Influence of Photosynthetic Cathodes on Anodic Microbial Communities in Acetate-Fed Microbial Fuel Cells Pre-Enriched Under Applied Voltage
Abstract
1. Introduction
2. Materials and Methods
2.1. MFC and pMFC Construction
2.2. Synthetic Wastewater and Cathode Media in MFC
2.3. Anodic Electrical Stimulation Experiments
2.4. Operation of pMFCs with Microalgae/Cyanobacteria
2.5. Measurement of Biomass Growth
2.6. Electrochemical Measurements
2.7. Chemical Analyses
2.8. SEM Analysis
2.9. Amplicon Sequencing and Bioinformatic Workflow
2.10. Functional Prediction
2.11. Real-Time Quantitative PCR
2.12. Experimental Replication and Data Processing
3. Results and Discussion
3.1. Effects of Anodic Stimulation on Electrochemical Performance
3.1.1. Start-Up, Voltage Output, Current and Power Density
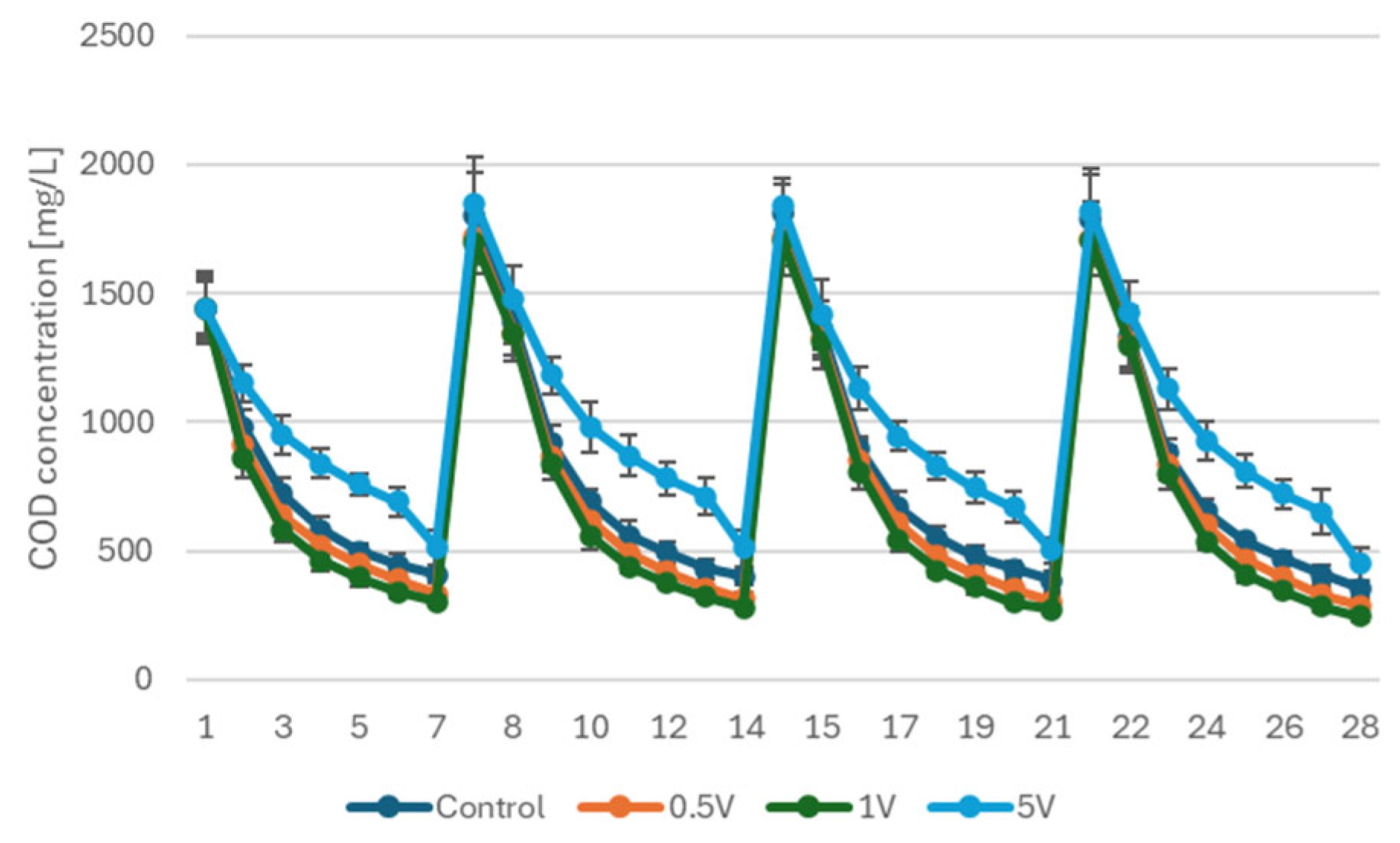
3.1.2. COD Removal Kinetics Under Different Stimulation Regimes
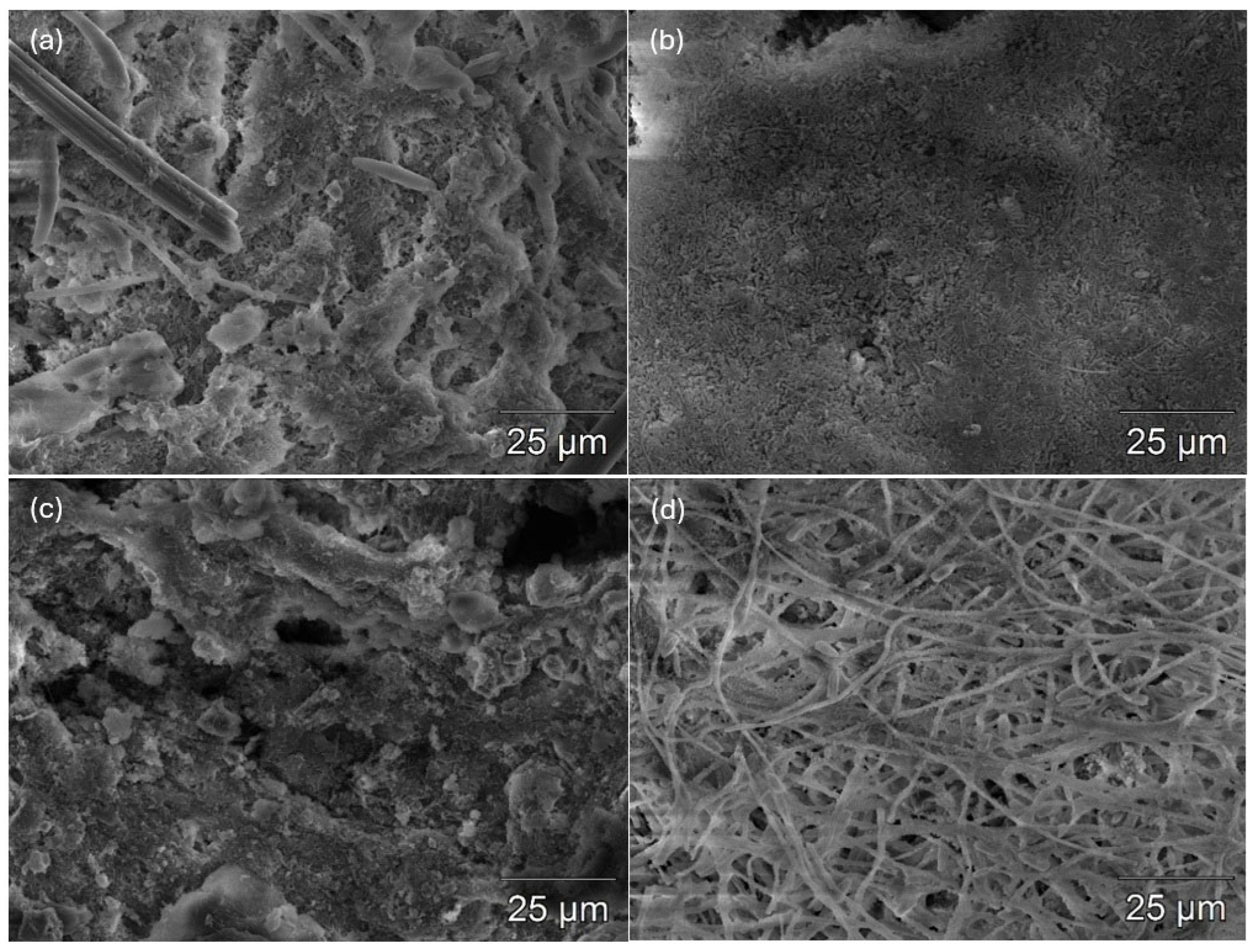
3.1.3. SEM-Based Evaluation of Biofilm Morphology
3.2. Effects of Microalgal/Cyanobacterial Cathodes on pMFC Performance
3.2.1. Algal Biomass Development and Stability
3.2.2. Electrochemical Performance of pMFCs
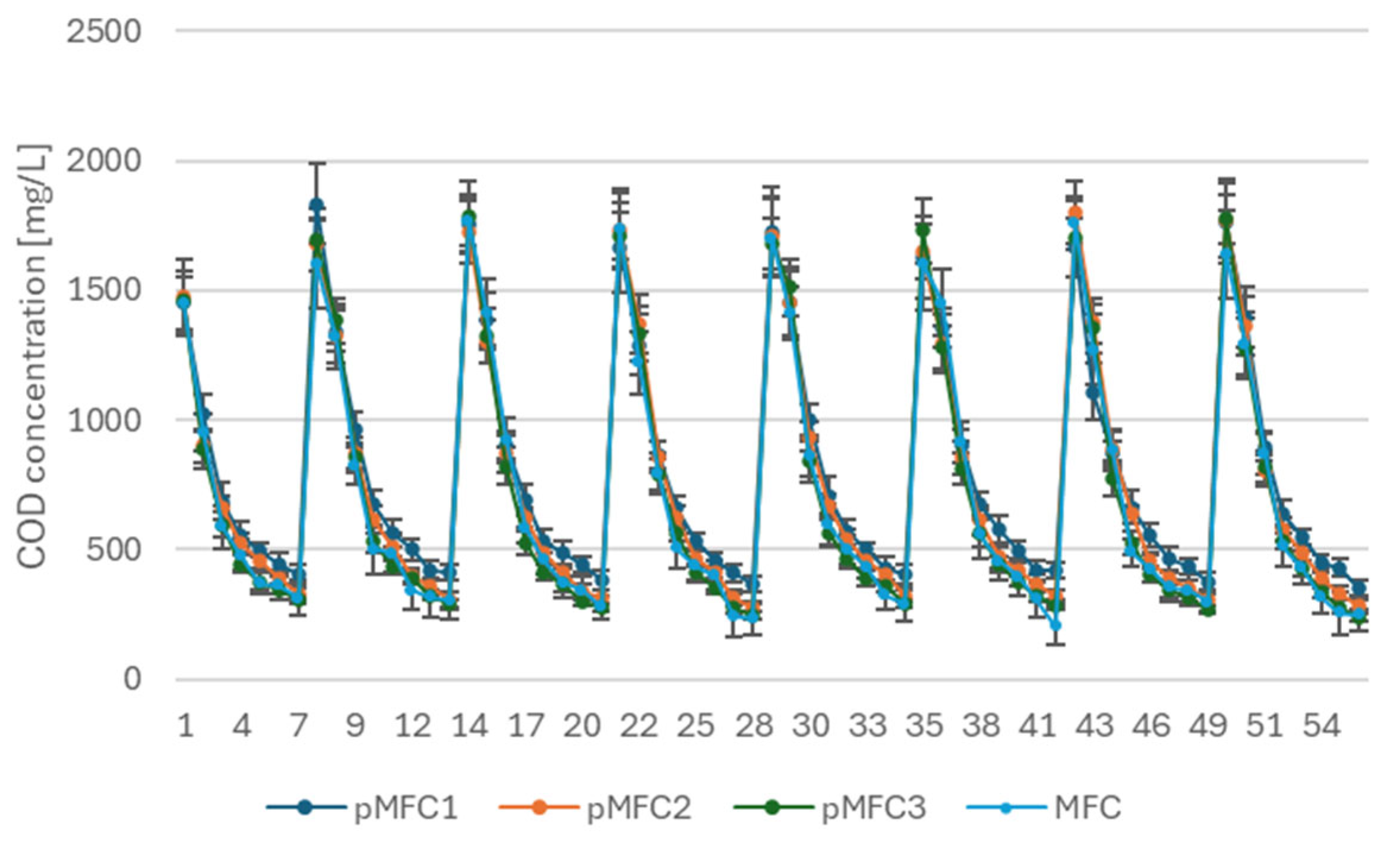
3.2.3. COD Removal in pMFCs Compared to Non-Phototrophic MFC
3.3. Microbial Community Structure in Anodic Biofilms
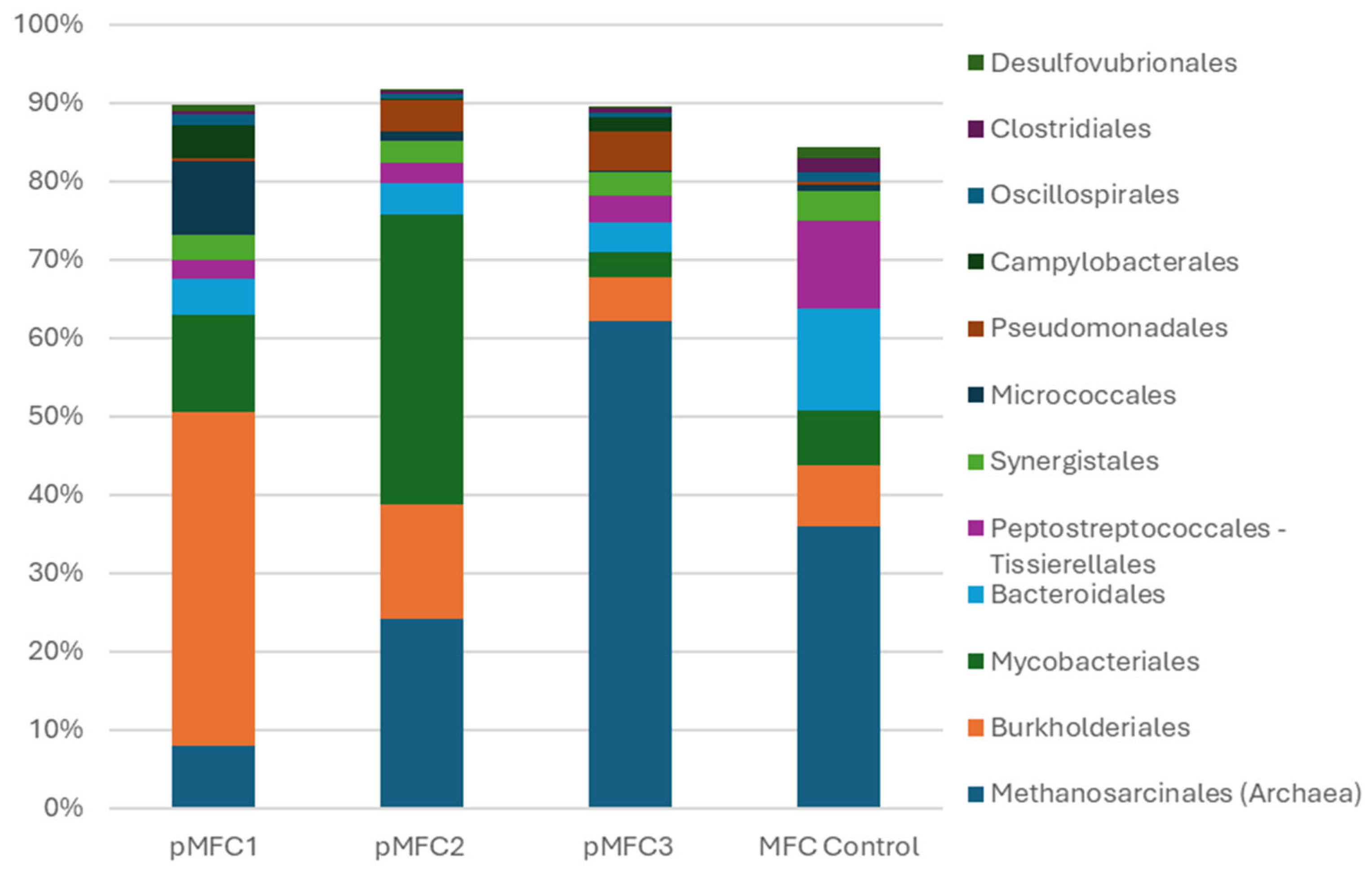
3.3.1. Community Composition at the Order Level
3.3.2. Alpha-Diversity
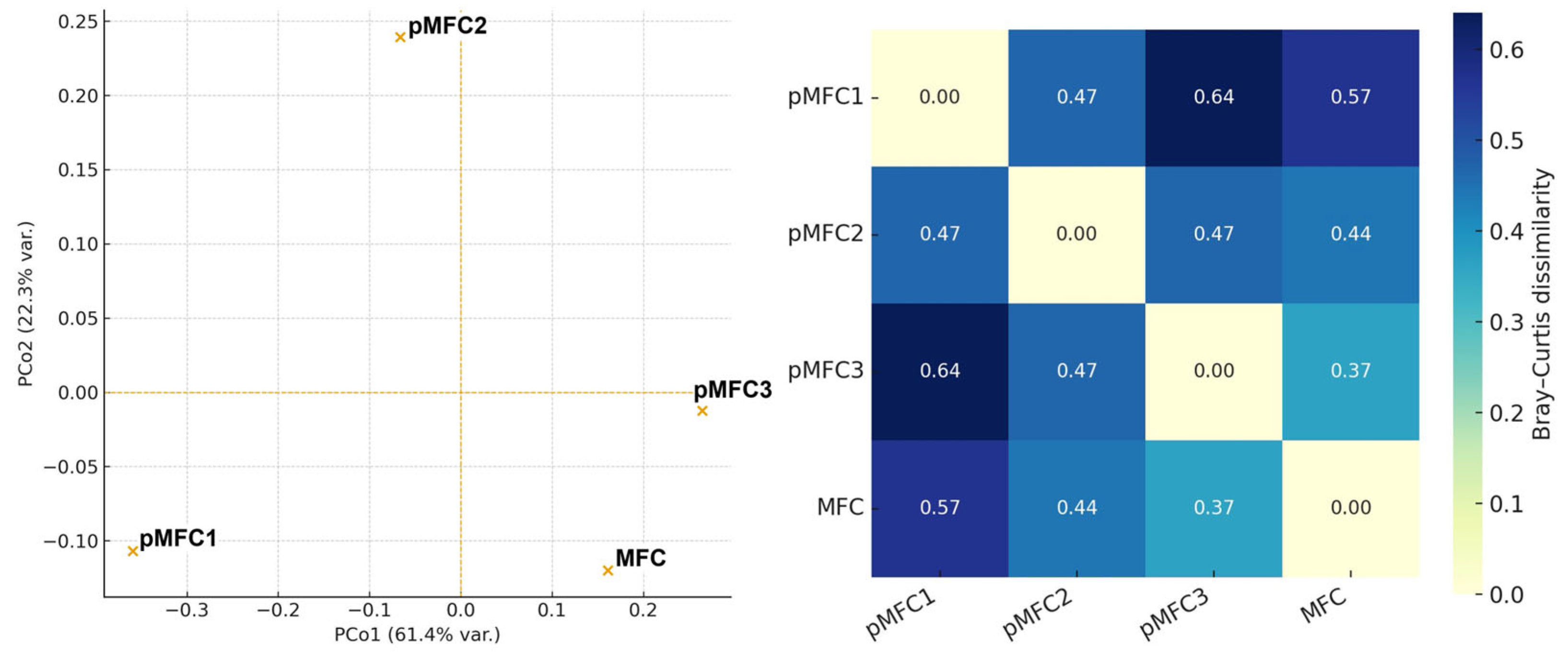
3.3.3. Beta-Diversity (PCoA)
3.4. Functional Metabolic Potential of Anodic Biofilms
3.4.1. Relative Abundance of Functional Categories
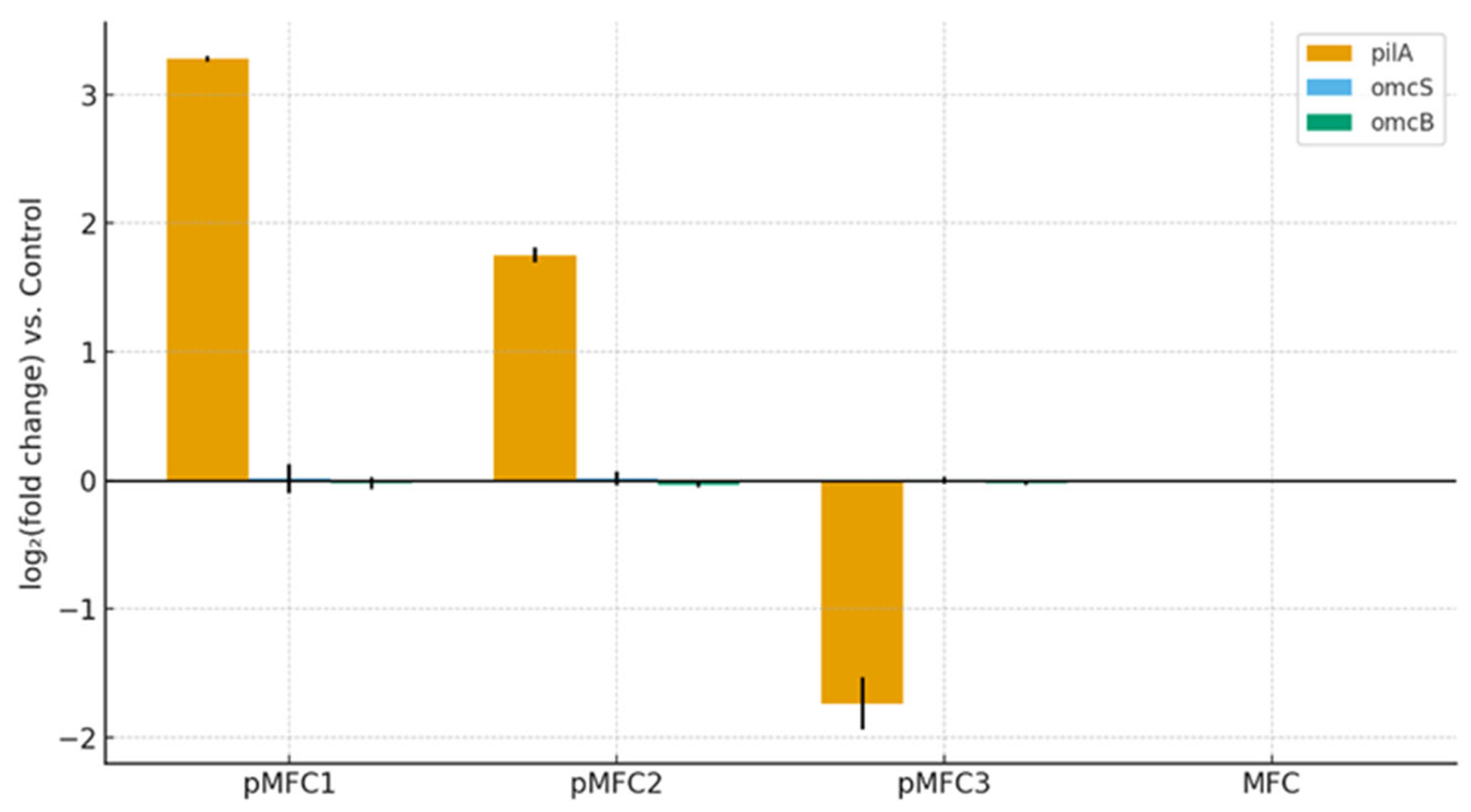
3.4.2. Relative Quantitative PCR
3.5. Study Limitations and Future Research Directions
4. Conclusions
- Short-term anodic stimulation strongly accelerates electroactive biofilm formation. Among the tested voltages, 1 V provided optimal activation, significantly improving start-up behavior, voltage output, power density and COD removal kinetics, while 5 V impaired biofilm integrity and system performance.
- SEM confirmed stimulation-dependent biofilm morphology. Dense, cohesive electroactive structures formed at 1 V, whereas high-voltage stimulation produced damaged, filamentous and sparsely colonized surfaces.
- Photosynthetic cathodes exert strong, species-specific effects on system performance. Chlorella sp. produced the highest voltage, current density and CE; Arthrospira platensis showed intermediate performance; Tetraselmis subcordiformis generated no current due to inhibitory saline conditions.
- Cathodic physiology reshapes anodic microbial communities. Chlorella sp. enriched facultative electroactive taxa and suppressed methanogenesis; Arthrospira platensis favored Actinobacteria and sulfur-oxidizing bacteria; Tetraselmis subcordiformis induced a methanogenesis-dominated biofilm incompatible with EET.
- Functional prediction and qPCR confirm shifts in electron-use pathways. Chlorella sp. increased the abundance of the EET marker gene pilA, while cytochrome-based pathways (omcS/omcB) remained minimal in all variants. Tetraselmis subcordiformis strongly increased all methanogenic pathways, redirecting electrons away from the anode.
- Synergy between 1 V stimulation and Chlorella sp. biocathodes provides the most electroactive configuration. This combination suppresses electron-dissipating processes (methanogenesis), supports redox-balanced conditions and enhances CE.
- Cathodic microalgae therefore function as top–down ecological selectors, modulating anodic redox gradients, metabolic potential and biofilm electroactivity through cross-chamber chemical and electrochemical coupling.
- The synergy between anodic stimulation and phototrophic oxygen production may facilitate fast establishment of conductive biofilms by balancing redox gradients and reducing competitive electron sinks.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, B.E. Microbial Fuel Cells; Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kannan, N.; Donnellan, P. Algae-Assisted Microbial Fuel Cells: A Practical Overview. Bioresour. Technol. Rep. 2021, 15, 100747. [Google Scholar] [CrossRef]
- Don, C.D.Y.A.; Babel, S. Comparing the Performance of Microbial Fuel Cell with Mechanical Aeration and Photosynthetic Aeration in the Cathode Chamber. Int. J. Hydrogen Energy 2021, 46, 16751–16761. [Google Scholar] [CrossRef]
- Kumar, A.; Hsu, L.H.; Kavanagh, P.; Barrière, F.; Lens, P.N.L.; Lapinsonnière, L.; Lienhard, V.J.H.; Schröder, U.; Jiang, X.; Leech, D. The Ins and Outs of Microorganism–Electrode Electron Transfer Reactions. Nat. Rev. Chem. 2017, 1, 0024. [Google Scholar] [CrossRef]
- Zieliński, M.; Rusanowska, P.; Dudek, M.; Starowicz, A.; Barczak, Ł.; Dębowski, M. Efficiency of Photosynthetic Microbial Fuel Cells (pMFC) Depending on the Type of Microorganisms Inhabiting the Cathode Chamber. Energies 2024, 17, 2296. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Ronen, A.; Lerman, S.; Balaish, M.; Ein-Eli, Y.; Dosoretz, C.G. Low Voltage Electric Potential as a Driving Force to Hinder Biofouling in Self-Supporting Carbon Nanotube Membranes. Water Res. 2018, 129, 143–153. [Google Scholar] [CrossRef]
- Baek, Y.; Yoon, H.; Shim, S.; Choi, J.; Yoon, J. Electroconductive Feed Spacer as a Tool for Biofouling Control in a Membrane System for Water Treatment. Environ. Sci. Technol. Lett. 2014, 1, 179–184. [Google Scholar] [CrossRef]
- Sun, D.; Cheng, S.; Zhang, F.; Logan, B.E. Current Density Reversibly Alters Metabolic Spatial Structure of Exoelectrogenic Anode Biofilms. J. Power Sources 2017, 356, 566–571. [Google Scholar] [CrossRef]
- Lin, H.; Wu, X.; Miller, C.; Zhu, J. Improved Performance of Microbial Fuel Cells Enriched with Natural Microbial Inocula and Treated by Electrical Current. Biomass Bioenergy 2013, 54, 170–180. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.Y.; Yoon, J. The Role of Reactive Oxygen Species in the Electrochemical Inactivation of Microorganisms. Environ. Sci. Technol. 2006, 40, 6117–6122. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Chin, K.J.; Esteve-Núñez, A.; Leang, C.; Lovley, D.R. Direct Correlation between Rates of Anaerobic Respiration and Levels of mRNA for Key Respiratory Genes in Geobacter sulfurreducens. Appl. Environ. Microbiol. 2004, 70, 5183–5189. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, J.; Yuan, Y.; Yang, G.; Xing, B. TiO2 Nanoparticle-Induced Nanowire Formation Facilitates Extracellular Electron Transfer. Environ. Sci. Technol. Lett. 2018, 5, 564–570. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; Converti, A.; Sato, S.; Carvalho, J.C.M. Fed-Batch Cultivation of Arthrospira (Spirulina) platensis: Potassium Nitrate and Ammonium Chloride as Simultaneous Nitrogen Sources. Bioresour. Technol. 2010, 101, 4491–4498. [Google Scholar] [CrossRef]
- Alavianghavanini, A.; Shayesteh, H.; Bahri, P.A.; Vadiveloo, A.; Moheimani, N.R. Microalgae Cultivation for Treating Agricultural Effluent and Producing Value-Added Products. Sci. Total Environ. 2024, 912, 169369. [Google Scholar] [CrossRef]
- Li, S.F.; Wang, C.C.; Taidi, B. Effective CO2 Capture by the Fed-Batch Culture of Chlorella vulgaris. J. Environ. Chem. Eng. 2023, 11, 110889. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Grigorenko, A.V.; Astafieva, A.P.; Vlaskin, M.S. Growth of Chlorella vulgaris under Atmospheric CO2 in Lab-Scale Photobioreactors: Effects of Aeration Rate and Growth Model Comparison. Bioengineering 2025, 12, 1294. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. The Effect of Autotrophic Cultivation of Platymonas subcordiformis in Waters from the Natural Aquatic Reservoir on Hydrogen Yield. Resources 2022, 11, 31. [Google Scholar] [CrossRef]
- Yao, C.H.; Ai, J.N.; Cao, X.P.; Xue, S. Characterization of Cell Growth and Starch Production in the Marine Green Microalga Tetraselmis subcordiformis under Extracellular Phosphorus-Deprived and Sequentially Phosphorus-Replete Conditions. Appl. Microbiol. Biotechnol. 2013, 97, 6099–6110. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.A.; Khan, S.; Jur, J.S.; Haug, A.T.; Weidner, J.W. Measuring Oxygen, Carbon Monoxide and Hydrogen Sulfide Diffusion Coefficients and Solubility in Nafion Membranes. Electrochim. Acta 2011, 54, 6850–6860. [Google Scholar] [CrossRef]
- Huang, D.; Song, B.Y.; Li, M.J.; Li, X.Y. Oxygen Diffusion in Cation-Form Nafion Membrane of Microbial Fuel Cells. Electrochim. Acta 2018, 276, 268–283. [Google Scholar] [CrossRef]
- Ramirez-Nava, J.; Martínez-Castrejón, M.; García-Mesino, R.L.; López-Díaz, J.A.; Talavera-Mendoza, O.; Sarmiento-Villagrana, A.; Rojano, F.; Hernández-Flores, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738. [Google Scholar] [CrossRef]
- Baik, K.D.; Hong, B.K.; Kim, M.S. Novel Technique for Measuring Oxygen Crossover through the Membrane in Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 8927–8933. [Google Scholar] [CrossRef]
- Yadav, A.K.; Nayak, S.K.; Acharya, B.C.; Mishra, B.K. Algal-Assisted Microbial Fuel Cell for Wastewater Treatment and Bioelectricity Generation. Energy Sources A Recovery Util. Environ. Eff. 2014, 37, 127–133. [Google Scholar]
- Bazdar, E.; Roshandel, R.; Yaghmaei, S.; Mardanpour, M.M. The Effect of Different Light Intensities and Light/Dark Regimes on the Performance of Photosynthetic Microalgae Microbial Fuel Cell. Bioresour. Technol. 2018, 261, 350–360. [Google Scholar] [CrossRef]
- Madhav, D.; Wang, J.; Keloth, R.; Mus, J.; Buysschaert, F.; Vandeginste, V. A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell. Energies 2024, 17, 998. [Google Scholar] [CrossRef]
- Foniok, K.; Drozdova, L.; Prokop, L.; Krupa, F.; Kedron, P.; Blazek, V. Mechanisms and Modelling of Effects on the Degradation Processes of a Proton Exchange Membrane (PEM) Fuel Cell: A Comprehensive Review. Energies 2025, 18, 2117. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors Affecting the Efficiency of a Bioelectrochemical System: A Review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhang, Z.; Wang, Y.; Liu, F.; Wang, H.; Bi, C.; Yu, C.; Zhou, Y. Effect of Cations (Na+, Co2+, Fe3+) Contamination in Nafion Membrane: A Molecular Simulations Study. Int. J. Hydrogen Energy 2024, 50, 635–649. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Microbial Fuel Cells—Challenges and Applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef]
- Nath, D.; Chakraborty, I.; Ghangrekar, M.M. Methanogenesis Inhibitors Used in Bio-Electrochemical Systems: A Review Revealing Reality to Decide Future Direction and Applications. Bioresour. Technol. 2021, 319, 124141. [Google Scholar] [CrossRef]
- Lin, W.C.; Coppi, M.V.; Lovley, D.R. Geobacter sulfurreducens Can Grow with Oxygen as a Terminal Electron Acceptor. Appl. Environ. Microbiol. 2004, 70, 2525–2528. [Google Scholar] [CrossRef]
- Speers, A.M.; Reguera, G. Competitive Advantage of Oxygen-Tolerant Bioanodes of Geobacter sulfurreducens in Bioelectrochemical Systems. Biofilm 2021, 3, 100052. [Google Scholar] [CrossRef]
- Yuan, Y.; Conrad, R.; Lu, Y. Responses of Methanogenic Archaeal Community to Oxygen Exposure in Rice Field Soil. Environ. Microbiol. Rep. 2009, 1, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Regan, J.M. Comparison of Anode Bacterial Communities and Performance in Microbial Fuel Cells with Different Electron Donors. Appl. Microbiol. Biotechnol. 2007, 77, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, F.; Liu, Z.; Liu, Y.; Liu, H. Enhancing the Electricity Generation and Nitrate Removal of Microbial Fuel Cells with a Novel Denitrifying Exoelectrogenic Strain EB-1. Front. Microbiol. 2018, 9, 2633. [Google Scholar] [CrossRef]
- Widera, B.; Tyszkiewicz, N.; Truu, J.; Rutkowski, P.; Młynarz, P.; Pasternak, G. Relationship between Biodiversity and Power Generated by Anodic Bacteria Enriched from Petroleum-Contaminated Soil at Various Potentials. Int. Biodeterior. Biodegrad. 2024, 194, 105849. [Google Scholar] [CrossRef]
- Mikkola, M.S.; Rockward, T.; Uribe, F.A.; Pivovar, B.S. The Effect of NaCl in the Cathode Air Stream on PEMFC Performance. Fuel Cells 2007, 7, 153–158. [Google Scholar] [CrossRef]
- Madhav, D.; Shao, C.; Mus, J.; Buysschaert, F.; Vandeginste, V. The Effect of Salty Environments on the Degradation Behavior and Mechanical Properties of Nafion Membranes. Energies 2023, 16, 2256. [Google Scholar] [CrossRef]
- Kaur, A.; Boghani, H.C.; Michie, I.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Inhibition of Methane Production in Microbial Fuel Cells: Operating Strategies Which Select Electrogens over Methanogens. Bioresour. Technol. 2014, 173, 75–81. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Arkatkar, A. Anodic Catalyst and Mediators for Tailoring the Biochemical Behaviour of Microbial Fuel Cell System. Discov. Electrochem. 2025, 2, 41. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass Transport through a Proton Exchange Membrane (Nafion) in Microbial Fuel Cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Logan, B. Exoelectrogenic Bacteria That Power Microbial Fuel Cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.G.; Rother, D.; Bardischewsky, F.; Quentmeier, A.; Fischer, J. Oxidation of Reduced Inorganic Sulfur Compounds by Bacteria: Emergence of a Common Mechanism? Appl. Environ. Microbiol. 2001, 67, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; ter Heijne, A.; Rijnaarts, H.; Chen, W.S. The Effect of Anode Potential on Electrogenesis, Methanogenesis and Sulfidogenesis in a Simulated Sewer Condition. Water Res. 2022, 226, 119229. [Google Scholar] [CrossRef]
- Walker, D.; Adhikari, R.; Holmes, D.; Ward, J.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Electrically Conductive Pili from Pilin Genes of Phylogenetically Diverse Microorganisms. ISME J. 2018, 12, 48–58. [Google Scholar] [CrossRef]
- Kim, B.; Leang, C.; Ding, Y.R.; Glaven, R.H.; Coppi, M.V.; Lovley, D.R. OmcF, a Putative c-Type Monoheme Outer Membrane Cytochrome Required for the Expression of Other Outer Membrane Cytochromes in Geobacter sulfurreducens. J. Bacteriol. 2005, 187, 4505–4513. [Google Scholar] [CrossRef]


| Component | Amount |
|---|---|
| NH4Cl (g·L−1) | 1.50 |
| Na2HPO4 (g·L−1) | 0.60 |
| KCl (g·L−1) | 0.10 |
| Na-acetate (g·L−1) | 15 |
| NaHCO3 (g·L−1) | 2.50 |
| Modified Wolin’s mineral solution (mL·L−1) | 10.00 |
| Wolin’s vitamin solution (10×) (mL·L−1) | 1.00 |
| Control | 0.5 V/4 h | 1 V/4 h | 5 V/4 h | |
|---|---|---|---|---|
| Start-up time (min) | 580 ± 25 | 410 ± 15 | 340 ± 15 | 910 ± 20 |
| Average voltage (V) | 0.185 ± 0.013 | 0.229 ± 0.029 | 0.253 ± 0.014 | 0.150 ± 0.021 |
| Current density (mA·cm−2) | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.05 ± 0.01 |
| Power density (mW·m−2) | 122 ± 17 | 187 ± 47 | 229 ± 25 | 80 ± 23 |
| CE (%) | 8.5 ± 0.7 | 10.0 ± 1.3 | 10.6 ± 0.7 | 7.6 ± 1.2 |
| Week | Variant | K (1·d−1) | V (mg·L−1·d−1) |
|---|---|---|---|
| Week 1 | Control | 0.5658 | 599.40 |
| 0.5 V | 0.6278 | 691.31 | |
| 1 V | 0.6969 | 790.09 | |
| 5 V | 0.2674 | 284.99 | |
| Week 2 | Control | 0.4337 | 661.28 |
| 0.5 V | 0.4046 | 628.99 | |
| 1 V | 0.4125 | 652.31 | |
| 5 V | 0.2847 | 413.05 | |
| Week 3 | Control | 0.4538 | 692.53 |
| 0.5 V | 0.4132 | 642.42 | |
| 1 V | 0.4299 | 680.61 | |
| 5 V | 0.3258 | 455.21 | |
| Week 4 | Control | 0.4478 | 681.13 |
| 0.5 V | 0.4117 | 641.31 | |
| 1 V | 0.4245 | 679.19 | |
| 5 V | 0.2914 | 428.53 |
| pMFC1 | pMFC2 | pMFC3 | MFC | |
|---|---|---|---|---|
| Average voltage (V) | 0.393 ± 0.064 | 0.276 ± 0.049 | 0 | 0.248 ± 0.053 |
| Current density (mA·cm−2) | 0.14 ± 0.02 | 0.10 ± 0.02 | 0 | 0.09 ± 0.02 |
| Power density (mW·m−2) | 552 ± 180 | 272 ± 97 | 0 | 220 ± 94 |
| CE (%) | 19.2 ± 3.0 | 13.4 ± 2.1 | 0 | 11.1 ± 3.0 |
| Sobs | Shannon (H’) | Simpson (1–D) | Chao1 | Evenness (J) | |
|---|---|---|---|---|---|
| pMFC1 (Chlorella sp.) | 112 | 2.3 | 0.782 | 112 | 0.486 |
| pMFC2 (Arthrospira platensis) | 100 | 2.09 | 0.778 | 100 | 0.454 |
| pMFC3 (Tetraselmis subcordiformis) | 125 | 1.87 | 0.602 | 125 | 0.387 |
| MFC (control) | 129 | 2.52 | 0.828 | 129 | 0.519 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Rusanowska, P.; Barczak, Ł.; Starowicz, A.; Głowacka, K.; Dębowski, M.; Zieliński, M. Influence of Photosynthetic Cathodes on Anodic Microbial Communities in Acetate-Fed Microbial Fuel Cells Pre-Enriched Under Applied Voltage. Energies 2026, 19, 41. https://doi.org/10.3390/en19010041
Rusanowska P, Barczak Ł, Starowicz A, Głowacka K, Dębowski M, Zieliński M. Influence of Photosynthetic Cathodes on Anodic Microbial Communities in Acetate-Fed Microbial Fuel Cells Pre-Enriched Under Applied Voltage. Energies. 2026; 19(1):41. https://doi.org/10.3390/en19010041
Chicago/Turabian StyleRusanowska, Paulina, Łukasz Barczak, Adam Starowicz, Katarzyna Głowacka, Marcin Dębowski, and Marcin Zieliński. 2026. "Influence of Photosynthetic Cathodes on Anodic Microbial Communities in Acetate-Fed Microbial Fuel Cells Pre-Enriched Under Applied Voltage" Energies 19, no. 1: 41. https://doi.org/10.3390/en19010041
APA StyleRusanowska, P., Barczak, Ł., Starowicz, A., Głowacka, K., Dębowski, M., & Zieliński, M. (2026). Influence of Photosynthetic Cathodes on Anodic Microbial Communities in Acetate-Fed Microbial Fuel Cells Pre-Enriched Under Applied Voltage. Energies, 19(1), 41. https://doi.org/10.3390/en19010041









