Advancing Electrochemical Energy Storage: A Review of Electrospinning Factors and Their Impact
Abstract
1. Introduction
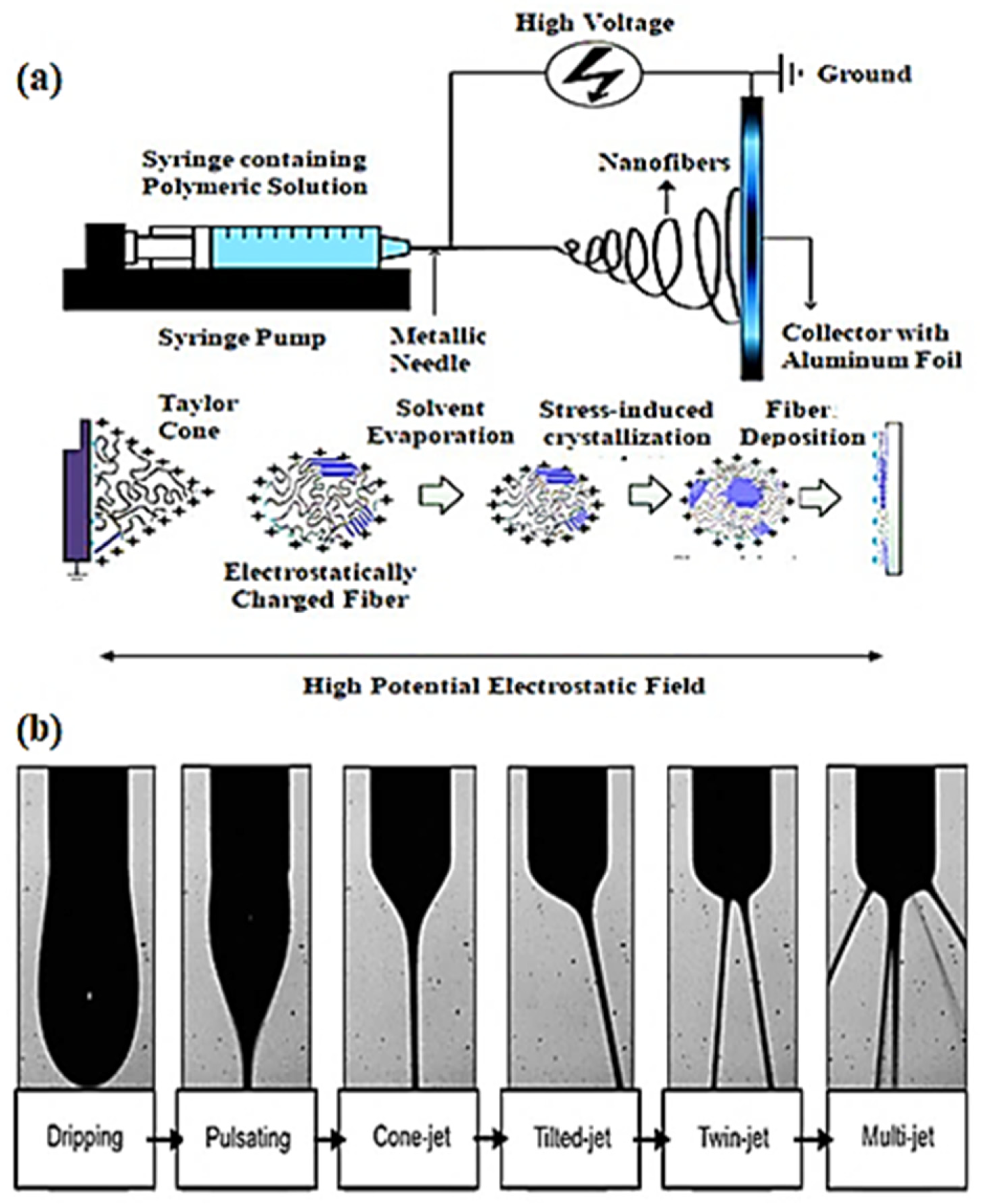
2. Fundamental Features of Electrospinning
- i.
- A syringe pump (including a syringe and syringe needle);
- ii.
- Metal collector (stationary or rotary);
- iii.
- High-voltage supply unit.
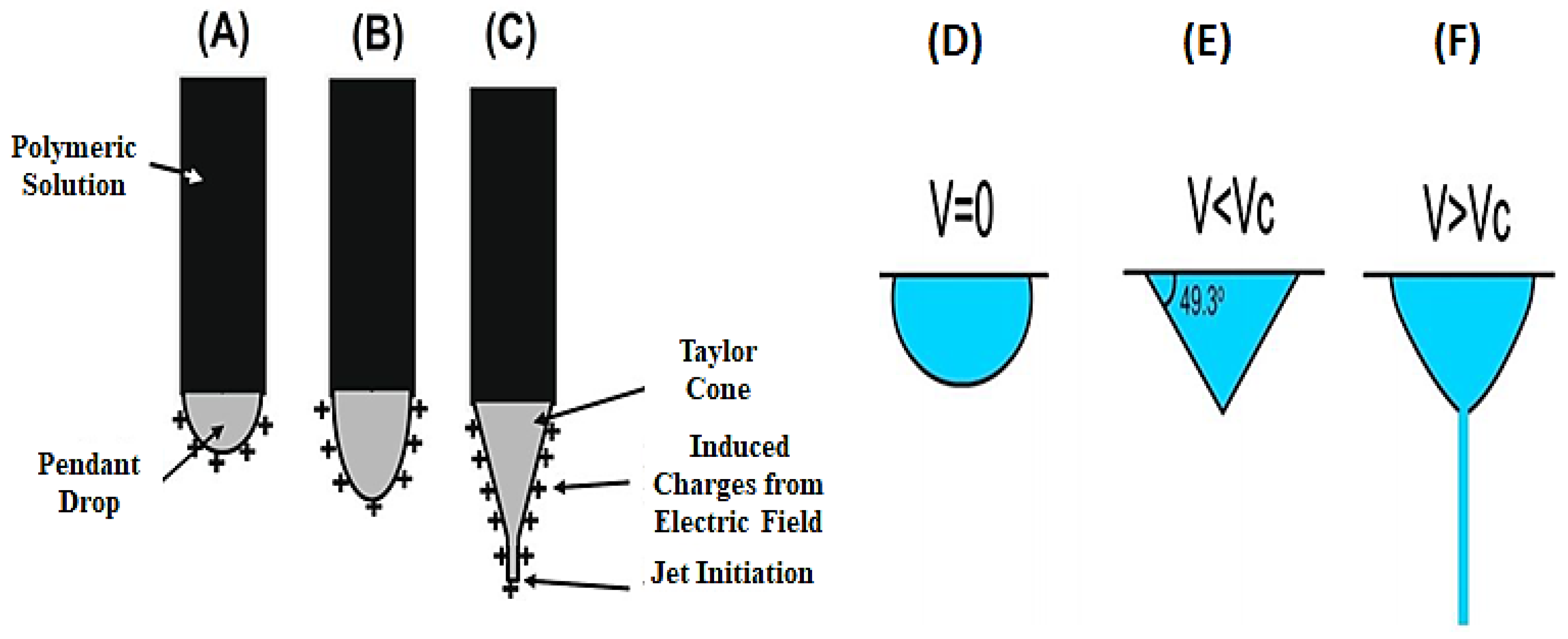
- i.
- Charging of liquid droplets and creation of Taylor’s cone;
- ii.
- Elongation of the charged jet;
- iii.
- Thinning of the jet in a high-potential electrostatic field;
- iv.
3. Approaches for the Fabrication of Electrospun Membranes
3.1. Single-Spinneret Electrospinning
3.2. Multi-Spinneret Electrospinning
3.3. Coaxial Electrospinning

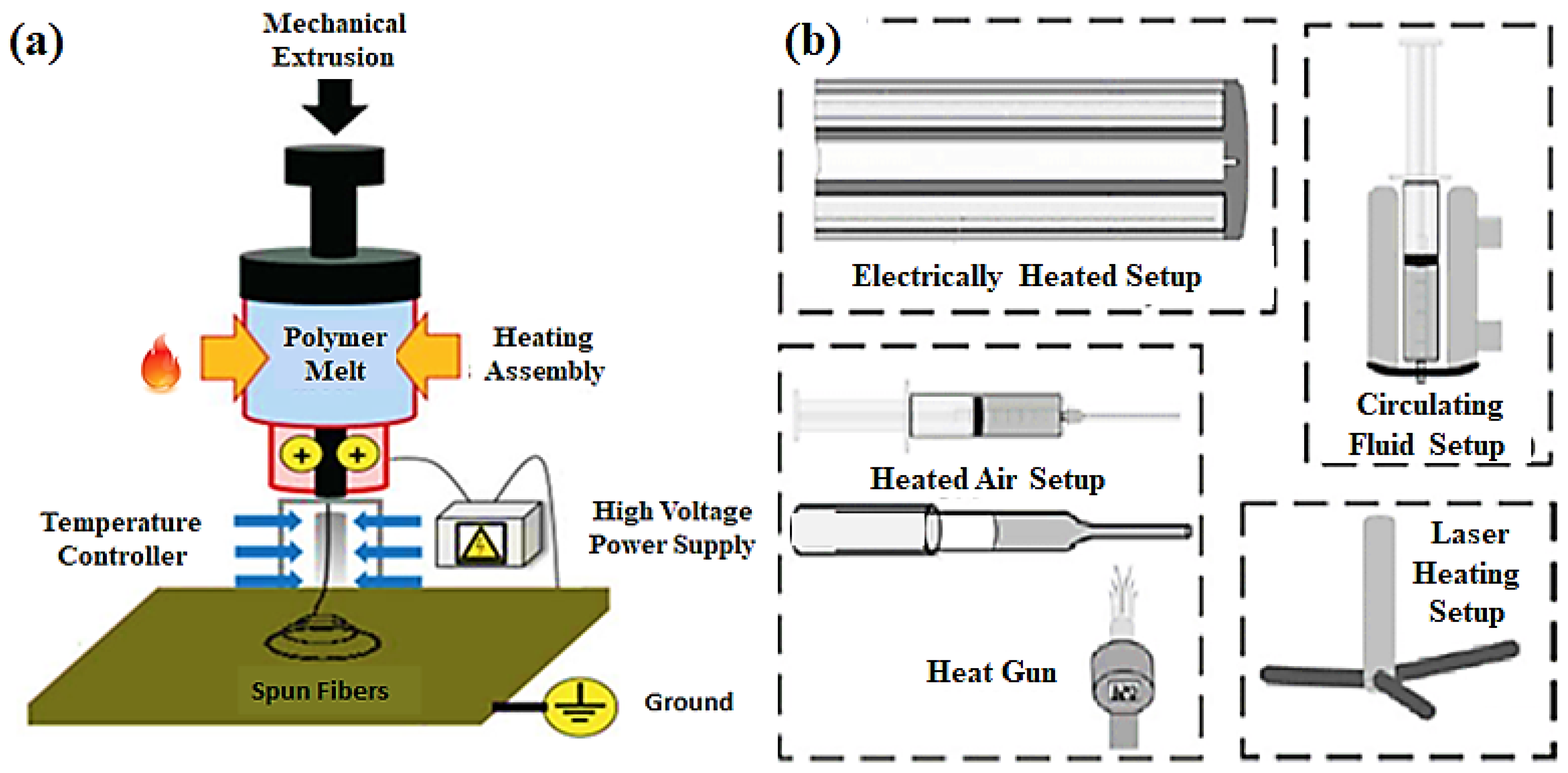
3.4. Melt Electrospinning
- i.
- Accessibility of various engineering thermoplastics, i.e., PP, PE, PC, etc. These are not soluble in normal solvents at room temperature, so they are difficult to execute using solution electrospinning.
- ii.
- Health protection is provided to the operators due to the lack of solvent involvement.
- iii.
- Ecofriendly, as there is no smoke or toxicity hazards released into the environment.
- iv.
- The surface of fibers compares to fibers produced by solution electrospinning, containing tiny pores on the exterior.
- v.
- Ease in the fabrication of microporous-based fibrous films that can be employed in various applications.
4. Factors Affecting the Fabrication of Electrospun Membranes
4.1. Operational Considerations
4.1.1. Applied Voltage/Electric Field
4.1.2. Solution/Melt Feed Rate (Flow Rate)
4.1.3. Tip-to-Collector Distance
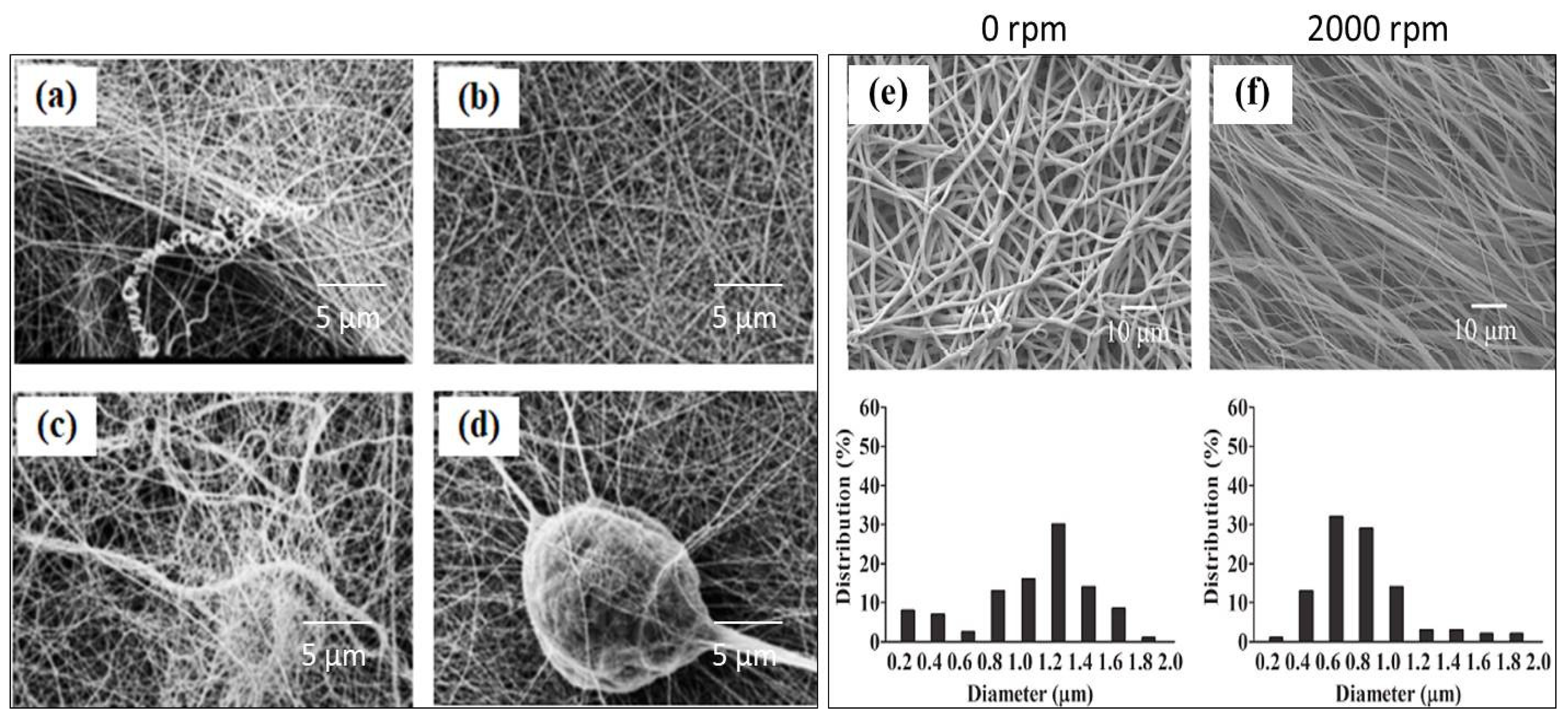
4.1.4. Collector Design and Drum Speed
4.2. Material Considerations
4.2.1. Solution Concentration
4.2.2. Polymer Molecular Weight
4.2.3. Solution Viscosity
4.2.4. Solution Conductivity
4.2.5. Surface Tension and Solvent Function
4.3. Environmental Considerations
4.3.1. Relative Humidity
4.3.2. Temperature
5. Electrospun Nanofibers in Energy Storage Applications
5.1. Application of Electrospun Nanofibers in Lithium-Based Batteries
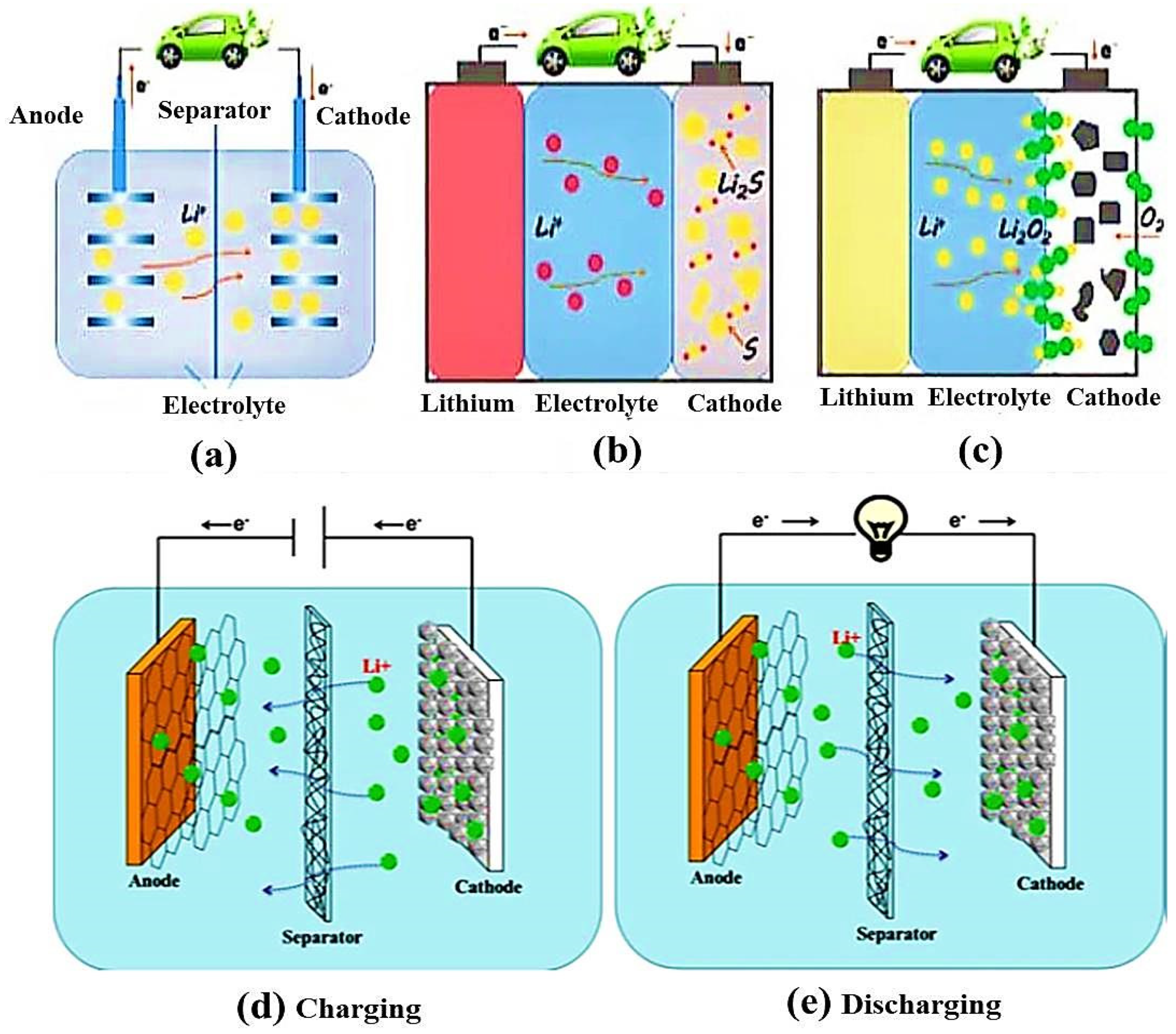
5.1.1. Electrospun Nanofibers as Separator Materials
5.1.2. Electrospun Nanofibers as Cathode Electrode Materials
- i.
- Great redox potential and enough storage of Li-ions to intercalate;
- ii.
- Rapid addition and taking of Li-ions;
- iii.
- Cost-effective, ecological, and easy to synthesize.
5.1.3. Electrospun Nanofibers as Anode Electrode Materials
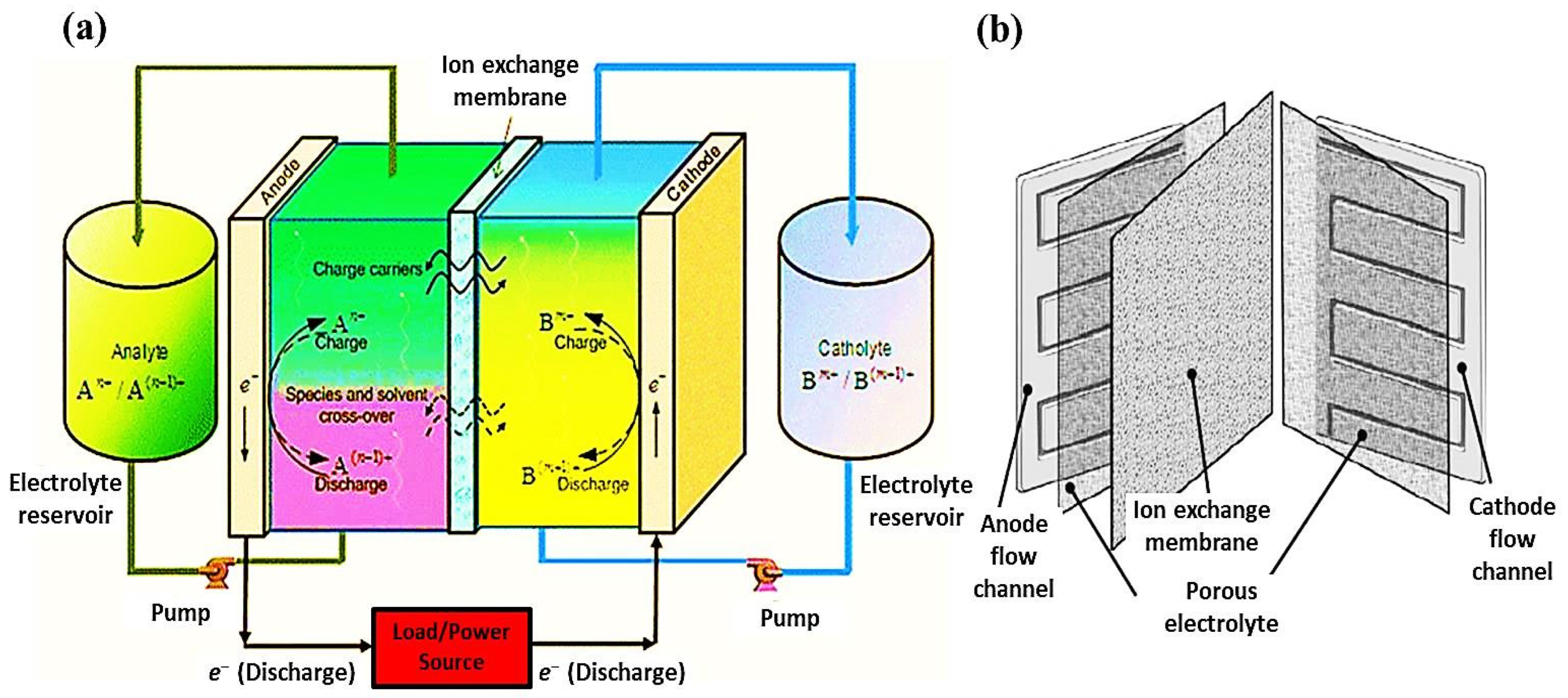
6. Electrospun Nanofibers in Redox Flow Batteries
7. Electrospun Nanofibers in Supercapacitors
8. Other Electrospun Nanofiber-Based Energy Storage Devices
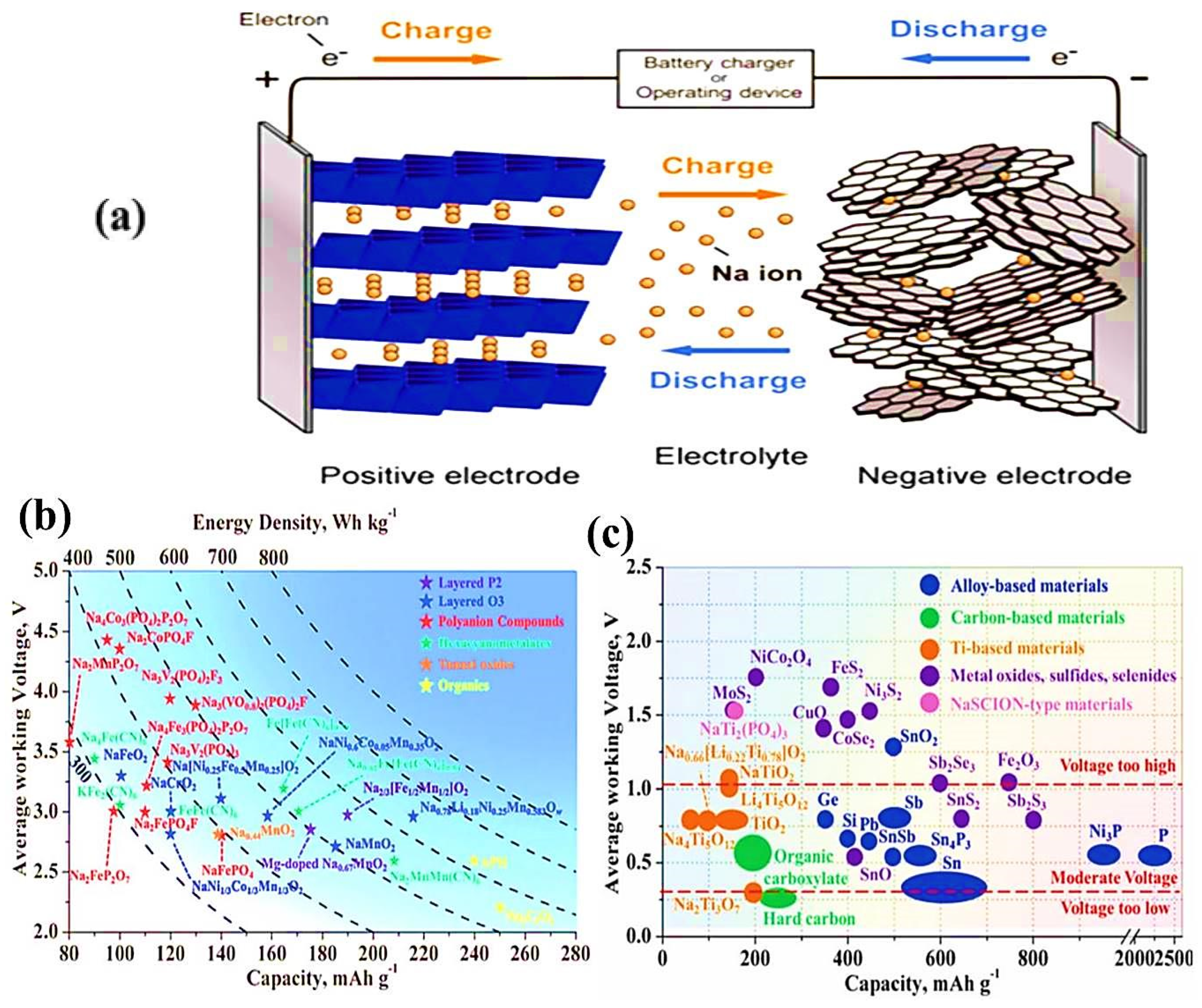
8.1. Sodium-Ion Batteries
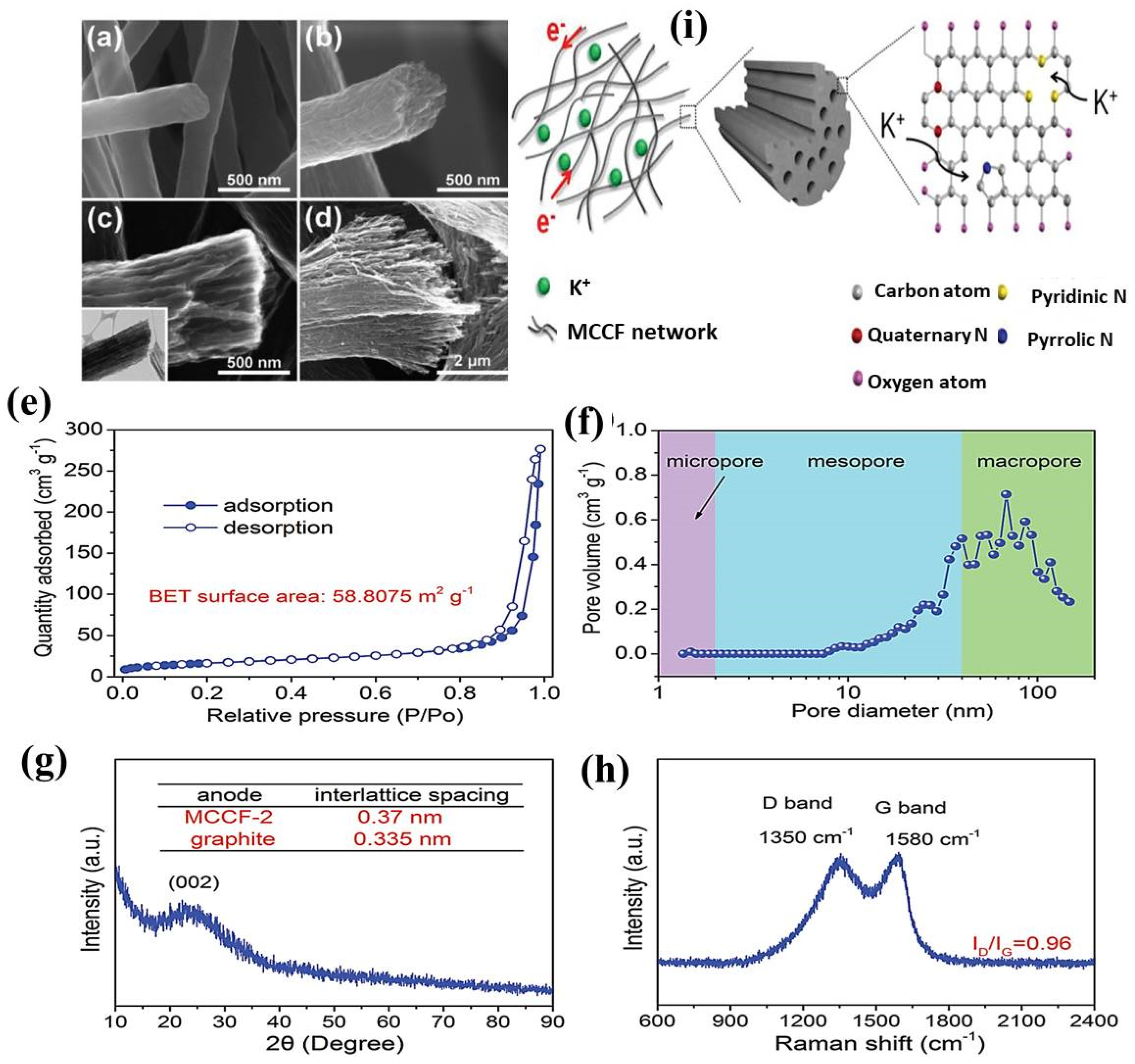
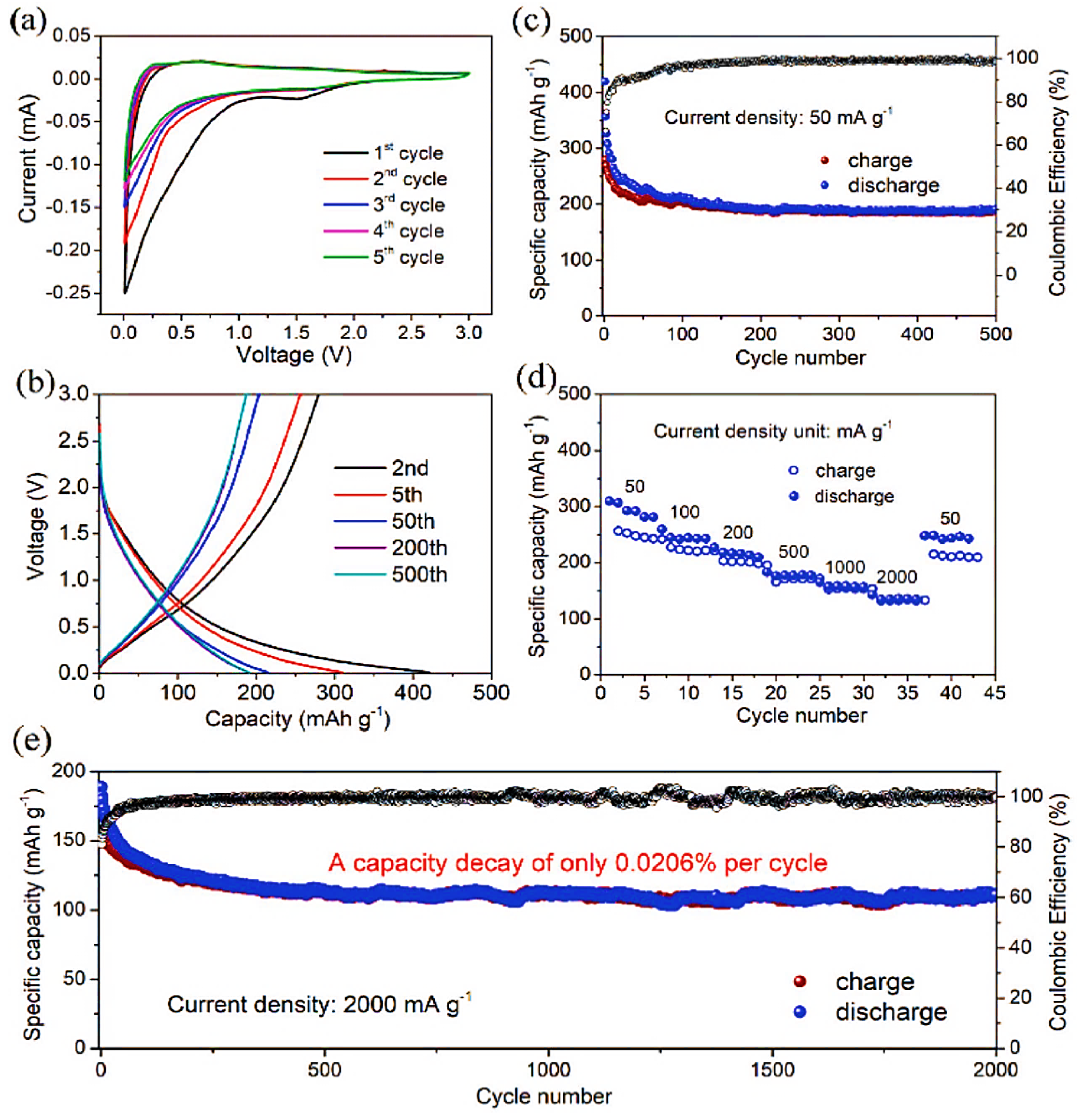
8.2. Potassium-Ion Batteries
8.3. Lithium–Sulfur Batteries (Li-S)
8.4. Lithium–Oxygen Batteries (Li-O2)/Li–Air Batteries (LABs):
9. Challenges and Perspectives
- i.
- Uniformity of fiber diameter: As the scale increases, maintaining uniformity in the fiber diameter becomes difficult, which eventually affects the structure. Uneven fiber properties can negatively impact the performance of an energy storage device, such as lower ionic conductivity or reduced specific capacity.
- ii.
- Material usage: Large-scale electrospinning requires a large amount of solvents and materials, which can be costly and environmentally unfriendly, especially if organic solvents are used. The large volumes needed for scaling up may also lead to difficulties in solvent recovery and reuse, which may raise both environmental concerns.
- iii.
- Process control: The electrospinning setup at the laboratory scale makes it much easier to operate parameters, i.e., voltage, flow rate, and needle-to-tip collector distance. Therefore, we can obtain the desired fiber properties. However, its use at a larger scale might differ in achieving the desired results because slight variations in these parameters can lead to significant differences in fiber characteristics.
- iv.
- Scaling equipment: Most electrospinning systems are designed for small-scale production. Adapting them to large-scale manufacturing requires specialized equipment. Maintaining a controlled environment, such as constant humidity and temperature, becomes more complex as the scale increases, which can affect the formation and quality of the fibers.
- v.
- Energy efficiency: Electrospinning processes often require high voltage, which can be energy-intensive when scaled up. The energy efficiency of the process is a fundamental part of industrial applications.
- vi.
- Cost-effectiveness: Electrospun nanofibers can offer significant advantages for energy storage devices. The materials and energy required at an industrial scale can be costly. This increases the total cost of production for energy storage devices, which makes them less competitive compared to traditional manufacturing methods.
- vii.
- Post-processing steps: After the electrospinning process, nanofibers often require post-processing steps, like annealing, crosslinking, or coating, to optimize their properties for energy storage applications. At the industrial scale, these steps can require more resources to accomplish.
10. Conclusions
Funding
Conflicts of Interest
References
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022, 301, 113908. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S. Strategic analysis of the renewable electricity transition: Power to the world without carbon emissions? Energies 2023, 16, 6183. [Google Scholar] [CrossRef]
- Fletcher, C.; Ripple, W.J.; Newsome, T.; Barnard, P.; Beamer, K.; Behl, A.; Bowen, J.; Cooney, M.; Crist, E.; Field, C. Earth at risk: An urgent call to end the age of destruction and forge a just and sustainable future. PNAS Nexus 2024, 3, pgae106. [Google Scholar] [CrossRef] [PubMed]
- Nwokediegwu, Z.Q.S.; Ibekwe, K.I.; Ilojianya, V.I.; Etukudoh, E.A.; Ayorinde, O.B. Renewable energy technologies in engineering: A review of current developments and future prospects. Eng. Sci. Technol. J. 2024, 5, 367–384. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Liu, J.; Wang, G. Science mapping the knowledge domain of electrochemical energy storage technology: A bibliometric review. J. Energy Storage 2024, 77, 109819. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Afroze, J.D.; Tong, L.; Abden, M.J.; Chen, Y. Multifunctional hierarchical graphene-carbon fiber hybrid aerogels for strain sensing and energy storage. Adv. Compos. Hybrid Mater. 2023, 6, 18. [Google Scholar] [CrossRef]
- Zhu, Q.; Ong, P.J.; Goh, S.H.A.; Yeo, R.J.; Wang, S.; Liu, Z.; Loh, X.J. Recent advances in graphene-based phase change composites for thermal energy storage and management. Nano Mater. Sci. 2024, 6, 115–138. [Google Scholar] [CrossRef]
- Sundar, S.; Nitin, S. Emerging Trends of Graphene-Based Nanomaterials as Digital Materials for Data Generation in Industry 4.0. In Advancements in Intelligent Process Automation; IGI Global Scientific Publishing: New York, NY, USA, 2025; pp. 325–358. [Google Scholar] [CrossRef]
- Monteiro, B.; Simões, S. Optimization of Al6061 Nanocomposites Production Reinforced with Multiwalled Carbon Nanotubes. J. Compos. Sci. 2024, 8, 381. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, S.; Ren, M.; Zhai, H.-J. Dispersed carbon nanotube paper tape loaded poly (3, 4-ethylenedioxythiophene) for flexible supercapacitors with significantly enhanced energy storage performance and deformation durability. J. Power Sources 2025, 625, 235684. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Jia, T.; Tao, B.; Hong, M.; Song, P.; Xu, M. Vertically aligned cellulose nanofiber/carbon nanotube aerogel-infused epoxy nanocomposites for highly efficient solar-thermal-electric conversion. J. Mater. Sci. Technol. 2025, 214, 313–321. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Yan, J.; Guan, C.; Wang, J. Electrospun nanofibers for new generation flexible energy storage. Energy Environ. Mater. 2021, 4, 502–521. [Google Scholar] [CrossRef]
- Anwar, M.T.; Naz, R.; Ahmed, A.; Ahmed, S.; Ashraf, G.A.; Rasheed, T. Energy applications of nanofibers and their composites. In Polymeric Nanofibers and Their Composites; Elsevier: Amsterdam, The Netherlands, 2025; pp. 255–272. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Wang, W.; Yang, H.; Wan, Y.; Zhang, Y.; Guan, L.; Yao, Y.; Teng, X.; Meng, C. Structural design of electrospun nanofibers for electrochemical energy storage and conversion. J. Alloys Compd. 2023, 935, 167920. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun polymer nanofibers: Processing, properties, and applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef]
- Thakur, D.; Tran, N.H.T.; Nguyen, P.K.T.; Devi, N.B.; Kosame, S.; Ta, Q.T.H.; Chhantyal, A.K.; Truong, T.T. Advanced nanostructures for energy applications. In Advances in Nanostructures; Elsevier: Amsterdam, The Netherlands, 2025; pp. 325–375. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Khomarloo, N.; Mohsenzadeh, E.; Gospodinova, D.N.; Neznakomova, M.; Salaün, F. PVA-Based Electrospun Materials—A Promising Route to Designing Nanofiber Mats with Desired Morphological Shape—A Review. Int. J. Mol. Sci. 2024, 25, 1668. [Google Scholar] [CrossRef] [PubMed]
- Chamanehpour, E.; Thouti, S.; Rubahn, H.G.; Dolatshahi-Pirouz, A.; Mishra, Y.K. Smart Nanofibers: Synthesis, Properties, and Scopes in Future Advanced Technologies. Adv. Mater. Technol. 2024, 9, 2301392. [Google Scholar] [CrossRef]
- Subeshan, B.; Atayo, A.; Asmatulu, E. Machine learning applications for electrospun nanofibers: A review. J. Mater. Sci. 2024, 59, 14095–14140. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Shi, Q.; Ning, X.; Zheng, J.; Long, Y.-Z. Research Progress and Prospect of Centrifugal Electrospinning and Its Application. J. Alloys Compd. 2024, 990, 174433. [Google Scholar] [CrossRef]
- Marjuban, S.M.H.; Rahman, M.; Duza, S.S.; Ahmed, M.B.; Patel, D.K.; Rahman, M.S.; Lozano, K. Recent advances in centrifugal spinning and their applications in tissue engineering. Polymers 2023, 15, 1253. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, M.H.; Pawar, K.K.; Bharath, S.P.; Kim, T.-U.; Kim, J.-Y.; Kim, S.S.; Kim, H.W. Metal oxide nanowires grown by a vapor–liquid–solid growth mechanism for resistive gas-sensing applications: An overview. Materials 2023, 16, 6233. [Google Scholar] [CrossRef]
- Mohammad, S.N.; Mohammad, S.N. Vapor–Solid Growth Mechanism. In Vapor–Solid Growth Mechanism; Synthesis of Nanomaterials: Mechanisms, Kinetics and Materials Properties; Springer: Berlin/Heidelberg, Germany, 2020; pp. 121–138. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Sun, Q.; Ye, X.; Ma, X.; Wang, J. Sol–gel routes toward ceramic nanofibers for high-performance thermal management. Chemistry 2022, 4, 1475–1497. [Google Scholar] [CrossRef]
- Larsen, G.; Gonzalez, D.; Noriega, S.; Ragusa, J. Twenty years of making inorganic ultrafine fibers via electrospinning and sol–gel techniques I: An update on catalysis, photocatalysis, and adsorption R&D opportunities. Chem. Eng. Commun. 2024, 211, 1469–1493. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Aslam, S.; Noor, A.E.; Jini, D.; Majeed, S.; Velusamy, P.; Alothman, A.A.; Alshgari, R.A.; Mushab, M.S.S. Hydrothermally synthesized Ag-TiO2 nanofibers (NFs) for photocatalytic dye degradation and antibacterial activity. Chemosphere 2023, 321, 138077. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Sun, X.; Li, J.; Huang, L.; Sun, H.; Gao, X. Enhanced superhydrophobicity of electrospun carbon nanofiber membranes by hydrothermal growth of ZnO nanorods for oil–water separation. Arab. J. Chem. 2023, 16, 104523. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Jin, X. Maneuvering the secondary surface morphology of electrospun poly (vinylidene fluoride) nanofibers by controlling the processing parameters. Mater. Res. Express 2019, 7, 015008. [Google Scholar] [CrossRef]
- Kashif, M.; Li, H.; Liu, J.; Rasul, S.; Ullah, Q.; Liu, Y. Competitive Behavior of Isotactic Polybutene-1 Polymorphs in Electrospun Membranes and Solution Cast Films via Cold Crystallization. J. Macromol. Sci. Part B 2022, 61, 897–913. [Google Scholar] [CrossRef]
- Javanifar, R.; Dabagh, S.; Kaya, M.; Sengel, S.B.; Ebrahimi, A.; Ghorbanpoor, H.; Avci, H. A novel green Microfiltration approach by developing a Lab-on-a-Chip System: A case study for Escherichia coli. Sep. Purif. Technol. 2025, 353, 128411. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, structures, and materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Wang, C.; Su, Y.; Xie, J. Advances in electrospun nanofibers: Versatile materials and diverse biomedical applications. Acc. Mater. Res. 2024, 5, 987–999. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of advanced electrospinning techniques: A critical review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Chang, P.-K.; Huang, S.-H.; Chen, J.-W.; Chun-Te Lin, J.; Hsiao, T.-C. Submicron PAN and nanofiber CTA air filters: Fabrication, optimization, and performance. Sep. Purif. Technol. 2025, 352, 128111. [Google Scholar] [CrossRef]
- Nirwan, V.P.; Amarjargal, A.; Hengsbach, R.; Fahmi, A. Electrospun Smart Hybrid Nanofibers for Multifaceted Applications. Macromol. Rapid Commun. 2024, 2400617. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, C.; Li, G.; Cheng, H.; Li, Y.; Liu, T.; Mao, Q.; Cho, H.; Gao, Q.; Gao, C. Recent developments of electrospun nanofibers for electrochemical energy storage and conversion. Energy Storage Mater. 2024, 65, 103111. [Google Scholar] [CrossRef]
- Ren, W.; Qin, M.; Zhou, Y.; Zhou, H.; Zhu, J.; Pan, J.; Zhou, J.; Cao, X.; Liang, S. Electrospun Na4Fe3 (PO4)2(P2O7) nanofibers as free-standing cathodes for ultralong-life and high-rate sodium-ion batteries. Energy Storage Mater. 2023, 54, 776–783. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, M.; Wen, Y.; Li, J.; Zhang, X.; Hui, J.; Zhu, Q.; Zhang, H. Tuning the Local Coordination of CoP1–x S x between NiAs-and MnP-Type Structures to Catalyze Lithium–Sulfur Batteries. ACS Nano 2023, 17, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Banjare, M.K.; Behera, K.; Banjare, R.K.; Tandon, M.; Pandey, S.; Ghosh, K.K. Electronics application of nanofibers and their composites. In Polymeric Nanofibers and Their Composites; Elsevier: Amsterdam, The Netherlands, 2025; pp. 497–519. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, Y.; Zhang, K.; Tang, X.; Huang, J.; Yuan, K.; Chen, Y. Coaxial electrospun free-standing and mechanically stable hierarchical porous carbon nanofiber membranes for flexible supercapacitors. Carbon 2020, 160, 80–87. [Google Scholar] [CrossRef]
- Yan, C.; Wei, J.; Guan, J.; Shao, Z.; Lv, S. Highly foldable and free-standing supercapacitor based on hierarchical and hollow MOF-anchored cellulose acetate carbon nanofibers. Carbon 2023, 213, 118187. [Google Scholar] [CrossRef]
- Ishita, I.; Radhakanth, S.; Sow, P.K.; Singhal, R. Nanofibers and their composites for supercapacitor applications. In Polymeric Nanofibers and Their Composites; Elsevier: Amsterdam, The Netherlands, 2025; pp. 539–568. [Google Scholar] [CrossRef]
- Iskandarani, B.; Rajabalizadeh Mojarrad, N.; Yurum, A.; Alkan Gürsel, S.; Yarar Kaplan, B. Electrospun nanofiber electrodes for boosted performance and durability at lower humidity operation of PEM fuel cells. Energy Fuels 2022, 36, 9282–9294. [Google Scholar] [CrossRef]
- Eskitoros-Togay, Ş.M.; Bulbul, Y.E.; Cınar, Z.K.; Sahin, A.; Dilsiz, N. Fabrication of PVP/sulfonated PES electrospun membranes decorated by sulfonated halloysite nanotubes via electrospinning method and enhanced performance of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2023, 48, 280–290. [Google Scholar] [CrossRef]
- Prajsner, M.; Winiarz, P. Application of the electrospinning technique in the preparation of selected electrode materials for solid oxide fuel cells. J. Phys. Conf. Ser. 2024, 2812, 012008. [Google Scholar] [CrossRef]
- Xu, Z.; Gong, Y.; Wang, J.; Ma, Z.; Yu, R.; Yang, J.; Liu, Y.; Guo, Q.; Zhou, E.; Tan, Z. Carbon nanofibers fabricated via electrospinning to guide crystalline orientation for stable perovskite solar cells with efficiency over 24%. Chem. Eng. J. 2023, 453, 139961. [Google Scholar] [CrossRef]
- Algabry, S.M.; Shoueir, K.R.; Kashyout, A.E.-H.B.; El-Kemary, M. Engineering electrospun of in-situ plasmonic AgNPs onto PANI@ PVDF nanofibrous scaffold as back surface support for enhancing silicon solar cells efficiency with the electrical model assessment. Sustain. Mater. Technol. 2022, 31, e00380. [Google Scholar] [CrossRef]
- Alli, Y.A.; Bamisaye, A.; Onawole, A.T.; Oladoye, P.O.; Bankole, O.M.; Koivisto, B.; Youssef, K. Coaxial electrospinning: Design, characterization, mechanistic insights and their emerging applications in solar cells. Nano Energy 2024, 131, 110203. [Google Scholar] [CrossRef]
- Mohammadi-Ganjgah, A.; Shaterian, M.; Bahrami, H.; Rasuli, R.; Yavari, S.; Ghasemi, R.; Parvizi, Z. Electrospun synthesis of polyaniline and titanium dioxide nanofibers as potential electrode materials in electrochemical hydrogen storage. Renew. Energy 2024, 226, 120439. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, M.; Yan, S.; Chen, X.; Li, W.; Xu, M.; Wang, C.; Lu, X. Partial oxidation of Rh/Ru nanoparticles within carbon nanofibers for high-efficiency hydrazine oxidation-assisted hydrogen generation. J. Colloid Interface Sci. 2025, 679, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sari, S.M.; Ang, B.C.; Daud, W.M.A.W.; Patah, M.F.A. Visible-light-driven photocatalytic hydrogen production on defective, sulfur self-doped g-C3N4 nanofiber fabricate via electrospinning method. J. Environ. Chem. Eng. 2023, 11, 109318. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. rGO-wrapped Ag-doped TiO2 nanofibers for photocatalytic CO2 reduction under visible light. J. Clean. Prod. 2022, 374, 134022. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Huang, Y.; Zhang, Y.; Akula, S. Recent advancements in the application of electrospun nanofibers for carbon dioxide capture and utilization. Appl. Energy 2024, 365, 123305. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, C.; Zhang, Z.; Zhang, J.; Wang, L.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Coaxial electrospinning prepared Cu species loaded SrTiO3 for efficient photocatalytic reduction of CO2 to CH3OH. J. Environ. Chem. Eng. 2024, 12, 111990. [Google Scholar] [CrossRef]
- Laghlimi, C.; Moutcine, A.; Ziat, Y.; Belkhanchi, H.; Koufi, A.; Bouyassan, S. Hydrogen, Chronology and Electrochemical Production. Sol. Energy Sustain. Dev. J. 2024, 22–37. [Google Scholar] [CrossRef]
- Machmudah, S.; Kanda, H.; Goto, M. Hydrolysis of biopolymers in near-critical and subcritical water. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–107. [Google Scholar]
- Sun, Z.; Deitzel, J.M.; Knopf, J.; Chen, X.; Gillespie, J.W., Jr. The effect of solvent dielectric properties on the collection of oriented electrospun fibers. J. Appl. Polym. Sci. 2012, 125, 2585–2594. [Google Scholar] [CrossRef]
- Liu, Y.M.T. Electrospun Polymer Nanofiber Separators and Electrolyte Membranes for Energy Storage and Conversion Applications. In Nano-Size Polymers; Springer: Cham, Switzerland, 2016; pp. 201–223. [Google Scholar] [CrossRef]
- Baghali, M.; Jayathilaka, W.; Ramakrishna, S. The role of electrospun nanomaterials in the future of energy and environment. Materials 2021, 14, 558. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Wang, K.; Kang, W.; Yang, G. Thermally robust hierarchical nanofiber triboelectric yarns for efficient energy harvesting in firefighting E-textiles. Chem. Eng. J. 2024, 499, 156188. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Siddiky, A.Y.; Islam, M.A.; Hossain, A.; Rimon, M.I.H.; Oliullah, M.S.; Khan, J.; Rahman, M.; Hossain, N.; Chowdhury, M.A. Progress and prospects of electrospun nanofibrous membranes for water filtration: A comprehensive review. Desalination 2024, 574, 117285. [Google Scholar] [CrossRef]
- Nonjola, P.T.; Mutangwa, N.; Luo, H. Membrane separators for electrochemical energy storage technologies. In Nanomaterials in Advanced Batteries and Supercapacitors; Springer: Berlin/Heidelberg, Germany, 2016; pp. 417–462. [Google Scholar] [CrossRef]
- Hawes, G.F.; Rehman, S.; Rangom, Y.; Pope, M.A. Advanced manufacturing approaches for electrochemical energy storage devices. Int. Mater. Rev. 2023, 68, 323–364. [Google Scholar] [CrossRef]
- Senthil, C.; Jung, H.Y. Nanostructured separators based on nonpolyolefin polymers. In Nanostructured Lithium-Ion Battery Materials; Elsevier: Amsterdam, The Netherlands, 2025; pp. 293–316. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y. Advances in Lithium-Ion Battery Separators: A Review of Engineering Polymeric Porous Membranes. In Prime Archives in Polymer Technology; VIDE LEAF: Telangana, India, 2024; p. 170. Available online: https://videleaf.com/wp-content/uploads/2024/05/Advances-in-Lithium-Ion-Battery-Separators-A-Review-of-Engineering-Polymeric-Porous-Membranes.pdf (accessed on 24 April 2025).
- Tong, B.; Li, X. Towards separator safety of lithium-ion batteries: A review. Mater. Chem. Front. 2024, 8, 309–340. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y. Engineering polymer-based porous membrane for sustainable lithium-ion battery separators. Polymers 2023, 15, 3690. [Google Scholar] [CrossRef]
- Babiker, D.M.; Usha, Z.R.; Wan, C.; Hassaan, M.M.E.; Chen, X.; Li, L. Recent progress of composite polyethylene separators for lithium/sodium batteries. J. Power Sources 2023, 564, 232853. [Google Scholar] [CrossRef]
- Gan, L.; Dong, Z.; Xu, H.; Lv, H.; Liu, G.; Zhang, F.; Huang, Z. Beyond conventional degradation: Catalytic solutions for polyolefin upcycling. CCS Chem. 2024, 6, 313–333. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Huang, C.; Jin, X. A mini review on the generation of crimped ultrathin fibers via electrospinning: Materials, strategies, and applications. Polym. Adv. Technol. 2020, 31, 1449–1462. [Google Scholar] [CrossRef]
- Ji, D.; Lin, Y.; Guo, X.; Ramasubramanian, B.; Wang, R.; Radacsi, N.; Jose, R.; Qin, X.; Ramakrishna, S. Electrospinning of nanofibres. Nat. Rev. Methods Primers 2024, 4, 1. [Google Scholar] [CrossRef]
- Uyar, M.; Cakmak, S. Three-dimensional macro/micro-porous curcumin releasing polycaprolactone/chitosan nanofiber scaffolds as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133573. [Google Scholar] [CrossRef]
- Borah, A.R.; Hazarika, P.; Duarah, R.; Goswami, R.; Hazarika, S. Biodegradable Electrospun Membranes for Sustainable Industrial Applications. ACS Omega 2024, 9, 11129–11147. [Google Scholar] [CrossRef]
- Lee, A.; Jin, H.; Dang, H.-W.; Choi, K.-H.; Ahn, K.H. Optimization of experimental parameters to determine the jetting regimes in electrohydrodynamic printing. Langmuir 2013, 29, 13630–13639. [Google Scholar] [CrossRef]
- Chen, S.; Yao, W.; Ding, Z.; Du, J.; Wang, T.; Xiao, X.; Zhang, L.; Yang, J.; Guan, Y.; Chen, C. Injectable Electrospun Fiber-Hydrogel Composite Delivery System for Prolonged and Nociceptive-Selective Analgesia. Adv. Fiber Mater. 2024, 6, 1428–1445. [Google Scholar] [CrossRef]
- Planchette, C.; Lamanna, G.; Pan, K.-L. Physics of droplets. Front. Phys. 2024, 12, 1386926. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zhang, Y.; Yu, H.; Tan, Z. Near-field electrospinning for 2D and 3D structuring: Fundamentals, methods, and applications. Mater. Today Adv. 2024, 21, 100461. [Google Scholar] [CrossRef]
- Coman, G. Energy-Space-Time Plateau-Rayleigh Instability, Quantum Phenomenon & the Fundamental Interactions. J. Electr. Electron. Eng. 2024, 3, 1–17. [Google Scholar] [CrossRef]
- Wang, J.; Han, J.; Wu, T.; Li, B.; Yu, K.; Xu, H.; Zhang, W. Bubble deformation and breakup in a non-uniform electric field. Chem. Eng. Sci. 2024, 287, 119741. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Zhang, Q.; Wang, X.-X.; Lu, Y.; Li, W.-B.; Peng, Q.-Y.; Xu, F.-Y. Review of Electrospinning in the Fabrication of Nanogenerators. ACS Appl. Nano Mater. 2024, 7, 4630–4652. [Google Scholar] [CrossRef]
- Li, W.; Yin, Y.; Zhou, H.; Fan, Y.; Yang, Y.; Gao, Q.; Li, P.; Gao, G.; Li, J. Recent Advances in Electrospinning Techniques for Precise Medicine. Cyborg Bionic Syst. 2024, 5, 0101. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, S.; Dixit, H.N.; Samavedi, S. Establishment of Unique Cone Shapes and Universal Shape Parameters toward Predicting Fiber Diameter in Polymer Electrospinning. Ind. Eng. Chem. Res. 2024, 63, 13238–13251. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, W.; Jiang, Z.; Amarjargal, A.; Park, C.-H.; Pant, H.R.; Im, I.-T.; Kim, C.S. Two-nozzle electrospinning of (MWNT/PU)/PU nanofibrous composite mat with improved mechanical and thermal properties. Curr. Appl. Phys. 2013, 13, 1247–1255. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The history of electrospinning: Past, present, and future developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. ChemPlusChem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Khan, J.; Khan, A.; Khan, M.Q.; Khan, H. Applications of co-axial electrospinning in the biomedical field. Next Mater. 2024, 3, 100138. [Google Scholar] [CrossRef]
- Alharbi, A.; Alarifi, I.M.; Khan, W.S.; Asmatulu, R. Synthesis and analysis of electrospun SrTiO3 nanofibers with NiO nanoparticles shells as photocatalysts for water splitting. Macromol. Symp. 2016, 365, 246–257. [Google Scholar] [CrossRef]
- Yue, T.; Wang, M.; Li, X.; Zheng, M.; Liu, J.; Lin, J.; Liu, Y. Core-sheath PVDF hollow porous fibers via coaxial wet spinning for energy harvesting. Compos. Commun. 2024, 50, 102019. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Islam, M.A.; Hosseini, S.A.; Shamaei, L.; Aktij, S.A.; Sadrzadeh, M. Melt electrospinning for membrane fabrication. In Electrospun and Nanofibrous Membranes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 27–51. [Google Scholar] [CrossRef]
- Zakaria, M.; Bhuiyan, M.R.; Hossain, M.S.; Khan, N.-M.U.; Salam, M.A.; Nakane, K. Advances of polyolefins from fiber to nanofiber: Fabrication and recent applications. Discov. Nano 2024, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.R.; Bertea, A.-P. Sensors from Electrospun Nanostructures. In Nanostructures in Energy Generation, Transmission and Storage; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Dalton, P.D. Melt electrospinning. Chem. Asian J. 2011, 6, 44–56. [Google Scholar] [CrossRef]
- Wubneh, A.; Kim, C.I.; Ayranci, C. A study on theoretical predictive model and experimental findings of melt-electrospinning process. Polym. Adv. Technol. 2024, 35, e6310. [Google Scholar] [CrossRef]
- Lim, J.; Moon, E.; Kim, H.S. Dynamic behavior of melt electrospinning of blended polypropylene. J. Text. Inst. 2024, 1–8. [Google Scholar] [CrossRef]
- Tijing, L.; Woo, Y.; Yao, M.; Ren, J.; Shon, H. Electrospinning for membrane fabrication: Strategies and applications. In Comprehensive Membrane Science and Engineering, 2nd ed.; Elsevier: Oxford, UK, 2017; pp. 418–444. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Ardakkyzy, A.; Nuraje, N.; Toktarbay, Z. Effects of Electrospinning Parameters on the Morphology of Electrospun Fibers. Eurasian Chem. Technol. J. 2024, 26, 105–111. [Google Scholar] [CrossRef]
- Aramide, B.; Kothandaraman, A.; Edirisinghe, M.; Jayasinghe, S.N.; Ventikos, Y. General computational methodology for modeling electrohydrodynamic flows: Prediction and optimization capability for the generation of bubbles and fibers. Langmuir 2019, 35, 10203–10212. [Google Scholar] [CrossRef]
- Palwai, S. Physics of Electrospinning. In Electrospinning-Theory, Applications, and Update Challenges; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Zhu, T.; Song, X.; Duan, Z.; Song, Y.; Hu, X.; Zhou, Y.; Han, Y.; Ran, X. Effects of electrostatic polarities on the morphology of electrospun oxide nanofibers: A case study on alumina-based nanofibers. Ceram. Int. 2024, 50, 20402–20409. [Google Scholar] [CrossRef]
- Yördem, O.; Papila, M.; Menceloğlu, Y.Z. Effects of electrospinning parameters on polyacrylonitrile nanofiber diameter: An investigation by response surface methodology. Mater. Des. 2008, 29, 34–44. [Google Scholar] [CrossRef]
- Bakar, S.; Fong, K.; Eleyas, A.; Nazeri, M. Effect of voltage and flow rate electrospinning parameters on polyacrylonitrile electrospun fibers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012076. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Chou, C.C.; Yu, P.H.; Lin, K.L. Effects of applied voltage on the morphology and phases of electrospun poly (vinylidene difluoride) nanofibers. Polym. Int. 2022, 71, 1176–1183. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Recent progress of membrane distillation using electrospun nanofibrous membrane. J. Membr. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A review on current nanofiber technologies: Electrospinning, centrifugal spinning, and electro-centrifugal spinning. Macromol. Mater. Eng. 2023, 308, 2200502. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Wang, H.; Ou, W.; He, J.; Liu, M.; Lu, Z.; Hu, J.; Zheng, G.; Wu, D. Response Surface Methodology to Explore the Influence Mechanism of Fiber Diameter in a New Multi-Needle Electrospinning Spinneret. Polymers 2024, 16, 2222. [Google Scholar] [CrossRef] [PubMed]
- Acik, G.; Cansoy, C.E.; Kamaci, M. Effect of flow rate on wetting and optical properties of electrospun poly (vinyl acetate) micro-fibers. Colloid Polym. Sci. 2019, 297, 77–83. [Google Scholar] [CrossRef]
- Zargham, S.; Bazgir, S.; Tavakoli, A.; Rashidi, A.S.; Damerchely, R. The effect of flow rate on morphology and deposition area of electrospun nylon 6 nanofiber. J. Eng. Fibers Fabr. 2012, 7, 155892501200700414. [Google Scholar] [CrossRef]
- De Prá, M.A.A.; Ribeiro-do-Valle, R.M.; Maraschin, M.; Veleirinho, B. Effect of collector design on the morphological properties of polycaprolactone electrospun fibers. Mater. Lett. 2017, 193, 154–157. [Google Scholar] [CrossRef]
- Ghelich, R.; Rad, M.K.; Youzbashi, A.A. Study on morphology and size distribution of electrospun NiO-GDC composite nanofibers. J. Eng. Fibers Fabr. 2015, 10, 155892501501000102. [Google Scholar] [CrossRef]
- Jabur, A.; Abbas, L.; Muhi Aldain, S. Effects of ambient temperature and needle to collector distance on PVA nanofibers diameter obtained from electrospinning technique. Eng. Technol. J. 2017, 35, 340–347. [Google Scholar] [CrossRef]
- Chagas, P.A.; Medeiros, G.B.; Silva, E.N.; Morais, S.; Mata, G.C.; Guerra, V.G.; Aguiar, M.L.; Oliveira, W.P. Design and Characterization of the Electrospun Nanofibers Mats. In Electrospun Nanofibres; CRC Press: Boca Raton, FL, USA, 2024; pp. 128–166. [Google Scholar] [CrossRef]
- Shao, J.; Zheng, Y.; Newton, M.A.A.; Li, Y.; Xin, B. Effect of Electric Field Induced by Collector Shape on the Electrospun Jet Motion and Fiber Structure Evolution. Fibers Polym. 2023, 24, 3435–3444. [Google Scholar] [CrossRef]
- Demirtaş, M.S.; Saha, M.C. Engineering highly aligned continuous nanofibers via electrospinning: A comprehensive study on collector design, electrode geometry, and collector speed. Express Polym. Lett. 2024, 18, 851–867. [Google Scholar] [CrossRef]
- Kashif, M.; Guo, H.; Ge, J.; Lv, X.; Rasul, S.; Liu, Y. Effect of different symmetries of electrospun poly (lactic acid) nanofibers on facemask filtration. ACS Appl. Polym. Mater. 2023, 5, 5995–6002. [Google Scholar] [CrossRef]
- Bakar, S.S.S.; Foong, K.M.; Halif, N.A.; Yahud, S. Effect of solution concentration and applied voltage on electrospun polyacrylonitrile fibers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012018. [Google Scholar] [CrossRef]
- Unverzagt, L.; Dolynchuk, O.; Lettau, O.; Wischke, C. Characteristics and Challenges of Poly (ethylene-co-vinyl acetate) Solution Electrospinning. ACS Omega 2024, 9, 18624–18633. [Google Scholar] [CrossRef] [PubMed]
- Colmenares-Roldán, G.J.; Quintero-Martínez, Y.; Agudelo-Gómez, L.M.; Rodríguez-Vinasco, L.F.; Hoyos-Palacio, L.M. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Rev. Fac. Ing. Univ. Antioq. 2017, 35–45. [Google Scholar] [CrossRef]
- Perez-Puyana, V.M.; Romero, A.; Guerrero, A.; Moroni, L.; Wieringa, P.A. Enabling low molecular weight electrospinning through binary solutions of polymer blends. Next Mater. 2025, 6, 100306. [Google Scholar] [CrossRef]
- Hajieghrary, F.; Ghanbarzadeh, B.; Pezeshki, A.; Dadashi, S.; Falcone, P.M. Development of Hybrid Electrospun Nanofibers: Improving Effects of Cellulose Nanofibers (CNFs) on Electrospinnability of Gelatin. Foods 2024, 13, 2114. [Google Scholar] [CrossRef]
- Tahir, M.; Vicini, S.; Sionkowska, A. Electrospun materials based on polymer and biopolymer blends—A review. Polymers 2023, 15, 1654. [Google Scholar] [CrossRef]
- Kasaai, M.R. How Do We Set Up or Choose An Optimal Procedure to Determine the Molecular Weight of Polymers with A Good Accuracy Using Size Exclusion Chromatography or Viscometry? A Mini-Review. J. Polym. Biopolym. Phys. Chem. 2024, 12, 8–14. [Google Scholar] [CrossRef]
- Dharmaraj, D.; Chavan, N.; Likhitha, U.; Nayak, U.Y. Electrospun nanofibers for dermatological delivery. J. Drug Deliv. Sci. Technol. 2024, 99, 105981. [Google Scholar] [CrossRef]
- Javed, K.; Krumme, A.; Viirsalu, M.; Krasnou, I.; Plamus, T.; Vassiljeva, V.; Tarasova, E.; Savest, N.; Mere, A.; Mikli, V. A method for producing conductive graphene biopolymer nanofibrous fabrics by exploitation of an ionic liquid dispersant in electrospinning. Carbon 2018, 140, 148–156. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers - ScienceDirect. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Araújo, E.S.; Nascimento, M.L.F.; de Oliveira, H.P. Electrospinning of polymeric fibres: An unconventional view on the influence of surface tension on fibre diameter. Fibres Text. East. Eur. 2016, 24, 22–29. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.; Bertea, A.; Bertea, A.; Popa, A. The influence of polymer solution on the properties of electrospun 3D nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012092. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid Interface Sci. 2020, 286, 102315. [Google Scholar] [CrossRef]
- Castro, C.; Juárez, D.A.; Arizmendi-Morquecho, A.; Gonzalez-Perez, G.; Estrada-Villegas, G.M. The effects of relative humidity and salt concentration on the diameter of hydrophilic polymeric nanofibers obtained by electrospinning: Synergistic effect study by Central Composite Design (CCD). React. Funct. Polym. 2024, 203, 106013. [Google Scholar] [CrossRef]
- Fashandi, H.; Karimi, M. Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer 2012, 53, 5832–5849. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology. Tissue Eng Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef]
- Vrieze, S.D.; Camp, T.V.; Nelvig, A.; Hagstrm, B.; Westbroek, P.; Clerck, K.D. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Kim, C.H.; Jung, Y.H.; Kim, H.Y.; Lee, D.R.; Dharmaraj, N.; Choi, K.E. Effect of collector temperature on the porous structure of electrospun fibers. Macromol. Res. 2006, 14, 59–65. [Google Scholar] [CrossRef]
- Pampal, E.S.; Stojanovska, E.; Simon, B.; Kilic, A. A review of nanofibrous structures in lithium ion batteries. J. Power Sources 2015, 300, 199–215. [Google Scholar] [CrossRef]
- Chen, S.; Ye, W.; Hou, H. Electrospun Fibrous Membranes as Separators of Lithium-Ion Batteries. In Electrospun Nanofibers for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Hasany, S.; Alam, T.; Dönmez, K.B. Advancement in High Energy Density Materials for Energy Storage Systems. In Materials for Boosting Energy Storage. Volume 3: Advances in Sustainable Energy Technologies; ACS Publications: Washington, DC, USA, 2024; pp. 101–122. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, M.; Chen, J.; Zhang, M.; Hong, W. Advances in biomass-based nanofibers prepared by electrospinning for energy storage devices. Fuel 2024, 355, 129534. [Google Scholar] [CrossRef]
- Haichao, L.; Haoyi, L.; Bubakir, M.; Weimin, Y.; Barhoum, A. Engineering nanofibers as electrode and membrane materials for batteries, supercapacitors, and fuel cells. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, L.; Liu, P.; Zhu, Y.; Li, J. Direct regeneration and upcycling of cathode material from spent lithium ion batteries: Recent advances and perspectives. Sep. Purif. Technol. 2024, 355, 129574. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, D.H.; Pyo, S.G. Eletrospinning: Improving the Performance of 1-D Nanofibers Used in Anodes, Cathodes, and Separators in Lithium-Ion Batteries. Int. J. Energy Res. 2024, 2024, 1847943. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, P.; Gao, Z.; Li, X.; Cui, W.; Li, R.; Ramakrishna, S.; Zhang, J.; Long, Y.-Z. Review on electrospinning anode and separators for lithium ion batteries. Renew. Sustain. Energy Rev. 2024, 189, 113939. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Y.; Li, D.; Zhao, K.; Wang, L.; Tan, P.; Dong, H.; Wang, Y.; Liang, J. A review of electrospun separators for lithium-based batteries: Progress and application prospects. Carbon Energy 2024, 6, e539. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.R.; Manaf, O.; Sundaran, S.P.; Sujith, A. Development of nanocomposite membranes by electrospun nanofibrous materials. In Nanocomposite Membranes for Water and Gas Separation; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Recent Development of Polyolefin-Based Microporous Separators for Li−Ion Batteries: A Review. Chem. Rec. 2020, 20, 570–595. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of nanofibers for energy applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef]
- Liang, Y.; Cheng, S.; Zhao, J.; Zhang, C.; Sun, S.; Zhou, N.; Qiu, Y.; Zhang, X. Heat treatment of electrospun Polyvinylidene fluoride fibrous membrane separators for rechargeable lithium-ion batteries. J. Power Sources 2013, 240, 204–211. [Google Scholar] [CrossRef]
- Padmaraj, O.; Venkateswarlu, M.; Satyanarayana, N. Characterization and Electrochemical Properties of P (VdF-co-HFP) Based Electrospun Nanocomposite Fibrous Polymer Electrolyte Membrane for Lithium Battery Applications. Electroanalysis 2014, 26, 2373–2379. [Google Scholar] [CrossRef]
- Mahant, Y.P.; Kondawar, S.B.; Nandanwar, D.V.; Koinkar, P. Poly (methyl methacrylate) reinforced poly (vinylidene fluoride) composites electrospun nanofibrous polymer electrolytes as potential separator for lithium ion batteries. Mater. Renew. Sustain. Energy 2018, 7, 5. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R. Rep. A Rev. J. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Mendez, S. Recent advances on materials for lithium-ion batteries. Energies 2021, 14, 3145. [Google Scholar] [CrossRef]
- Zhu, J.; Yildirim, E.; Aly, K.; Shen, J.; Chen, C.; Lu, Y.; Jiang, M.; Kim, D.; Tonelli, A.E.; Pasquinelli, M.A. Hierarchical multi-component nanofiber separators for lithium polysulfide capture in lithium–sulfur batteries: An experimental and molecular modeling study. J. Mater. Chem. A 2016, 4, 13572–13581. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Lu, Y.; Zang, J.; Jiang, M.; Kim, D.; Zhang, X. Highly porous polyacrylonitrile/graphene oxide membrane separator exhibiting excellent anti-self-discharge feature for high-performance lithium–sulfur batteries. Carbon 2016, 101, 272–280. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Shi, R.; Dirican, M.; Zhu, P.; Yan, C.; Jia, H.; Zang, J.; He, J.; Zhang, X. Ultrafine and polar ZrO2-inlaid porous nitrogen-doped carbon nanofiber as efficient polysulfide absorbent for high-performance lithium-sulfur batteries with long lifespan. Chem. Eng. J. 2018, 349, 376–387. [Google Scholar] [CrossRef]
- Fan, C.; Yang, R.; Shi, Z.; Mao, L.; Li, L.; Yan, Y.; Zou, Y.; Kang, E.; Zhong, L.; Xu, Y. NC-Co3O4 polyhedron embedded multifunctional separator based on structural engineering towards stable and durable lithium-sulfur battery. J. Alloys Compd. 2023, 968, 171969. [Google Scholar] [CrossRef]
- Zhu, C.; Wen, Y.; Aken, P.A.V.; Maier, J.; Yu, Y. High Lithium Storage Performance of FeS Nanodots in Porous Graphitic Carbon Nanowires. Adv. Funct. Mater. 2015, 25, 2335–2342. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Kaushik, S.; Mehta, T.; Chand, P.; Sharma, S.; Kumar, G. Recent advancements in cathode materials for high-performance Li-ion batteries: Progress and prospects. J. Energy Storage 2024, 97, 112818. [Google Scholar] [CrossRef]
- Koech, A.K.; Mwandila, G.; Mulolani, F.; Mwaanga, P. Lithium-ion Battery Fundamentals and Exploration of Cathode Materials: A Review. S. Afr. J. Chem. Eng. 2024, 50, 321–339. [Google Scholar] [CrossRef]
- Teh, P.F.; Pramana, S.S.; Sharma, Y.; Ko, Y.W.; Madhavi, S. Electrospun Zn1–x MnxFe2O4 nanofibers as anodes for lithium-ion batteries and the impact of mixed transition metallic oxides on battery performance. Acs Appl. Mater. Interfaces 2013, 5, 5461–5467. [Google Scholar] [CrossRef]
- Luo, L.; Xu, W.; Xia, Z.; Fei, Y.; Zhu, J.; Chen, C.; Lu, Y.; Wei, Q.; Qiao, H.; Zhang, X. Electrospun ZnO–SnO2 composite nanofibers with enhanced electrochemical performance as lithium-ion anodes. Ceram. Int. 2016, 42, 10826–10832. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, Y.; Chen, C.; Ge, Y.; Jasper, S.; Leary, J.D.; Li, D.; Jiang, M.; Zhang, X. Porous one-dimensional carbon/iron oxide composite for rechargeable lithium-ion batteries with high and stable capacity. J. Alloys Compd. 2016, 672, 79–85. [Google Scholar] [CrossRef]
- Wang, X.L.; Kim, E.M.; Senthamaraikannan, T.G.; Lim, D.-H.; Jeong, S.M. Porous hollow high entropy metal oxides (NiCoCuFeMg) 3O4 nanofiber anode for high-performance lithium-ion batteries. Chem. Eng. J. 2024, 484, 149509. [Google Scholar] [CrossRef]
- Qiu, P.; Jin, R.; Son, Y.; Ju, A.; Jiang, W.; Wang, L.; Luo, W. Mesoporous nanofibers from extended electrospinning technique. Adv. Fiber Mater. 2024, 6, 658–685. [Google Scholar] [CrossRef]
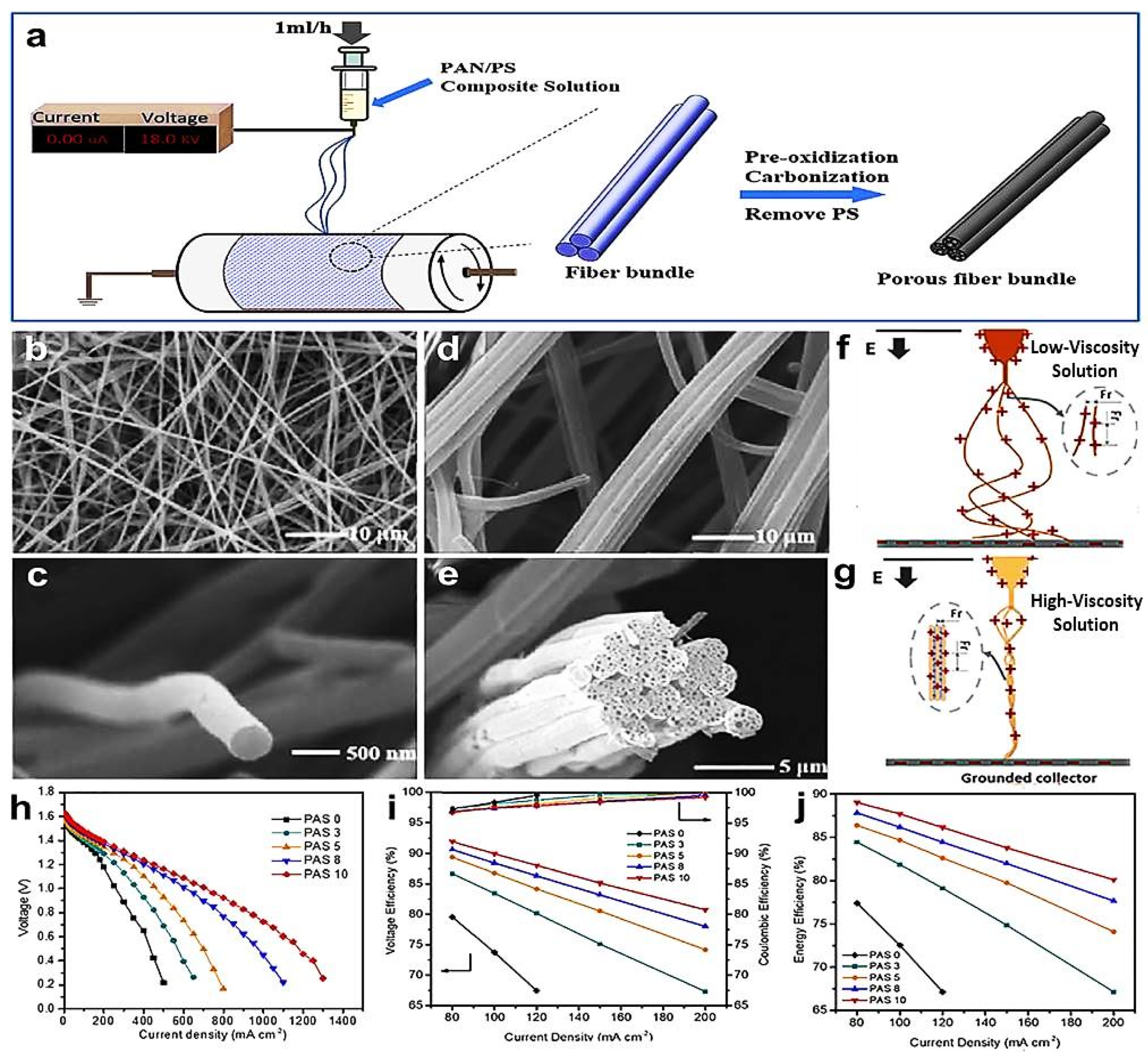
- Han, Z.; Wang, T.; Cai, Y.; Rong, S.; Ma, J.; Hou, L.; Ji, Y. Electrospun porous carbon nanofiber-based electrodes for redox flow batteries: Progress and opportunities. Carbon 2024, 222, 118969. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Lin, D.; Wang, Q.; Liu, C.; Chu, P.; Leung, P. Electrospun Carbon Nanofiber Composite Electrode with Gradient Porous Structure for Rapid Ion Transport in an All-Vanadium Redox Flow Battery. Energy Technol. 2024, 12, 2400825. [Google Scholar] [CrossRef]
- Li, J.; Al-Yasiri, M.; Pham, H.; Park, J. Redox flow batteries. Adv. Mater. Battery Sep. 2024, 327–347. [Google Scholar] [CrossRef]
- Available online: http://www.energystorage.org (accessed on 24 April 2025).
- Xu, D.; Zhang, C.; Li, Y. Molecular engineering redox-active organic materials for nonaqueous redox flow battery. Curr. Opin. Chem. Eng. 2022, 37, 100851. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, L.; Jiang, H.; Chao, C.; Zhao, T. Formation of electrodes by self-assembling porous carbon fibers into bundles for vanadium redox flow batteries. J. Power Sources 2018, 405, 106–113. [Google Scholar] [CrossRef]
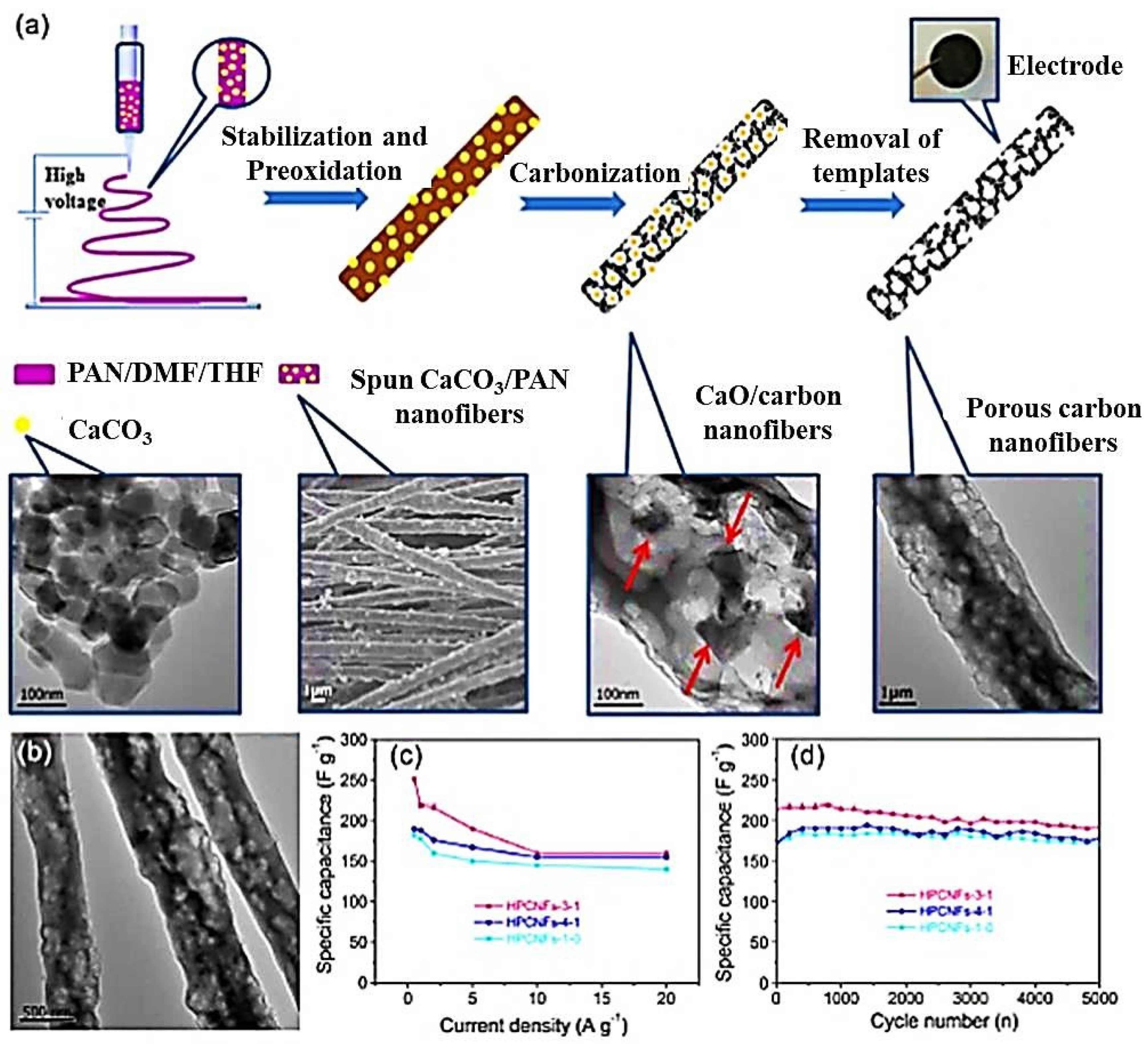
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Chettiannan, B.; Dhandapani, E.; Arumugam, G.; Rajendran, R.; Selvaraj, M. Metal-organic frameworks: A comprehensive review on common approaches to enhance the energy storage capacity in supercapacitor. Coord. Chem. Rev. 2024, 518, 216048. [Google Scholar] [CrossRef]
- Bejjanki, D.; Puttapati, S.K. Supercapacitor Basics (EDLCs, Pseudo, and Hybrid). In Multidimensional Nanomaterials for Supercapacitors: Next Generation Energy Storage; Bentham Science Publishers: Boston, MA, USA, 2024; pp. 29–48. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Liu, Y.; Meng, F.; Cao, Z. Dual storage mechanism of charge adsorption desorption and Faraday redox reaction enables aqueous symmetric supercapacitor with 1.4 V output voltage. Chem. Eng. J. 2024, 479, 147906. [Google Scholar] [CrossRef]
- Xiangye, L.; Tianjiao, B.; Xin, W.; Bing, Z.; Zhenzhen, W.; Tieshi, H. Application of Electrospun Fibers in Supercapacitors. Prog. Chem. 2021, 33, 1159–1174. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Sahatiya, P.; Badhulika, S. Template-assisted electrospinning of bubbled carbon nanofibers as binder-free electrodes for high-performance supercapacitors. ChemElectroChem 2018, 5, 531–539. [Google Scholar] [CrossRef]
- Wanison, R.; Syahputra, W.N.H.; Kammuang-lue, N.; Sakulchangsatjatai, P.; Chaichana, C.; Shankar, V.U.; Suttakul, P.; Mona, Y. Engineering aspects of sodium-ion battery: An alternative energy device for Lithium-ion batteries. J. Energy Storage 2024, 100, 113497. [Google Scholar] [CrossRef]
- Cui, R.; Ma, Y.; Gao, X.; Wang, W.; Wang, J.; Xing, Z.; Ju, Z. Research progress of co-intercalation mechanism electrolytes in sodium-ion batteries. Energy Storage Mater. 2024, 71, 103627. [Google Scholar] [CrossRef]
- Xu, G.L.; Amine, R.; Abouimrane, A.; Che, H.; Dahbi, M.; Ma, Z.F.; Saadoune, I.; Alami, J.; Mattis, W.L.; Pan, F. Challenges in Developing Electrodes, Electrolytes, and Diagnostics Tools to Understand and Advance Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702403.1702401–1702403.1702463. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
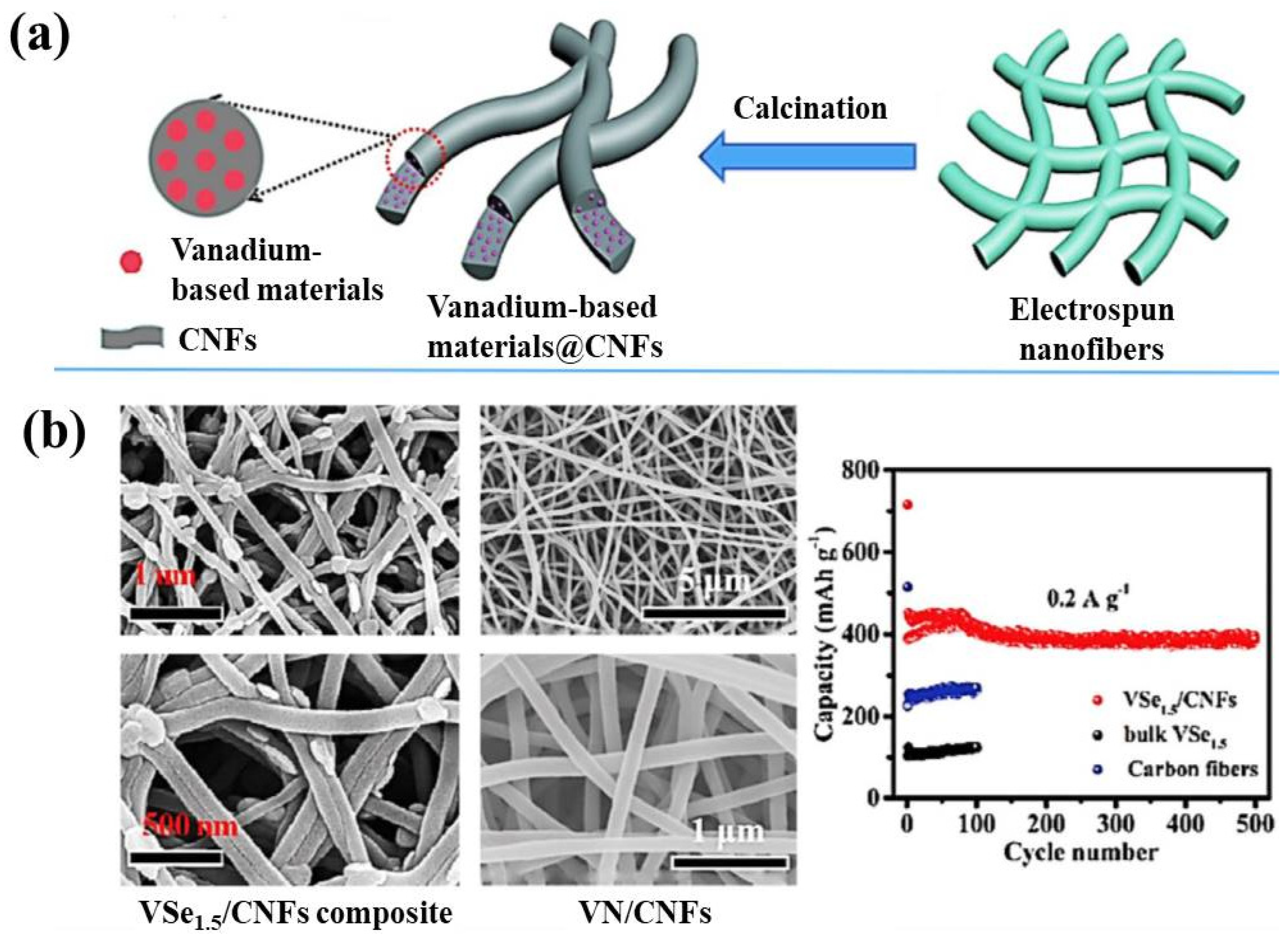
- Xu, L.; Xiong, P.; Zeng, L.; Fang, Y.; Liu, R.; Liu, J.; Luo, F.; Chen, Q.; Wei, M.; Qian, Q. Electrospun VSe 1.5/CNF composite with excellent performance for alkali metal ion batteries. Nanoscale 2019, 11, 16308–16316. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiong, P.; Zeng, L.; Liu, R.; Liu, J.; Luo, F.; Li, X.; Chen, Q.; Wei, M.; Qian, Q. Facile fabrication of a vanadium nitride/carbon fiber composite for half/full sodium-ion and potassium-ion batteries with long-term cycling performance. Nanoscale 2020, 12, 10693–10702. [Google Scholar] [CrossRef]
- Zha, J.; Zheng, D.; Wang, Y.; Xie, Z.; Wu, G.; Qi, J.; Wei, F.; Meng, Q.; Xue, X.; Zhao, D. Coaxial electrospinning synthesis free-standing Sn/TiO2 flexible carbon fibers with sheath/core structure for advanced flexible lithium/potassium-ion batteries. Electrochim. Acta 2024, 500, 144745. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, Q.; Yang, H.; Zhang, J.; Wang, W. The Enormous Potential of Sodium/Potassium-Ion Batteries as The Mainstream Energy Storage Technology for Large-Scale Commercial Applications. Adv. Mater. 2024, 36, 2405989. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Lan, H.; Kuang, C.; Wang, H.; Guo, L. Superior potassium storage in chitin-derived natural nitrogen-doped carbon nanofibers. Carbon 2018, 128, 224–230. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, T.; Zhao, Y.; Yao, H.; Yang, J.; Zheng, S. Constructing multichannel carbon fibers as freestanding anodes for potassium-ion battery with high capacity and long cycle life. Adv. Mater. Interfaces 2020, 7, 1901829. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Wang, Z.; Zhao, L.; Li, P.; Yang, Y.; Yang, C.; Huang, H.; Guo, S. Short-range order in mesoporous carbon boosts potassium-ion battery performance. Adv. Energy Mater. 2018, 8, 1701648. [Google Scholar] [CrossRef]
- Tai, Z.; Zhang, Q.; Liu, Y.; Liu, H.; Dou, S. Activated carbon from the graphite with increased rate capability for the potassium ion battery. Carbon 2017, 123, 54–61. [Google Scholar] [CrossRef]
- Qi, X.; Huang, K.; Wu, X.; Zhao, W.; Wang, H.; Zhuang, Q.; Ju, Z. Novel fabrication of N-doped hierarchically porous carbon with exceptional potassium storage properties. Carbon 2018, 131, 79–85. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Q.; Luo, S.; Wang, Z.; Zhang, Y.; Liu, Y.; Hao, A.; Guo, R. High performance potassium-ion battery anode based on biomorphic N-doped carbon derived from walnut septum. J. Power Sources 2019, 415, 165–171. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.-Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.-C.; Chung, S.-H. Stability Enhancement of Lithium–Sulfur Batteries Using Electrospun Separator/Electrolyte Membranes. ACS Sustain. Chem. Eng. 2024, 12, 14230–14238. [Google Scholar] [CrossRef]
- Barghamadi, M.; Kapoor, A.; Wen, C. A Review on Li-S Batteries as a High Efficiency Rechargeable Lithium Battery. J. Electrochem. Soc. 2013, 160, A1256–A1263. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Shi, L.; Mu, D.; Xu, H.; Wu, B. A hierarchical carbon fiber/sulfur composite as cathode material for Li–S batteries. Carbon 2015, 86, 146–155. [Google Scholar] [CrossRef]
- Yang, Y.; McDowell, M.T.; Jackson, A.; Cha, J.J.; Hong, S.S.; Cui, Y. New nanostructured Li2S/silicon rechargeable battery with high specific energy. Nano Lett. 2010, 10, 1486–1491. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G. Lithium–sulfur batteries meet electrospinning: Recent advances and the key parameters for high gravimetric and volume energy density. Adv. Sci. 2022, 9, 2103879. [Google Scholar] [CrossRef]
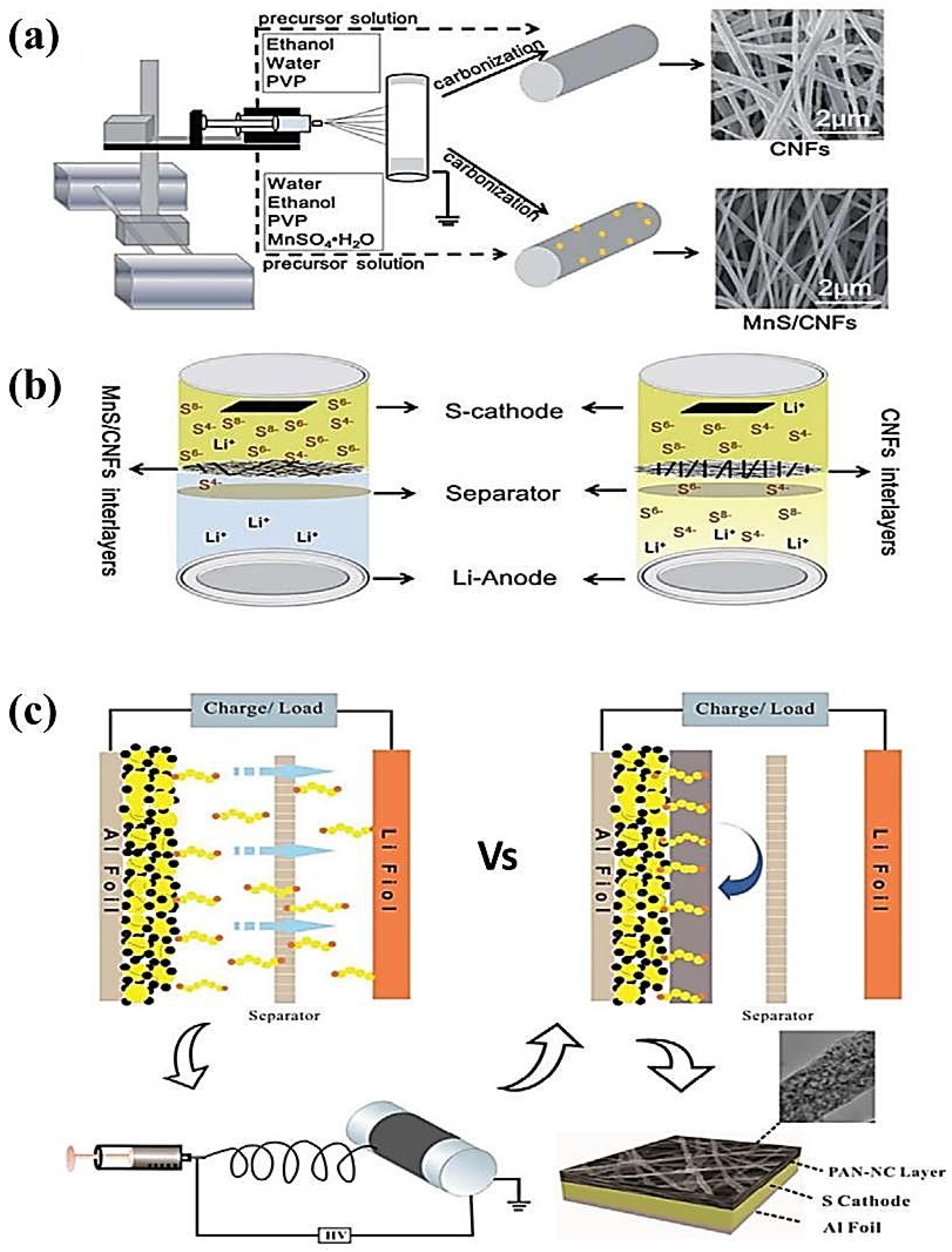
- Wang, X.; Zhao, X.; Ma, C.; Yang, Z.; Chen, G.; Wang, L.; Yue, H.; Zhang, D.; Sun, Z. Electrospun carbon nanofibers with MnS sulfiphilic sites as efficient polysulfide barriers for high-performance wide-temperature-range Li–S batteries. J. Mater. Chem. A 2020, 8, 1212–1220. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Wang, Y.; Shen, X.; Wang, F.; Li, H.; Hwang, B.-J.; Zhao, J. Directly coating a multifunctional interlayer on the cathode via electrospinning for advanced lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 29804–29811. [Google Scholar] [CrossRef]
- Zhao, T.; Ye, Y.; Lao, C.Y.; Divitini, G.; Coxon, P.R.; Peng, X.; He, X.; Kim, H.K.; Xi, K.; Ducati, C. A Praline-Like Flexible Interlayer with Highly Mounted Polysulfide Anchors for Lithium–Sulfur Batteries. Small 2017, 13, 1700357. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yang, J.-N.; Wang, K.-X.; Chen, J.-S. Optimization Strategies for Cathode Materials in Lithium–Oxygen Batteries. Acc. Mater. Res. 2024, 5, 1496–1506. [Google Scholar] [CrossRef]
- Wen, B.; Zhu, Z.; Li, F.-J. Advances and Challenges on Cathode Catalysts for Lithium-Oxygen Batteries. J. Electrochem. 2023, 29, 3. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Ogasawara, T.; Debart, A.; Holzapfel, M.; Novak, P.; Bruce, P.G. Rechargeable Li2O2 Electrode for Lithium Batteries. J. Am. Chem. Soc. 2006, 128, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, M.; Tao, S. Advanced electrospinning nanomaterials: From spinning fabrication techniques to electrochemical applications. Nano Res. 2024, 17, 7077–7116. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zheng, J.P.; Liang, R.; Zhang, C.; Wang, B.; Hendrickson, M.; Plichta, E.J. Lithium–Air Batteries Using SWNT/CNF Buckypapers as Air Electrodes. J. Electrochem. Soc. 2010, 157, A953–A956. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, M.; Orzech, M.W.; Niu, Y.; Tang, W.; Chen, J.; Marlow, M.N.; Puhan, D.; Zhao, Y.; Tan, R. In-situ fabrication of carbon-metal fabrics as freestanding electrodes for high-performance flexible energy storage devices. Energy Storage Mater. 2020, 30, 329–336. [Google Scholar] [CrossRef]
- Débart, A.; Bao, J.; Armstrong, G.; Bruce, P.G. An O2 cathode for rechargeable lithium batteries: The effect of a catalyst. J. Power Sources 2007, 174, 1177–1182. [Google Scholar] [CrossRef]
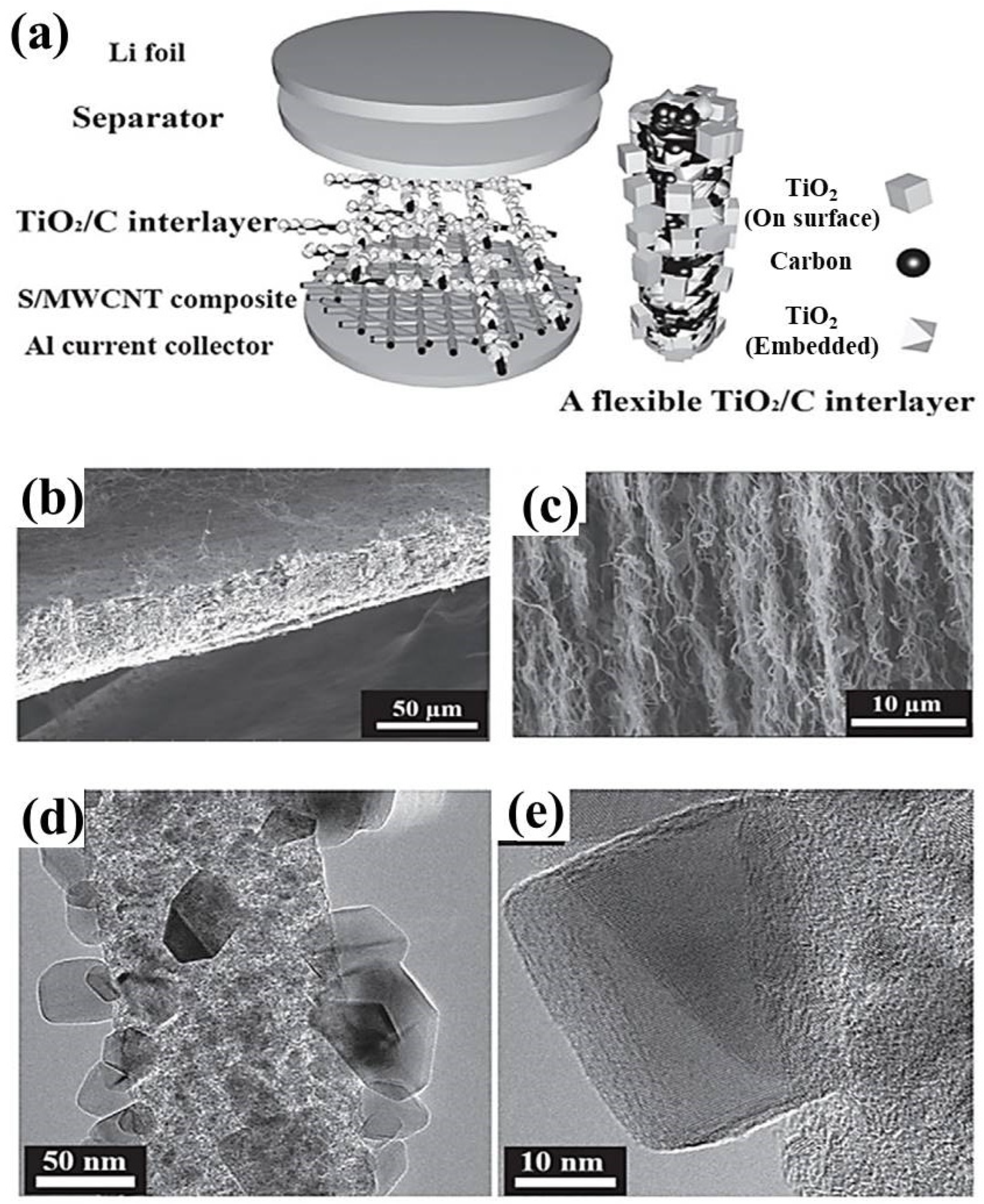
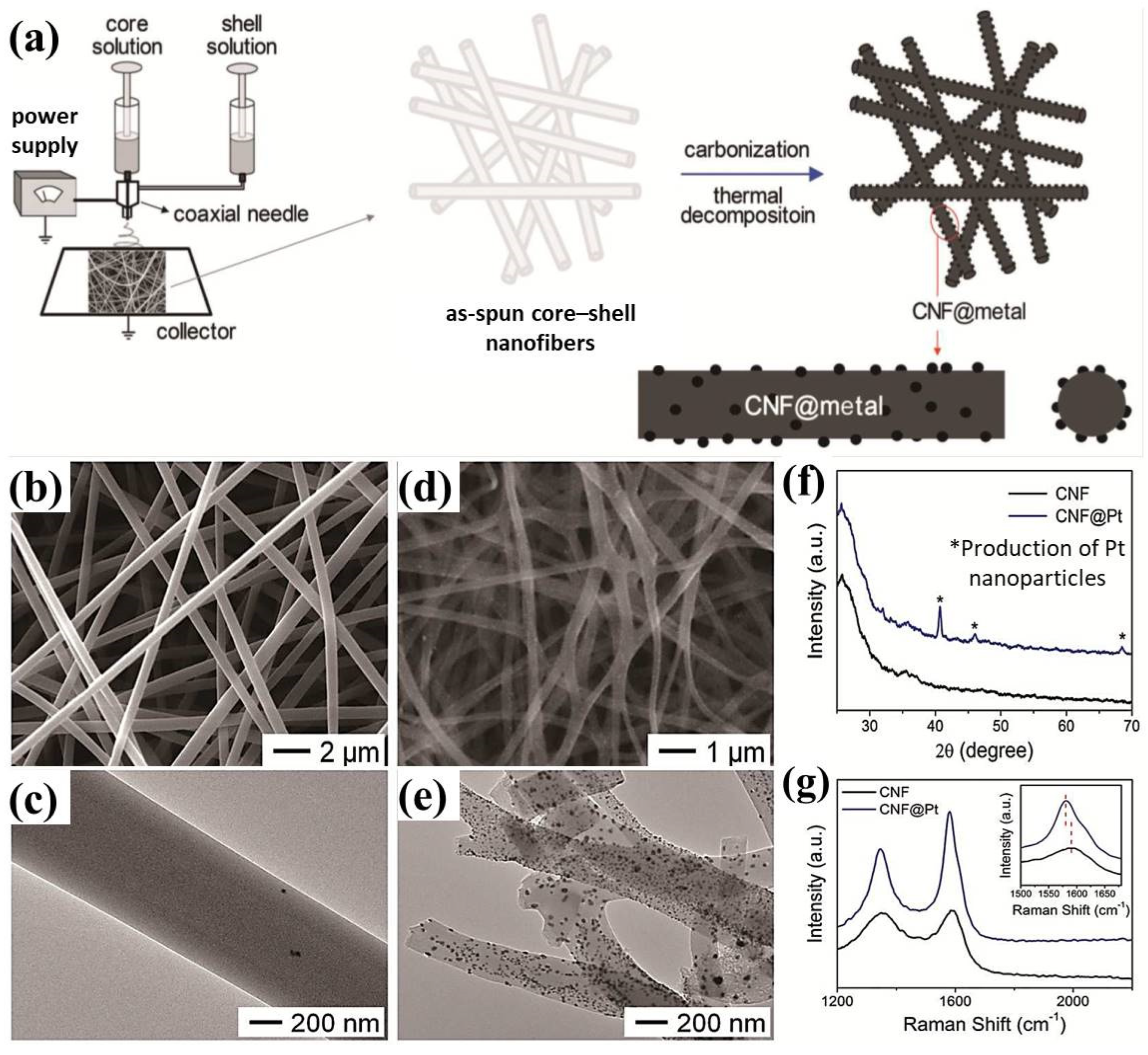
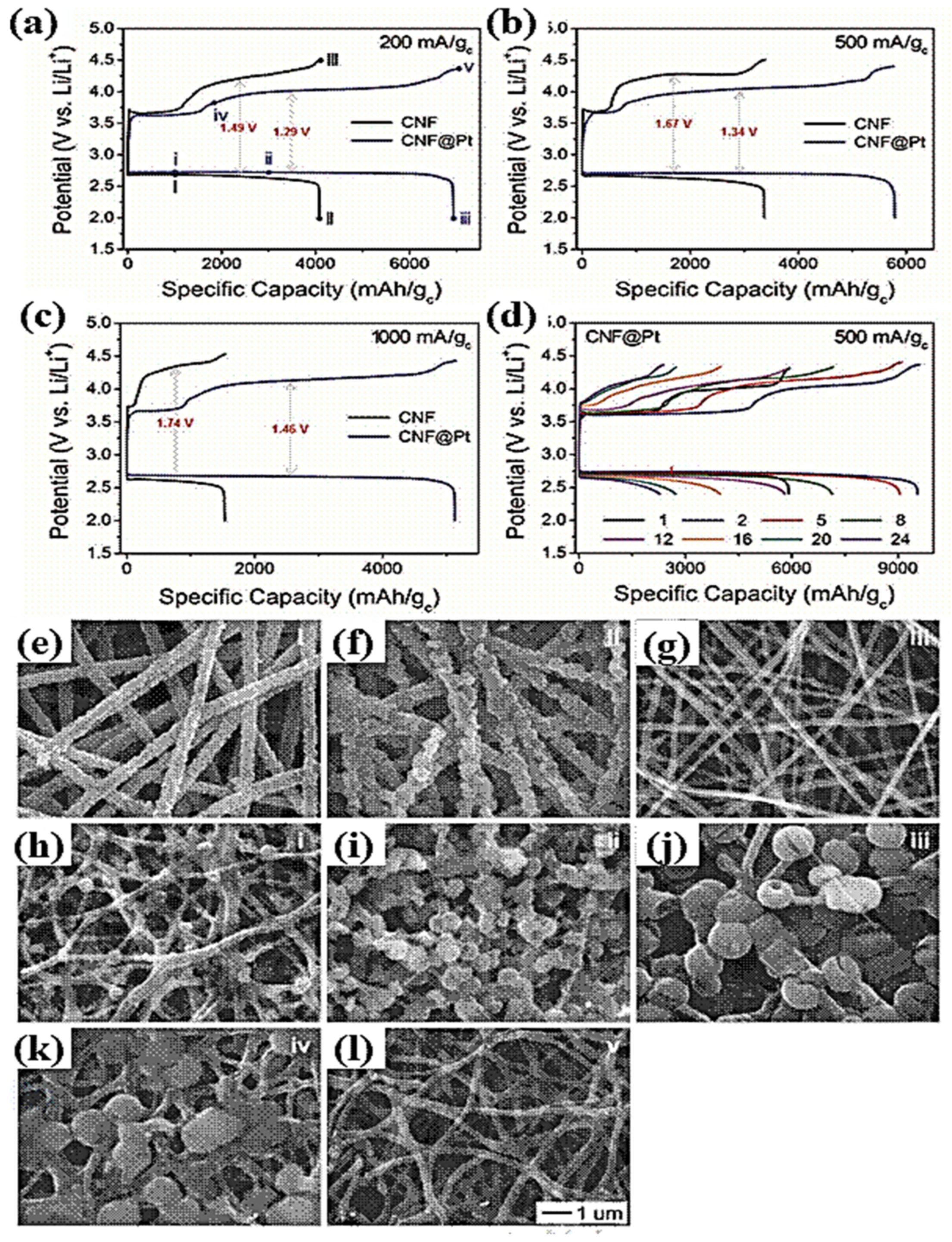
- Bui, H.T.; Kim, D.W.; Suk, J.; Kang, Y. Carbon nanofiber@ platinum by a coaxial electrospinning and their improved electrochemical performance as a Li− O2 battery cathode. Carbon 2018, 130, 94–104. [Google Scholar] [CrossRef]
- Nie, H.; Xu, C.; Zhou, W.; Wu, B.; Li, X.; Liu, T.; Zhang, H. Free-standing thin webs of activated carbon nanofibers by electrospinning for rechargeable Li–O2 batteries. ACS Appl. Mater. Interfaces 2016, 8, 1937–1942. [Google Scholar] [CrossRef]
- Zhou, Y.; Candelaria, S.L.; Liu, Q.; Uchaker, E.; Cao, G. Porous carbon with high capacitance and graphitization through controlled addition and removal of sulfur-containing compounds. Nano Energy 2015, 12, 567–577. [Google Scholar] [CrossRef]

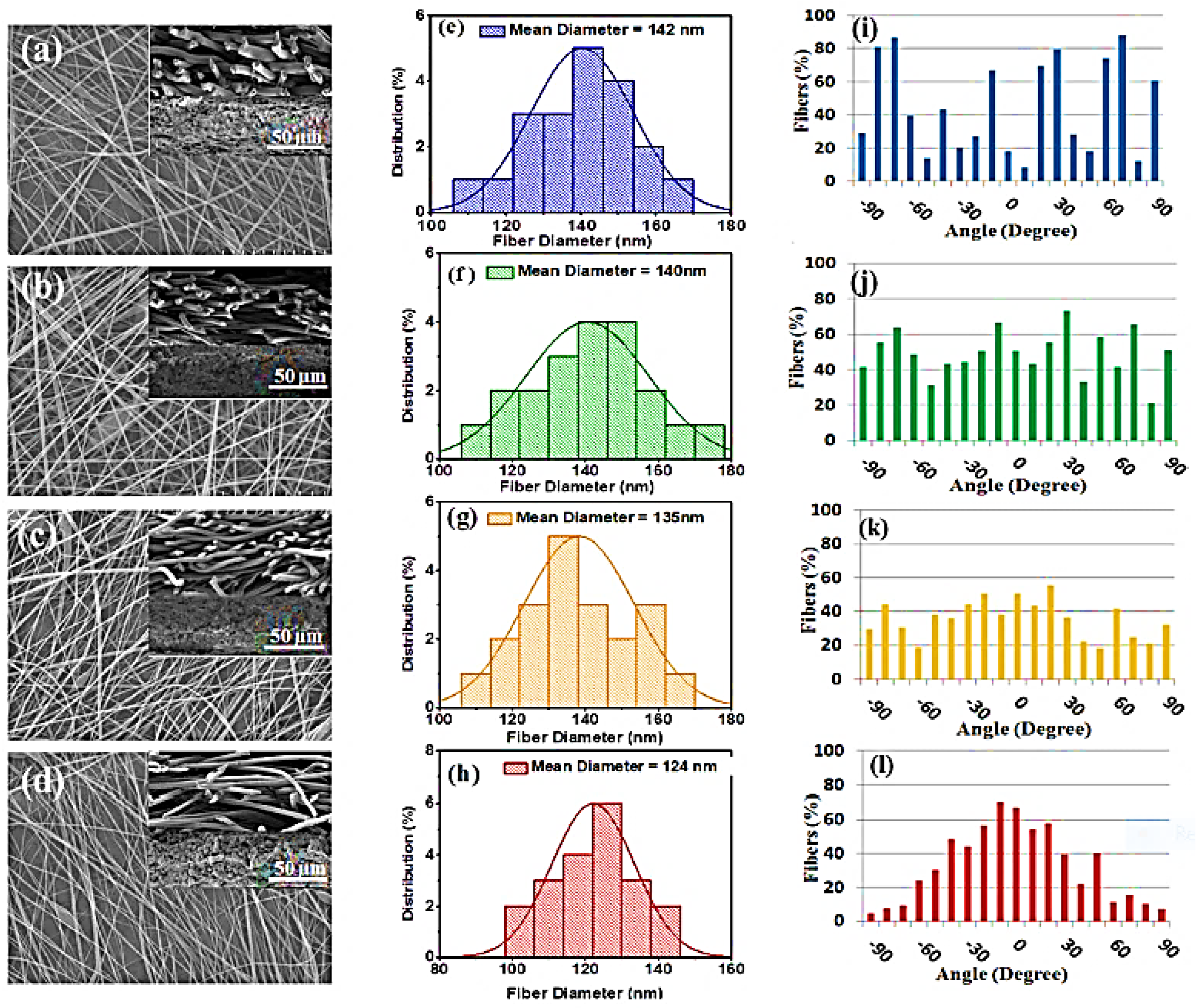
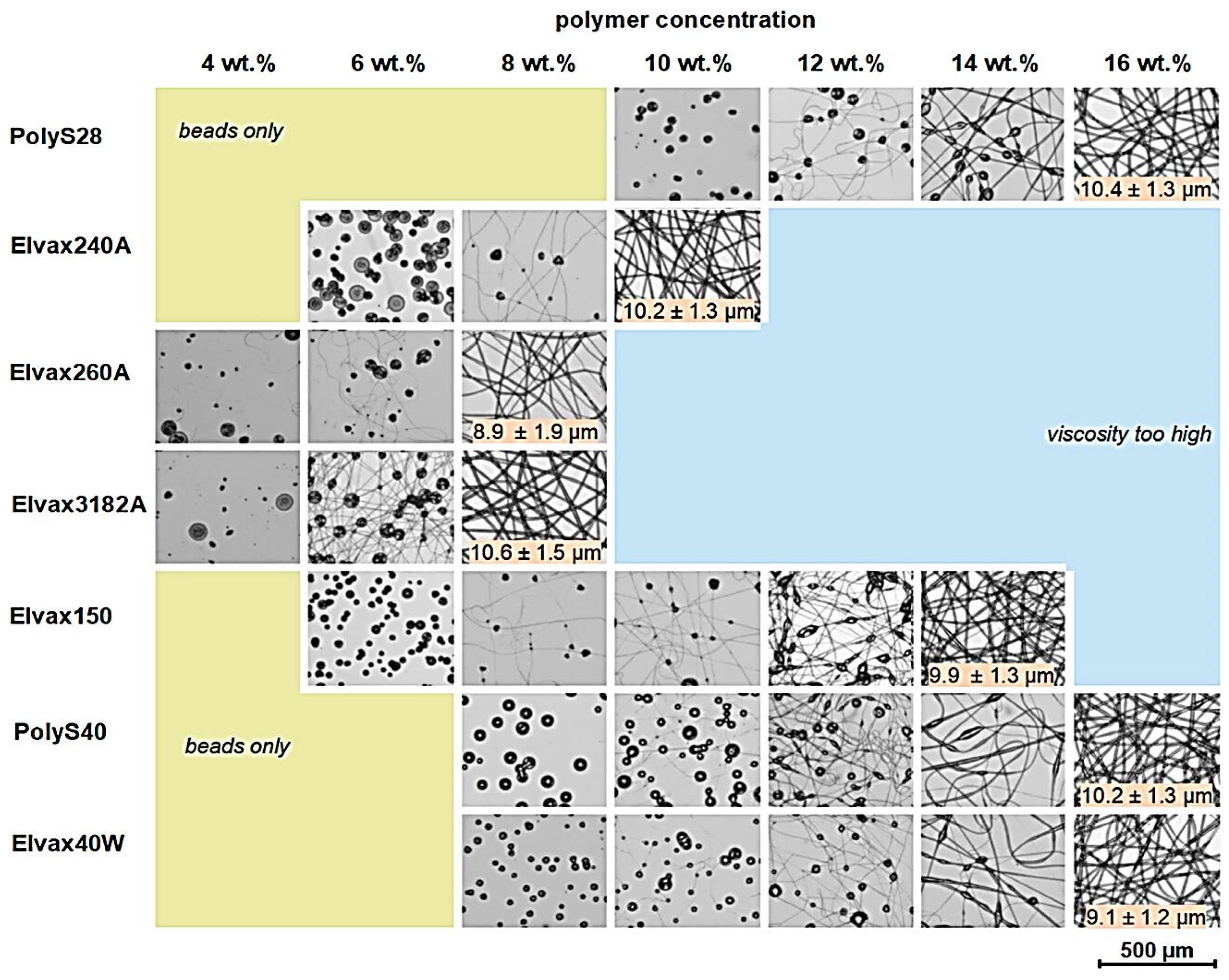
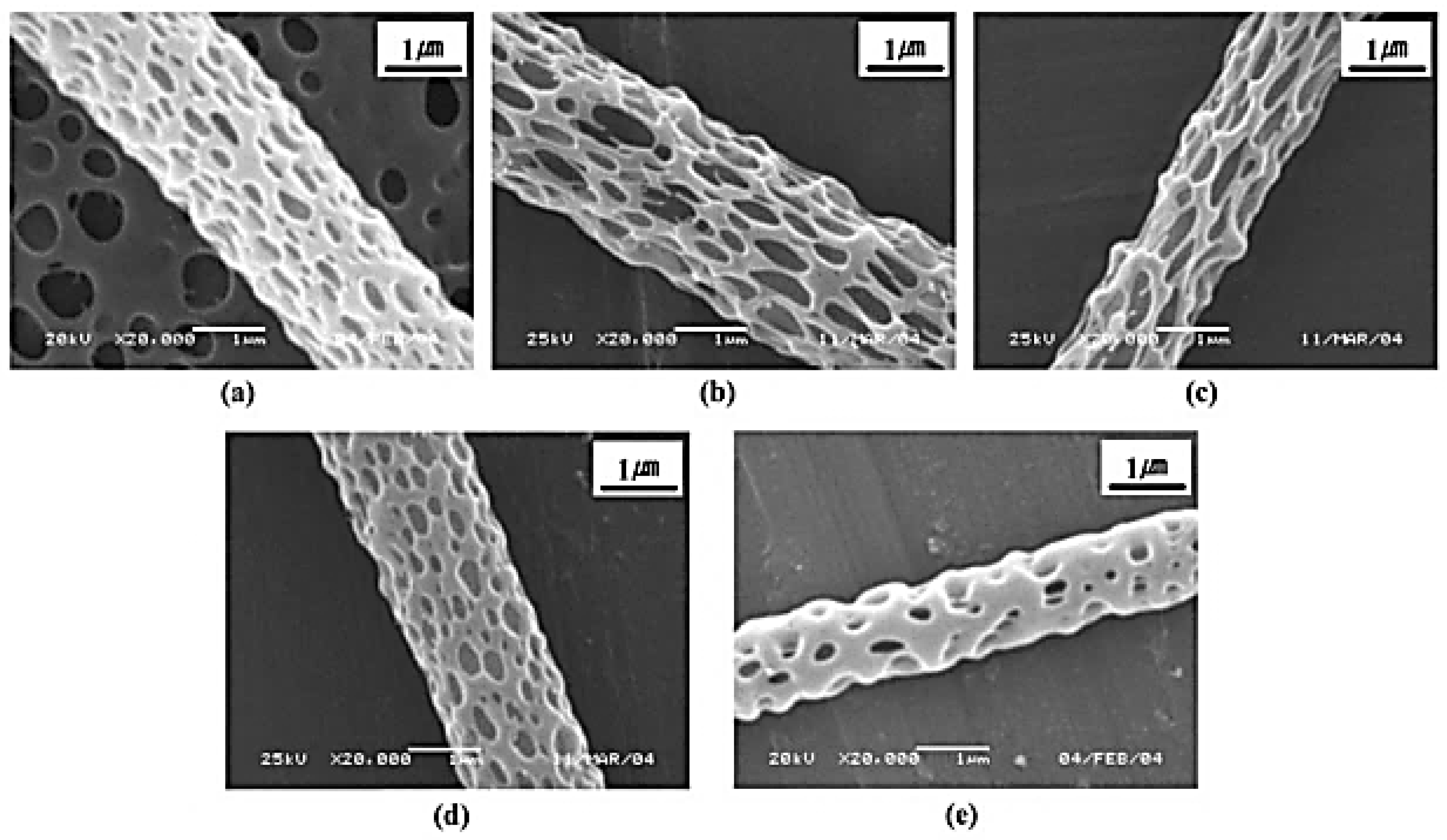
| Mode of Electrospinning | Fiber Size | Key Factors | Benefits | Drawbacks |
|---|---|---|---|---|
| Solution electrospinning | 5 to 500 nm | Solubility of solution and properties, static voltage, needle-tip-to-collector distance | Versatile, cost-effective, easy-to-operate energy storage, and filtration applications | Environmental pollution, solvent availability |
| Melt electrospinning | ≥500 nm | Viscosity of melt, static voltage, ambient temperature | Ecofriendly, safety, biomedical applications | Complicated device, expensive, large diameter of fibers |
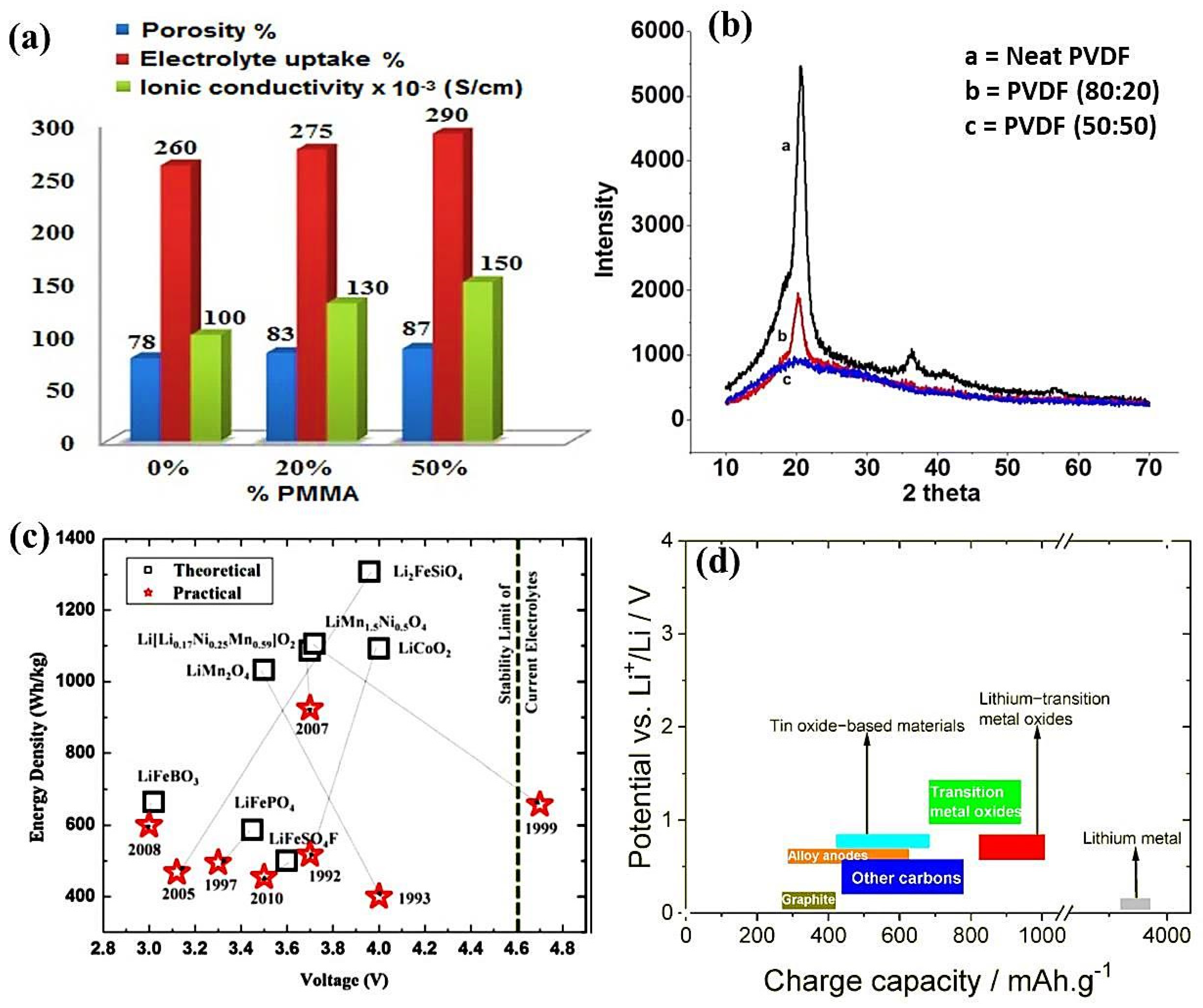
| Membrane Blend Composition | Melting Enthalpy ΔHf (J/g) | Crystallinity Xc (%) | Bulk Resistance Rb (Ω) | Ionic Conductivity σ (S/cm) |
|---|---|---|---|---|
| PVDF (100/0) | 53.29 | 50.75 | 0.50 | 0.10 |
| PVDF/PMMA (80/20) | 43.84 | 41.75 | 0.29 | 0.13 |
| PVDF/PMMA (50/50) | 25.33 | 24.12 | 0.22 | 0.15 |
| Battery Type | Cyclic Life | Energy Eff (%) | Installation Cost (USD/kWh) | Environmental Influences | Response Time | Discharge Behavior | Installation and Maintenance Cost (Approx USD/kWh) |
|---|---|---|---|---|---|---|---|
| Lead–acid | 500 | 45 | 5500 | Moderate | Good | Bad | 3860 |
| Nickel–cadium | 800 | 70 | 1700 | Moderate | Good | Bad | 2833 |
| Zinc–bromine | 2500 | 68 | 520 | Serious | Good | Good | 3191 |
| Sodium–sulfur | 3000 | 80–85 | 1000 | Moderate | Good | Good | 4639 |
| Lithium-ion | 2000 | 90–95 | 3000 | Slight | Good | Bad | 6346 |
| All-vanadim Redox flow | 13000 | 75–85 | 989 | slight | Good | Good | 1327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashif, M.; Rasul, S.; Mohideen, M.M.; Liu, Y. Advancing Electrochemical Energy Storage: A Review of Electrospinning Factors and Their Impact. Energies 2025, 18, 2399. https://doi.org/10.3390/en18092399
Kashif M, Rasul S, Mohideen MM, Liu Y. Advancing Electrochemical Energy Storage: A Review of Electrospinning Factors and Their Impact. Energies. 2025; 18(9):2399. https://doi.org/10.3390/en18092399
Chicago/Turabian StyleKashif, Muhammad, Sadia Rasul, Mohamedazeem M. Mohideen, and Yong Liu. 2025. "Advancing Electrochemical Energy Storage: A Review of Electrospinning Factors and Their Impact" Energies 18, no. 9: 2399. https://doi.org/10.3390/en18092399
APA StyleKashif, M., Rasul, S., Mohideen, M. M., & Liu, Y. (2025). Advancing Electrochemical Energy Storage: A Review of Electrospinning Factors and Their Impact. Energies, 18(9), 2399. https://doi.org/10.3390/en18092399