Preparation and Characterization of Tetra-Imidazolium Hydroxide Polyphenylene Membranes via Nickel Catalyzed C–C Coupling Polymerization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of 1,4-Dichloro-2,5-Dibenzoylbenzene (PBP)
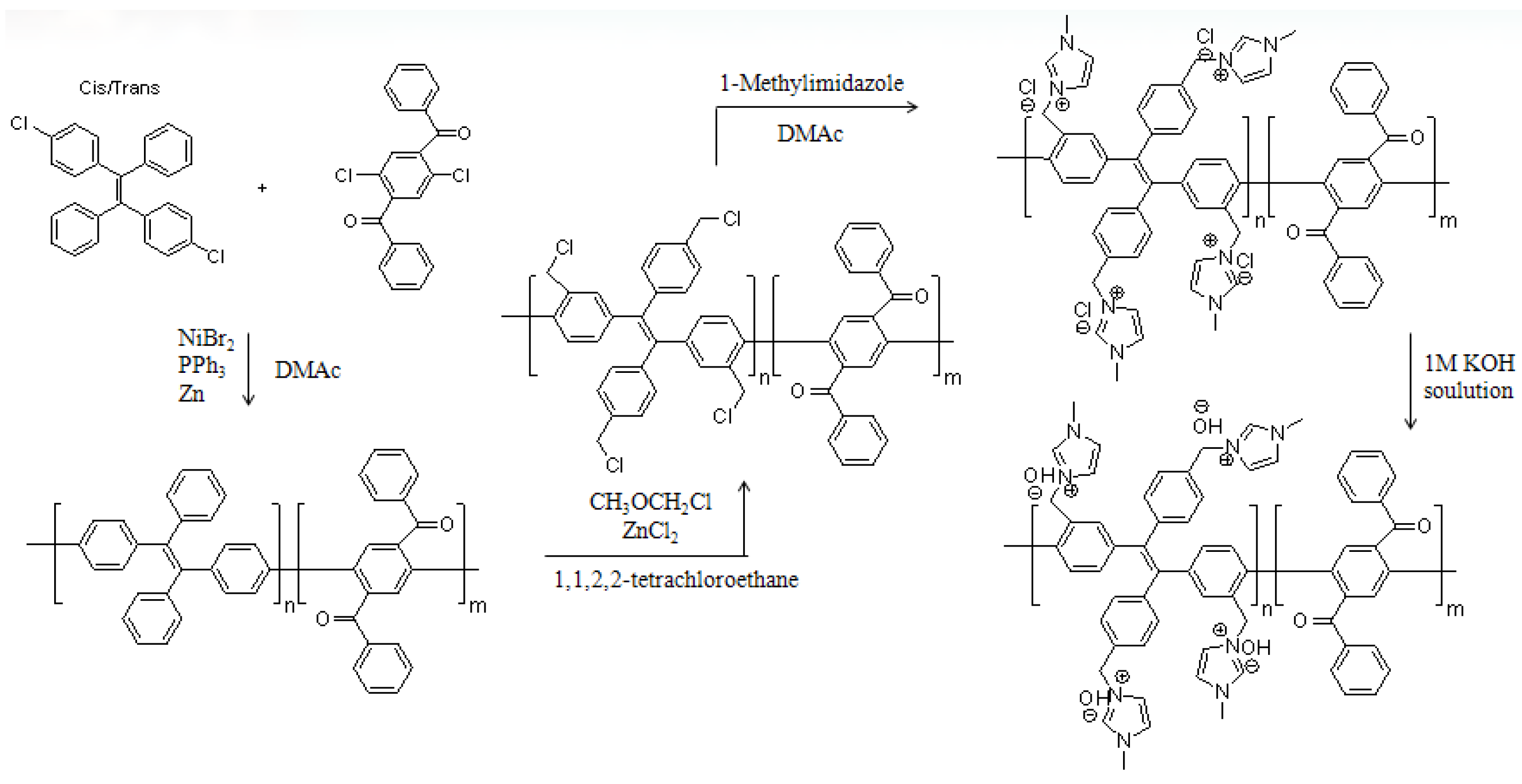
2.3. Preparation of Poly(tetraphenylethylene)-co-(dibenzoylphenylene) (PTPEDBP)
2.4. Preparation of Chloromethylated Polymer (PTPEDBP-Cl)
2.5. Preparation of Tetra-Imidazolium Chloride Polymer (PTPEDBP-I/Cl)
2.6. Preparation of Tetra-Imidazolium Hydroxide Polymer (PTPEDBP-I/OH)
2.7. Characterization of Membranes
3. Results and Discussion
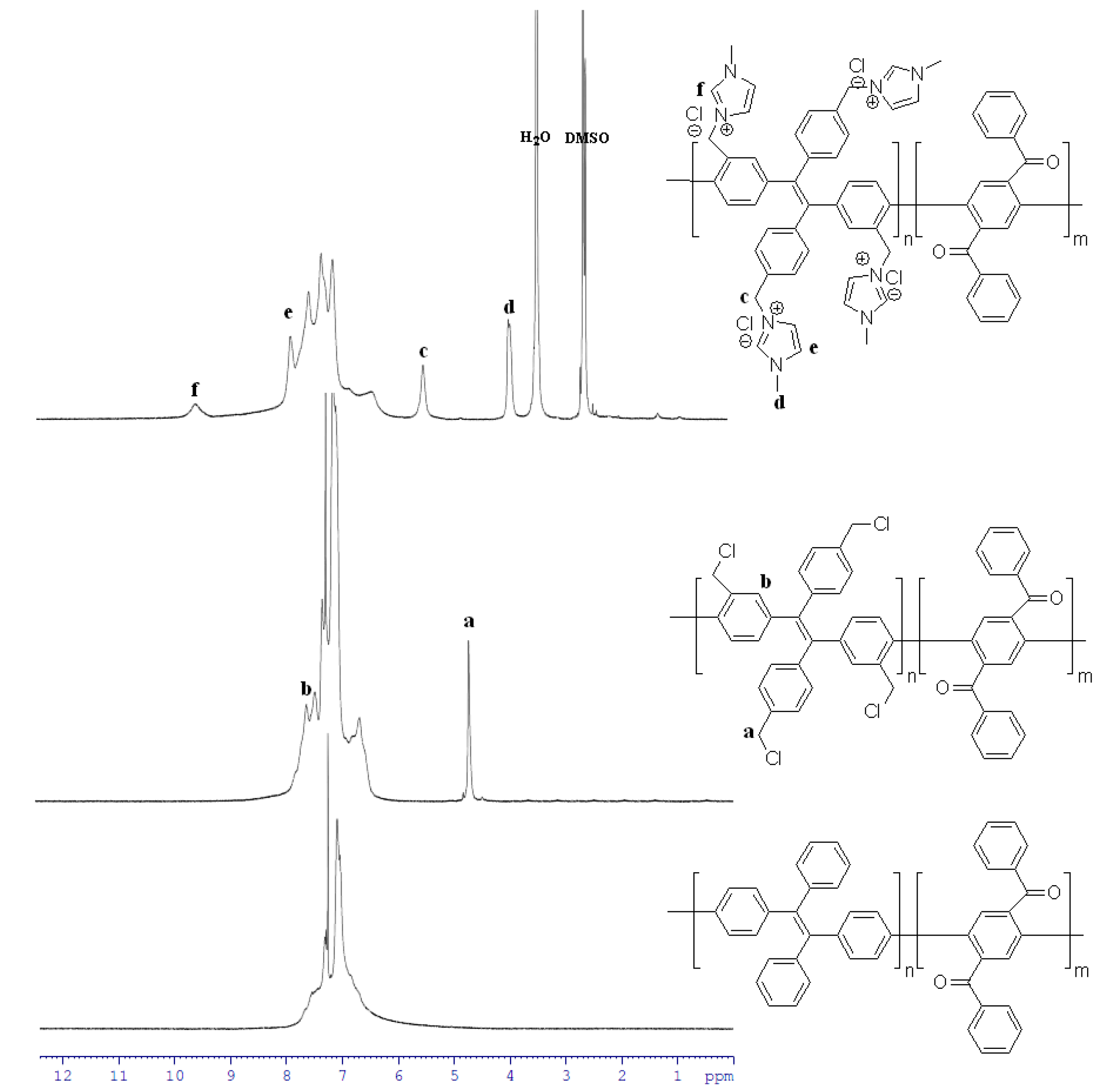
3.1. Preparation of Polymers
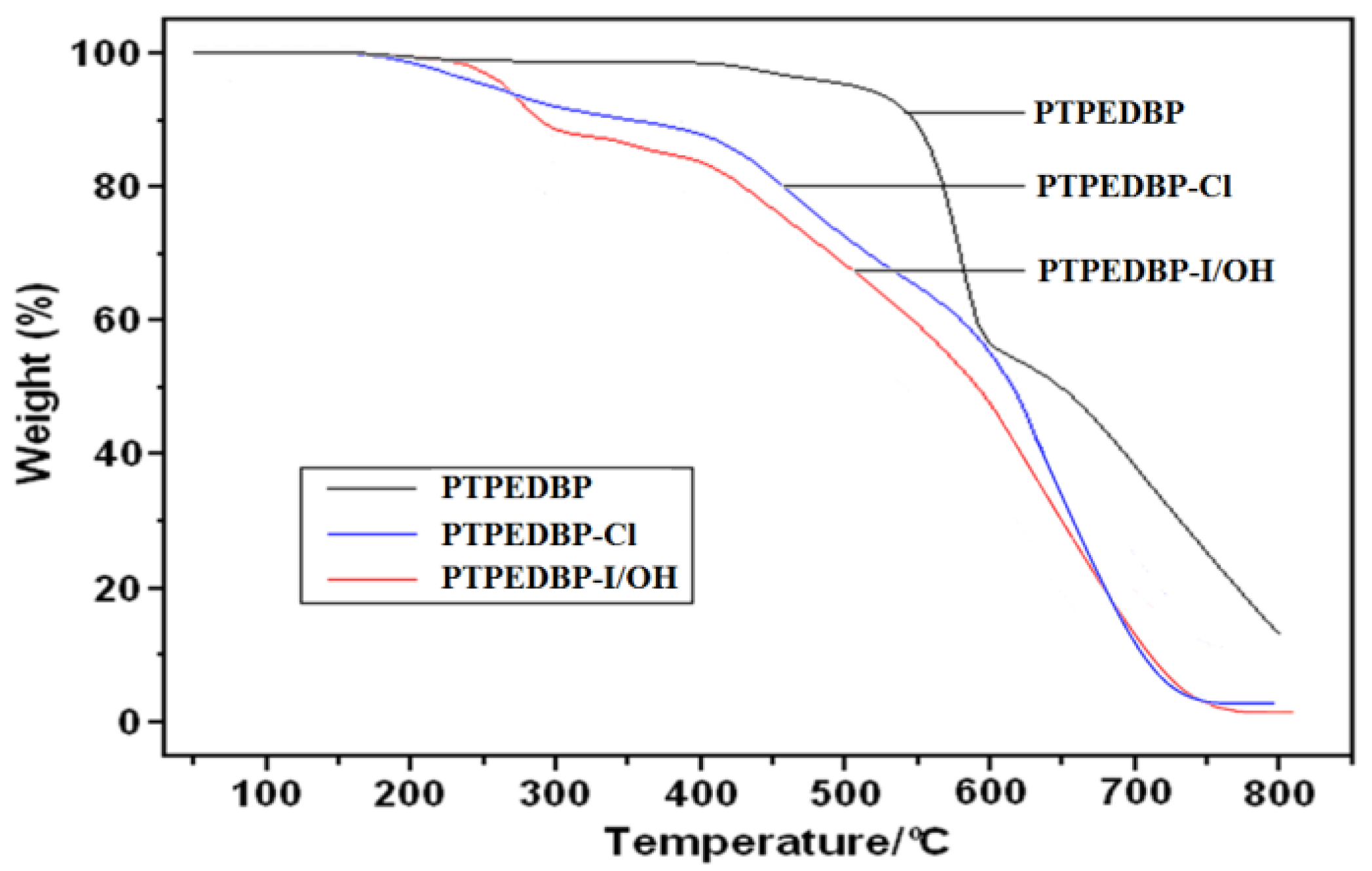
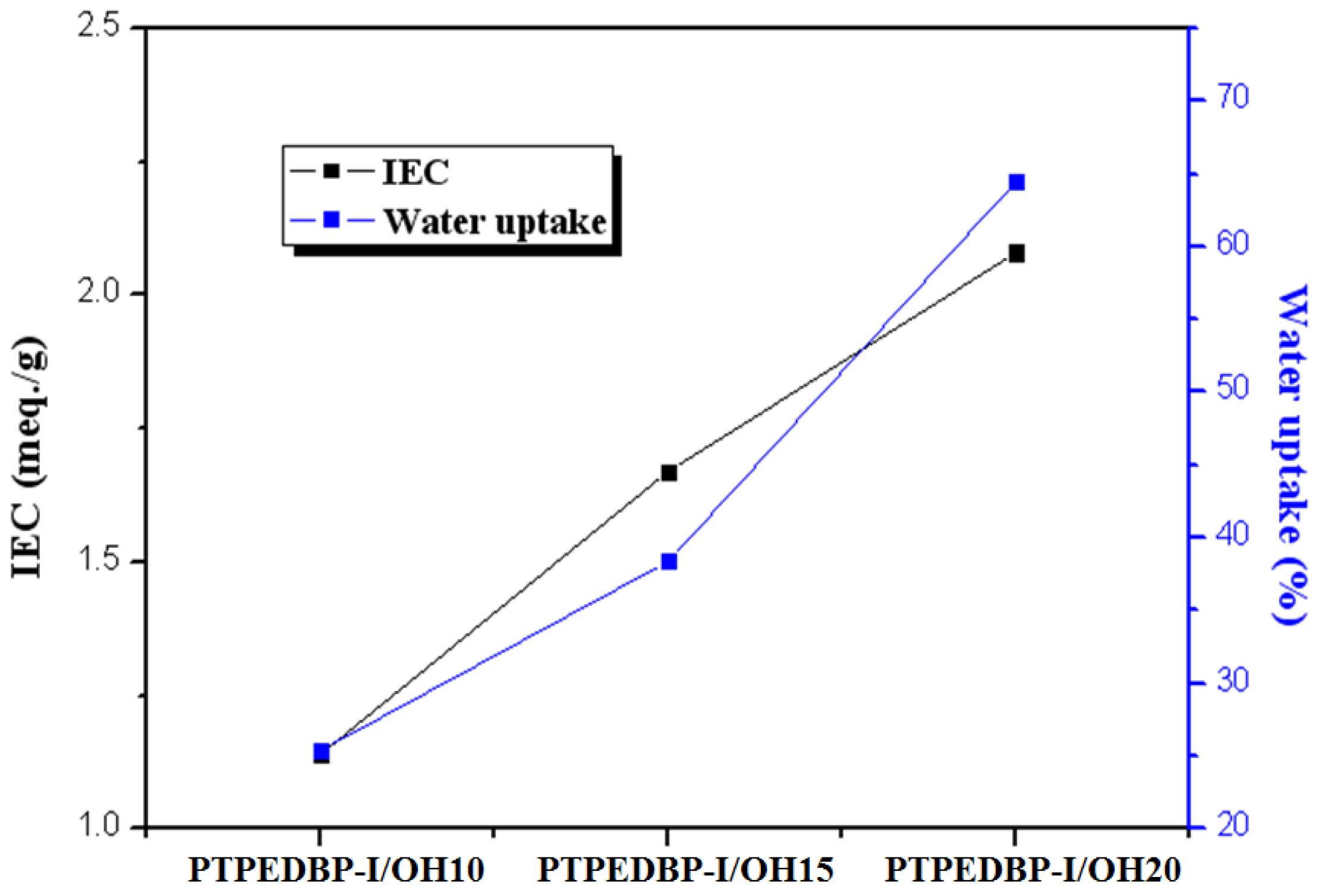
3.2. Properties of Anion Exchange Membrane
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vogel, C.; Komber, H.; Quetschke, A.; Butwilowski, W.; Potschke, A.; Schlenstedt, K.; Haack, J.M. Side-chain sulfonated random and multiblock poly(ether sulfone)s for PEM applications. React. Funct. Polym. 2011, 71, 828–842. [Google Scholar] [CrossRef]
- Stoica, D.; Ogier, L.; Akrour, L.; Alloin, F.; Fauvarque, J.F. Anionic membrane based on polyepichlorhydrin matrix for alkaline fuel cell: Synthesis, physical and electrochemical properties. Electrochim. Acta 2007, 53, 1596–1603. [Google Scholar] [CrossRef]
- Lin, B.; Qiu, L.B.; Peng, Y.; Yan, F. Alkaline Stable Imidazolium-based Ionomers Containing Poly(arylene ether sulfone) Side Chains for Alkaline Anion Exchange Membranes. Macromolecules 2011, 44, 9642–9649. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion Conductive Block Poly(arylene ether)s: Synthesis, Properties, and Application in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Chempath, S.; Einsla, B.R.; Pratt, L.R.; Macomber, C.S.; Boncella, J.M.; Rau, J.A.; Pivovar, B.S. Mechanism of Tetraalkylammonium Headgroup Degradation in Alkaline Fuel Cell Membranes. J. Phys. Chem. C 2008, 112, 3179–3182. [Google Scholar] [CrossRef]
- Wang, G.; Weng, Y.; Zhao, J.; Chu, D.; Xie, D.; Chen, R. Developing a novel alkaline anion exchange membrane derived from poly (ether-imide) for improved ionic conductivity. Polym. Adv. Technol. 2010, 21, 554–560. [Google Scholar] [CrossRef]
- Dao, P.T.; Jing, P.; Shan, F.L.; Lin, Z.; Jun, T.L. Alkaline polymer electrolyte fuel cells: Principle, challenges, and recent progress. Sci. China Chem. 2010, 53, 357–364. [Google Scholar]
- Switzer, E.E.; Olson, T.S.; Datye, A.K.; Atanassov, P.; Hibbs, M.R.; Fujimoto, C.; Cornelius, C.J. Novel KOH-free anion exchange membrane fuel cell: Performance comparison of alternative anion-exchange ionomers in catalyst ink. Electochim. Acta 2010, 55, 3404–3408. [Google Scholar] [CrossRef]
- Wang, G.; Weng, Y.; Chu, D.; Chen, R.; Xie, D. Developing a polysulfone-based alkaline anion exchange membrane for improved ionic conductivity. J. Memb. Sci. 2009, 332, 63–68. [Google Scholar] [CrossRef]
- Tanaka, M.; Koike, M.; Miyatake, M.; Watanabe, M. Synthesis and properties of anion conductive ionomers containing fluorenyl groups for alkaline fuel cell applications. Polym. Chem. 2011, 2, 99–106. [Google Scholar] [CrossRef]
- Vega, J.A.; Chartier, C.; Mustain, W.E. Effect of hydroxide and carbonate alkaline media on anion exchange membranes. J. Power Sources 2010, 195, 7176–7180. [Google Scholar] [CrossRef]
- Zhou, J.; Unlu, M.; Vega, J.A.; Kohl, P.A. Anionic polysulfone ionomers and membranes containing fluorenyl groups for anionic fuel cells. J. Power Sources 2009, 190, 285–292. [Google Scholar] [CrossRef]
- Yan, J.; Hickner, M.A. Polysulfone ionomers and membranes containing fluorenyl groups for anionic fuel cells. Macromolecules 2010, 43, 2349–2356. [Google Scholar] [CrossRef]
- Vinodh, R.; Ilakkiya, A.; Elamathi, S.; Sangeetha, D. A novel anion exchange membrane from polystyrene (ethylenebutylene) polystyrene: Synthesis and characterization. Mater. Sci. Eng. B 2010, 167, 43–50. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Bauer, B.; Strathmann, H.; Effenberger, F. Anion-exchange membranes with improved alkaline stability. Desalination 1990, 79, 125–144. [Google Scholar] [CrossRef]
- Kim, D.S.; Labouriau, A.; Guiver, M.D.; Kim, Y.S. Guanidinium functionalized anion exchange polymer electrolytes via activated fluorophenyl amine reaction. Chem. Mater. 2011, 23, 3795–3797. [Google Scholar] [CrossRef]
- Hou, H.Y.; Sun, G.Q.; He, R.H.; Sun, B.; Jin, W.; Liu, H.; Xin, Q. Alkali doped polybenzimidazole membrane for alkaline direct methanol fuel cell. Int. J. Hydrogen Energy 2008, 33, 7172–7176. [Google Scholar] [CrossRef]
- Rao, A.H.N.; Kim, H.J.; Nam, S.; Kim, T.H. Cardo poly(arylene ether sulfone) block copolymers with pendant imidazolium side chains as novel anion exchange membranes for direct methanol alkaline fuel cell. Polymer 2013, 54, 6918–6928. [Google Scholar] [CrossRef]
- Deavin, O.I.; Murphy, S.; Ong, A.L.; Poynton, S.D.; Zeng, R.; Herman, H.; Varcoe, J.R. Anion-exchange membranes for alkaline polymer electrolyte fuel cells: Comparison of pendent benzyltrimethylammonium- and benzylmethylimidazolium-head-groups. Energy Environ. Sci. 2012, 5, 8584–8597. [Google Scholar] [CrossRef]
- Wang, J.; Wei, H.; Yang, S.; Fang, H.; Xu, P.; Ding, Y. Constructing pendent imidazolium-based poly(phenylene oxide)s for anion exchange membranes using a click reaction. RSC Advance 2015, 5, 93415–93422. [Google Scholar] [CrossRef]
- Clark, T.J.; Robertson, N.J.; Kostalik, I.V.H.A.; Lobkovsky, E.B.; Mutolo, P.F.; Abruna, H.C.D.; Coates, G.W. A ring-opening metathesis polymerization route to alkaline anion exchange membranes: Development of hydroxide-conducting thin films from an ammonium-functionalized monomer. J. Am. Chem. Soc. 2009, 131, 12888–12889. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.H.; Hickner, M.A.; Cornelius, C.J.; Loy, D.A. Ionomeric poly(phenylene) prepared by Diels-Alder polymerization: Synthesis and physical properties of a novel polyelectrolyte. Macromolecules 2005, 38, 5010–5016. [Google Scholar] [CrossRef]
- Lim, Y.D.; Lee, H.C.; Lee, S.H.; Jang, H.H.; Hossain, M.A.; Cho, Y.G.; Kim, T.H.; Hong, Y.T.; Kim, W.G. Synthesis and properties of sulfonated poly(phenylene sulfone)s without ether linkage by Diels-Alder reaction for PEMFC application. Electrochimica Acta 2014, 119, 16–23. [Google Scholar] [CrossRef]
- Ghassemi, H.; McGrath, J.E. Synthesis and properties of new sulfonated poly(p-phenylene) derivatives for proton exchange membranes. I. Polymer 2004, 45, 5847–5854. [Google Scholar] [CrossRef]
- Jang, H.H.; Hong, T.H.; Yoo, J.H.; Lee, S.H.; Pyo, J.S.; Sutradhar, S.C.; Ju, H.C.; Kim, W.G. Preparation and characterization of sulfonated poly(phenylene)s membranes containing conjugated moiety via nickel catalyzed carbon-carbon coupling polymerization. Int. J. Hydrogen Energy 2015, 40, 14364–14370. [Google Scholar] [CrossRef]
- Guzmman-Gutierrez, T.M.; Daniel, R.N.; Serguei, F.; Salvador, L.M.; Mikhail, G.Z.; Carmen, G.M.; Hernandez, M.; Kricheldorf, H.; Edward, S.W. Dramatic enhancement of superacid-catalyzed polyhydroxyalkylation reactions. Macromolecules 2011, 44, 194–202. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, Y.D.; Hossain, M.A.; Jang, H.H.; Jeon, Y.T.; Lee, S.Y.; Jin, L.; Kim, W.G. Synthesis and properties of grafting sulfonated polymer containing isatin by super acid-catalyzed polyhydroxyalkylation reaction for PEMFC. Renewable Energy 2015, 79, 72–77. [Google Scholar]
- Lim, Y.D.; Seo, D.W.; Lee, S.H.; Ju, H.H.; Hong, T.W.; Kim, D.M.; Ju, H.C.; Kim, W.G. Synthesis and properties of cis/trans mixtures of bis(4-hydroxyphenyl)-1,2-diphenylethylene containing sulfonated poly(ethersulfone)s proton exchange membranes. Int. J. Hydrogen Energy 2013, 38, 7667–7673. [Google Scholar]
- Fu, H.; Yun, H.; Kwei, T.K.; Okamoto, Y.; Blumstengel, S.; Walser, A.; Dorsinville, R. Blue Photo- and Electroluminescence Based on Poly(2-benzoyl-1,4-phenylene) and Poly(2,5-dibenzoyl-1,4-phenylene. Polym. Adv. Technol. 1999, 10, 259–264. [Google Scholar]
- Seo, D.W.; Sarker, S.; Nath, N.C.D.; Choi, S.W.; Ahammad, A.J.S.; Lee, J.J.; Kim, W.G. Synthesis of a novel imidazolium-based electrolytes and application for dye-sensitized solar cells. Electrochim. Acta 2010, 55, 1483–1488. [Google Scholar]
- Basuli, U.; Jose, J.; Lee, R.H.; Yoo, Y.H.; Jeong, K.U.; Ahn, J.H.; Nah, C.W. Properties and Degradation of the Gasket Component of a Proton Exchange Membrane Fuel Cell—A Review. J. Nanosci. Nanotechnol. 2012, 12, 7641–7657. [Google Scholar]
- Gou, M.; Fang, J.; Xu, H.; Li, W.; Lu, X.; Lan, C.; Li, K. Synthesis and characterization of novel anion exchange membranes based on imidazolium-type ionic liquid for alkaline fuel cells. J. Memb. Sci. 2010, 362, 97–104. [Google Scholar]
- Henkensmeier, D.; Cho, H.; Brela, M.; Michalak, A.; Dyck, A.; Germer, W.; Duong, N.M.H.; Jang, J.H.; Kim, H.J.; Woo, N.S.; Lim, T.H. Anion conducting polymers based on ether linked polybenzimidazole (PBI-OO). Int. J. Hydrogen Energy 2014, 39, 2842–2853. [Google Scholar]
- Yanagi, H.; Fukuta, K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). ECS Transactions 2008, 16, 257–262. [Google Scholar]
- Wang, J.; Zhao, Z.; Gong, F.; Li, S.; Zhang, S. Synthesis of soluble poly(arylene ether sulfone) ionomers with pendant quaternary ammonium groups for anion exchange membranes. Macromolecules 2009, 42, 8711–8717. [Google Scholar]
- Price, S.C.; Williams, K.S.; Beyer, F.L. Relationships between structure and alkaline stability of imidazolium cations for fuel cell membrane applications. ACS Macro Lett. 2014, 3, 160–165. [Google Scholar]
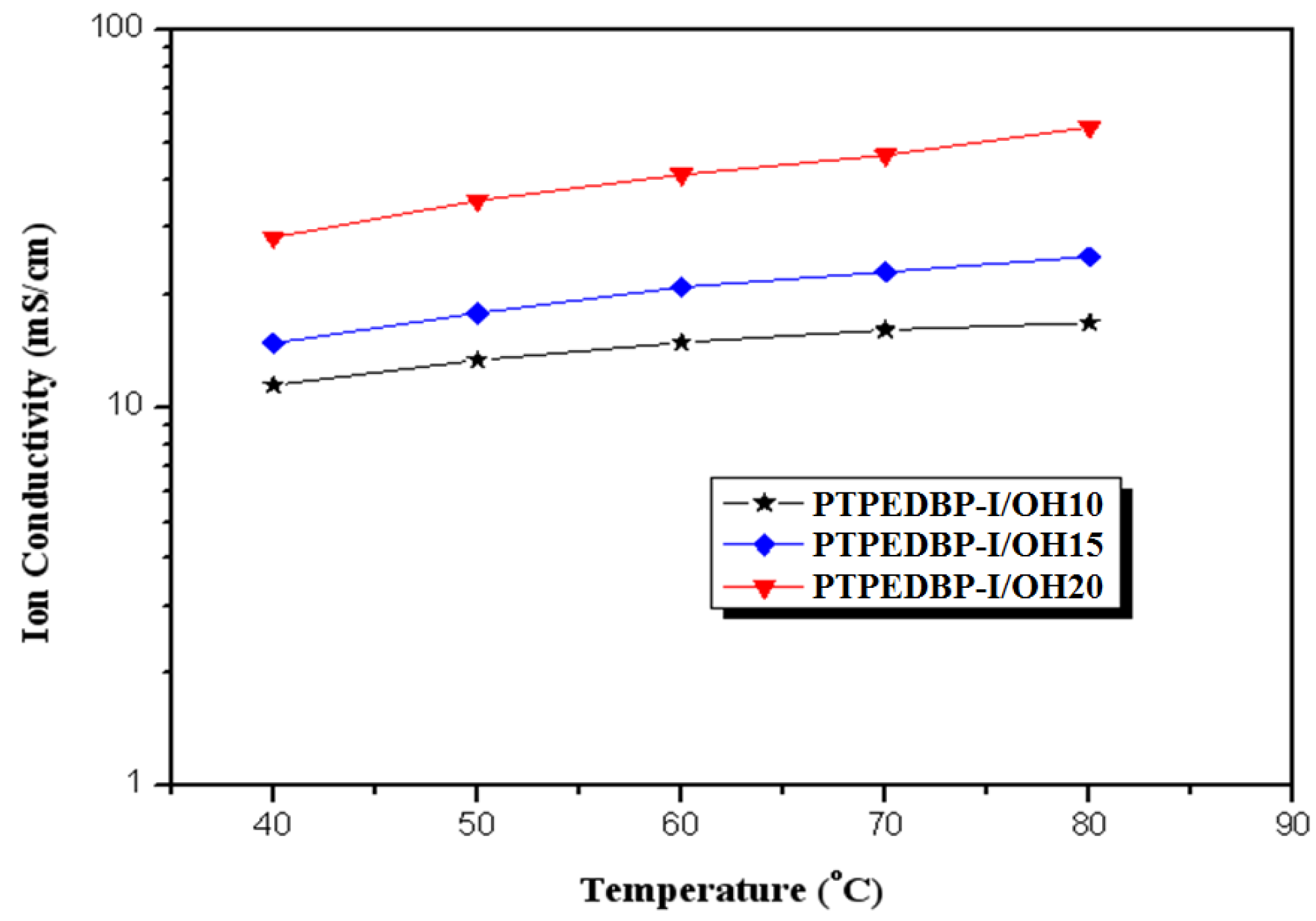
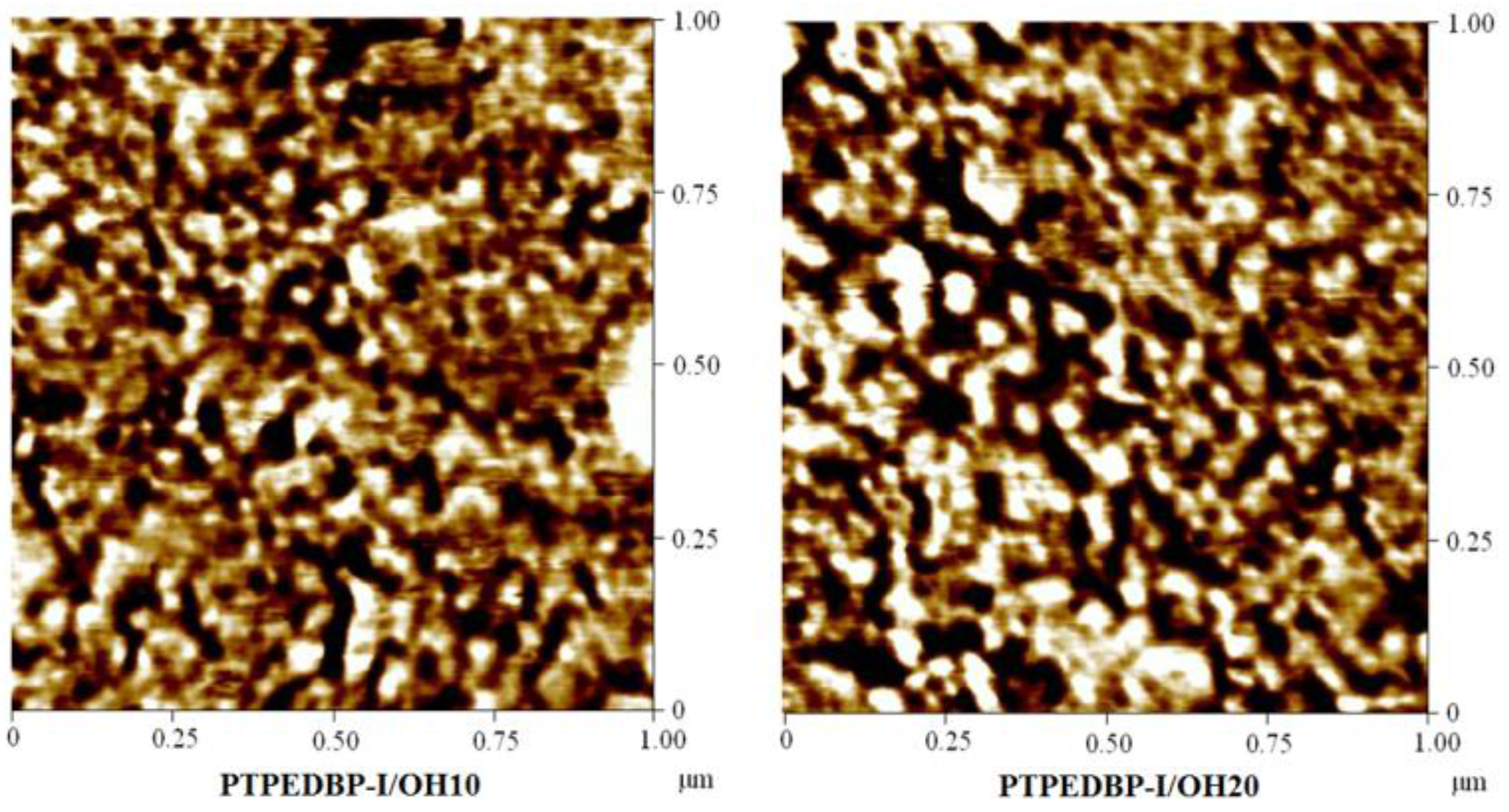
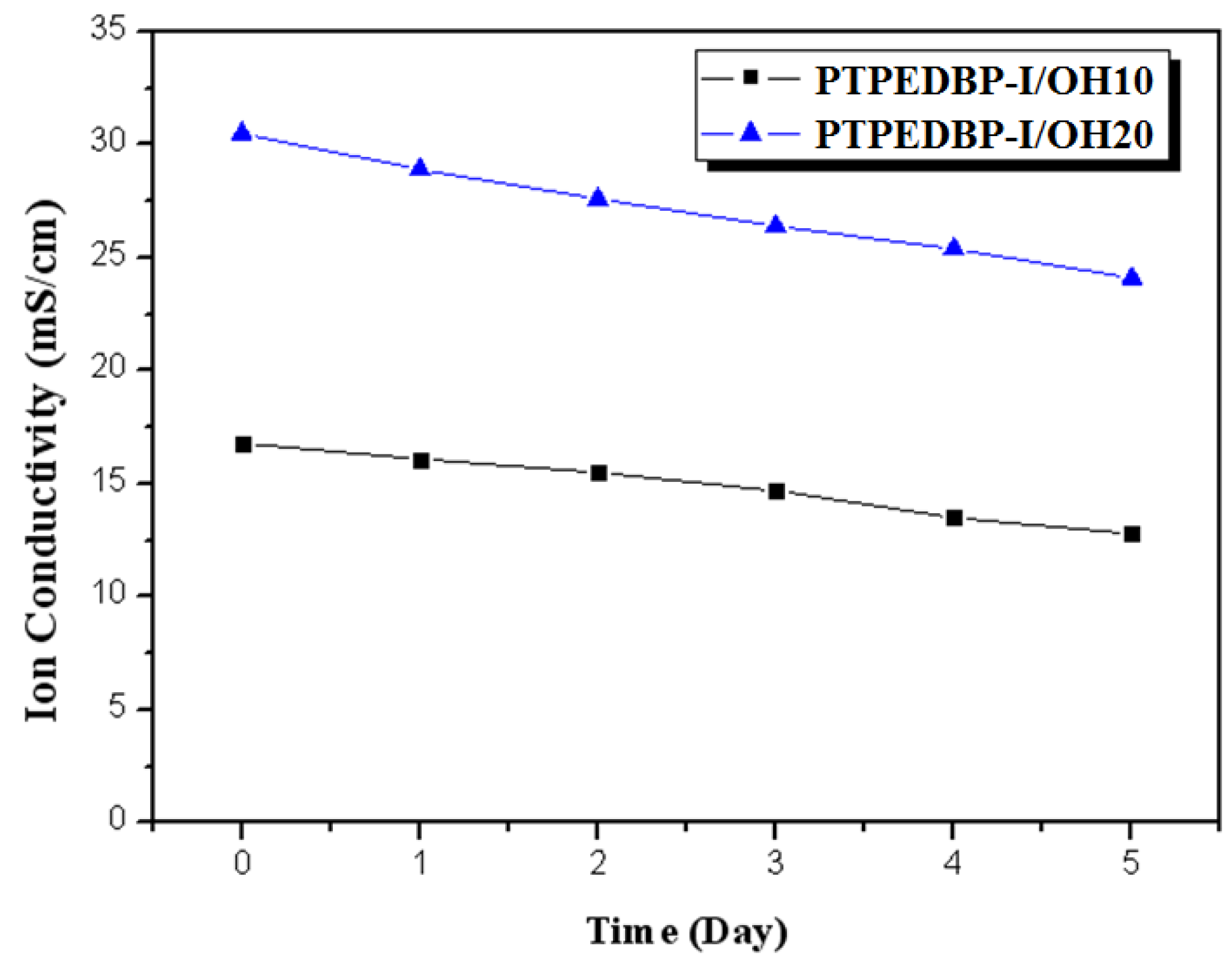
| Polymer | Theoretical IEC (meq./g) | Titrated IEC (meq./g) | Water Uptake 1 (%) | Proton Conductivity 2 (mS/cm) |
|---|---|---|---|---|
| PTPEDBP-I/OH 10 | 1.32 | 1.14 | 25.4 | 16.8 |
| PTPEDBP-I/OH 15 | 1.78 | 1.67 | 38.4 | 24.5 |
| PTPEDBP-I/OH 20 | 2.24 | 2.08 | 64.5 | 30.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Lee, S.; Ha, J.; Choi, K.; Ryu, T.; Kim, K.; Jeon, H.-S.; Kim, W. Preparation and Characterization of Tetra-Imidazolium Hydroxide Polyphenylene Membranes via Nickel Catalyzed C–C Coupling Polymerization. Energies 2016, 9, 271. https://doi.org/10.3390/en9040271
Jang H, Lee S, Ha J, Choi K, Ryu T, Kim K, Jeon H-S, Kim W. Preparation and Characterization of Tetra-Imidazolium Hydroxide Polyphenylene Membranes via Nickel Catalyzed C–C Coupling Polymerization. Energies. 2016; 9(4):271. https://doi.org/10.3390/en9040271
Chicago/Turabian StyleJang, Hohyoun, Soonho Lee, Jaeseong Ha, Kunyoung Choi, Taewook Ryu, Kyunghwan Kim, Heung-Seok Jeon, and Whangi Kim. 2016. "Preparation and Characterization of Tetra-Imidazolium Hydroxide Polyphenylene Membranes via Nickel Catalyzed C–C Coupling Polymerization" Energies 9, no. 4: 271. https://doi.org/10.3390/en9040271
APA StyleJang, H., Lee, S., Ha, J., Choi, K., Ryu, T., Kim, K., Jeon, H.-S., & Kim, W. (2016). Preparation and Characterization of Tetra-Imidazolium Hydroxide Polyphenylene Membranes via Nickel Catalyzed C–C Coupling Polymerization. Energies, 9(4), 271. https://doi.org/10.3390/en9040271






