A Highly Flexible Supercapacitor Based on MnO2/RGO Nanosheets and Bacterial Cellulose-Filled Gel Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Colloidal Suspensions of MnO2, RGO and Their Mixture
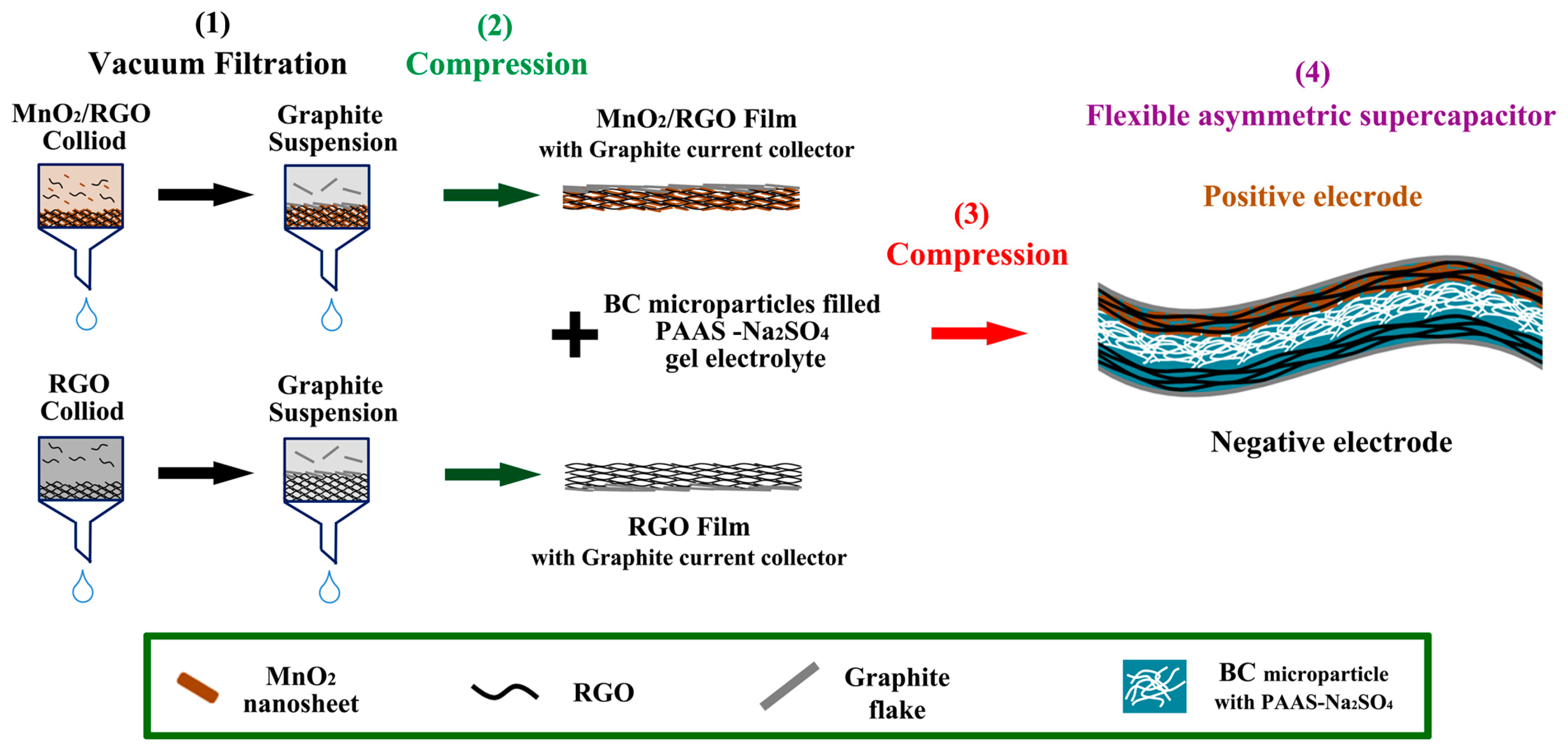
2.2. Preparation of the Flexible RGO and MnO2/RGO Hydrogel Film Electrodes
2.3. Preparation of the BC/PAAS-Na2SO4 Gel Electrolyte
2.4. Fabrication of the Flexible SC Devices
2.5. Characterization
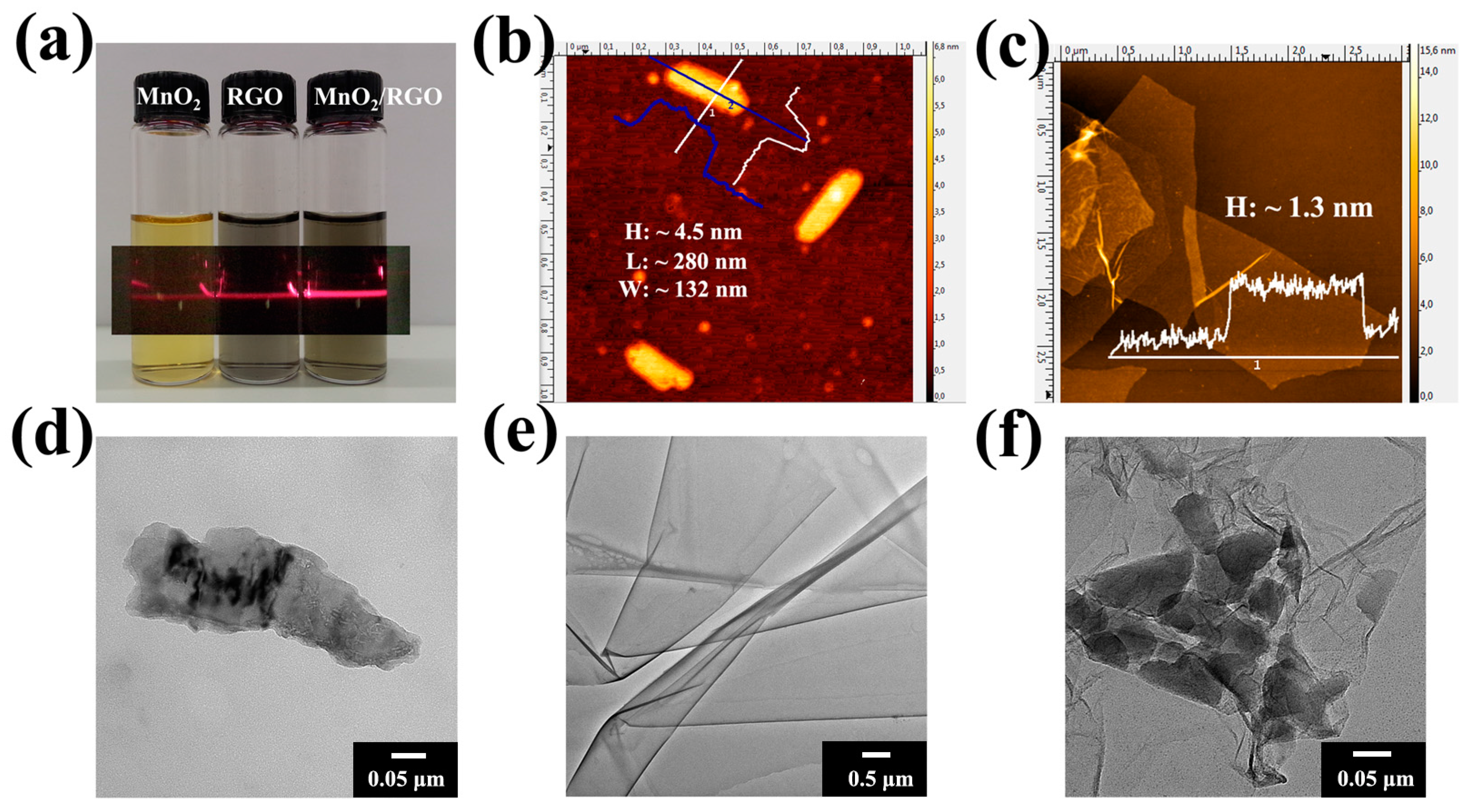
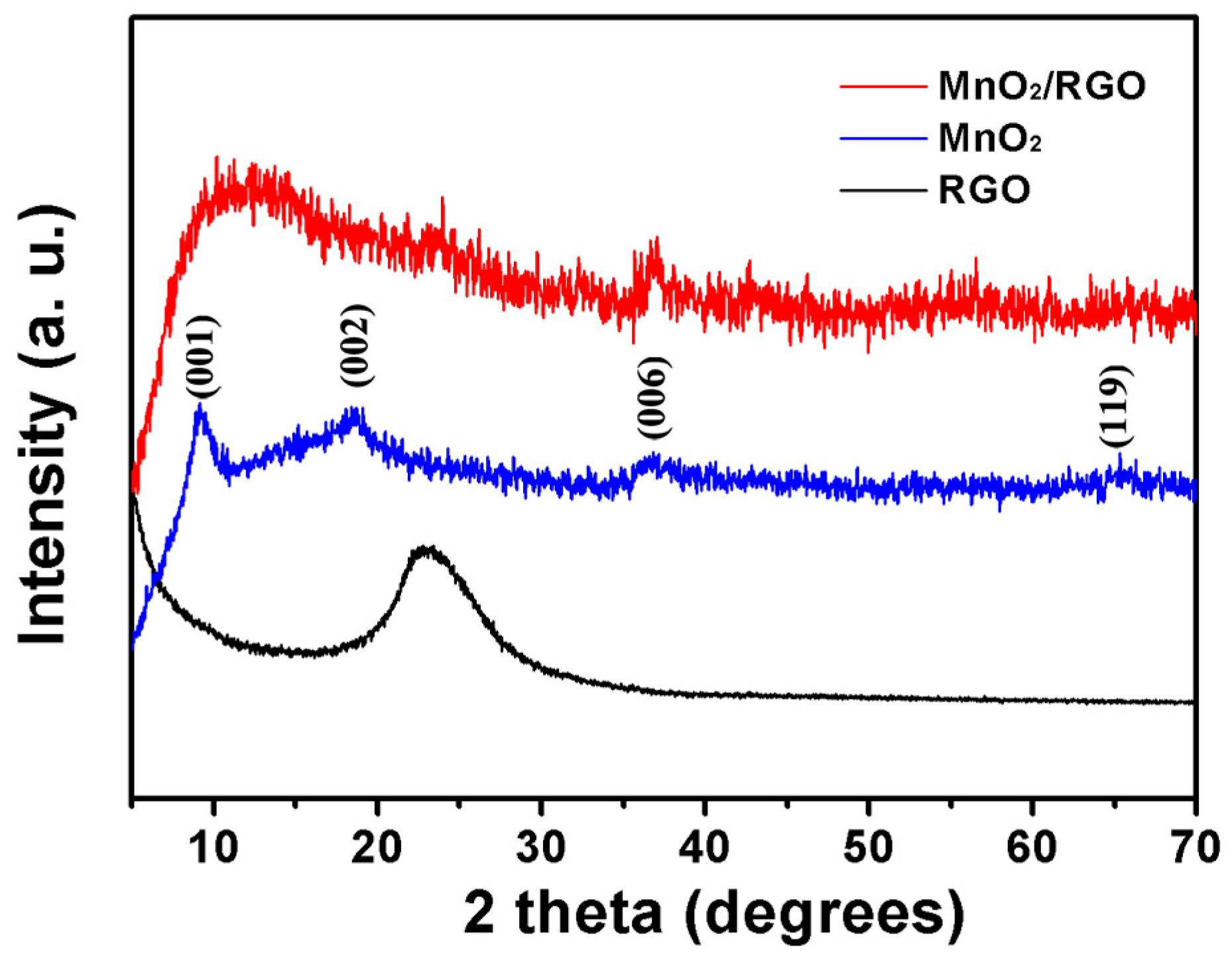
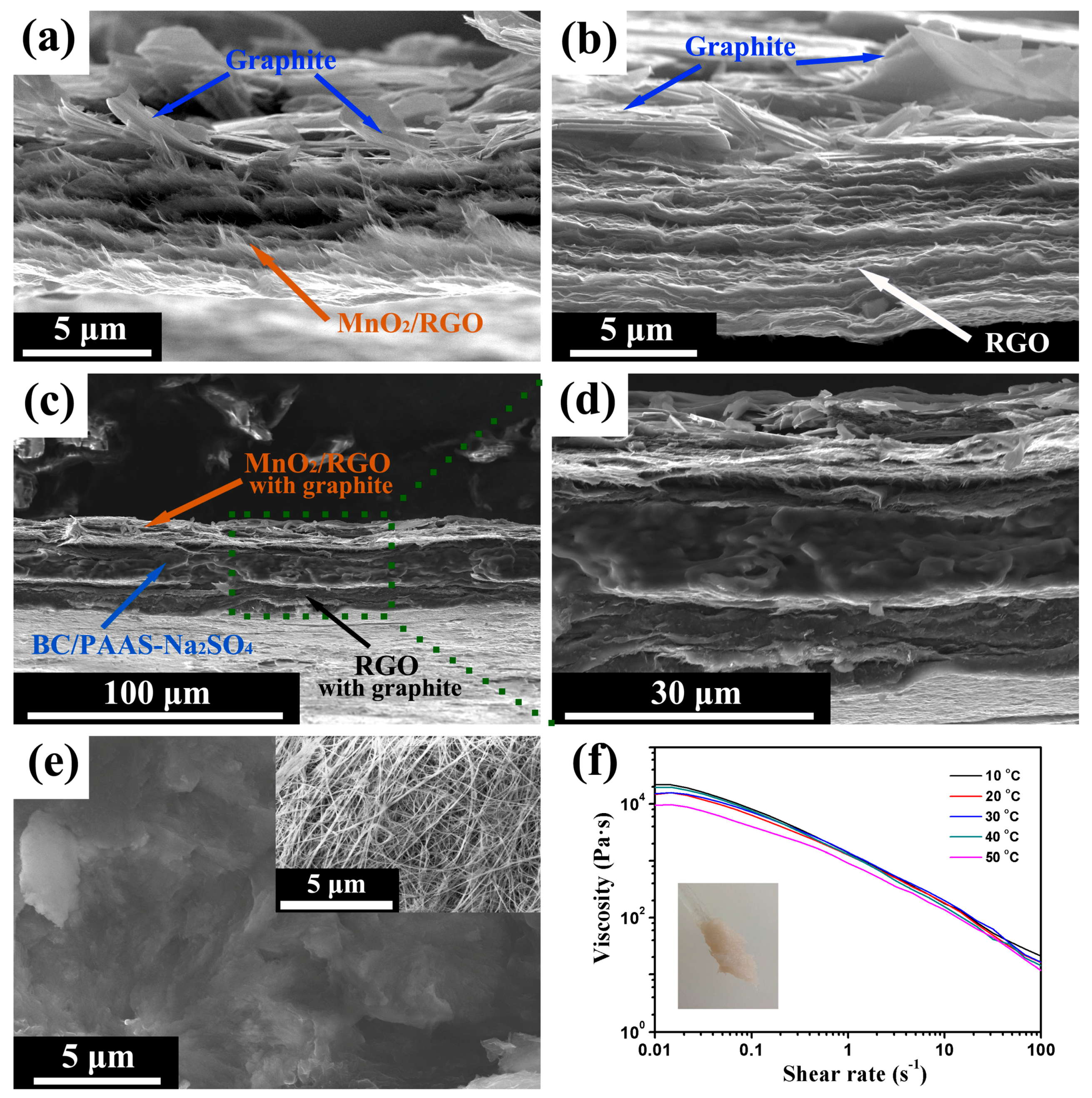
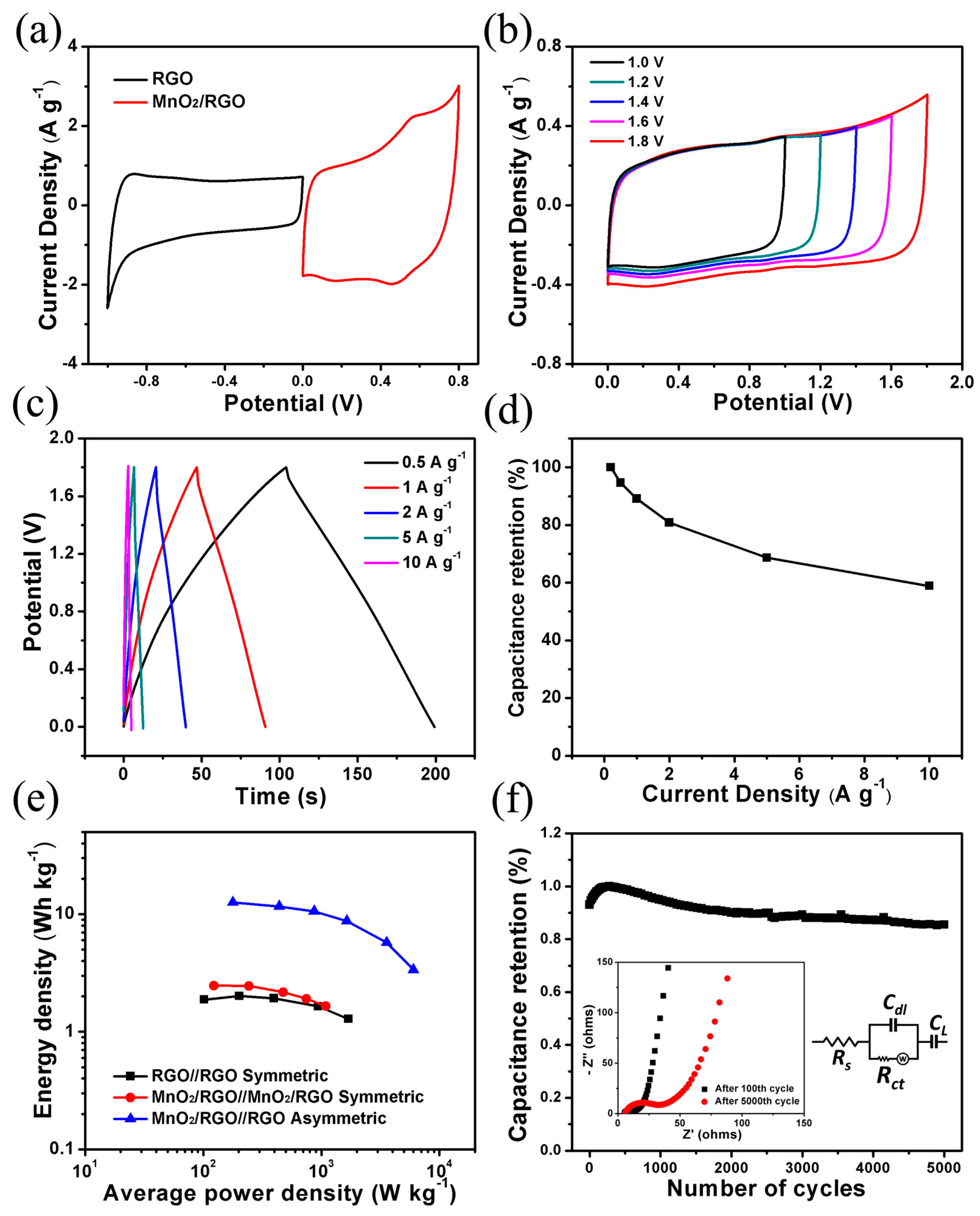
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jang, H.; Park, Y.J.; Chen, X.; Das, T.; Kim, M.-S.; Ahn, J.-H. Graphene-based flexible and stretchable electronics. Adv. Mater. 2016, 28, 4184–4202. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Choi, K.-H.; Choi, W.-S.; Kwon, Y.H.; Jung, H.-R.; Shin, H.-C.; Kim, J.Y. Progress in flexible energy storage and conversion systems, with a focus on cable-type lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2414. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible energy-storage devices: Design consideration and recent progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef] [PubMed]
- Scalia, A.; Bella, F.; Lamberti, A.; Bianco, S.; Gerbaldi, C.; Tresso, E.; Pirri, C.F. A flexible and portable powerpack by solid-state supercapacitor and dye-sensitized solar cell integration. J. Power Sources 2017, 359, 311–321. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, T.; Lemordant, D.; Claude-Montigny, B. Are room temperature ionic liquids able to improve the safety of supercapacitors organic electrolytes without degrading the performances? J. Power Sources 2012, 201, 353–359. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, Y.; Li, M.; Wang, X.; Wu, Y. Green energy storage chemistries based on neutral aqueous electrolytes. J. Mater. Chem. A 2014, 2, 10739–10755. [Google Scholar] [CrossRef]
- Virya, A.; Lian, K. Li2SO4-polyacrylamide polymer electrolytes for 2.0V solid symmetric supercapacitors. Electrochem. Commun. 2017, 81, 52–55. [Google Scholar] [CrossRef]
- Dai, Z.; Peng, C.; Chae, J.H.; Ng, K.C.; Chen, G.Z. Cell voltage versus electrode potential range in aqueous supercapacitors. Sci. Rep. 2015, 5, 9854. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte. Energy Environ. Sci. 2012, 5, 9611. [Google Scholar] [CrossRef]
- Long, J.W.; Bélanger, D.; Brousse, T.; Sugimoto, W.; Sassin, M.B.; Crosnier, O. Asymmetric electrochemical capacitors—Stretching the limits of aqueous electrolytes. MRS Bull. 2011, 36, 513–522. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Piñero, E.; Frackowiak, E.; Béguin, F. High-voltage asymmetric supercapacitors operating in aqueous electrolyte. Appl. Phys. A 2005, 82, 567–573. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020–14027. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, L.; Liu, L.; Zhu, B.; Dong, H.; Chen, X. All-solid-state flexible ultrathin micro-supercapacitors based on graphene. Adv. Mater. 2013, 25, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Li, F.; Cheng, H.-M. Carbon nanotubes and graphene for flexible electrochemical energy storage: From materials to devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiao, Y.; Lu, Z. Fully printed ultraflexible supercapacitor supported by a single-textile substrate. ACS Appl. Mater. Interfaces 2016, 8, 32317–32323. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Peng, X.; Liu, B.; Wu, C.; Xie, Y.; Yu, G. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett. 2013, 13, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lu, X.; Peng, L.; Xu, K.; Peng, X.; Huang, J.; Yu, G.; Xie, Y. Two-dimensional vanadyl phosphate ultrathin nanosheets for high energy density and flexible pseudocapacitors. Nat. Commun. 2013, 4, 2431. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Ma, R.; Sakai, N.; Bai, X.; Li, S.; Sasaki, T. Redox Active Cation Intercalation/Deintercalation in Two-dimensional layered MnO2 nanostructures for high-rate electrochemical energy storage. ACS Appl. Mater. Interfaces 2017, 9, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 2008, 112, 4406–4417. [Google Scholar] [CrossRef]
- Omomo, Y.; Sasaki, T.; Zhou, L.; Watanabe, M. Redoxable nanosheet crystallites of MnO2 derived via delamination of a layered manganese oxide. J. Am. Chem. Soc. 2003, 125, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Athouël, L.; Moser, F.; Dugas, R.; Crosnier, O.; Bélanger, D.; Brousse, T. Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte. J. Phys. Chem. C 2008, 112, 7270–7277. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Ruoff, R.S.; Wen, Z.; Liu, Q. Self-assembly of mesoporous nanotubes assembled from interwoven ultrathin birnessite-type MnO2 nanosheets for asymmetric supercapacitors. Sci. Rep. 2014, 4, 3878. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, X.; Wang, Y.; Walsh, F.C.; Ouyang, J.-H.; Jia, D.; Zhou, Y. Materials and fabrication of electrode scaffolds for deposition of MnO2 and their true performance in supercapacitors. J. Power Sources 2015, 293, 657–674. [Google Scholar] [CrossRef]
- Ko, Y.; Kwon, M.; Bae, W.K.; Lee, B.; Lee, S.W.; Cho, J. Flexible supercapacitor electrodes based on real metal-like cellulose papers. Nat. Commun. 2017, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Hall, A.S.; Kim, J.-D.; Mallouk, T.E. A Facile and Template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem. Mater. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. Flexible all-solid-state asymmetric supercapacitors based on free-standing carbon nanotube/graphene and Mn3O4 nanoparticle/graphene paper electrodes. ACS Appl. Mater. Interfaces 2012, 4, 7020–7026. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. Fabrication of two-dimensional reduced graphene oxide supported V2O5 networks and their application in supercapacitors. Mater. Chem. Phys. 2016, 170 (Suppl. C), 266–275. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992. [Google Scholar] [CrossRef]
- Xiang, C.; Young, C.C.; Wang, X.; Yan, Z.; Hwang, C.-C.; Cerioti, G.; Lin, J.; Kono, J.; Pasquali, M.; Tour, J.M. Large flake graphene oxide fibers with unconventional 100% knot efficiency and highly aligned small flake graphene oxide fibers. Adv. Mater. 2013, 25, 4592–4597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Shi, G. Size fractionation of graphene oxide sheets by pH-assisted selective sedimentation. J. Am. Chem. Soc. 2011, 133, 6338–6342. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, H.C.; Yi, C.; Wang, K.; Gong, X. Polyaniline-modified oriented graphene hydrogel film as the free-standing electrode for flexible solid-state supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 23932–23940. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Z.; Huang, X.; Liu, Y.; Huang, Y.; Duan, X. Flexible solid-state supercapacitors based on three-dimensional graphene hydrogel films. ACS Nano 2013, 7, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Qiu, L.; Li, D. Revisiting the capacitance of polyaniline by using graphene hydrogel films as a substrate: The importance of nano-architecturing. Energy Environ. Sci. 2013, 6, 477–481. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, I.Y.; Kim, T.W.; Lee, J.M.; Hwang, S.J. Mixed colloidal suspensions of reduced graphene oxide and layered metal oxide nanosheets: Useful precursors for the porous nanocomposites and hybrid films of graphene/metal oxide. Chem. Eur. J. 2012, 18, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Sun, M.; Yu, S.; Wang, G. Preparation and supercapacitance performance of manganese oxide nanosheets/graphene/carbon nanotubes ternary composite film. Electrochim. Acta 2014, 125, 488–496. [Google Scholar] [CrossRef]
- Kai, K.; Yoshida, Y.; Kageyama, H.; Saito, G.; Ishigaki, T.; Furukawa, Y.; Kawamata, J. Room-temperature synthesis of manganese oxide monosheets. J. Am. Chem. Soc. 2008, 130, 15938–15943. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Eigler, S.; Enzelberger-Heim, M.; Grimm, S.; Hofmann, P.; Kroener, W.; Geworski, A.; Dotzer, C.; Rockert, M.; Xiao, J.; Papp, C.; et al. Wet chemical synthesis of graphene. Adv. Mater. 2013, 25, 3583–3587. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lian, K. Proton-conducting polymer electrolytes and their applications in solid supercapacitors: A review. RSC Adv. 2014, 4, 33091–33113. [Google Scholar] [CrossRef]
- Lv, X.; Li, G.; Li, D.; Huang, F.; Liu, W.; Wei, Q. A new method to prepare no-binder, integral electrodes-separator, asymmetric all-solid-state flexible supercapacitor derived from bacterial cellulose. J. Phys. Chem. Solids 2017, 110, 202–210. [Google Scholar] [CrossRef]
- Sacco, A.; Bella, F.; De La Pierre, S.; Castellino, M.; Bianco, S.; Bongiovanni, R.; Pirri, C.F. Electrodes/Electrolyte Interfaces in the Presence of a Surface-Modified Photopolymer Electrolyte: Application in Dye-Sensitized Solar Cells. ChemPhysChem 2015, 16, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Brousse, T.; Taberna, P.-L.; Crosnier, O.; Dugas, R.; Guillemet, P.; Scudeller, Y.; Zhou, Y.; Favier, F.; Bélanger, D.; Simon, P. Long-term cycling behavior of asymmetric activated carbon/MnO2 aqueous electrochemical supercapacitor. J. Power Sources 2007, 173, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. High-performance asymmetric supercapacitor based on graphene hydrogel and nanostructured MnO2. ACS Appl. Mater. Interfaces 2012, 4, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.-H.; Kang, F.; Yang, Q.-H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Chen, Y.C.; Lin, Y.G.; Chen, L.C.; Chen, K.H. Reversible phase transformation of MnO2 nanosheets in an electrochemical capacitor investigated by in situ Raman spectroscopy. Chem. Commun. 2011, 47, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Electrochemical cyclability mechanism for MnO2 electrodes utilized as electrochemical supercapacitors. J. Power Sources 2009, 186, 543–550. [Google Scholar] [CrossRef]
- Koo, M.; Park, K.-I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable inorganic thin-film battery for fully flexible electronic systems. Nano Lett. 2012, 12, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Ma, E.Y.; Gleskova, H.; Wagner, S. Mechanics of rollable and foldable film-on-foil electronics. Appl. Phys. Lett. 1999, 74, 1177–1179. [Google Scholar] [CrossRef]
- Gleskova, H.; Cheng, I.C.; Wagner, S.; Suo, Z. Mechanical theory of the film-on-substrate-foil structure: Curvature and overlay alignment in amorphous silicon thin-film devices fabricated on free-standing foil substrates. In Flexible Electronics: Materials and Applications; Wong, W.S., Salleo, A., Eds.; Springer Science & Business Media: New York, NY, USA, 2009; pp. 29–50. ISBN 978-0-387-74362-2. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, H.; Saha, N.; Kazantseva, N.; Moucka, R.; Cheng, Q.; Saha, P. A Highly Flexible Supercapacitor Based on MnO2/RGO Nanosheets and Bacterial Cellulose-Filled Gel Electrolyte. Materials 2017, 10, 1251. https://doi.org/10.3390/ma10111251
Fei H, Saha N, Kazantseva N, Moucka R, Cheng Q, Saha P. A Highly Flexible Supercapacitor Based on MnO2/RGO Nanosheets and Bacterial Cellulose-Filled Gel Electrolyte. Materials. 2017; 10(11):1251. https://doi.org/10.3390/ma10111251
Chicago/Turabian StyleFei, Haojie, Nabanita Saha, Natalia Kazantseva, Robert Moucka, Qilin Cheng, and Petr Saha. 2017. "A Highly Flexible Supercapacitor Based on MnO2/RGO Nanosheets and Bacterial Cellulose-Filled Gel Electrolyte" Materials 10, no. 11: 1251. https://doi.org/10.3390/ma10111251
APA StyleFei, H., Saha, N., Kazantseva, N., Moucka, R., Cheng, Q., & Saha, P. (2017). A Highly Flexible Supercapacitor Based on MnO2/RGO Nanosheets and Bacterial Cellulose-Filled Gel Electrolyte. Materials, 10(11), 1251. https://doi.org/10.3390/ma10111251




