Easy Fabrication of Highly Thermal-Stable Cellulose Nanocrystals Using Cr(NO3)3 Catalytic Hydrolysis System: A Feasibility Study from Macro- to Nano-Dimensions
Abstract
:1. Introduction
2. Results and Discussion
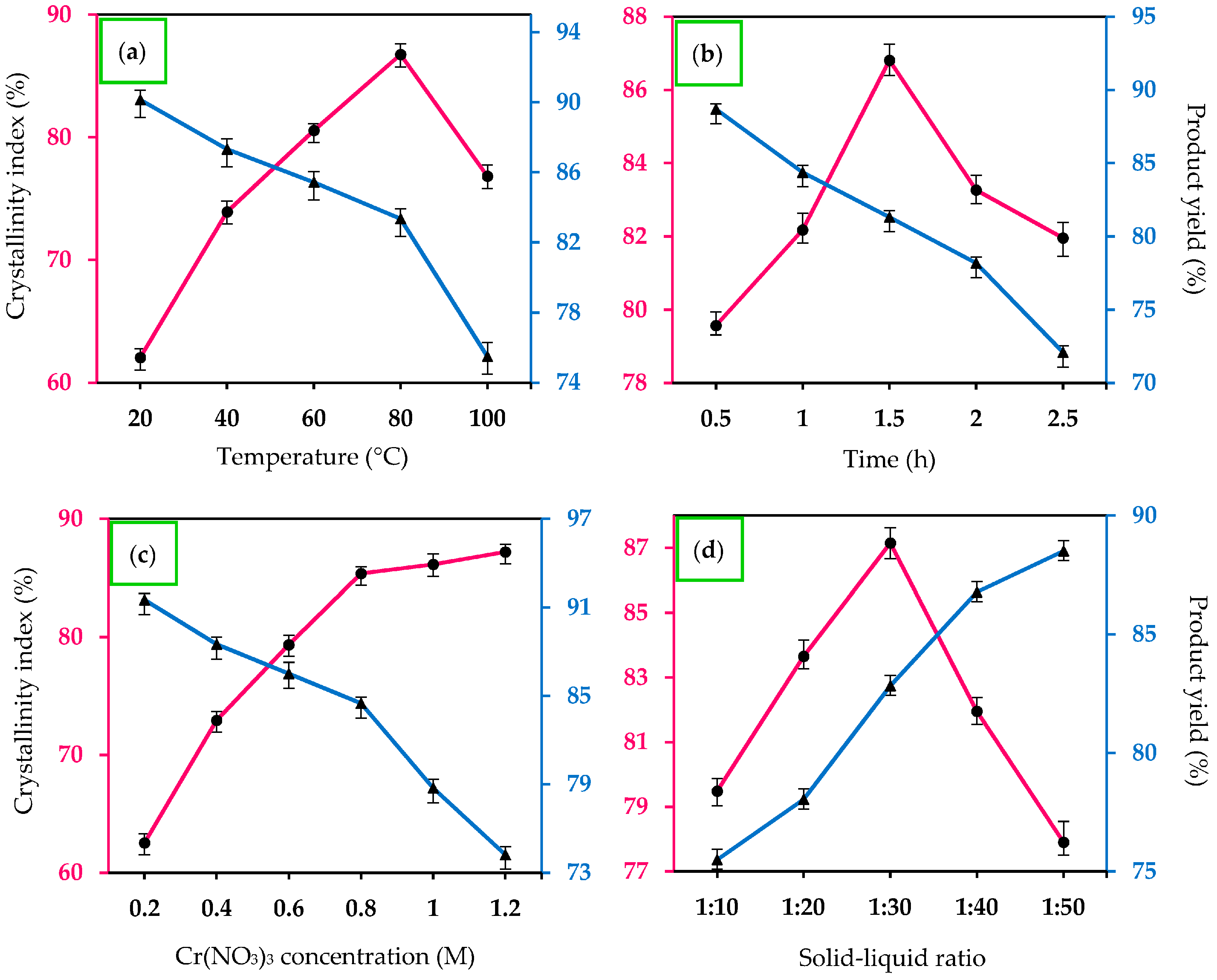
2.1. Effect of Reaction Temperature
2.2. Effects of Hydrolysis Time
2.3. Effects of Metal Salt Concentration
2.4. Effects of Water Content
2.5. Crystallinity Study—XRD Investigation
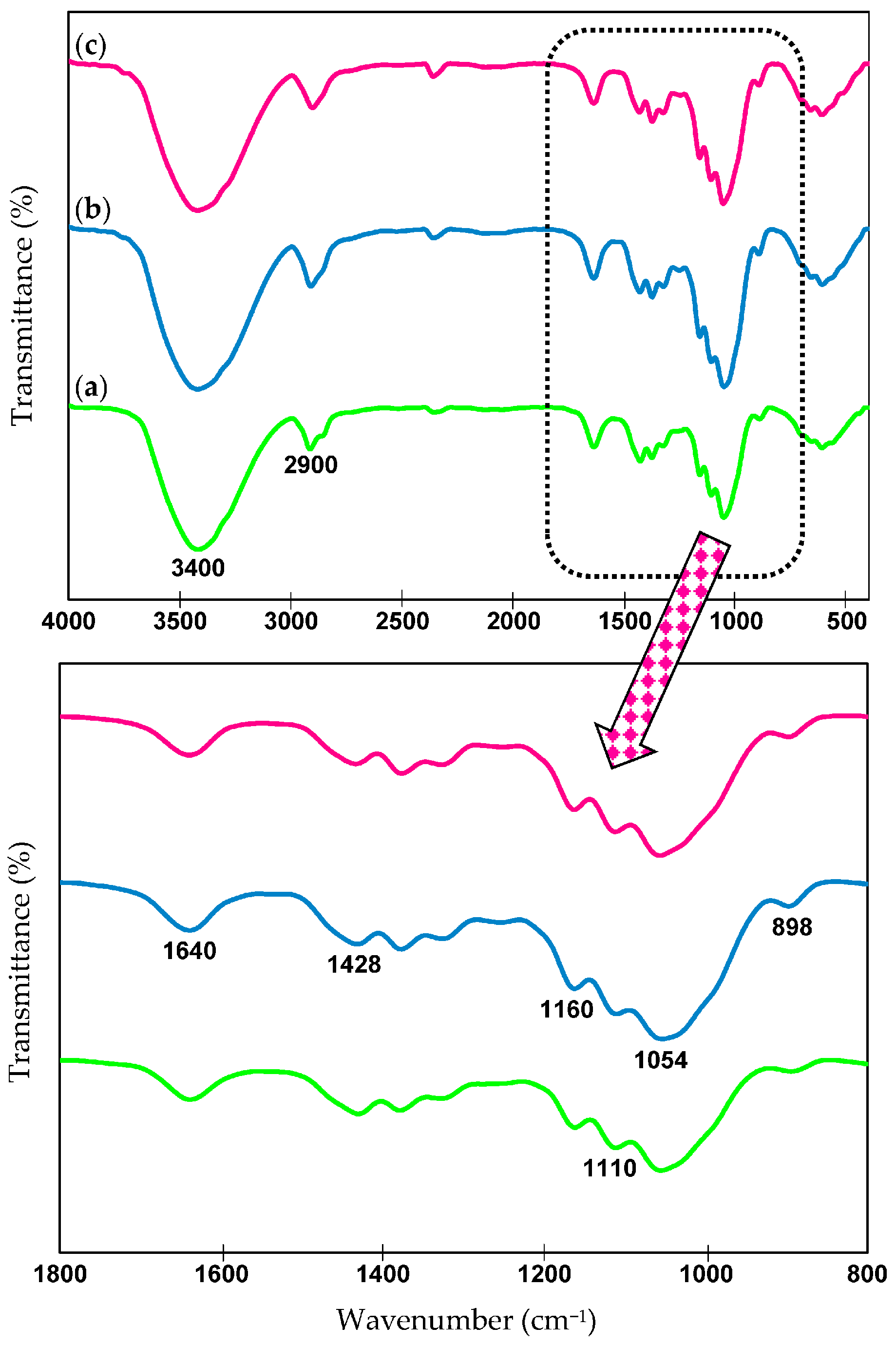
2.6. Chemical Structures—FTIR Analysis
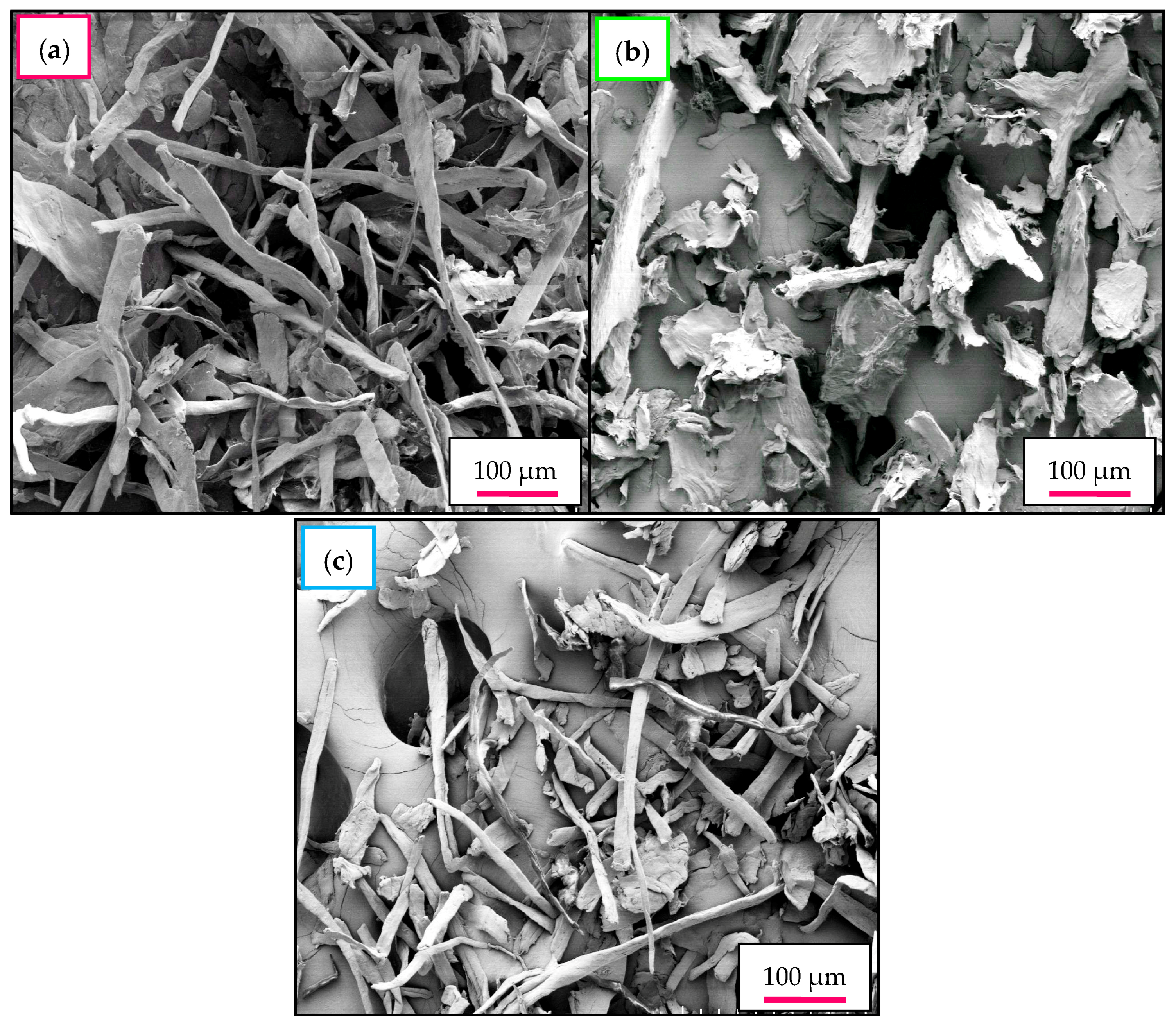
2.7. Morphological Investigation—FESEM-EDX
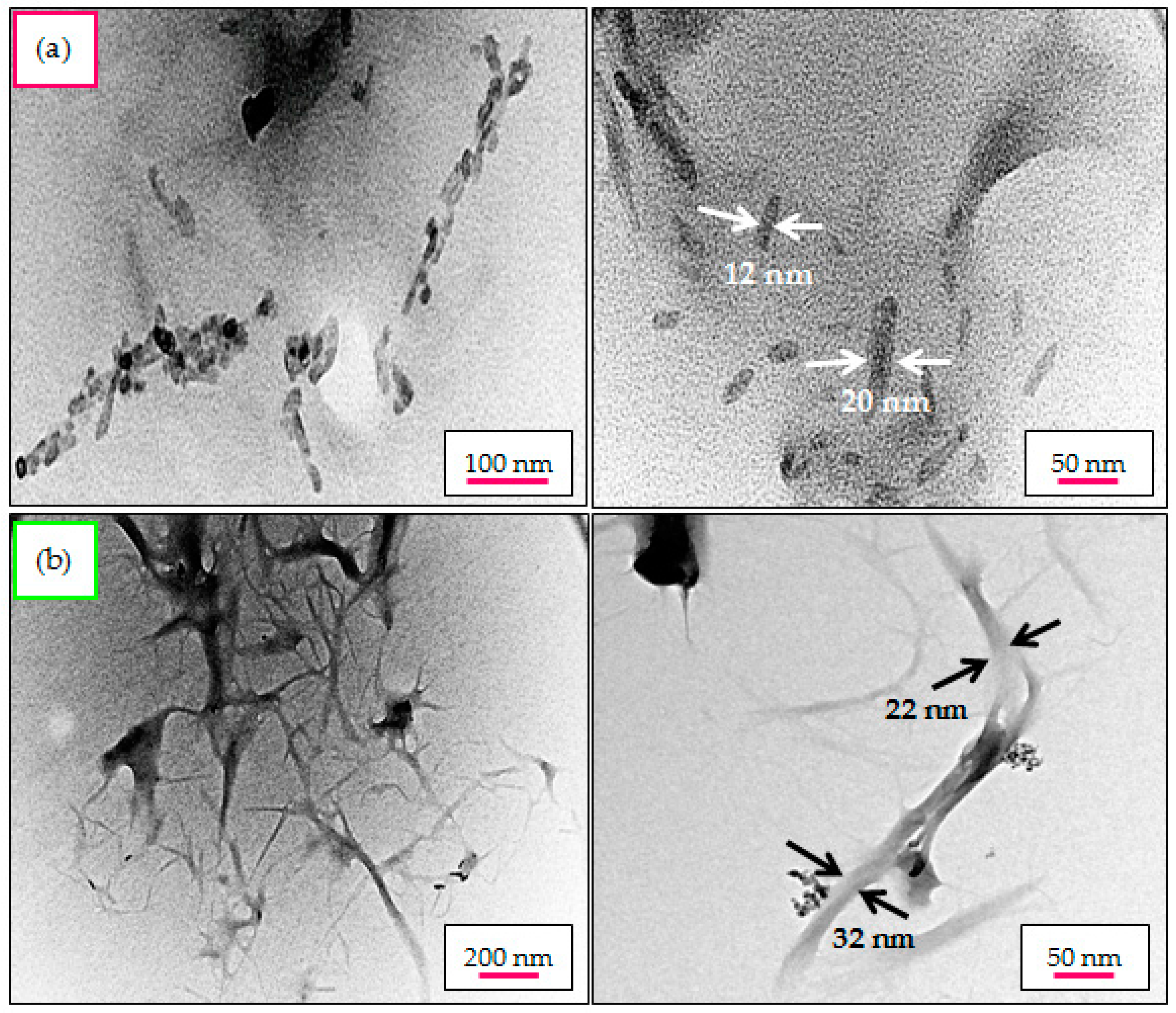
2.8. Structural Morphology and Particle Size Distribution—TEM Analysis
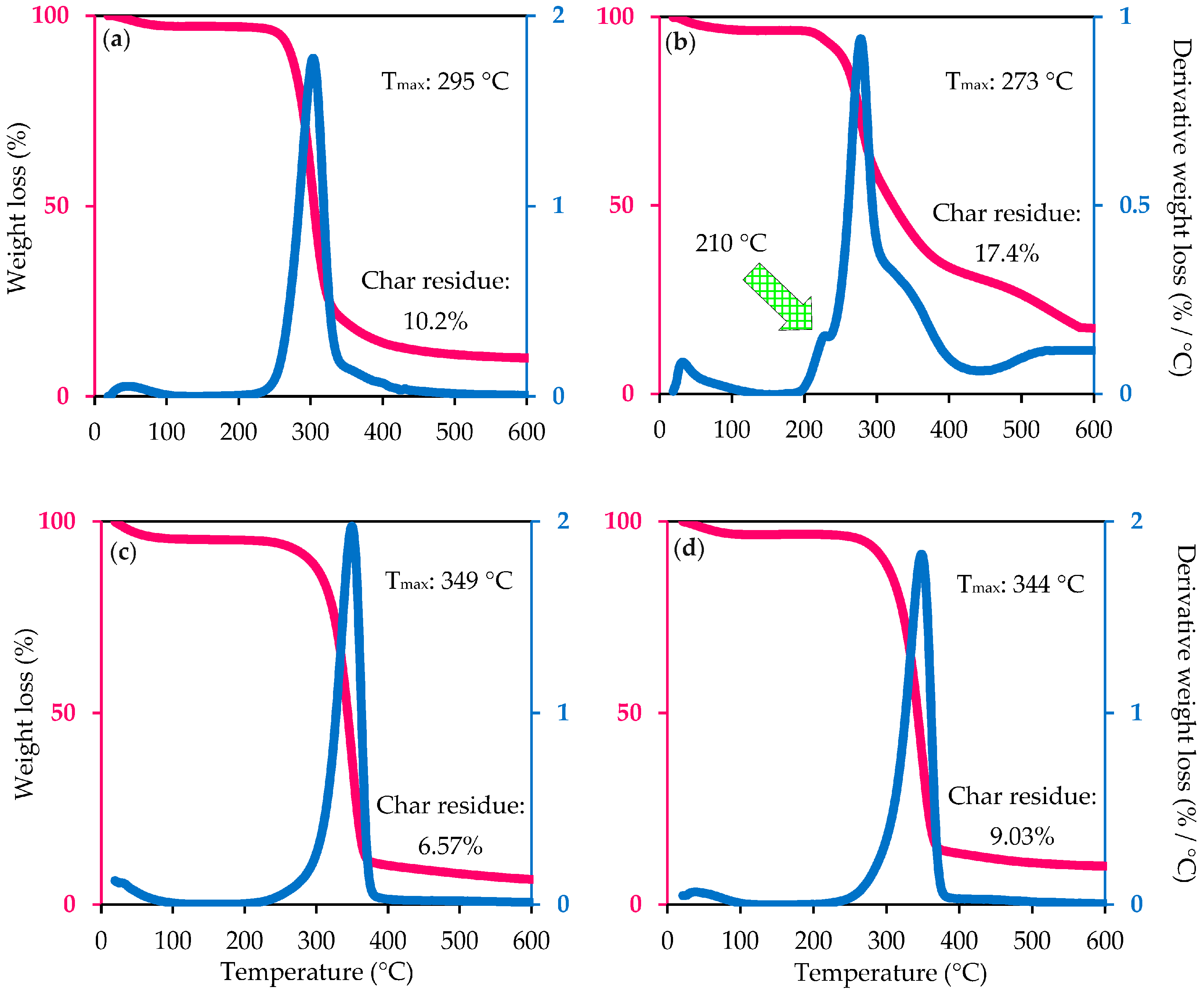
2.9. Thermostability Analysis
- (i)
- The replacement of hydroxyl groups (O–H) by active sulfate groups (O–SO3H) via either esterification or direct catalysis would initiate the dehydration reaction to occur on the sulfated nanocellulose fiber, leading to the water formation, further catalyzing the decomposition of remaining cellulose fiber into smaller particles. On the other hand, the unsulfated crystals tend to collapse at higher decomposition temperature [53];
- (ii)
- It was believed that the concentrated H2SO4 not only removes the loosely-packed defective parts but also has high potential to damage crystalline domains, making the molecules more susceptible to the degradation step in response to increased temperature [18];
- (iii)
- The rapid reduction of molecular weight of nanocellulose during hydrolysis compared to that of its starting material contributed to its early decomposition during heating treatment [54];
- (iv)
- The short nanocellulose chains provide a high specific surface area and result in the formation of a large number of free-end-chains in the surface that tended to decompose easily at a lower temperature. The high surface area of nanocellulose plays a significant role in diminishing their thermal stability due to the increased exposure to heat source [55].
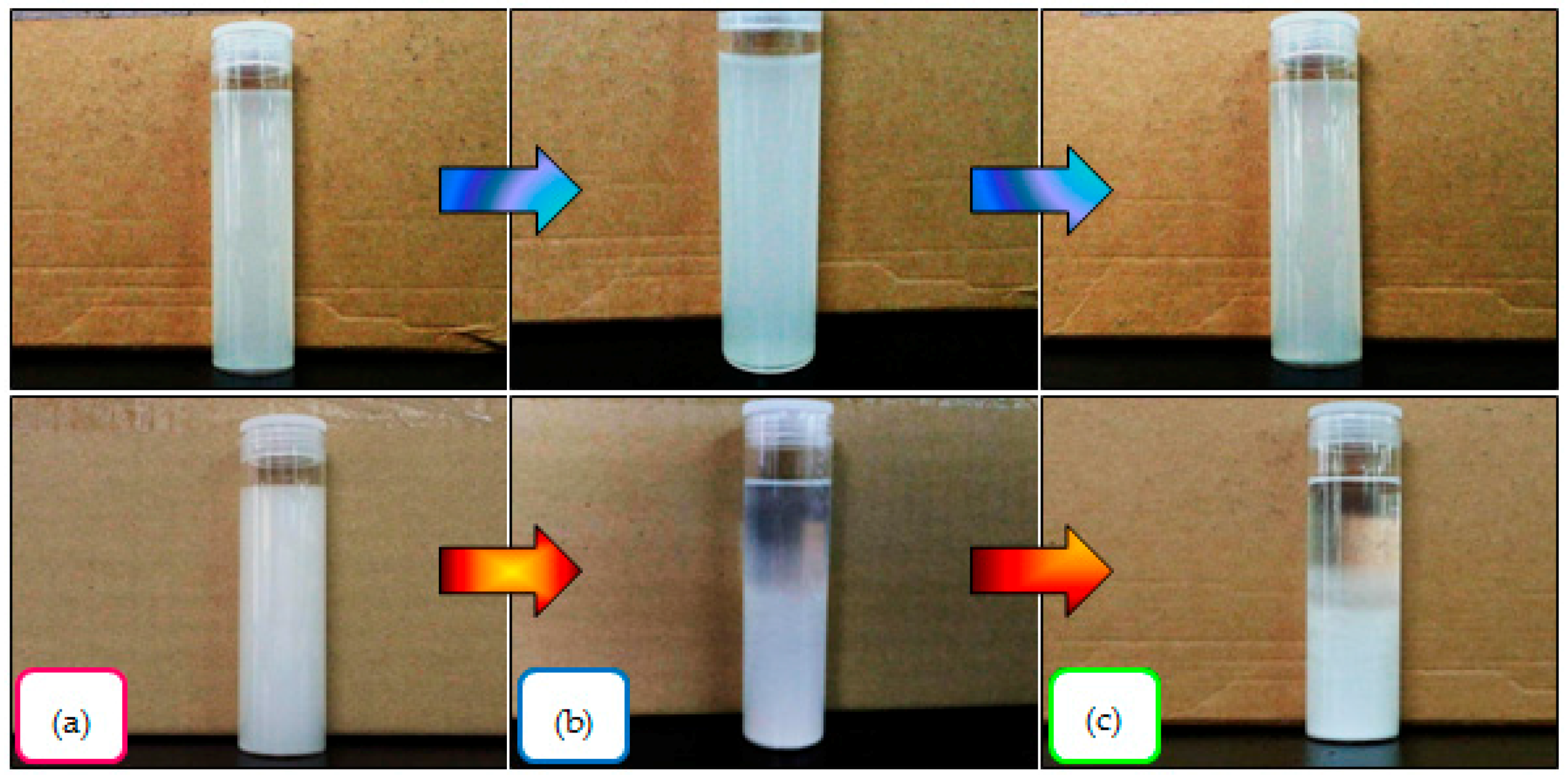
2.10. Visual Appearance of Nanocellulose
2.11. Comparison between H2SO4 and Cr(NO3)3 Hydrolysis System
3. Materials and Methods
3.1. Materials
3.2. Preparation of CNCCr(NO3)3 via Cr(NO3)3 Catalyzed Hydrolysis
3.3. Isolation of CNCH2SO4 via H2SO4 Catalyzed Hydrolysis
3.4. Products Characterization Analysis
3.4.1. Chemical Structure: Fourier-Transformed Infrared Spectral Analysis
3.4.2. Crystal Structure: X-ray Diffraction Studies
3.4.3. Morphological Analysis: FESEM and TEM Analysis
3.4.4. Thermal Property Investigation: Thermogravimetric Analysis (TGA) Study
3.4.5. Visual Examination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, X.; Meng, X.; Zhao, J.; Miao, M.; Shi, L.; Zhang, S.; Fang, J. Extraction and preparation of cellulose nanocrystals from dealginate kelp residue: Structures and morphological characterization. Cellulose 2015, 22, 1763–1772. [Google Scholar] [CrossRef]
- Abraham, R.; Wong, C.; Puri, M. Enrichment of cellulosic waste hemp (cannabis sativa) hurd into non-toxic microfibres. Materials 2016, 9, 562. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Deshmane, V.G. Intensification of enzymatic hydrolysis of cellulose using high-frequency ultrasound: An investigation of the effects of process parameters on glucose yield. Energy Fuels 2015, 29, 4998–5006. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure-properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Puangsin, B.; Yang, Q.; Saito, T.; Isogai, A. Comparative characterization of tempo-oxidized cellulose nanofibril films prepared from non-wood resources. Int. J. Biol. Macromol. 2013, 59, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (cncs) by ionic liquid solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Tan, B.K.; Ching, Y.C.; Gan, S.N.; Rozali, S. Biodegradable mulches based on poly (vinyl alcohol), kenaf fiber, and urea. BioResources 2015, 10, 5532–5543. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Wang, S.; Yin, Y. Effects of ultrasonic treatment during acid hydrolysis on the yield, particle size and structure of cellulose nanocrystals. Carbohydr. Polym. 2016, 135, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.Z.; Chowdhury, Z.Z.; Hamid, S.B.A.; Ali, M.E. Statistical optimization for acid hydrolysis of microcrystalline cellulose and its physiochemical characterization by using metal ion catalyst. Materials 2014, 7, 6982–6999. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of new cellulose nanomaterial from red algae marine biomass gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Saito, T.; Isogai, A. Cellulose nanofibrils prepared from softwood cellulose by tempo/naclo/naclo 2 systems in water at ph 4.8 or 6.8. Int. J. Biol. Macromol. 2012, 51, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils—An enzymatic approach. Bioresources 2007, 1, 176–188. [Google Scholar]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.B.; Lee, H.V.; Hamid, S.B.A. Preparation of nanocellulose via transition metal salt-catalyzed hydrolysis pathway. BioResources 2015, 10, 7627–7639. [Google Scholar] [CrossRef]
- Goh, K.Y.; Ching, Y.C.; Chuah, C.H.; Abdullah, L.C.; Liou, N.-S. Individualization of microfibrillated celluloses from oil palm empty fruit bunch: Comparative studies between acid hydrolysis and ammonium persulfate oxidation. Cellulose 2016, 23, 379–390. [Google Scholar] [CrossRef]
- Correa, A.C.; de Morais Teixeira, E.; Pessan, L.A.; Mattoso, L.H.C. Cellulose nanofibers from curaua fibers. Cellulose 2010, 17, 1183–1192. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr. Polym. 2014, 110, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.B.A.; Zain, S.K.; Das, R.; Centi, G. Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohydr. Polym. 2015, 138, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wang, S.; Han, Q. Novel process for isolating fibrils from cellulose fibers by high-intensity ultrasonication. Ii. Fibril characterization. J. Appl. Polym. Sci. 2010, 115, 2756–2762. [Google Scholar] [CrossRef]
- Tian, C.; Yi, J.; Wu, Y.; Wu, Q.; Qing, Y.; Wang, L. Preparation of highly charged cellulose nanofibrils using high-pressure homogenization coupled with strong acid hydrolysis pretreatments. Carbohydr. Polym. 2016, 136, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Su, J.; Lin, Y.; Huang, Z.; Lu, Y.; Sun, G.; Yang, M.; Huang, A.; Hu, H.; et al. A green and efficient technology for the degradation of cellulosic materials: Structure changes and enhanced enzymatic hydrolysis of natural cellulose pretreated by synergistic interaction of mechanical activation and metal salt. Bioresour. Technol. 2015, 177, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Brown, H.M.; Huang, X.; Zhou, X.-D.; Amonette, J.E.; Zhang, Z.C. Single-step conversion of cellulose to 5-hydroxymethylfurfural (hmf), a versatile platform chemical. Appl. Catal. A Gen. 2009, 361, 117–122. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Zhang, J.; Zhuang, J.; Zhang, B.; Gong, Y. Catalytic conversion of cellulose to levulinic acid by metal chlorides. Molecules 2010, 15, 5258–5272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, H.; Zheng, R.; Lin, Z.; Huang, H. The enhancement of pretreatment and enzymatic hydrolysis of corn stover by feso4 pretreatment. Biochem. Eng. J. 2011, 56, 158–164. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Wang, X.; Meng, L.; Wang, H.; Peng, G.; Wang, X.; Mu, X. Effective saccharification of lignocellulosic biomass over hydrolysis residue derived solid acid under microwave irradiation. Green Chem. 2012, 14, 2162–2167. [Google Scholar] [CrossRef]
- Karim, Z.; Chowdhury, Z.Z.; Hamid, S.B.A.; Ali, E. Optimizing pretreatment process conditions using lewis acid catalyst for higher crystallinity of α-cellulose. Sci. Adv. Mater. 2016, 8, 534–544. [Google Scholar] [CrossRef]
- Saelee, K.; Yingkamhaeng, N.; Nimchua, T.; Sukyai, P. An environmentally friendly xylanase-assisted pretreatment for cellulose nanofibrils isolation from sugarcane bagasse by high-pressure homogenization. Ind. Crops Prod. 2016, 82, 149–160. [Google Scholar] [CrossRef]
- Li, J.; Xiu, H.; Zhang, M.; Wang, H.; Ren, Y.; Ji, Y. Enhancement of cellulose acid hydrolysis selectivity using metal ion catalysts. Curr. Org. Chem. 2013, 17, 1617–1623. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.; Zattera, A. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Huang, B.; Huang, M.; Ai, X. Isolation and characteristics of cellulose and nanocellulose from lotus leaf stalk agro-wastes. BioResources 2014, 10, 684–696. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, Y.; Cha, R.; Yang, J.; Jiang, X. Water-soluble nanocrystalline cellulose films with highly transparent and oxygen barrier properties. Nanoscale 2016, 8, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Lee, H.V.; Hamid, S.B.A. A response surface methodology study: Effects of trivalent Cr3+ metal ion-catalyzed hydrolysis on nanocellulose crystallinity and yield. BioResources 2016, 11, 4645–4662. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Hamid, S.B.A. Preparation of nanostructured cellulose via Cr(iii)-and Mn(ii)-transition metal salt catalyzed acid hydrolysis approach. BioResources 2016, 11, 7224–7241. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Abd Hamid, S.B. Preparation and characterization of cellulose crystallites via Fe(iii)-, Co(ii)-and Ni(ii)-assisted dilute sulfuric acid catalyzed hydrolysis process. J. Nano Res. 2016, 41, 96–109. [Google Scholar] [CrossRef]
- He, W.; Jiang, S.; Zhang, Q.; Pan, M. Isolation and characterization of cellulose nanofibers from bambusa rigida. BioResources 2013, 8, 5678–5689. [Google Scholar] [CrossRef]
- Mohamed, M.; Salleh, W.; Jaafar, J.; Asri, S.; Ismail, A. Physicochemical properties of “green” nanocrystalline cellulose isolated from recycled newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (scb) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Abd Hamid, S.B. Facile production of nanostructured cellulose from elaeis guineensis empty fruit bunch via one pot oxidative-hydrolysis isolation approach. Carbohydr. Polym. 2017, 157, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Puglia, D.; Monti, M.; Peponi, L.; Santulli, C.; Kenny, J.; Torre, L. Extraction of cellulose nanocrystals from phormium tenax fibres. J. Polym. Environ. 2013, 21, 319–328. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, T.; Chang, P.R.; Li, K.; Wu, Q. Starch composites reinforced by bamboo cellulosic crystals. Bioresour. Technol. 2010, 101, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G. Preparation and characterisation of cellulose nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Lavoie, J.-M. Isolation of nanocrystalline cellulose: A technological route for valorizing recycled tetra pak aseptic multilayered food packaging wastes. Waste Biomass Valoriz. 2016. [Google Scholar] [CrossRef]
- Castro-Guerrero, C.F.; Gray, D.G. Chiral nematic phase formation by aqueous suspensions of cellulose nanocrystals prepared by oxidation with ammonium persulfate. Cellulose 2014, 21, 2567–2577. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose nanocrystals from forest residues as reinforcing agents for composites: A study from macro- to nano-dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Moriana, R.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Improved thermo-mechanical properties by the addition of natural fibres in starch-based sustainable biocomposites. Comp. Part A Appl. Sci. Manuf. 2011, 42, 30–40. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Effect of post-treatments and concentration of cotton linter cellulose nanocrystals on the properties of agar-based nanocomposite films. Carbohydr. Polym. 2015, 134, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Tammelin, T.; Österberg, M. Cellulose—Model films and the fundamental approach. Chem. Soc. Rev. 2006, 35, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shen, X.; Zhang, J.; Guo, D.; Kong, F.; Zhang, N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015, 125, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Jonoobi, M.; Mathew, A.P.; Oksman, K. Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind. Crops Prod. 2012, 40, 232–238. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Gray, D.G. Recent advances in chiral nematic structure and iridescent color of cellulose nanocrystal films. Nanomaterials 2016, 6, 213. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arabian J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Ge, S.; Xiong, L.; Sun, Q. Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind. Crops Prod. 2016, 83, 346–352. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jiang, X.; Sun, F.; Xu, X. Extraction and characterization of cellulose nanofibers from phyllostachys nidularia munro via a combination of acid treatment and ultrasonication. BioResources 2014, 9, 6876–6887. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Sabo, R.; Luo, X. Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem. 2011, 13, 1339–1344. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohydr. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]

| Element | CNCH2SO4 | CNCCr(NO3)3 |
|---|---|---|
| Mass Fraction (%) | Mass Fraction (%) | |
| C | 45.46 | 48.12 |
| O | 54.04 | 51.88 |
| S | 0.50 | n/d |
| Raw Materials | Tmax Value (°C) | Hydrolysis Method | Reference | |
|---|---|---|---|---|
| Cellulose | Nanomaterial | |||
| MCC | 295 | 344 | 0.8 M Cr(NO3)3 | This study |
| 295 | 210, 273 | 64 wt % H2SO4 | ||
| Cannabis sativa | 315 | 265, 327 | 6 M H2SO4 | [2] |
| Bamboo pulp | 338 | 348 | Ball milling + phosphotungstic acid | [53] |
| Bleached corncob residue | 320 | 360 | 0.5% HCl + 88% formic acid | [22] |
| 320 | 336 | PFI refining | ||
| Industrial sludge | 330 | 335 | Ultra-fine grinding | [62] |
| Wood (Needle fir) | 331 | 333 | Ultrasonication | [63] |
| Source | Hydrolysis System | Width (nm) a | Length (nm) a | CrI (%) * | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| MCC | 0.8 M Cr(NO3)3 | 29.1 ± 7.8 | 455.73 ± 38.1 | 86.5 ± 0.3 ** | 83.6 ± 0.6 | This study |
| 64 wt % H2SO4 | 9.9 ± 3.2 | 34.8 ± 14.2 | 81.4 ± 0.1 ** | 54.7 ± 0.3 | ||
| Hardwood pulp | Phosphotungstic acid | 15–25 | 600–800 nm | 85 | 60 | [20] |
| Flax stem | Ultrasonication treatment | 15–100 | ns | 81.6 | ns | [63] |
| Wood (Abies nephrolepis) | 10–20 | 71.0 | ||||
| Moso Bamboo | 10–40 | 64.9 | ||||
| Wheat straw stem | 15–35 | 63.4 | ||||
| MCC | Ultrasonic-assisted enzymatic hydrolysis | <10 | 40–50 | 82.26 ± 0.11 | 22.57 | [66] |
| Eucalyptus pulp | H2SO4 hydrolysis | 3.6 | ns | 81 | ns | [50] |
| Multi-pass high pressure grinding | 20 | 64.4 | ||||
| Bamboo pulp | Ball milling + phosphotungstic acid | 25∓50 | 200∓300 | 79.6 | 88.4 | [53] |
| Groundnut shells | 65 wt % H2SO4 | 5‒18 | 67‒172 | 74 ** | 31.3 | [67] |
| Lotus leaf stalks | High intensity ultrasonication | 20 ± 5 | Micron scale | 70 | ns | [35] |
| Phyllostachys nidularia Munro | 64 wt % H2SO4 + low intensity ultrasonication | 5‒20 | ns | 69.32 | ns | [68] |
| Sugarcane bagasse | High-pressure homogenization | <10 | ns | 68.10 ± 0.18 | ns | [32] |
| Eucalyptus wood pulp | 64 wt % H2SO4 | 5‒10 | 100‒300 | 64.4 | ns | [24] |
| 37 wt % HCl | Up to tens of nm | Several microns nm | 61.8 | ns | ||
| Bambusa rigida | 33 wt % H2SO4 + ultrasonication | 10‒30 | ns | 61.21 | ns | [41] |
| Old corrugated container | Phosphoric acid + enzymatic hydrolysis + sonication | 15–80 | 100–400 | 57.8 | 23.98 | [60] |
| Kraft eucalyptus pulp | Enzymatic hydrolysis + homogenization | 20 | 500 | 57 *** | ns | [69] |
| Corncob residue | 64 wt % H2SO4 | 5.5 ± 1.9 | 198 ± 51 | 55.9 | 34.5 | [22] |
| Component | Test Method | Percentage (%) |
|---|---|---|
| α-cellulose | ASTM D 1103-55T | 93.6 |
| Hemicellulose | ASTM D 1104-56 | 0.8 |
| Lignin | ASTM D 1106-56 | <0.3 |
| Ash | ASTM D 5360 | 10.4 |
| Moisture content | ASTM D 2495 | 2.3 |
| Extractive | TAPPI T257 cm-02 | n/d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.W.; Tan, T.H.; Lee, H.V.; Abd Hamid, S.B. Easy Fabrication of Highly Thermal-Stable Cellulose Nanocrystals Using Cr(NO3)3 Catalytic Hydrolysis System: A Feasibility Study from Macro- to Nano-Dimensions. Materials 2017, 10, 42. https://doi.org/10.3390/ma10010042
Chen YW, Tan TH, Lee HV, Abd Hamid SB. Easy Fabrication of Highly Thermal-Stable Cellulose Nanocrystals Using Cr(NO3)3 Catalytic Hydrolysis System: A Feasibility Study from Macro- to Nano-Dimensions. Materials. 2017; 10(1):42. https://doi.org/10.3390/ma10010042
Chicago/Turabian StyleChen, You Wei, Thean Heng Tan, Hwei Voon Lee, and Sharifah Bee Abd Hamid. 2017. "Easy Fabrication of Highly Thermal-Stable Cellulose Nanocrystals Using Cr(NO3)3 Catalytic Hydrolysis System: A Feasibility Study from Macro- to Nano-Dimensions" Materials 10, no. 1: 42. https://doi.org/10.3390/ma10010042
APA StyleChen, Y. W., Tan, T. H., Lee, H. V., & Abd Hamid, S. B. (2017). Easy Fabrication of Highly Thermal-Stable Cellulose Nanocrystals Using Cr(NO3)3 Catalytic Hydrolysis System: A Feasibility Study from Macro- to Nano-Dimensions. Materials, 10(1), 42. https://doi.org/10.3390/ma10010042






