Microalgae Isolation and Selection for Prospective Biodiesel Production
Abstract
:1. Introduction
2. Advanced Microalgae Biodiesel Production
3. Biodiesel Conversion from Microalgae
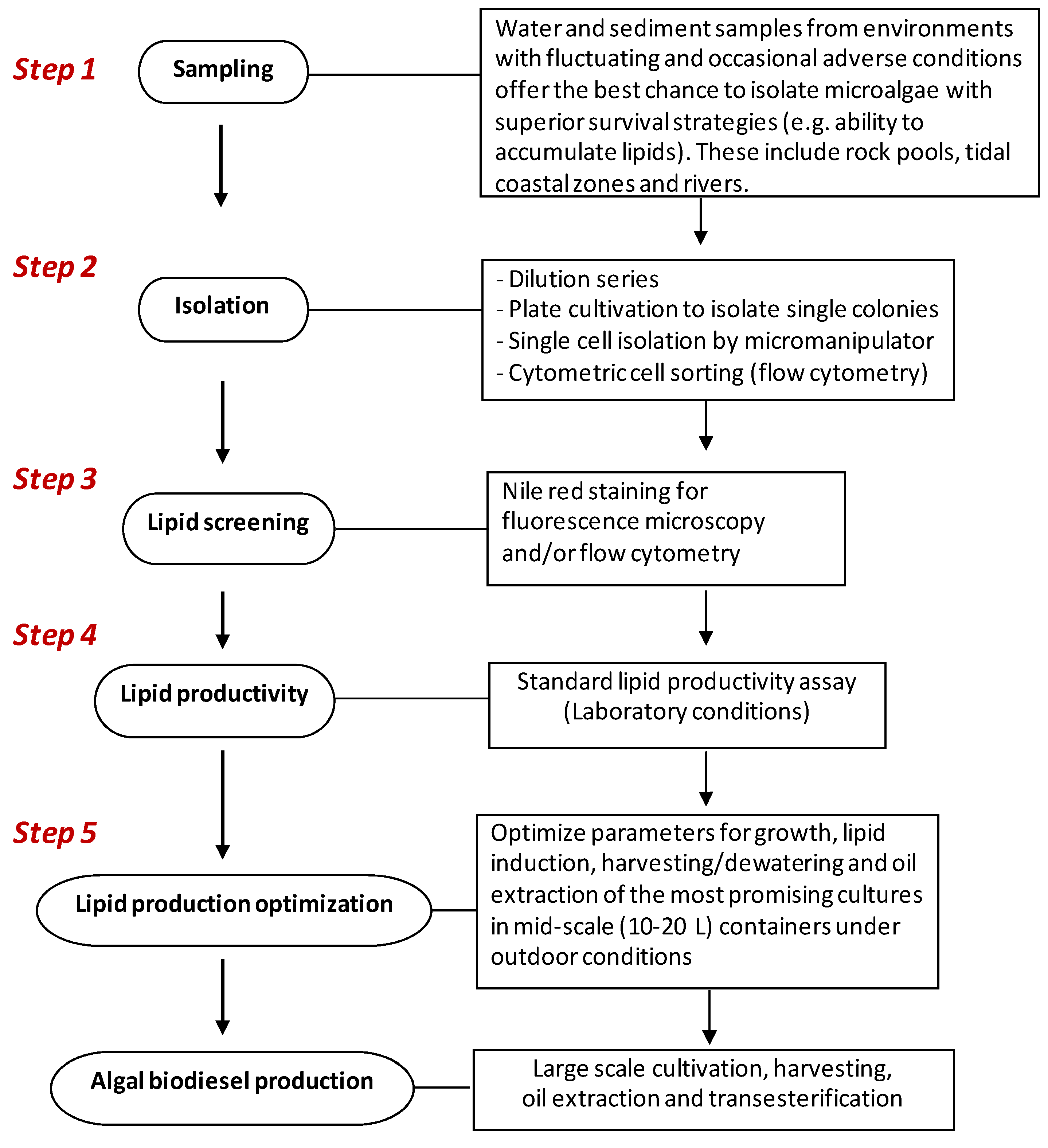
4. Isolation and Selection Criteria for Microalgae with Potential for Biodiesel Production
4.1. Sampling and Isolation of Pure Cultures
| Primer name | Forward (5’–3’) | Primer name | Reverse (5’–3’) | Species | References |
|---|---|---|---|---|---|
| TH18S5’ | GGTAACGAATTGTTAG | TH18S3’ | GTCGGCATAGTTTATG | Thalassiosira pseudonana | [21] |
| P45 | ACCTGGTTGATCCTGCCAGT | P47 | TCTCAGGCTCCCTCTCCGGA | Chlorella vulgaris | [22] |
| GTCAGAGGTGAAATTCTTGGATTTA | AGGGCAGGGACGTAATCAACG | Dunaliella salina | [23] | ||
| SS5 | GGTGATCCTGCCAGTAGTCATATGCTTG | SS3 | GATCCTTCCGCAGGTTCACCTACGGAAACC | Navicula sp. Chlorella sp. | [24] |
| GAAGTCGTAACAAGGTTTCC | TCCTGGTTAGTTTCTTTTCC | Chlamydomonas coccoides Tetraselmis suecicaNannochloris atomus | [25] | ||
| CCAACCTGGTTGATCCTGCCAGTA | CCTTGTTACGACTTCACCTTCCTCT | Nannochloropsis sp. | [26] |
4.2. Lipid Determination
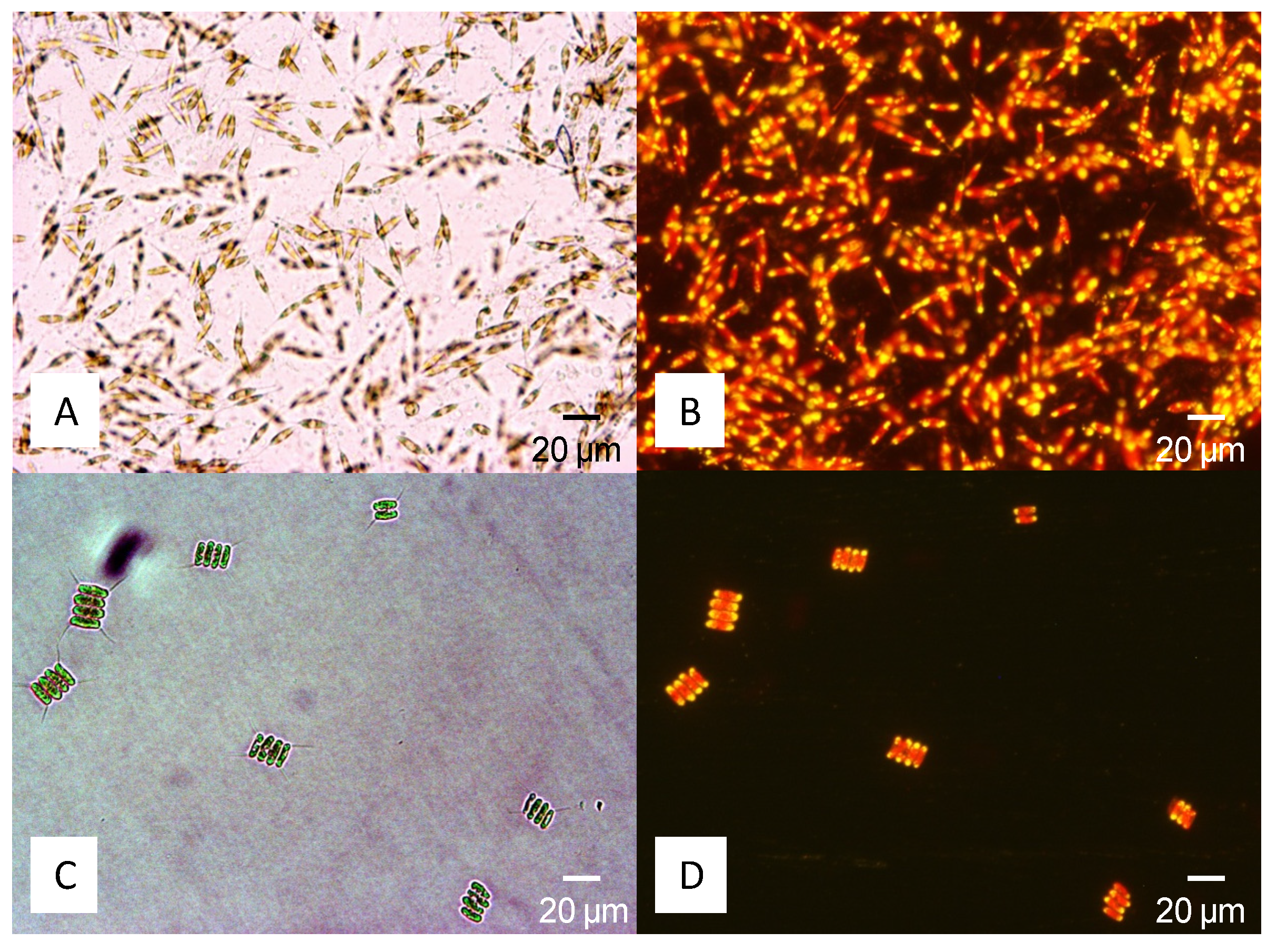
4.3. Cultivation and Biomass Production
4.4. Testing at Larger Scale
| Steps | Desirable traits |
|---|---|
| Screening | High oil |
| High saturated fatty acids | |
| Low unsaturated fatty acids | |
| High omega 3 fatty acids | |
| Rapid and synchronized lipid production | |
| Radiation tolerance/pigment synthesis | |
| Antioxidants, sterols, carotenoids, astaxanthins and other pigments | |
| Low starch contents | |
| High protein contents | |
| Cultivation | Rapid growth |
| Salinity/freshwater tolerance | |
| High/low temperature tolerance | |
| Reduced antennal pigments (for improved photosynthesis in bioreactor) | |
| Flagella properties/possession | |
| Sheering resistance | |
| Harvesting | Cell size and cell wall properties amenable for autoflocculation |
| Sinking speed | |
| Foam fractionation properties | |
| Structure and cell wall properties | |
| Extraction | Cell wall properties amenable for oil extraction |
| Lipid extraction efficiency |
5. Lipid Content in Microalgae
| Species | Total lipids (% dry weight) | PUFA (% total lipids) | PUFA (% dry weight) |
|---|---|---|---|
| Isochrysis galbana | 25.6 | 17 | 4.3 |
| Nanaochloropsis sp. | 5.6 | 2.8 | 0.2 |
| Chaetoceros calcitrans | 11.8 | 8.7 | 0.9 |
| Tetreselmis suecica | 2.5 | 20.9 | 0.2 |
| Skeletonema costatum | 9.7 | 5.1 | 0.5 |
| Phaeodactylum tricornutum | 30 | ||
| Porphyridium cruentum | 1.5 | 17.1 | 0.3 |
| Crypthecodinium cohnii | 20 | ||
| Botryococcus braunii | 25.0–75.0 | ||
| Chlorella sp. | 10.0–48.0 |
6. Cultivation and Lipid Extraction Properties of Microalgae
| Species | Eicosapentaenoic acid (EPA) (% of total fatty acids) | Docosahexaenoic acid (DHA) (% of total fatty acids) |
|---|---|---|
| Isochrysis galbana | 0.9 | |
| Nannochloropsis sp. | 30.1 | |
| Chaetoceros calcitrans | 34 | |
| Tetraselmis suecica | 6.2 | |
| Chaetoceros muelleri | 12.8 | 0.8 |
| Pavlova salina | 19.1 | 1.5 |
| Skeletonema costatum | 40.7 | 2.3 |
| Porphyridium cruentum | 30.7 | |
| Crypthecodinium cohnii | 30 | |
| Chroomonas salina | 12.9 | 7.1 |
| Chaetoceros constriccus | 18.8 | 0.6 |
| Tetraselmis viridis | 6.7 |
7. Conclusions
Acknowledgements
References
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science Ltd.: Hudson County, NJ, USA, 2004. [Google Scholar]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- CSIRO. Australian national algae culture collection. Available online: http://www.csiro.au/Organisation-Structure/National-Facilities/Australian-National-Algae-Culture-Collection.aspx (accessed on 19 January 2012).
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef] [PubMed]
- European Biodiesel Board. The EU biodiesel industry. Available online: http://www.ebb-eu.org/stats.php (accessed on 18 January 2012).
- Carriquiry, M. U.S. Bidiesel production: Recent developments and prospects. Iowa Agric. Rev. Online 2007, 13, 8–9. [Google Scholar]
- TUSNBB. Production statistics. Available online: http://www.biodiesel.org/production/production-statistics (accesssed on 18 January 2012).
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae; National Renewable Energy Laboratory: Golden, Colorado, USA, 1998. [Google Scholar]
- Delucchi, M.A. A Lifecycle Emission Model (LEM): Lifecycle Emissions from Transportation Fuels; Motor Vehicles, Transportation Modes, Electricity Use, Heating and Cooking Fuels; Institute of Transport Studies, University of California: Davis, CA, USA, 2003. [Google Scholar]
- Paulson, N.D.; Ginder, R.D. The Growth and Direction of Biodiesel Industry in the United States; Center for Agricultural and Rural Development: Iowa State University, IA, USA, 2007. [Google Scholar]
- Laboratory, N.R.E. Biodiesel Handling and Use Guide; The U.S. Department of Energy: Golden, Colorado, USA, 2009. [Google Scholar]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, N.P.; Boocock, D.G.B.; Konar, S.K. Liquid hydrocarbons from catalytic pyrolysis of sewage sludge lipid and canola oil: Evaluation of fuel properties. Energy Fuels 1995, 9, 248–256. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.; Hicks, K.B.; Jung, H.-J.G.; Lamb, J.F.S. Production of bio-oil from alfalfa stems by fluidized-Bed fast pyrolysis. Ind. Eng. Chem. Res. 2008, 47, 4115–4122. [Google Scholar] [CrossRef]
- Davey, H.M.; Kell, D.B. Flow cytometry and cell sorting of heterogeneous microbial populations: The importance of single-cell analyses. Microbiol. Rev. 1996, 60, 641–696. [Google Scholar] [PubMed]
- Reckermann, M. Flow sorting in aquatic ecology. Sci. Mar. 2000, 64, 235–246. [Google Scholar] [CrossRef]
- Dinh, L.T.T.; Guo, Y.; Mannan, M.S. Sustainability evaluation of biodiesel production using multicriteria decision-making. Environ. Prog. Sustain. Energy 2009, 28, 38–46. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Haznedaroglu, B.Z.; Bibby, K.; Peccia, J. Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: Pathway description and gene discovery for production of next-generation biofuels. BMC Genomics 2011, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Tonon, T.; Harvey, D.; Qing, R.; Li, Y.; Larson, T.R.; Graham, I.A. Identification of a fatty acid Δ11-desaturase from the microalga Thalassiosira pseudonana. FEBS Lett. 2004, 563, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Berard, A.; Dorigo, U.; Humbert, J.F.; Martin-Laurent, F. Microalgae community structure analysis based on 18S rDNA amplification from DNA extracted directly from soil as a potential soil bioindicator. Agronomie 2005, 25, 1–7. [Google Scholar]
- Rasoul-Amini, S.; Ghasemi, Y.; Morowvat, M.H.; Mohagheghzadeh, A. PCR amplification of 18S rRNA, single cell protein production and fatty acid evaluation of some naturally isolated microalgae. Food Chem. 2009, 116, 129–136. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sugiyama, H.; Maeda, Y.; Sato, R.; Tanaka, T.; Matsunaga, T. Marine diatom, Navicula sp. strain JPCC DA0580 and marine green alga, Chlorella sp. strain NKG400014 as potential sources for biodiesel production. Appl. Biochem. Biotechnol. 2010, 161, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Timmins, M.; Thomas-Hall, S.R.; Darling, A.; Zhang, E.; Hankamer, B.; Marx, U.C.; Schenk, P.M. Phylogenetic and molecular analysis of hydrogen-producing green algae. J. Exp. Bot. 2009, 60, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, B.; You, W. Identification of the alga known as Nannochloropsis Z-1 isolated from a prawn farm in Hainan, China as Chlorella. World J. Microbiol. Biotechnol. 2007, 23, 207–210. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, H.; Lim, D.K.Y.; Schenk, P.M. Perspectives on metabolic engineering for increased lipid contents in microalgae. Biofuels 2012, 3, 71–86. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Eltgroth, M.L.; Watwood, R.L.; Wolfe, G.V. Production and cellular localization of neutral long-chain lipids in the haptophyte algae Isochrysis galbana and Emiliania huxleyi. J. Phycol. 2005, 41, 1000–1009. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red—A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hu, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sommerfeld, M.; Hu, Q. Microwave-assisted Nile red method for in vivo quantification of neutral lipids in microalgae. Bioresour. Technol. 2011, 102, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Chan, K.W. Rapid immunofluorescence staining of human renal biopsy speciments using microwave irradiation. J. Clin. Pathol. 1987, 40, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, T.E.; Giberson, R.T.; Demaree, R.; Day, J.R. Microwave-assisted immunostaining: A new approach yields fast and consistent results. J. Neurosci. Methods 2004, 137, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, B.W. A Nile red staining method for the fluorescence detection of lipid in algae utilizing a FlowCAM: Biofuels digest. Available online: http://www.biofuelsdigest.com/bdigest/2010/05/05/a-nile-red-staining-method-for-the-fluorescence-detection-of-lipid-in-algae-utilizing-a-flowcam/ (accessed on 31 January 2012).
- Schenk, P.; Thomas-Hall, S.; Stephens, E.; Marx, U.; Mussgnug, J.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenerg. Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, J.G. Comparison of growth and lipid content in three Botryococcus braunii strains. J. Appl. Phycol. 2005, 17, 6. [Google Scholar]
- Thomas, W.H.; Tornabene, T.G.; Weissman, J. Screening for Lipid Yielding Microalgae: Activities for 1983; Solar Energy Research Institute: Golden, Colorado, USA, 1984. [Google Scholar]
- Al-Hasan, R.; Ali, A.; Ka’wash, H.; Radwan, S. Effect of salinity on the lipid and fatty acid composition of the halophyte Navicula sp.: Potential in mariculture. J. Appl. Phycol. 1990, 2, 215–222. [Google Scholar] [CrossRef]
- Pulz, O.P. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Knights, B.A.; Conway, E. Hydrocarbon content and its relationship to physiological state in the green alga Botryococcus braunii. Phytochemistry 1969, 8, 5. [Google Scholar]
- Huerlimann, R.; de Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Yan, L.; Schenk, P.M. Selection of Cultured Microalgae for Producing Omega-3 Bio-Lipid Oil; Report for Queensland Sea Scallop Trading Pty Ltd.; The University of Queensland: Queensland, Austrilia, 2011; p. 36. [Google Scholar]
- Scholz, M.; Hoshino, T.; Johnson, D.; Riley, M.R.; Cuello, J. Flocculation of wall-deficient cells of Chlamydomonas reinhardtii mutant cw15 by calcium and methanol. Biomass Bioenerg. 2011, 35, 4835–4840. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Recycling algae to improve species control and harvest efficiency from a high rate algal pond. Water Res. 2011, 45, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duong, V.T.; Li, Y.; Nowak, E.; Schenk, P.M. Microalgae Isolation and Selection for Prospective Biodiesel Production. Energies 2012, 5, 1835-1849. https://doi.org/10.3390/en5061835
Duong VT, Li Y, Nowak E, Schenk PM. Microalgae Isolation and Selection for Prospective Biodiesel Production. Energies. 2012; 5(6):1835-1849. https://doi.org/10.3390/en5061835
Chicago/Turabian StyleDuong, Van Thang, Yan Li, Ekaterina Nowak, and Peer M. Schenk. 2012. "Microalgae Isolation and Selection for Prospective Biodiesel Production" Energies 5, no. 6: 1835-1849. https://doi.org/10.3390/en5061835
APA StyleDuong, V. T., Li, Y., Nowak, E., & Schenk, P. M. (2012). Microalgae Isolation and Selection for Prospective Biodiesel Production. Energies, 5(6), 1835-1849. https://doi.org/10.3390/en5061835





