A Comparative Study of Open and Closed Heat-Engines for Small-Scale CHP Applications
Abstract
:1. Introduction
2. Externally Heated Versus Internally Heated Engine Cycles
2.1. Comparison of Thermal Efficiency
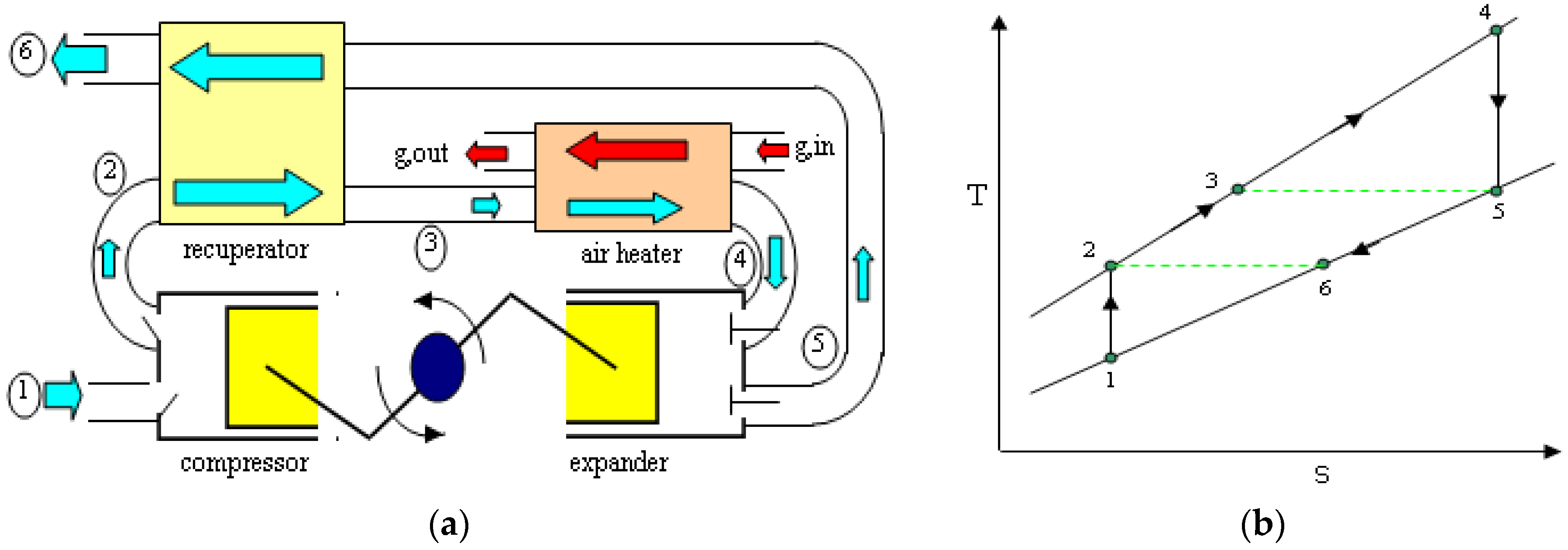
2.2. The Recuperated Constant Pressure Heat Engine Cycle
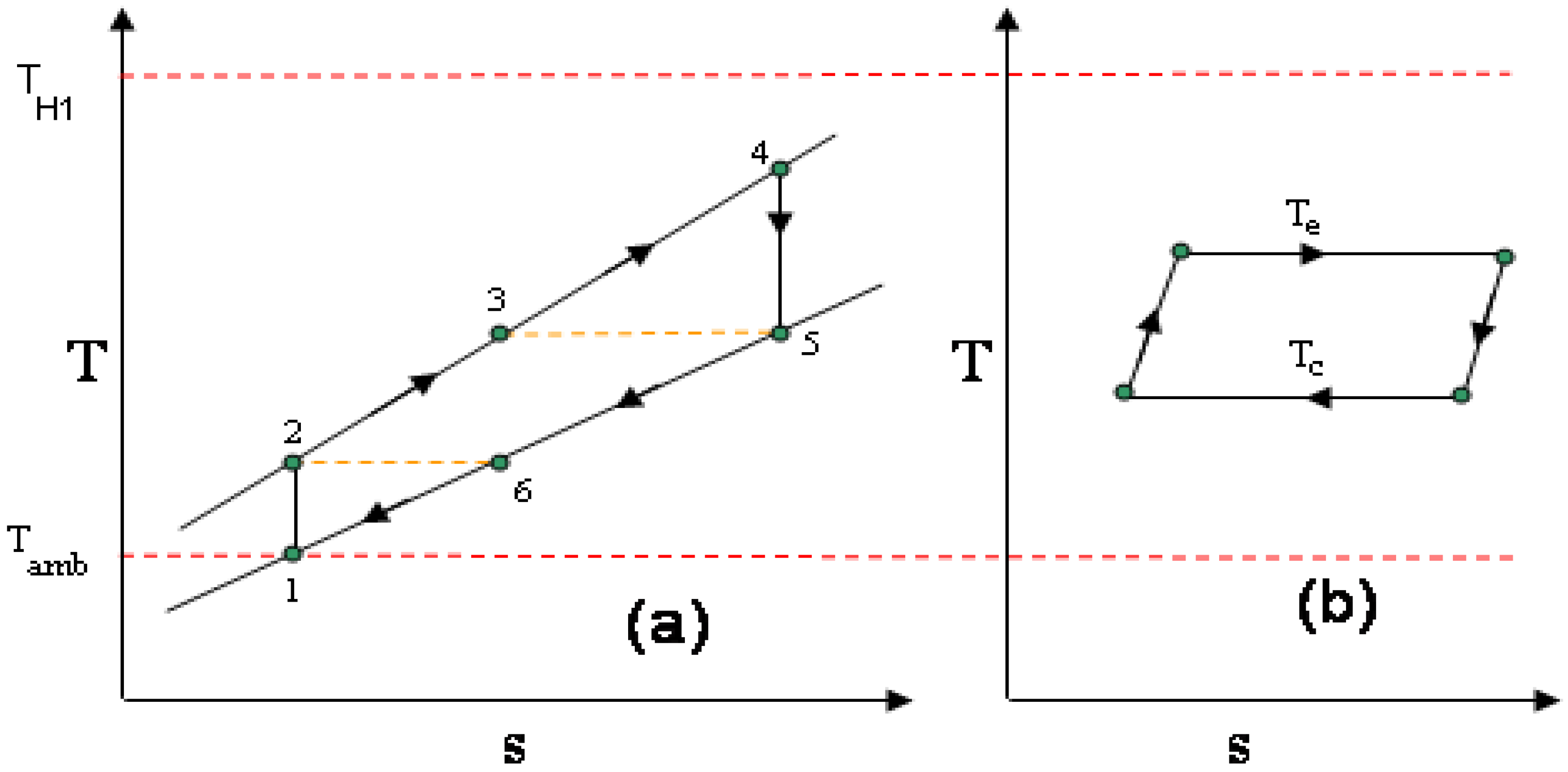
3. Comparison of the Externally Heated RJC (Recuperated Joule Cycle) and Stirling Cycle
- (1)
- Both the RJC and Stirling cycles are internally reversible.
- (2)
- Both cycles are heated externally via identical heat exchangers using hot combustion flue-gas: a variable temperature heat source.
- (3)
- In both engines the combustion gases are assumed to enter the high-temperature heat exchanger at the same temperature (TH1) and leave at the same temperature (TH2).
- (4)
- Both cycles are assumed to absorb heat at the same rate (J/s).
- (5)
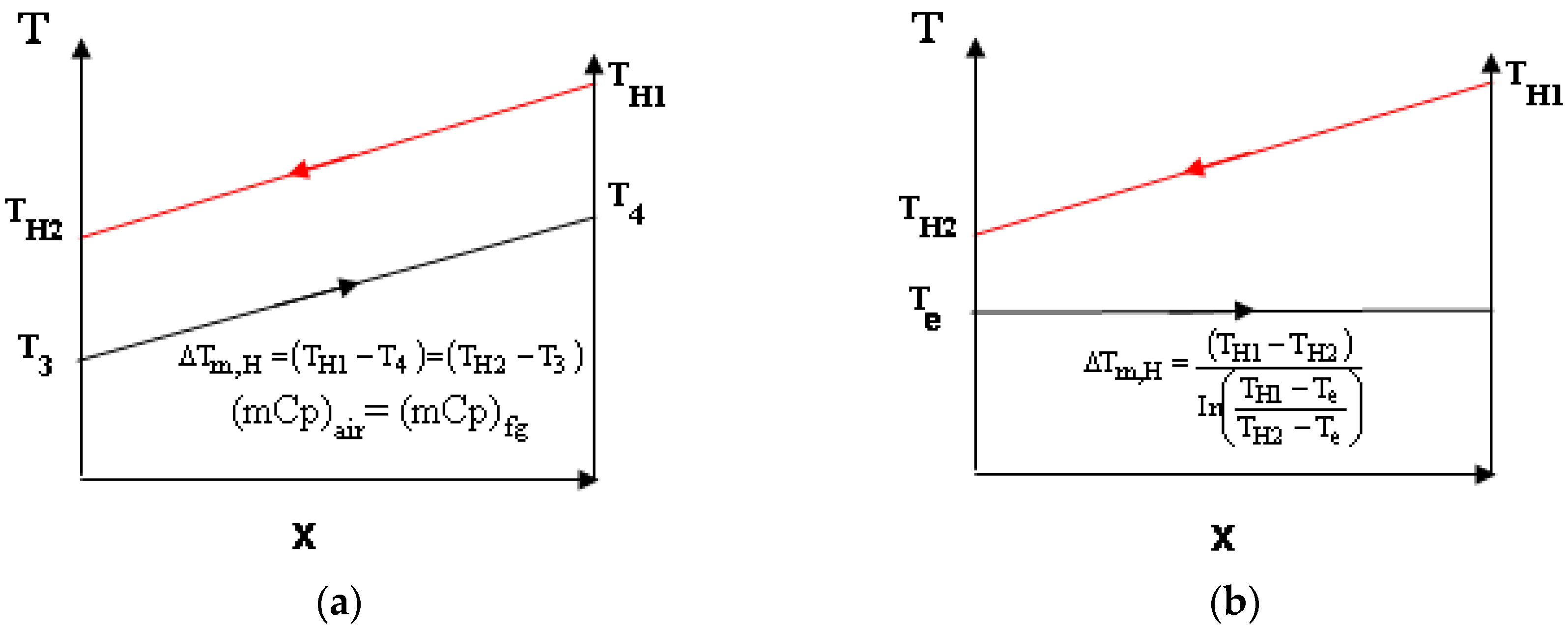
- Either ∆Tm,H,RJC = ∆Tm,H,SC or ATDH,RJC = ATDH,RC (both possibilities were investigated).
- (6)
- For the RJC the heat capacity rate, (CH), of its working fluid equals that of the combustion gases.
- (7)
- The working fluid within both cycles is dry-air, which is assumed to be a perfect gas.
- (8)
- (9)
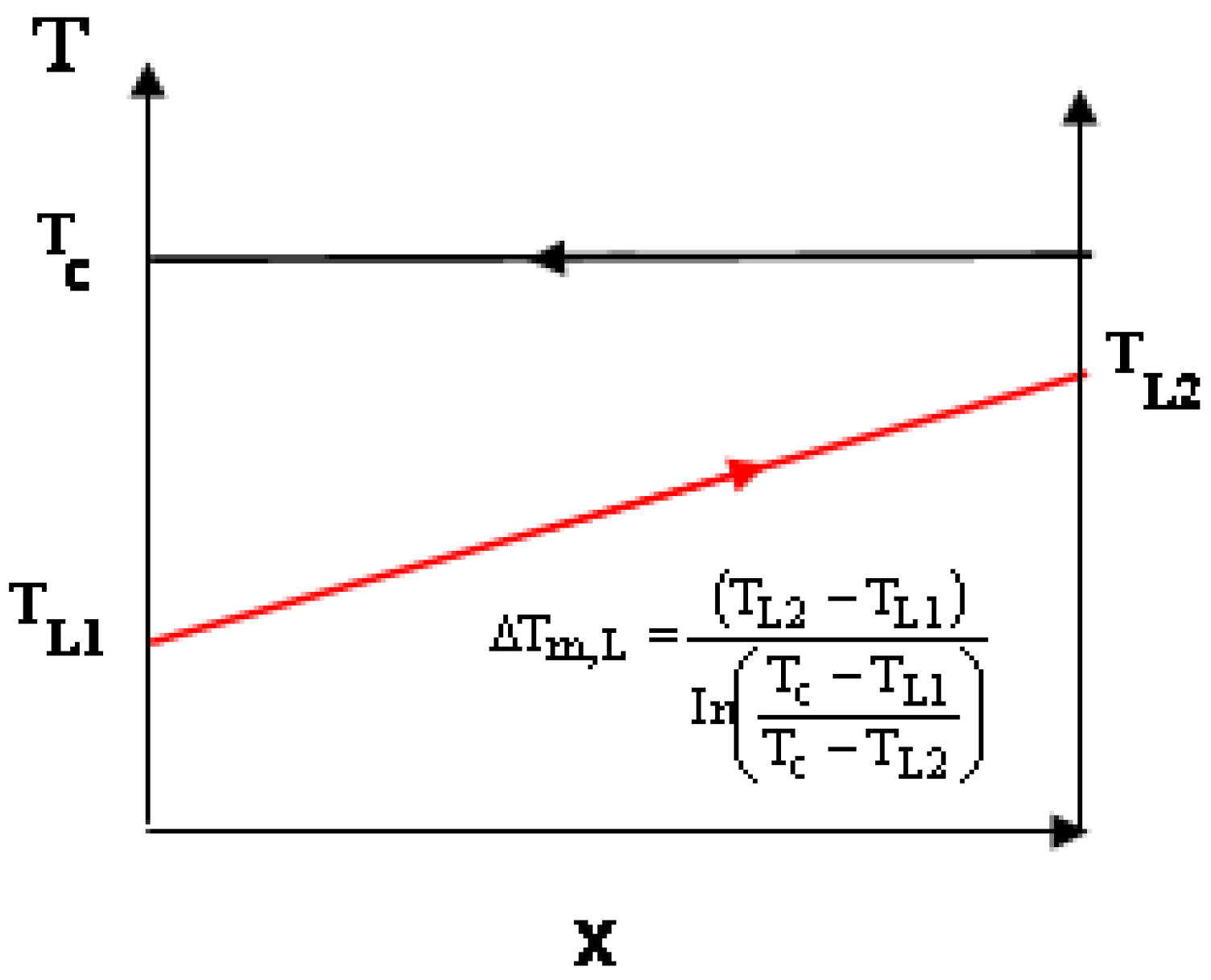
- The temperature of the air leaving the Stirling cycle’s low-temperature heat exchanger equals that leaving the recuperator of the RJC: T6 in Figure 2a equals TL2 in Figure 5. This is thought to be a reasonable assumption if the waste heat from both cycles is to be utilized for heating purposes because it would mean its temperature in both cases would be equal.
- (10)
- For the purpose of analysis the ambient air temperature is assumed to be 300 K.
3.1. Comparison of Efficiency Based on Equal ∆Tm,H Values
3.2. Comparison of Efficiency Based on Equal ATDH Values
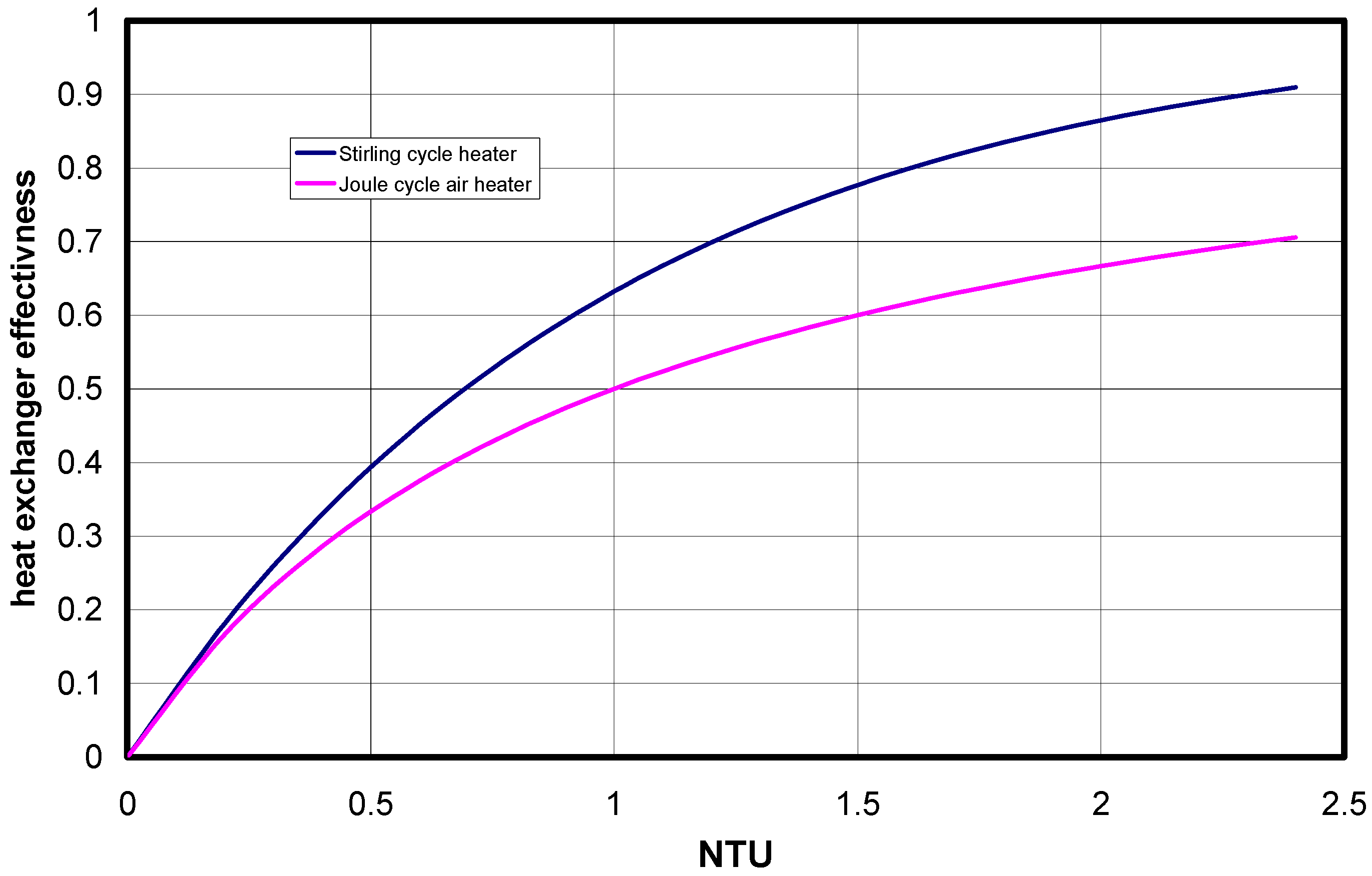
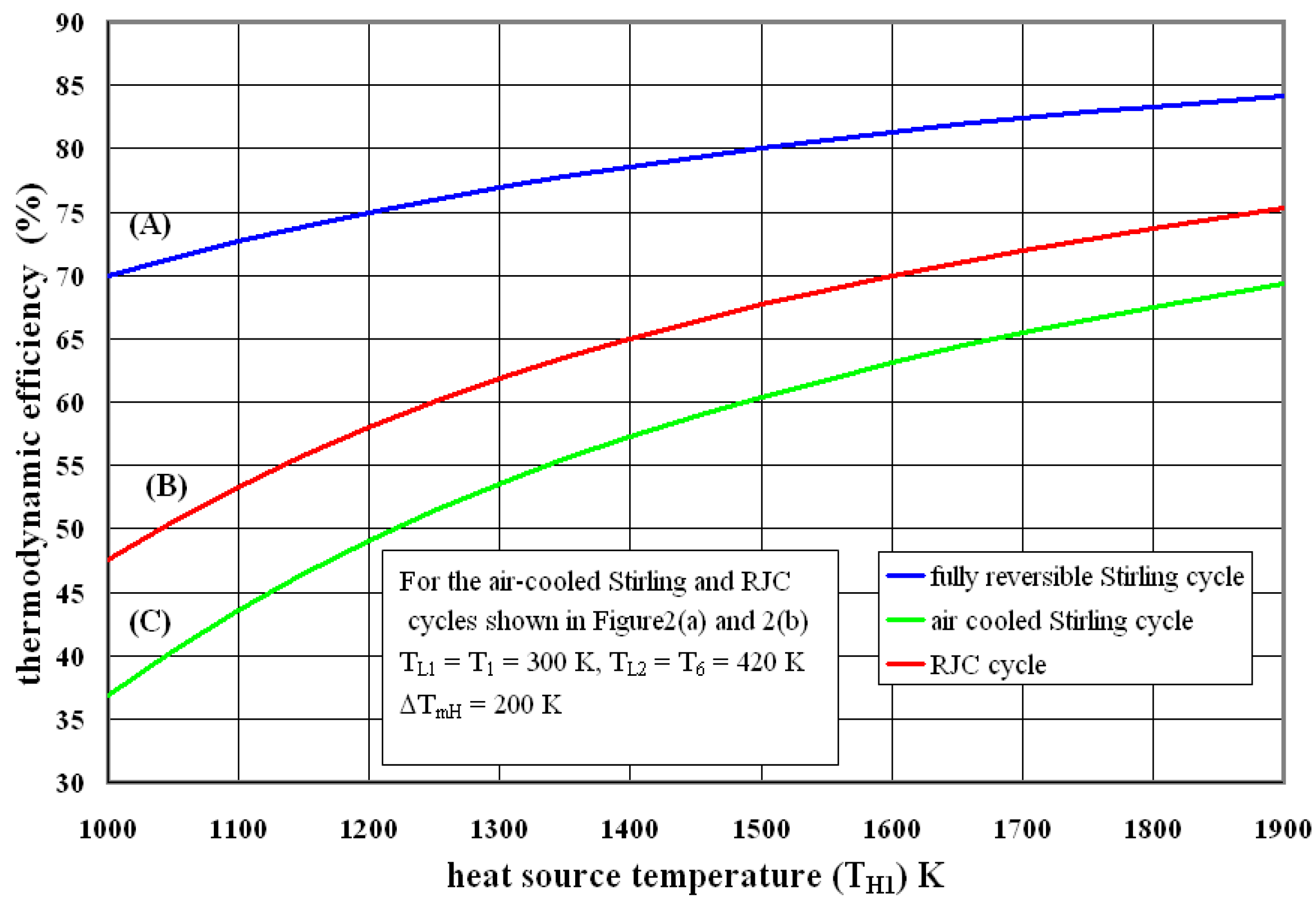
3.3. Some Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
Nomenclature
| A | heat transfer area (m2) |
| ATD | approach temperature difference (K) |
| C | heat capacity rate = mCp (W/K) |
| CHP | combined heat and power |
| Cp | specific heat capacity at constant pressure (J/kg·K) |
| m | mass flow (kg/s) |
| NTU | number of heat transfer units |
| Pr | RJC cycle pressure ratio (−) |
| Qinput | heat input rate (W) |
| RJC | recuperated Joule cycle |
| s | specific entropy (J/kg·K) |
| T | temperature (K) |
| Tc | isothermal compression temperature (K) |
| Te | isothermal expansion temperature (K) |
| ΔTm | area-weighted (or log-mean) temperature difference (oC) |
| U | overall heat transfer coefficient (W/m2·K) |
| Greek letters | |
| α | isentropic compression temperature ratio (−) |
| ε | heat exchange effectiveness (−) |
| θ | cycle temperature ratio, TH1/TL1 (−) |
| θ* | cycle temperature ratio for an internally reversible engine |
| isentropic compression efficiency (−) | |
| isentropic expansion efficiency (−) | |
| thermodynamic efficiency (−) | |
| Subscripts | |
| Amb | ambient air |
| SC | Stirling cycle |
| fg | flue-gas |
| H | heat source temperature |
| H1 | heat source inlet temperature |
| L | heat sink temperature |
| L1 | heat sink inlet temperature |
| opt | optimum value |
| r | recuperator |
| RJC | recuperated Joule cycle |
| SC | Stirling cycle |
References
- Organ, A.J.; Finkelstein, T. Air Engines: The History, Science, and Reality of the Perfect Engine; ASME: New York, NY, USA, 2009. [Google Scholar]
- Cardozo, E.; Erlich, C.; Malmquist, A.; Alejo, L. Integration of a wood pellet burner and a Stirling engine to produce residential heat and power. Appl. Therm. Eng. 2014, 73, 671–680. [Google Scholar] [CrossRef]
- Conroy, G.; Duffy, A.; Ayompe, L.M. Economic, energy and GHG emissions performance evaluation of a WhisperGen Mk IV Stirling engine l-CHP unit in a domestic dwelling. Energy Convers. Manag. 2014, 81, 465–474. [Google Scholar] [CrossRef]
- Eckardt, D. Gas Turbine Powerhouse; De Gruyter Oldenbourg: Berlin, Germany, 2014. [Google Scholar]
- Joule, J.P. On Air-Engines; Report of the British Association: Manchester, UK, 19 June 1851. [Google Scholar]
- Brayton, G. Improvements in Gas-Engines. U.S. Patent 125166A, 2 April 1871. [Google Scholar]
- Moss, R.; Roskilly, A.; Nanda, S. Reciprocating Joule cycle engine for domestic CHP system. Appl. Energy 2005, 80, 169–185. [Google Scholar] [CrossRef]
- Rogers, G.; Mayhew, Y. Engineering Thermodynamics, 4th ed.; Longman Group Limited: London, UK, 1995. [Google Scholar]
- Lewitt, E.H. Thermodynamics Applied to Heat Engines, 5th ed.; Sir Isaac Pitman and Sons Limited: London, UK, 1953. [Google Scholar]
- AsjaGen. Available online: http://www.asjagen.com/totem-2 (accessed on 17 April 2015).
- Helec Ltd. Available online: http://helec.co.uk (accessed on 16 April 2015).
- Baxi Ltd. Available online: http://www.baxi.co.uk/renewables/combined-heat-and-power/ecogen.htm (accessed on 3 March 2015).
- Ecogen: The Baxi Ecogen Dual Energy System; Baxi Limited: Warwick, UK, 2010.
- Jones, O.; Wardle, R.; Matthews, P. Customer-Led Network Revolution—Micro-CHP Trial Report; Customer Network-Led Revolution report L086: London, UK, 17 November 2014. [Google Scholar]
- Cohen, H.; Rogers, G.F.C.; Saravanamuttoo, H.I.H. Gas Turbine Theory, 2nd ed.; Longman Group Limited: London, UK, 1972. [Google Scholar]
- Goodger, E.M. Principles of Engineering Thermodynamics; The Macmillan Press: London, UK, 1974. [Google Scholar]
- Wright-Baker, H. Inchley’s Theory of Heat Engines; Longmans, Green and Co Ltd.: London, UK, 1945. [Google Scholar]
| Manufacturer | System Name | Engine Cycle | Output | Thermo Efficiency | Reference |
|---|---|---|---|---|---|
| asjaGen | TOTEM 10 | Otto | 10 kW | 30% | [10] |
| Helec Ltd | Energimizer | Otto | 7.5 kW | 25% | [11] |
| Helec Ltd | Powerbox 7500QSE | Stirling | 7.5 kW | 18% | [12] |
| Baxi Ltd | Ecogen | Stirling | 1 kW | 13% | [12,13,14] |
| State-Points | Process |
|---|---|
| 1–2 | Compression of ambient air |
| 2–3 | Recuperative heating |
| 3–4 | External heating |
| 4–5 | Expansion |
| 5–6 | Recuperative cooling |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eames, I.W.; Evans, K.; Pickering, S. A Comparative Study of Open and Closed Heat-Engines for Small-Scale CHP Applications. Energies 2016, 9, 130. https://doi.org/10.3390/en9030130
Eames IW, Evans K, Pickering S. A Comparative Study of Open and Closed Heat-Engines for Small-Scale CHP Applications. Energies. 2016; 9(3):130. https://doi.org/10.3390/en9030130
Chicago/Turabian StyleEames, Ian W., Kieran Evans, and Stephen Pickering. 2016. "A Comparative Study of Open and Closed Heat-Engines for Small-Scale CHP Applications" Energies 9, no. 3: 130. https://doi.org/10.3390/en9030130
APA StyleEames, I. W., Evans, K., & Pickering, S. (2016). A Comparative Study of Open and Closed Heat-Engines for Small-Scale CHP Applications. Energies, 9(3), 130. https://doi.org/10.3390/en9030130





