Synthesis and Characterization of Sulfonated Poly(Phenylene) Containing a Non-Planar Structure and Dibenzoyl Groups
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization of Membrane
2.3. Synthesis of 1,4-Dichloro-2,5-dibenzoylbenzene
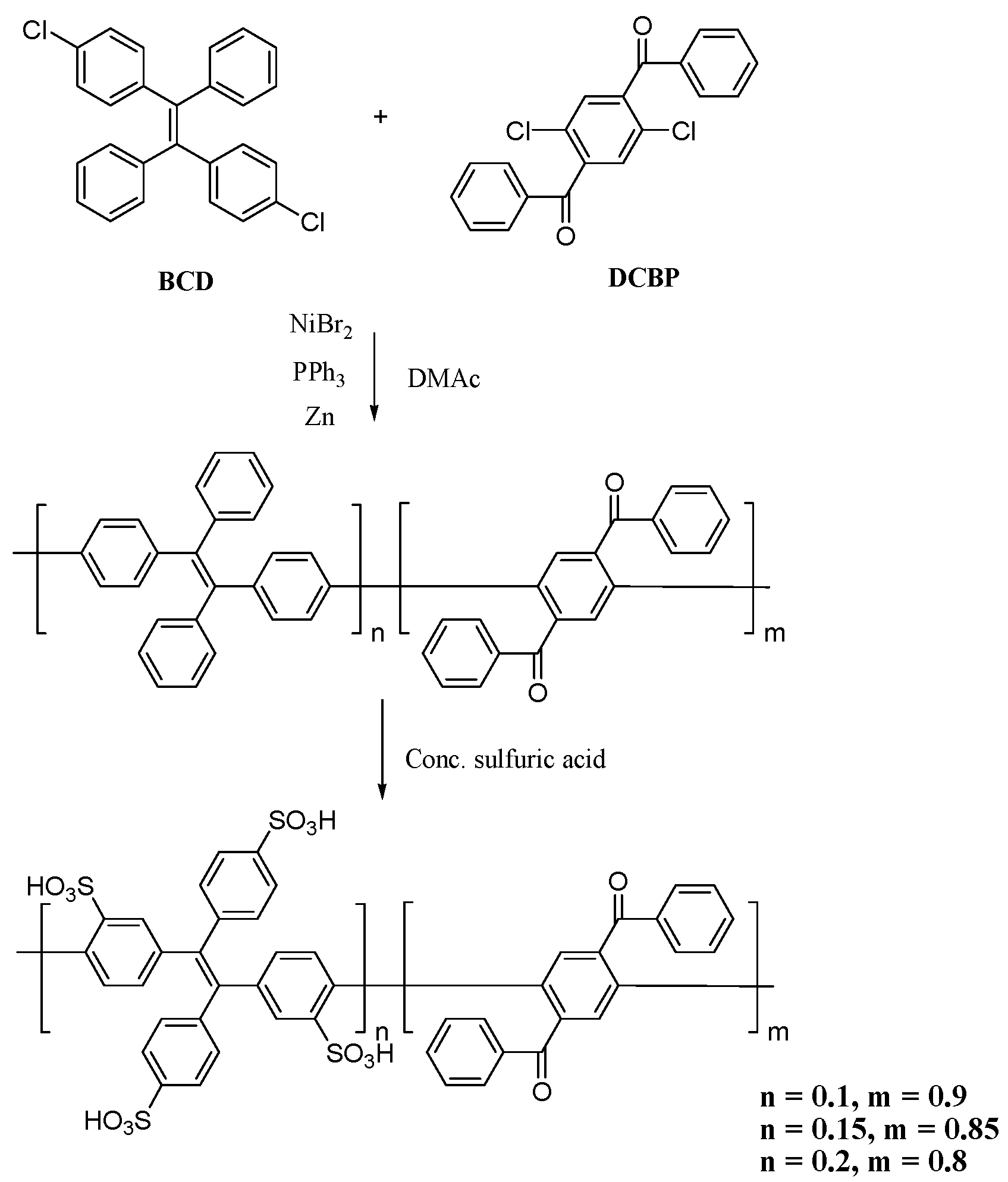
2.4. Synthesis of Polymers
2.5. Synthesis of Sulfonated Polymers
2.6. MEA Fabrication and Testing
3. Results and Discussion
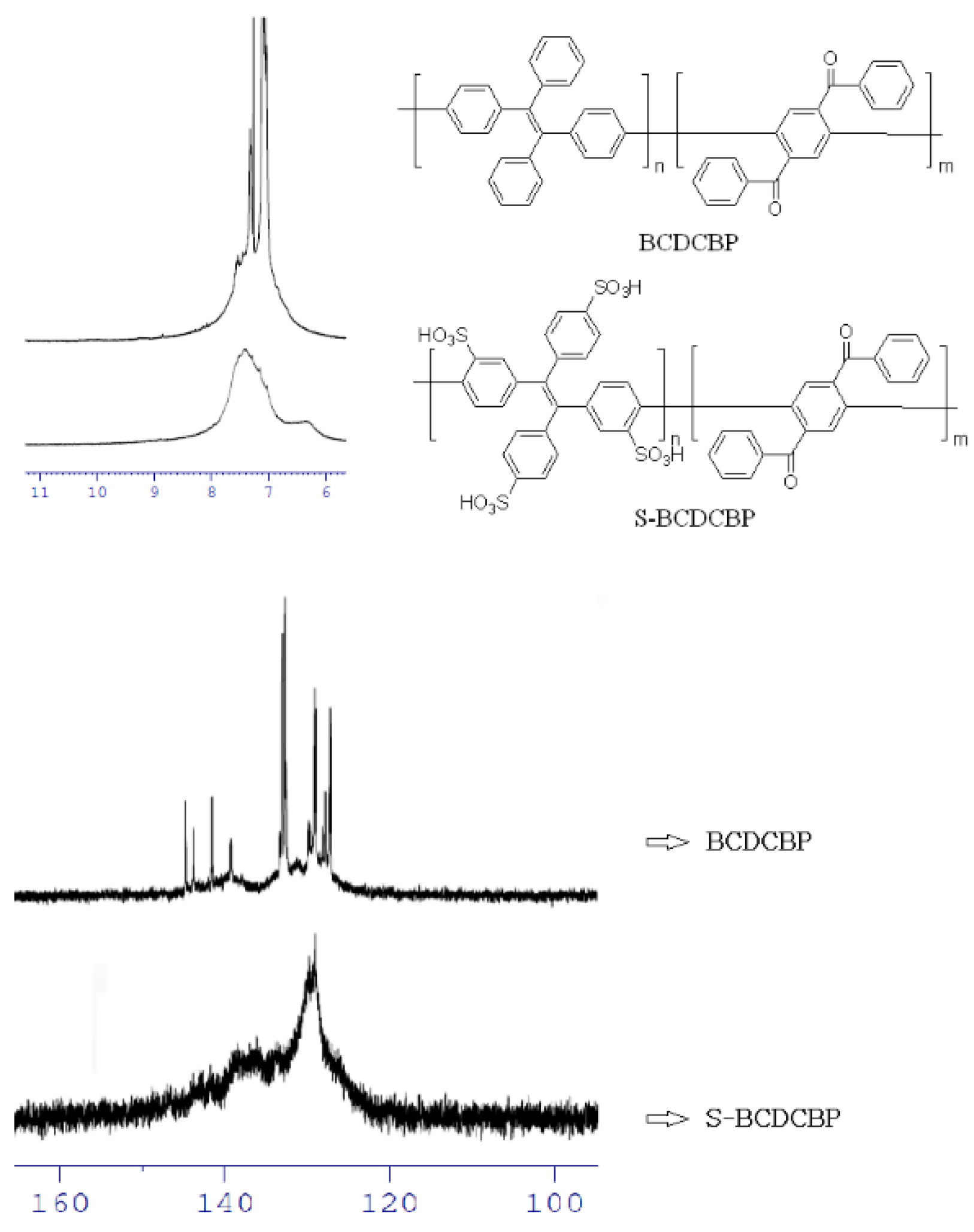
3.1. Preparation of the Polymers
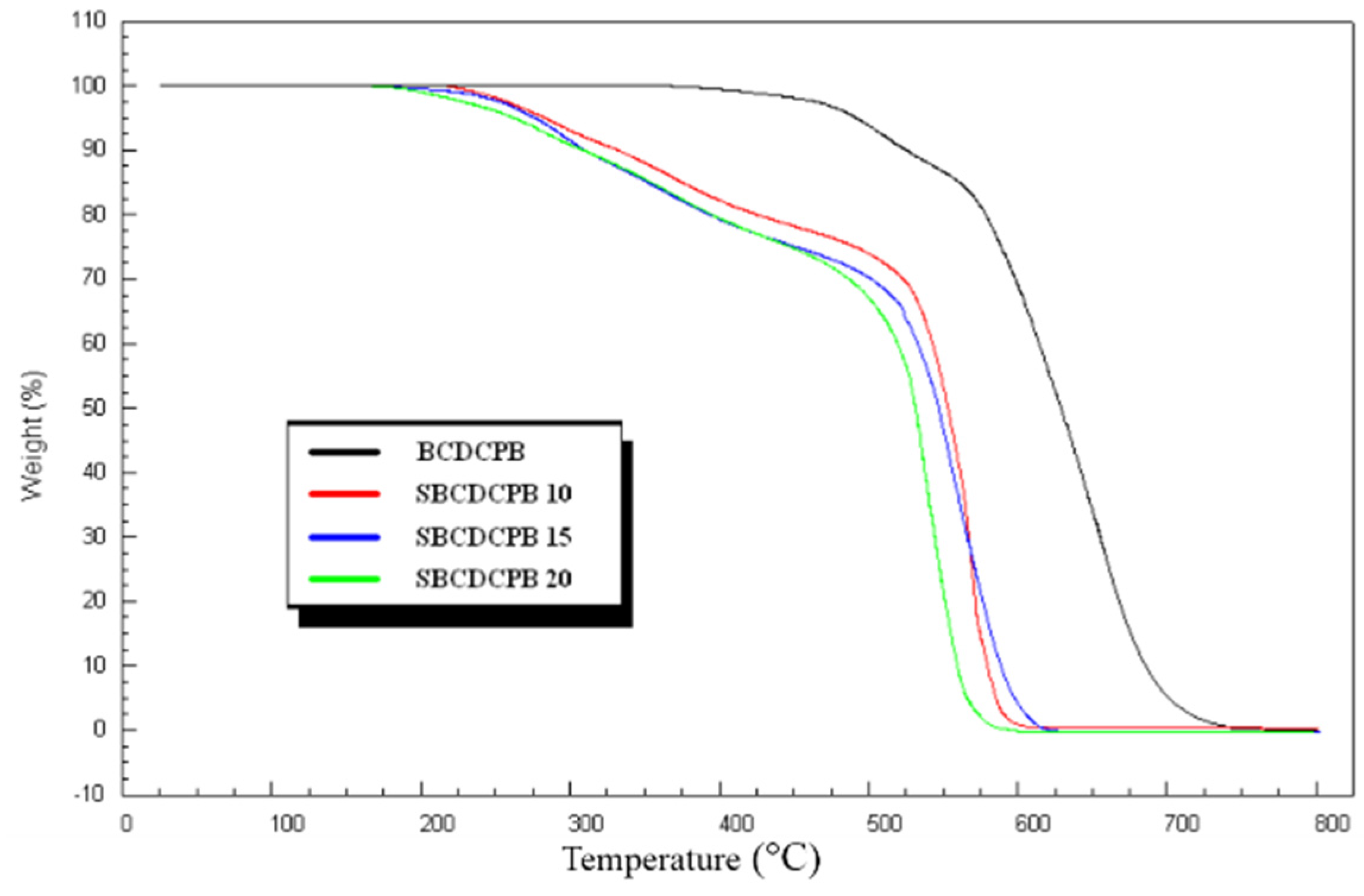
3.2. Properties of the Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, C.Y.; Sung, C.C. A review of the performance and analysis of proton exchange membrane fuel cell membrane electrode assemblies. J. Power Sour. 2012, 220, 348–353. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchangemembranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Gencoglu, M.T.; Ural, Z. Design of a PEM fuel cell system for residential application. Int. J. Hydrog. Energy 2009, 34, 5242–5248. [Google Scholar] [CrossRef]
- Jannasch, P. Recent developments in high-temperature proton conducting polymer electrolyte membranes. Curr. Opin. Collod Interface Sci. 2003, 8, 96–102. [Google Scholar] [CrossRef]
- Chen, S.L.; Krishnan, L.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Ion exchange resin/polystyrene sulfonate composite membranes for PEM fuel cells. J. Membr. Sci. 2004, 243, 327–333. [Google Scholar] [CrossRef]
- Hacivelioglu, F.; Okutan, E.; Celik, S.U.; Yesilot, S.; Bozkurt, A.; Kilic, A. Controlling phosphonic acid substitution degree on proton conducting polyphosphazenes. Polymer 2012, 53, 3659–3668. [Google Scholar] [CrossRef]
- Hacivelioglu, F.; Ozden, S.; Celik, S.U.; Yesilot, S.; Kilic, A.; Bozkurt, A. Azole substituted polyphosphazenes as nonhumidified proton conducting membranes. J. Mater. Chem. 2011, 21, 1020–1027. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jung, J.W.; Han, J.Y.; Kim, H.J.; Jang, J.H.; Lee, H.J.; Cho, E.A.; Henkensmeier, D.; Kim, J.Y.; Yoo, S.J.; et al. Effect of membrane electrode assembly fabrication method on the single cell performances of polybenzimidazole-based high temperature polymer electrolyte membrane fuel cells. Macromol. Res. 2014, 22, 1214–1220. [Google Scholar] [CrossRef]
- Lee, M.B.; Park, J.K.; Lee, H.S.; Lane, O.; Moore, R.B.; McGrath, J.E.; Barid, D.G. Effects of block length and solution-casting conditions on the final morphology and properties of disulfonated poly(arylene ether sulfone) multiblock copolymer films for proton exchange membranes. Polymer 2009, 50, 6129–6138. [Google Scholar] [CrossRef]
- Lufrano, F.; Squadrito, G.; Patti, A.; Passalacqua, E. Sulfonated polysulfone as promising membranes for polymer electrolyte fuel cells. J. Appl. Polym. Sci. 2000, 77, 1250–1256. [Google Scholar] [CrossRef]
- Sengul, E.; Erdener, H.; Akay, R.G.; Yucel, H.; Bac, N.; Eroglu, I. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrog. Energy 2009, 34, 4645–4652. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric proton conducting membranes for medium temperature fuel cells (110–160 °C). J. Membr. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Chen, L.K.; Wu, C.S.; Chen, M.C.; Hsu, K.L.; Li, H.C.; Hsieh, C.H.; Hsiao, M.H.; Chang, C.L.; Chu, P.P. Cross-linked norbornene sulfonated poly(ether ketone)s for proton exchange membrane. J. Membr. Sci. 2010, 361, 143–153. [Google Scholar] [CrossRef]
- Hickner, M.A.; Pivovar, B.S. The chemical and structural nature of proton exchange membrane fuel cell properties. Fuel Cells 2005, 5, 213–229. [Google Scholar] [CrossRef]
- Wang, F.; Hickner, M.; Kim, Y.S.; Zawodzinski, T.A.; McGrath, J.E. Direct polymerization of sulfonated poly(arylene ether sulfone) random (statistical) copolymers: Candidates for new proton exchange membranes. J. Membr. Sci. 2002, 197, 231–242. [Google Scholar] [CrossRef]
- Chen, J.; Ramasubramaniam, R.; Xue, C.; Liu, H. A Versatile, molecular engineering approach to simultaneously enhanced, multifunctional carbon nanotube-polymer composites. Adv. Funt. Mater. 2006, 16, 114–119. [Google Scholar] [CrossRef]
- Poppe, D.; Frey, H.; Kreuer, K.D.; Heinzel, A.; Mulhaupt, R. Carboxylated and sulfonated poly(arylene-co-arylene sulfone)s: Thermostable polyelectrolytes for fuel cell applications. Macromolecules 2002, 35, 7936–7941. [Google Scholar] [CrossRef]
- Ghassemi, H.; McGrath, J.E. Synthesis and properties of new sulfonated poly(p-phenylene) derivatives for proton exchange membranes. I. Polymer 2004, 45, 5847–5854. [Google Scholar] [CrossRef]
- Ninivin, C.L.; Longeau, A.B.; Demattei, D.; Coutanceau, C.; Lamy, C.; Leger, J.M. Sulfonated derivatives of polyparaphenylene as proton conducting membranes for direct methanol fuel cell application. J. Appl. Electrochem. 2004, 34, 1159–1170. [Google Scholar] [CrossRef]
- Phillips, R.W.; Sheares, V.V.; Samulski, E.T.; Desimone, J.M. Isomeric poly(benzophenone)s: Synthesis of highly crystalline poly(4,4′-benzophenone) and amorphous poly(2,5-benzophenone), a soluble poly (p-phenylene) derivative. Macromolecules 1994, 27, 2354–2356. [Google Scholar] [CrossRef]
- Lim, Y.D.; Seo, D.W.; Lee, S.H.; Ju, H.H.; Hong, T.W.; Kim, D.M.; Ju, H.C.; Kim, W.G. Synthesis and properties of cis/trans mixtures of bis(4-hydroxyphenyl)-1,2-diphenylethylene containing sulfonated poly(ethersulfone)s proton exchange membranes. Int. J. Hydrog. Energy 2013, 38, 7667–7673. [Google Scholar] [CrossRef]
- Fu, H.; Yun, H.; Kwei, T.K.; Okamoto, Y.; Blumstengel, S.; Walser, A.; Dorsinville, R. Blue photo and electroluminescence based on poly(2-benzoyl-1,4-phenylene) and poly(2,5-dibenzoyl-1,4-phenylene). Polym. Adv. Technol. 1999, 10, 259–264. [Google Scholar] [CrossRef]
- Guzmman-Gutierrez, T.M.; Daniel, R.N.; Serguei, F.; Salvador, L.M.; Mikhail, G.Z.; Carmen, G.M.; Hernandez, M.; Kricheldorf, H.; Edward, S.W. Dramatic enhancement of superacid-catalyzed polyhydroxyalkylation reactions. Macromolecules 2011, 44, 194–202. [Google Scholar] [CrossRef]
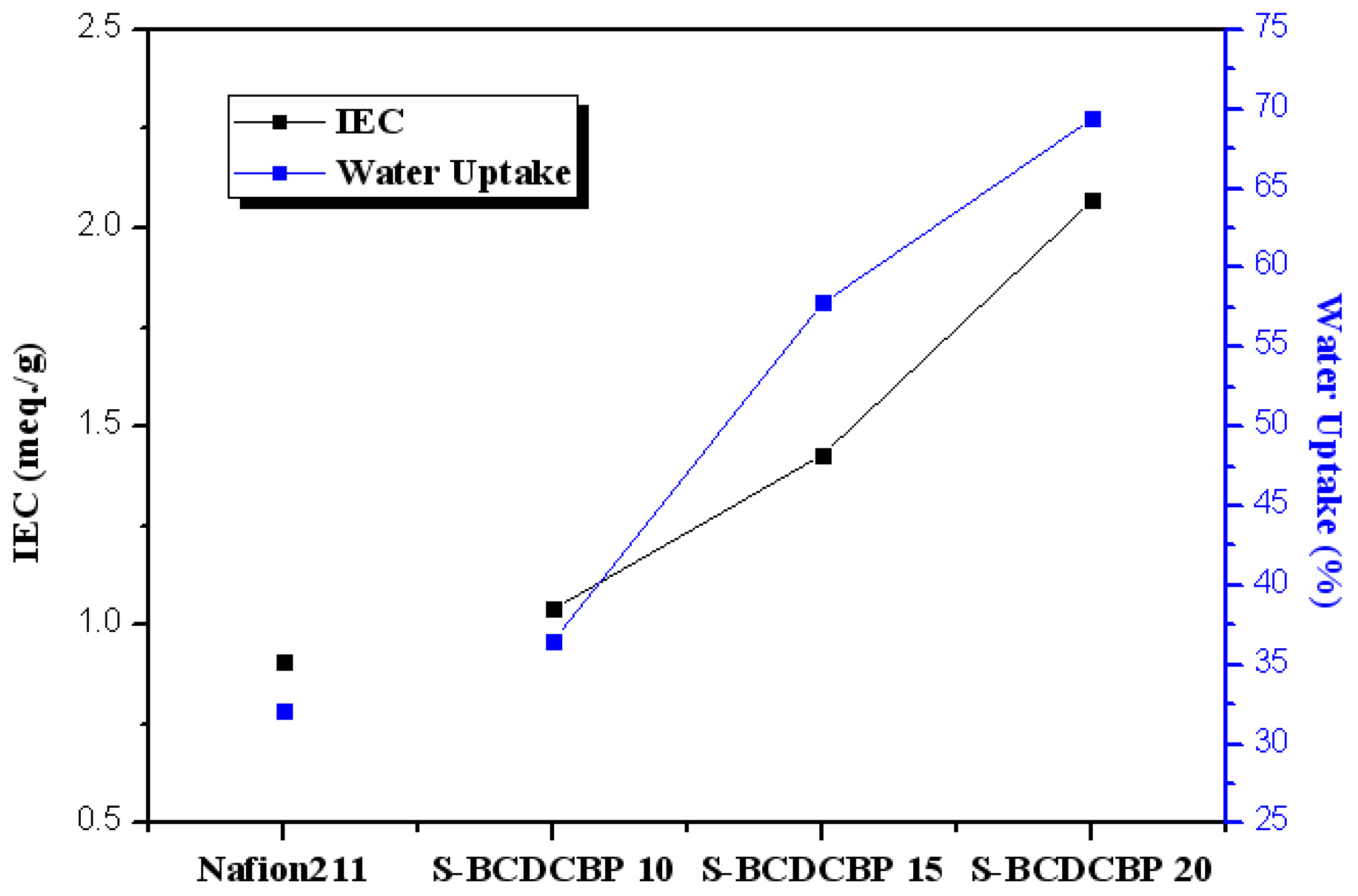

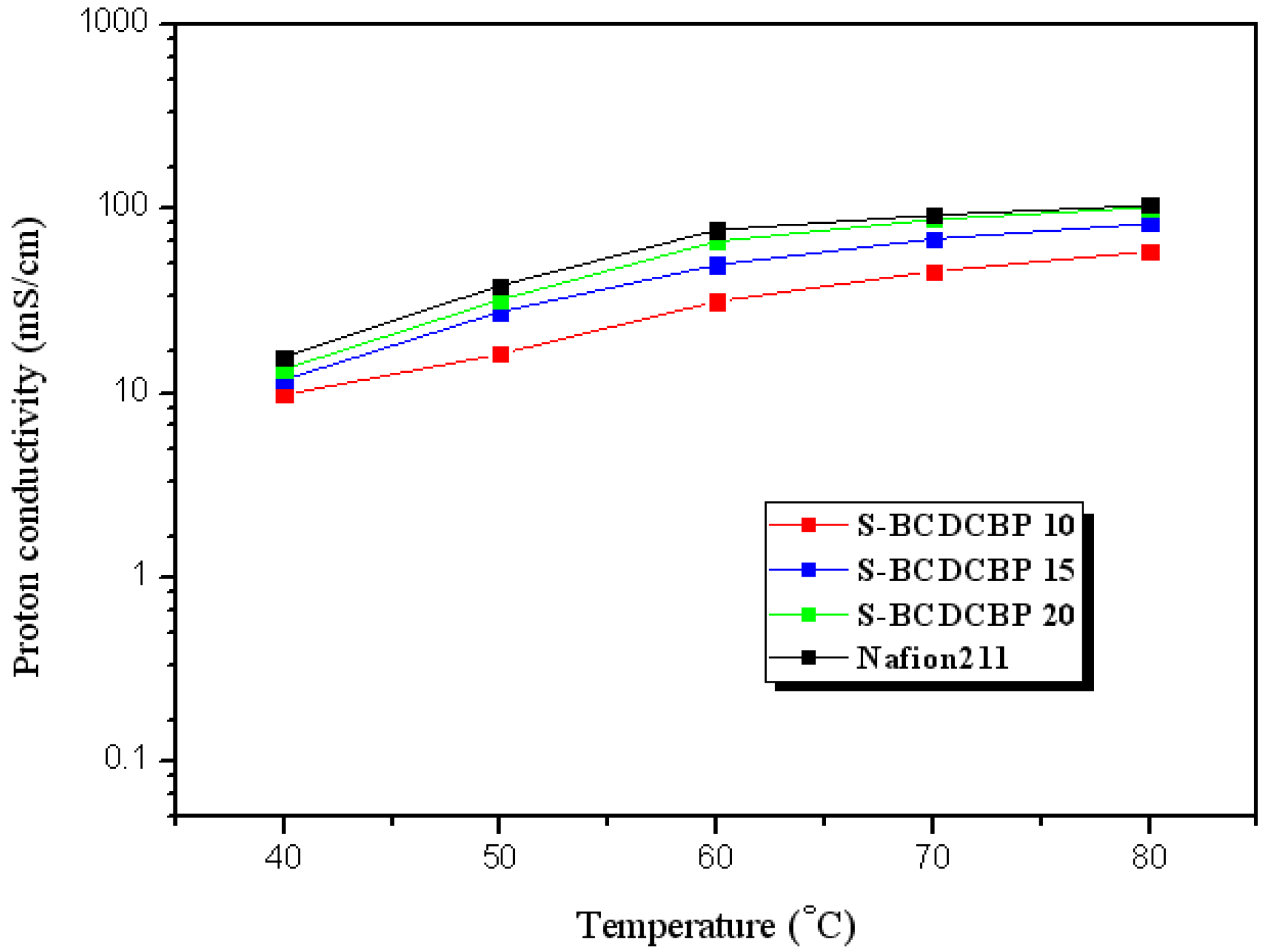

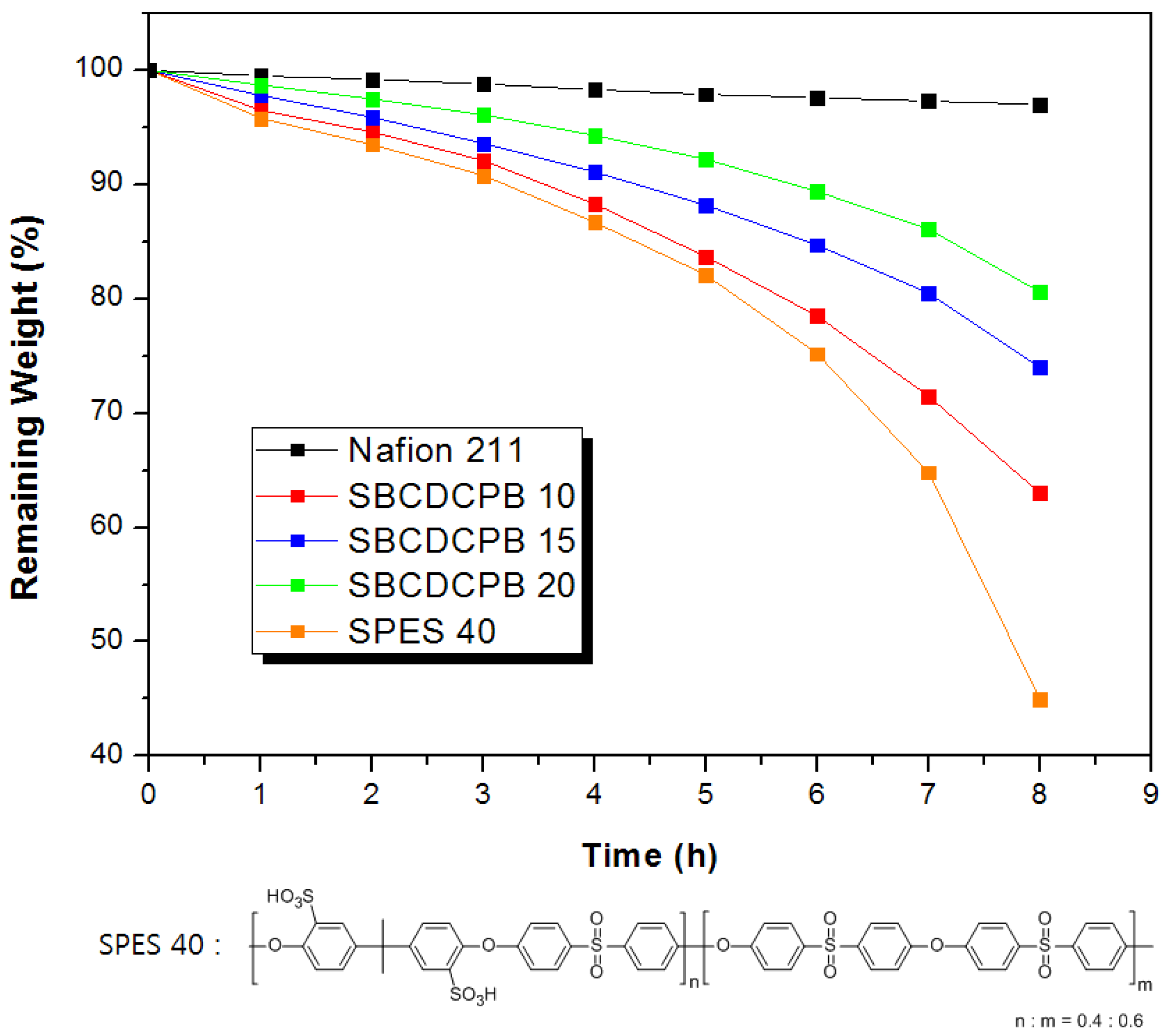
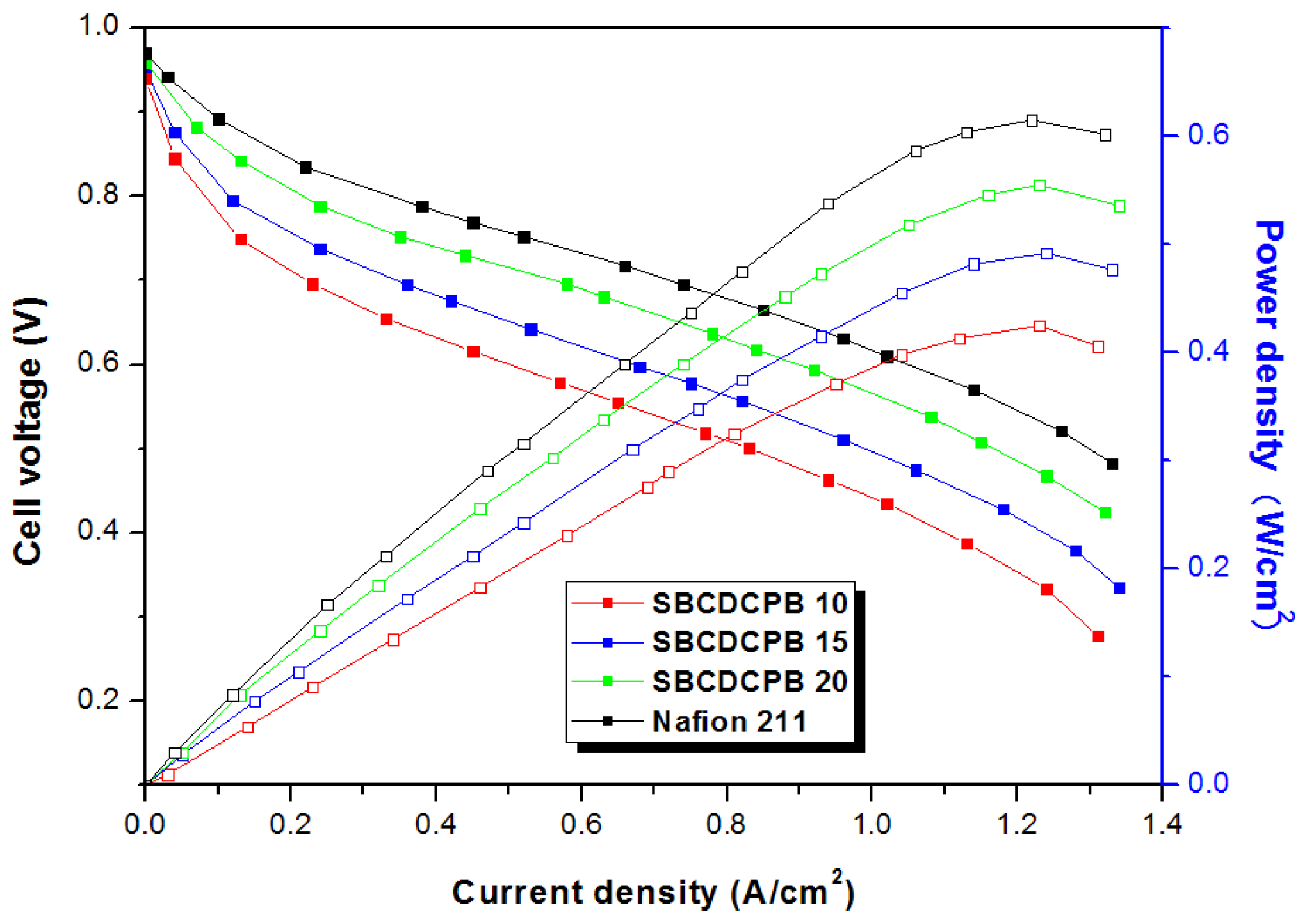
| Polymer | Theoretical IEC (meq./g) | Titrated IEC (meq./g) | Water Uptake 1 (%) | Proton Conductivity 2 (mS/cm) |
|---|---|---|---|---|
| SBCDCPB 10 | 1.24 | 1.04 | 36.5 | 58.7 |
| SBCDCPB 15 | 1.76 | 1.43 | 57.8 | 83.4 |
| SBCDCPB 20 | 2.23 | 2.07 | 69.4 | 101.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Sutradhar, S.C.; Yoo, J.; Ha, J.; Pyo, J.; Lee, C.; Ryu, T.; Kim, W. Synthesis and Characterization of Sulfonated Poly(Phenylene) Containing a Non-Planar Structure and Dibenzoyl Groups. Energies 2016, 9, 115. https://doi.org/10.3390/en9020115
Jang H, Sutradhar SC, Yoo J, Ha J, Pyo J, Lee C, Ryu T, Kim W. Synthesis and Characterization of Sulfonated Poly(Phenylene) Containing a Non-Planar Structure and Dibenzoyl Groups. Energies. 2016; 9(2):115. https://doi.org/10.3390/en9020115
Chicago/Turabian StyleJang, Hohyoun, Sabuj Chandra Sutradhar, Jiho Yoo, Jaeseong Ha, Jaeseung Pyo, Chaekyun Lee, Taewook Ryu, and Whangi Kim. 2016. "Synthesis and Characterization of Sulfonated Poly(Phenylene) Containing a Non-Planar Structure and Dibenzoyl Groups" Energies 9, no. 2: 115. https://doi.org/10.3390/en9020115
APA StyleJang, H., Sutradhar, S. C., Yoo, J., Ha, J., Pyo, J., Lee, C., Ryu, T., & Kim, W. (2016). Synthesis and Characterization of Sulfonated Poly(Phenylene) Containing a Non-Planar Structure and Dibenzoyl Groups. Energies, 9(2), 115. https://doi.org/10.3390/en9020115







