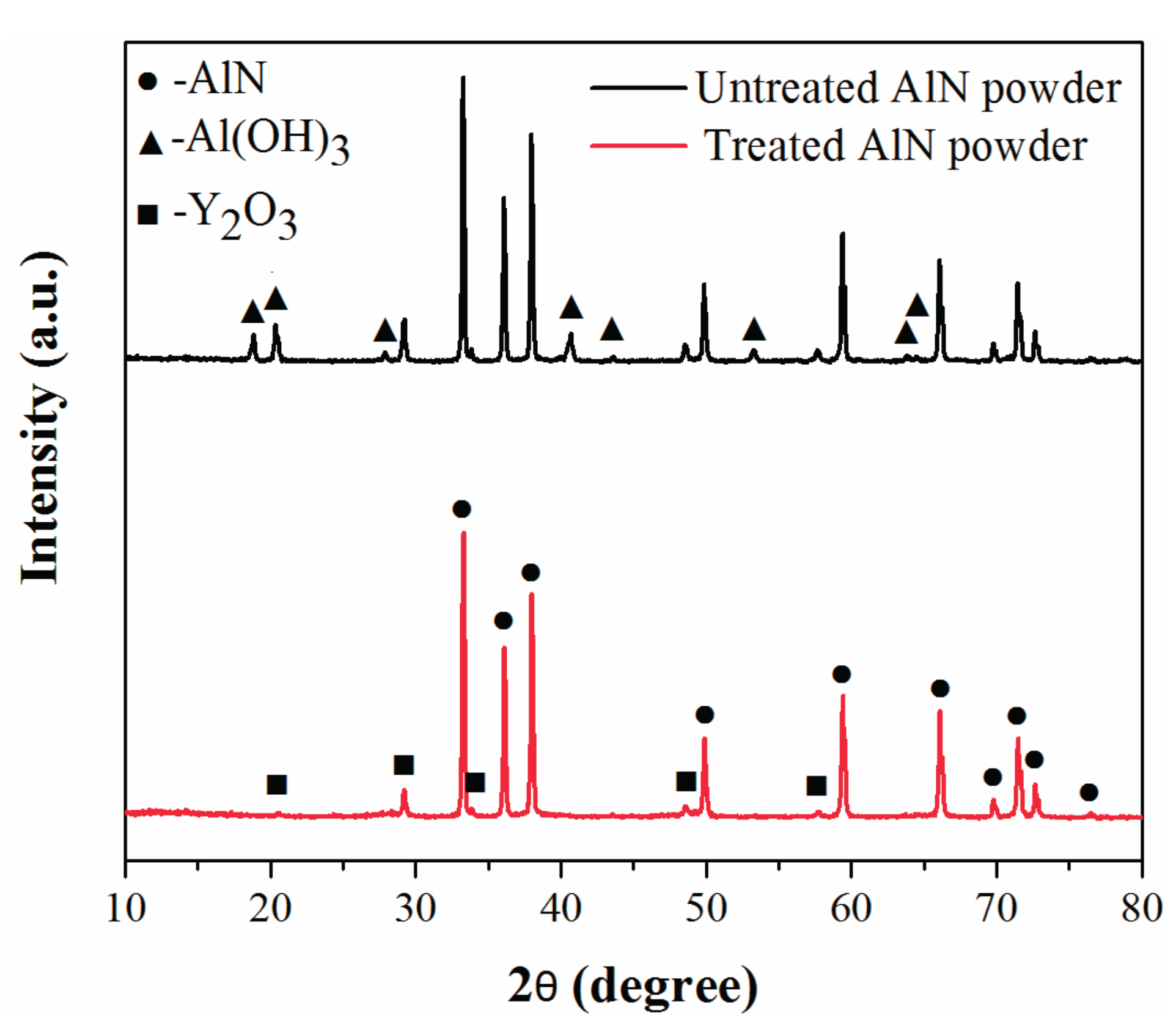
2.1. Powder Treatment
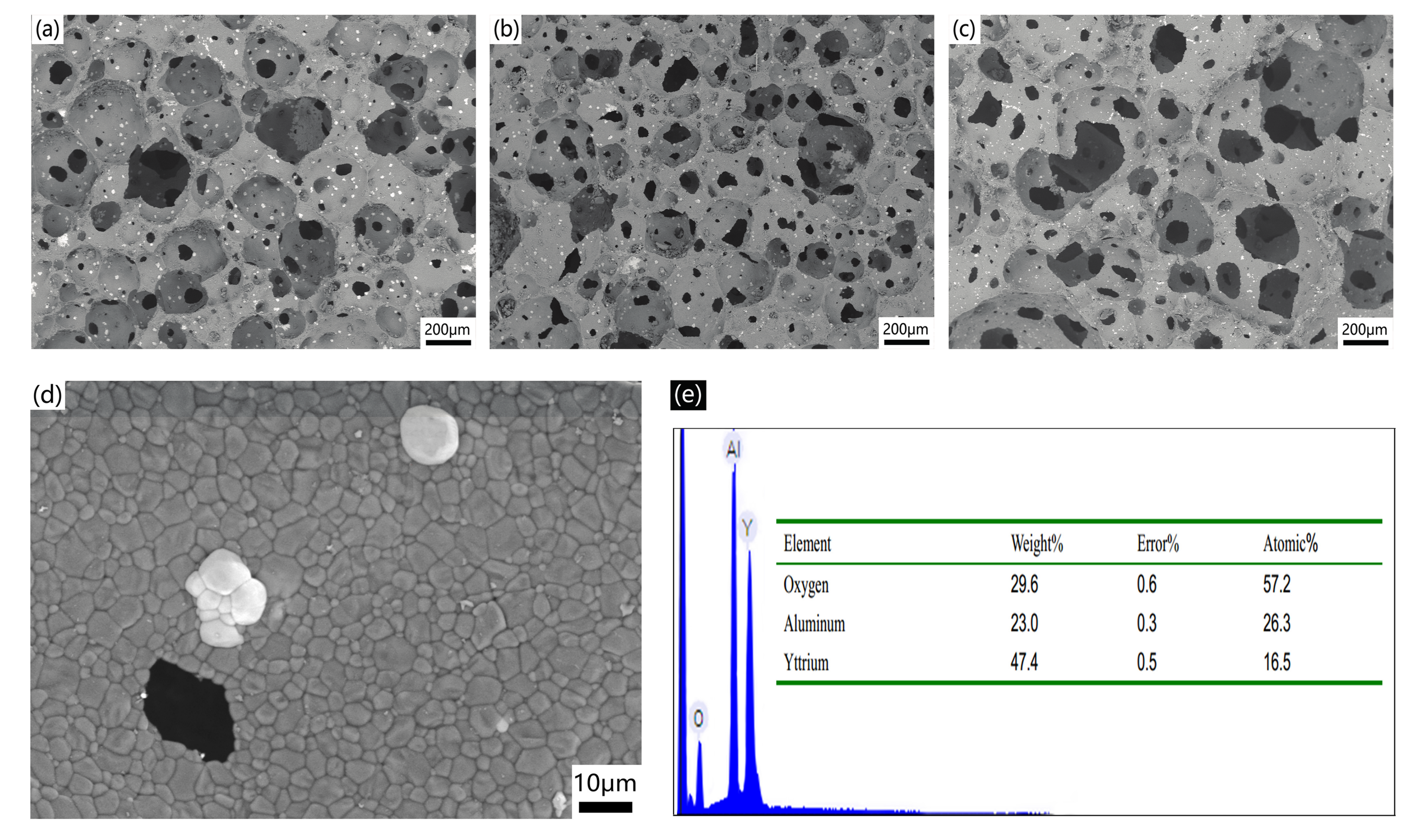
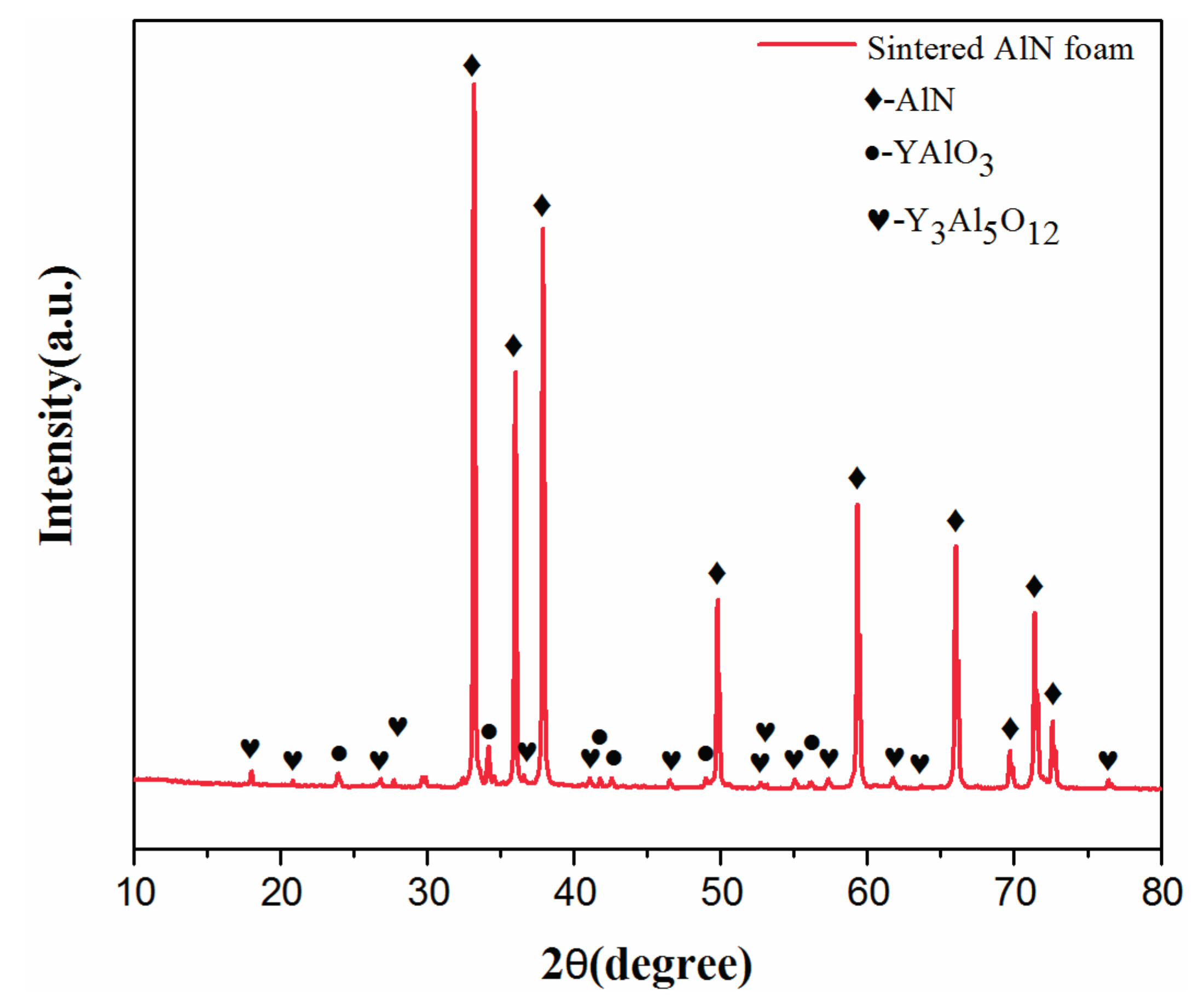
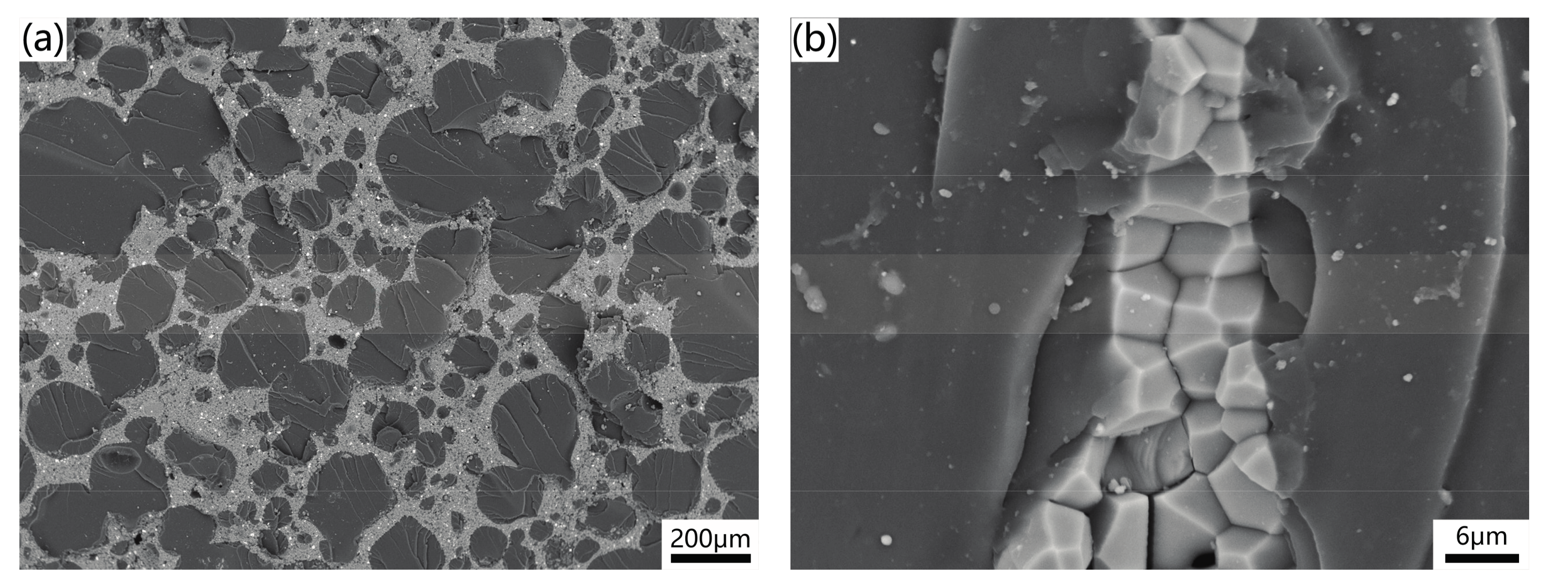
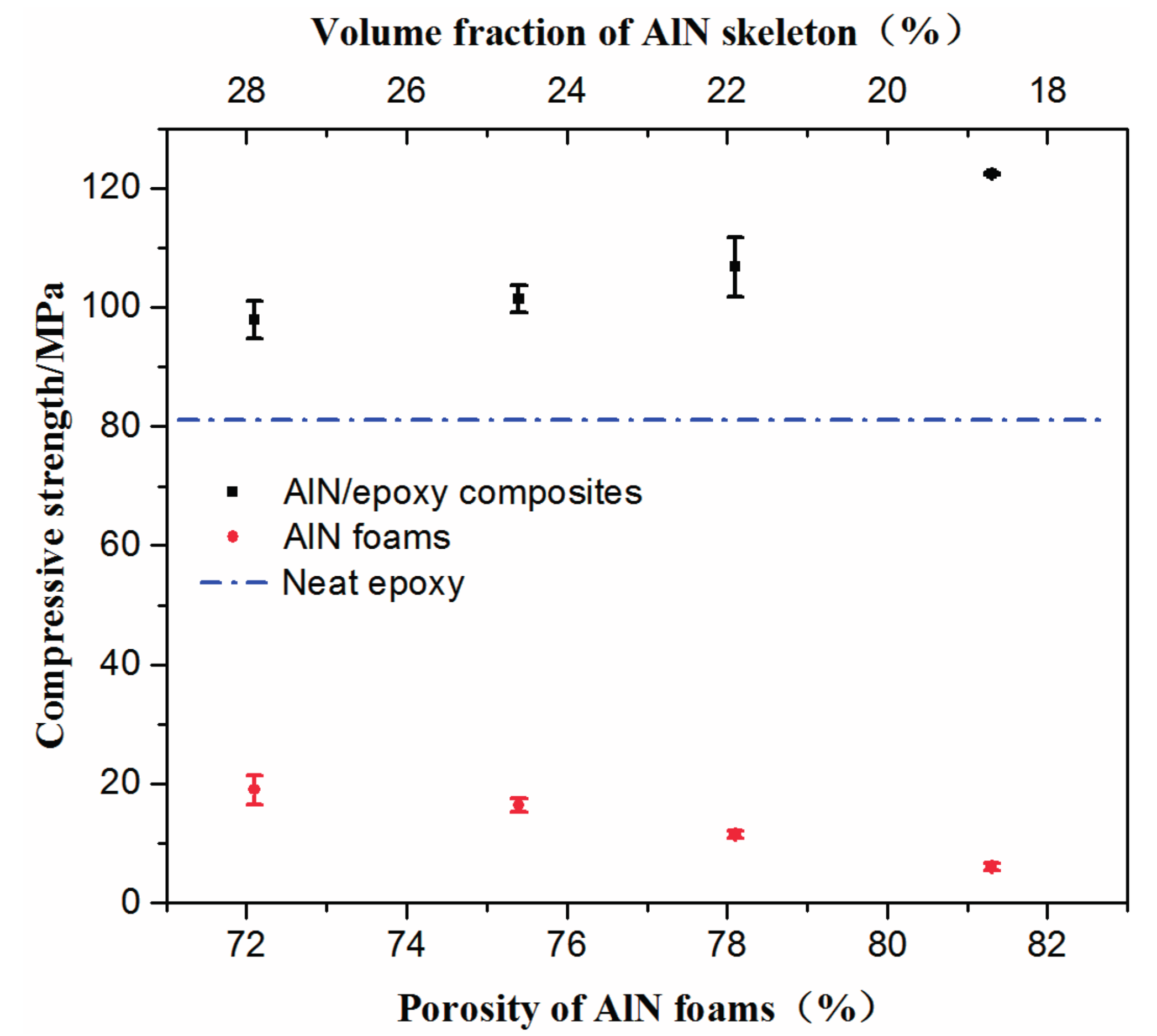
2.2. Properties of AlN Porous Ceramics
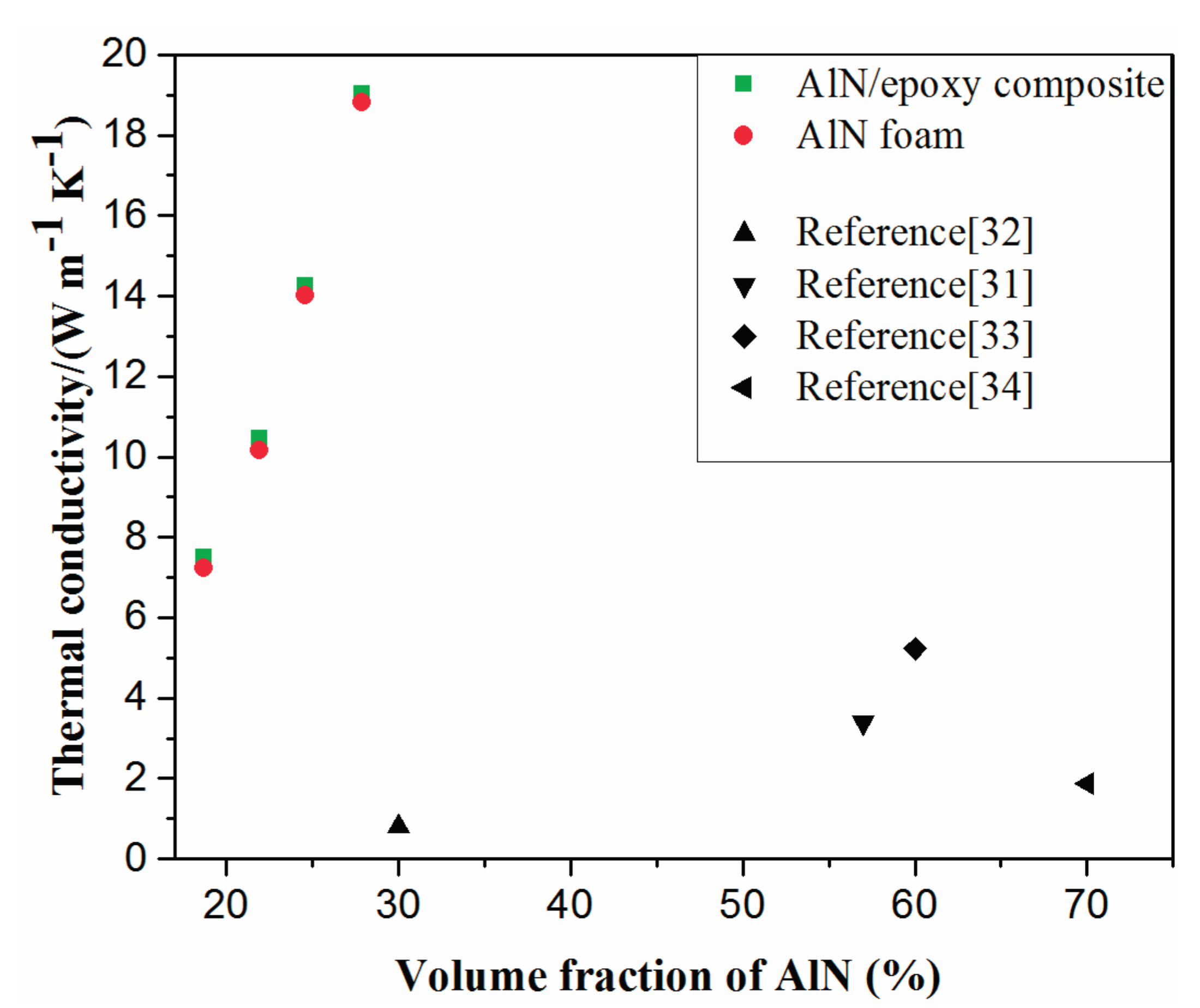
2.3. Properties of AlN/Epoxy Composite
3. Experiment and Characterization
3.1. Experimental Procedure
3.2. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yin, L.; Zhou, X.; Yu, J.; Wang, H.; Ran, C. Fabrication of a polymer composite with high thermal conductivity based on sintered silicon nitride foam. Compos. Part A 2016, 90, 626–632. [Google Scholar] [CrossRef]
- Hatui, G.; Bhattacharya, P.; Sahoo, S.; Dhibar, S.; Das, C.K. Combined effect of expanded graphite and multiwall carbon nanotubes on the thermo mechanical, morphological as well as electrical conductivity of in situ bulk polymerized polystyrene composites. Compos. Part A 2014, 56, 181–191. [Google Scholar] [CrossRef]
- Gu, J.; Lv, Z.; Wu, Y.; Zhao, R.; Tian, L.; Zhang, Q. Enhanced thermal conductivity of SiCp/PS composites by electrospinning-hot press technique. Compos. Part A 2015, 79, 8–13. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.C.; Wu, Z.J. The fabrication and properties of a graphite nanosheet/polystyrene composite based on graphite nanosheets treated with supercritical water. Compos. Sci Technol. 2015, 112, 50–57. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, S.; Li, H.; Shao, S. Study on insulating thermal conductive BN/HDPE composites. Thermochim. Acta 2007, 452, 36–42. [Google Scholar] [CrossRef]
- Gu, J.; Guo, Y.; Lv, Z.; Geng, W.; Zhang, Q. Highly thermally conductive POSS-g-SiCp/UHMWPE composites with excellent dielectric properties and thermal stabilities. Compos. Part A 2015, 78, 95–101. [Google Scholar] [CrossRef]
- Yu, A.P.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet-epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Sciamanna, V.; Nait-Ali, B.; Gonon, M. Mechanical properties and thermal conductivity of porous alumina ceramics obtained from particle stabilized foams. Ceram. Int. 2015, 41, 2599–2606. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Wang, K.; Feng, A. The effect of modified AlN on the thermal conductivity, mechanical and thermal properties of AlN/polystyrene composites. RSC Adv. 2016, 6, 102542–102548. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Wan, J.; Xiao, Y.; Fan, X. Preparation of AlN microspheres/UHMWPE composites for insulating thermal conductors. RSC Adv. 2016, 6, 80262–80267. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. Part B 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Xu, Y.S.; Chung, D.D.L.; Mroz, C. Thermally conducting aluminum nitride polymer-matrix composites. Compos. Part A 2001, 32, 1749–1757. [Google Scholar] [CrossRef]
- He, H.; Fu, R.; Shen, Y.; Han, Y.; Song, X. Preparation and properties of Si3N4/PS composites used for electronic packaging. Compos. Sci. Technol. 2007, 67, 2493–2499. [Google Scholar] [CrossRef]
- Zeng, J.; Fu, R.; Shen, Y.; He, H.; Song, X. High thermal conductive epoxy molding compound with thermal conductive pathway. J. Appl. Polym. Sci. 2009, 113, 2117–2125. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Okamoto, K. Toward High Thermal Conductivity Nano Micro Epoxy Composites with Sufficient Endurance Voltage. J. Int. Counc. Electron. Eng. 2014, 2, 90–98. [Google Scholar] [CrossRef]
- Donnay, M.; Tzavalas, S.; Logakis, E. Boron nitride filled epoxy with improved thermal conductivity and dielectric breakdown strength. Compos. Sci. Technol. 2015, 110, 152–158. [Google Scholar] [CrossRef]
- Ohashi, M.; Kawakami, S.; Yokogawa, Y.; Lai, G.-C. Spherical Aluminum Nitride Fillers for Heat-Conducting Plastic Packages. J. Am. Ceram. Soc. 2005, 88, 2615–2618. [Google Scholar] [CrossRef]
- Gu, J.; Du, J.; Dang, J.; Geng, W.; Hu, S.; Zhang, Q. Thermal conductivities, mechanical and thermal properties of graphite nanoplatelets/polyphenylene sulfide composites. RSC Adv. 2014, 4, 22101–22105. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Yang, Y.; Shimai, S.; Wang, S.W. Room-temperature gelcasting of alumina with a water-soluble copolymer. J. Mater. Res. 2013, 28, 1512–1516. [Google Scholar] [CrossRef]
- Yang, Y.; Shimai, S.; Sun, Y.; Dong, M.; Kamiya, H.; Wang, S. Fabrication of porous Al2O3 ceramics by rapid gelation and mechanical foaming. J. Mater. Res. 2013, 28, 2012–2016. [Google Scholar] [CrossRef]
- Olhero, S.M.; Novak, S.; Oliveira, M.; Krnel, K.; Kosmac, T.; Ferreira, J.M.F. A thermo-chemical surface treatment of AlN powder for the aqueous processing of AlN ceramics. J. Mater. Res. 2004, 19, 746–751. [Google Scholar] [CrossRef]
- Kocjan, A.; Krnel, K.; Kosmac, T. The influence of temperature and time on the AlN powder hydrolysis reaction products. J. Eur. Ceram. Soc. 2008, 28, 1003–1008. [Google Scholar] [CrossRef]
- Novak, S.; Kosmac, T. Preparation of alumina ceramics from aqueous suspensions employing the hydrolysis of aluminum nitride. J. Mater. Res. 2002, 17, 445–450. [Google Scholar] [CrossRef]
- Nakano, H.; Watari, K.; Urabe, K. Grain boundary phase in AlN ceramics fired under reducing N2 atmosphere with carbon. J. Eur. Ceram. Soc. 2003, 23, 1761–1768. [Google Scholar] [CrossRef]
- Shang, Q.S.; Wang, Z.J.; Li, J.; Zhou, G.H.; Zhang, H.L.; Wang, S.W. Gel-tape-casting of aluminum nitride ceramics. J. Adv. Ceram. 2017, 6, 67–72. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, W.; Ge, Y.Y.; Chen, K.X.; Xie, Z.P. Carbothermal synthesis of spherical AlN granules: Effects of synthesis parameters and Y2O3 additive. Ceram. Int. 2015, 41, 6715–6721. [Google Scholar] [CrossRef]
- Carolina, T.; Chayuda, C.; David, E.D.; George, V.F. Mechanical strength and damage tolerance of highly porous alumina ceramics produced from sintered particle stabilized foams. Ceram. Int. 2016, 42, 8478–8487. [Google Scholar]
- Rana, D.; Sauvant, V.; Halary, J.L. Molecular analysis of yielding in pure and antiplasticized epoxy-amine thermosets. J. Mater. Sci. 2002, 37, 5267–5274. [Google Scholar] [CrossRef]
- Rana, D.; Mounach, H.; Halary, J.L.; Monnerie, L. Differences in mechanical behavior between alternating and random styrene-methyl methacrylate copolymers. J. Mater. Sci. 2005, 40, 943–953. [Google Scholar] [CrossRef]
- Hu, M.; Feng, J.; Ng, K.M. Thermally conductive PP/AlN composites with a 3-D segregated structure. Compos. Sci. Technol. 2015, 110, 26–34. [Google Scholar] [CrossRef]
- Dang, T.M.L.; Kim, C.Y.; Zhang, Y.; Yang, J.F.; Masaki, T.; Yoon, D.H. Enhanced thermal conductivity of polymer composites via hybrid fillers of anisotropic aluminum nitride whiskers and isotropic spheres. Compos. Part B 2017, 114, 237–246. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, S.M.; Shanefield, D.J.; Cannon, W.R. Enhanced thermal conductivity of polymer matrix composite via high solids loading of aluminum nitride in epoxy resin. J. Am. Ceram. Soc. 2008, 91, 1169–1174. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Wang, L.; Yu, K.; Lin, Z.; He, L.; Bai, Y. Fabrication and characterization of aluminum nitride polymer matrix composites with high thermal conductivity and low dielectric constant for electronic packaging. Mater. Sci. Eng. 2012, 177, 892–896. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yang, C.; Li, J.; Mao, X.; Zhang, H.; Hu, S.; Wang, S. Development of AlN/Epoxy Composites with Enhanced Thermal Conductivity. Materials 2017, 10, 1442. https://doi.org/10.3390/ma10121442
Xu Y, Yang C, Li J, Mao X, Zhang H, Hu S, Wang S. Development of AlN/Epoxy Composites with Enhanced Thermal Conductivity. Materials. 2017; 10(12):1442. https://doi.org/10.3390/ma10121442
Chicago/Turabian StyleXu, Yonggang, Chi Yang, Jun Li, Xiaojian Mao, Hailong Zhang, Song Hu, and Shiwei Wang. 2017. "Development of AlN/Epoxy Composites with Enhanced Thermal Conductivity" Materials 10, no. 12: 1442. https://doi.org/10.3390/ma10121442
APA StyleXu, Y., Yang, C., Li, J., Mao, X., Zhang, H., Hu, S., & Wang, S. (2017). Development of AlN/Epoxy Composites with Enhanced Thermal Conductivity. Materials, 10(12), 1442. https://doi.org/10.3390/ma10121442